Abstract
Coronavirus Disease 2019 (COVID-19) is a recent public health issue worldwide. Also, diabetes is a frequent condition with high mortality. There is a strong relationship between COVID-19 and diabetes. This article analyses the intricate relationship between COVID-19 and hepcidin. Hepcidin increases in aged non-insulin diabetic patients. Hepcidin is the last target treatment of several medications commonly used. Viral diseases, especially SARS-CoV19, can activate the hepcidin pathway leading to an elevation in the iron load. This increased iron is released into the bloodstream and results in cell death through ferroptosis, like free iron. Excess iron has pro-coagulative and toxic effects. Hepcidin overexpression and iron overload are associated with COVID-19 infection and can be considered potential targets for treatment. Several studies have shown dalteparin (anti-Hepcidin) could improve the symptoms of COVID-19 in diabetics by appropriately modulating and decreasing oxidative stress and inflammation. This finding can be leading to enhancing the existing knowledge about Therapeutic measures for reducing Covid-19 impairments in diabetics and is suggested as a possible therapeutic agent in diabetes.
Keywords: Diabetes mellitus, COVID-19, Iron, Antihepcidin agents, Hyperferritinemic, Inflammation, Hepcidin peptide
Introduction
SARS-CoV-2 the reason of COVID-19 disease is belongs to coronavirus family. Several hypotheses and findings have been reported to indicate why the virus causing the COVID-19 outbreak is more deadly in some cases [1]. The disease burden of COVID-19 has been increasing and over one million confirmed cases and over 45 thousand deaths have been reported globally [2]. Because of the high prevalence of diabetes, understanding the special aspects of COVID-19 infection in diabetics is important. Diabetes and related complications enhance the risk of mortality and morbidity during acute infections because of inhibited innate and humoral immune functions. Diabetes in COVID-19 patients has correlated with COVID-19 severity and a two-fold increase in deaths than non-diabetics. So, more studies are needed on the pathogenic mechanisms, as well as therapeutic implications in diabetics. COVID-19 presents with many physiological and biochemical changes, including [3], elevated levels of CD4+ pro-inflammatory and CD8+ cytotoxic T cells [4], hemoglobin damage [5], increased liberation of cytokines (cytokine storm) [6], elevated coagulation state [7], and dysregulated iron homeostasis [8] like iron overload that could be as an important element in the COVID-19 pathogenesis [9, 10]. Liu and his colleagues suggested that the most important poisonous molecular step of coronavirus is attacking hemoglobin and detachment of the iron from heme to form porphyrins, as well as the release of iron into circulation. Therefore, hemoglobin can lose its capacity for binding to oxygen and hinder its transference to main organs resulting in damage to these organs. Also, excess free iron in the blood circulation can result in oxidative stress damage to the lungs and different organs. Moreover, the excess load of iron can cause inflammation and immune dysfunction [11, 12] which in turn results in elevated storage and uptake of iron by proteins binding to iron [13]. This fact is confirmed by ferritin levels elevation in the plasma of patients with COVID-19. Ferritin is the main iron storage molecule in the body [10]. Amplified iron load causes enhanced blood viscosity along with diffuse and recurrent macro and microcirculatory thrombosis; therefore, can be explained as one of the causes of unanticipated deterioration and death in some patients [14, 15]. In this review, we went through the medical literature around iron and hepcidin’s possible involvement in this context, which has indicated evidence of the probable virus interaction with iron metabolism and hepcidin [13]. According to the translation medicine view, in the current narrative review, based on the potential link between iron overload in COVID-19 and disease severity and/or mortality, we speculated a decrease in hepcidin levels with dalteparin (anti-hepcidin) can be a treatment strategy in covid-19 diabetic patients.
Hepcidin and COVID-19
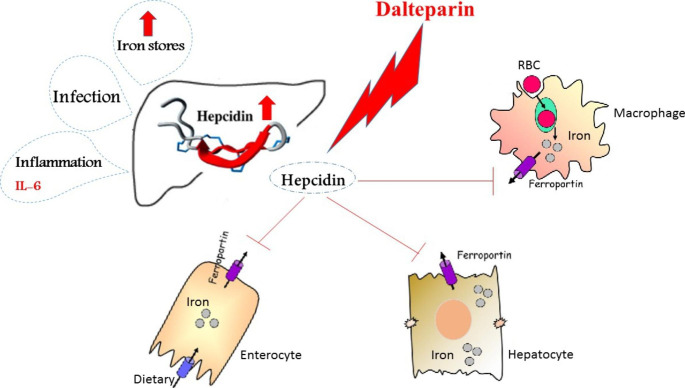
Hepcidin as an important iron regulative peptide can sequester iron in the macrophages and enterocytes, resulting in augmentation of ferritin in these cells, and so inhibiting iron outflow from them [16]. There is an association between elevated hepcidin and transferrin receptors in the blood and the prognosis of COVID-19. A recently published study showed a relationship between hyperferritinemia and COVID-19. Moreover, Sonnweber et al. have shown a link between elevated ferritin and proinflammatory cytokines mRNA expression in white blood cells in coronavirus affected patients. Also, their study indicated that cases with severe COVID-19 had higher concentrations of soluble transferrin receptor and hepcidin. Ehsani et al. have tried to answer a question about the possible similarity, and an evolutionary association between hepcidin and the viral spike protein in COVID-19 [17]. Recently, it was suggested that iron metabolism (lactoferrin and iron chelation, etc.) can regulate the treatment of COVID-19 [18]. As a result, we speculated high blood ferritin due to COVID-19-induced hyper inflammation implies a defective cycle of events, in which high amounts of ferritin maybe cause additional lesions in tissues (Fig. 1).
Fig. 1.
Effect of dalteparin on iron modulation
Viral intrusion and iron metabolism
Iron metabolism in an individual includes finely-tuning regulation of intestinal absorption, storage in the spleen and liver, transportation in circulation, iron usage (mostly for erythropoiesis in bone marrow), and recycle of iron by macrophages. Dietary Iron absorbs in enterocytes of the duodenum and is released into the blood by ferroportin, an iron transporter protein. The expression of ferroportin is done on the basolateral membrane of the duodenum cells, as well as in tissue-resident macrophages, and manages iron recycling and release [19]. Ferroportin-hepcidin axis plays a key role in the homeostasis of systemic iron. Hepatocytes express and secrete hepcidin that binds to ferroportin as its sole receptor. Following the binding of hepcidin, the internalization and degrading of ferroportin is done, resulting in the suppression of iron uptake in the duodenal cells and a decrease in the iron release from macrophages [20]. Iron absorption is happen upon the binding of transferrin-bound iron (TBI) and transferrin receptor 1(TfR1) [21]. Release of hepcidin is controlled by the accessibility of systemic iron (iron overload or deficiency), proinflammatory cytokines (IL-1β and IL-6), bone morphogenetic proteins (BMP6 and BMP2), as well as erythropoiesis signaling molecules (GDF15, ERFE, and TWSG1) [21]. Patients infected by SARS-CoV-2 have high amounts of inflammatory cytokines such as IFN-γ and IL1β, IL6, in serum, along with lung inflammation and considerable pulmonary impairment [22]. The coronavirus infection causes high levels of IFN-γ, IL1-β, interferon-inducible protein 10 (IP10 ), and monocyte chemotactic protein 1 (MCP1 ) which possibly induces T-helper-1 cell response [23]. Therefore, stimulation of such cytokines may induce hepcidin synthesis resulting in the accumulation of iron in macrophages; however, more studies are needed in this field. Notably, macrophages can be affected by coronavirus [24]. So, execs storage of iron may lead to viral replication within macrophages. Also, viruses are able to manage other iron-associated proteins to comfort replication and distribution itself. Regarding human cytomegalovirus (HCMV) infection HFE (Homeostatic Iron Regulator), a homeostatic iron regulator protein and an antagonist of TfR1 for binding to transferrin results in cellular iron overload [25]. In Human Immunodeficiency Viruses (HIV-1) infected macrophages the interaction between Nef (Negative Regulatory Factor) protein and HFE causes cellular iron overload [26]. Also, TfR1 is involved in the entry of many types of viruses [27].
COVID-19 and transferrin receptor
TfR is the main transferrin receptor and is homodimeric. TfR can facilitate the entrance of iron carried by transferrin into the cells. In general, TfR protein concentration in the lungs is significantly elevated in viral pneumonia. These observations may partly explain the vulnerability of lung tissue against inflammatory insults and can have implications for the Acute Respiratory Distress Syndrome (ARDS) pathogenesis, particularly regarding the role of iron [28]. The expression of TfR on cells facilitates the absorption of iron-loaded transferrin as well as the expression of cellular apo-ferritin for intracellularly storing iron [28]. At high levels of cellular iron, provocation of ferritin generation occurs along with the destabilization of TfR mRNA resulting in a decrease in TfR synthesis. On the contrary, at high iron levels, the reverse process is observed. The expression of proteins engaged in iron metabolism is controlled after transcription by IRP-1 and IRP-2 (iron regulatory proteins 1 and 2) [29]. Activated IRP is attached to iron-responsive elements in the noncoding areas of TfR and ferritin mRNA. IRP activation by ROS and iron signaling-independent inflammatory mediators are involved in iron regulation. Theoretically, several molecular mechanisms are involved in the iron metabolism of Covid-19 that increase ferritin over-production [30]. Moreover, the TfR is a proper portal for several viruses’ entrance into the cells which may be also considered in Covid-19 patients. However, in clinical ARDS, TfR protein levels have shown a significant increase in the lungs. A similar pathogenesis is expected in Covid-19 pneumonia [29].
In Covid-19 pneumonia, possibly there is a significant increase in iron levels in the lungs. Hepcidin can coordinate the level of iron bound to Tf as well as its amount entering the circulation[31]. The viral infection causes systemic iron loading with elevated Tf saturation, hepatic iron and serum ferritin because of the dysregulation of hepcidin production by the liver. Therefore, the cells in the reticuloendothelial system and the liver can collect iron overload during inflammation. Ferroportin exports iron from the cell and hepcidin arranged this process. Also, increased hepcidin decreases the level of available iron to attach to Tf, and conversely, a decrease in hepcidin is linked to increased Tf saturation. Higher hepcidin concentrations are linked to lower ferroportin concentrations, but the latter increases the intracellular iron storage of macrophages and hepatocytes [30]. Also, TfR can be considered as the gate of the virus. Indeed, blockage of TfR and antibody-mediated neutralization against TfR decreases the entry of SARS CoV-2into the cell [31].
The role of hepcidin in severity and mortality of COVID-19
The hallmark of the severity of COVID-19 is the activated innate immune system that is correlated with a hypercoagulable condition [32]. The COVID-19 clinical spectrum ranges from patients with no symptoms to respiratory failure. Inflammatory cytokines, particularly IL1β, IL6 and Tumor Necrosis Factor alpha (TNF-α), affect iron metabolism through the generation of hepcidin secreted by the liver that can limit iron accessibility by blockage of the iron export channel, ferroportin [33]. Increased hepcidin induced by inflammation leads to anemia through the reduction in plasma iron, red blood cell formation and hemoglobin synthesis impairment. [33]. Also, iron deficiency can affect immune system function and hypoxia sensing. Little is known about the potential role of disturbing iron homeostasis in COVID-19 [34, 35]. An investigation on 50 COVID-19 patients has indicated most of them had extremely low levels of serum iron, which was defined as a predictor of mortality [34]. Moreover, changes in iron metabolism were linked to hypoxemia in severe COVID-19 cases in the intensive care unit (ICU) [34, 36]. Hepcidin possibly indicates the association between COVID-19 severity and iron deficiency. Nonetheless, the role of hepcidin enhancement in the disruption of iron homeostasis in COVID-19 should be more studied [37]. In the mentioned study, blood sampling was done upon admission, followed by retrieving plasma immediately and freezing until analysis of the levels of iron and hepcidin as the main controller of iron homeostasis, as well as proinflammatory markers like ferritin and C-reactive protein (CRP), and also cytokines associated with hepcidin modulation like IL1β, IL6, TNFα and interferon-gamma (IFNγ). In 93.7% of patients, the iron level was below the normal range. Conversely, 61.3% of patients were found with significantly increased hepcidin levels [37]. However, because hypoferremia inhibits hepcidin expression, even hepcidin at normal ranges is improperly high in most patients. In addition, patients with higher hepcidin concentrations were markedly older and were found with higher levels of inflammation (ferritin and CRP) and cell damage markers. Interestingly, plasma hepcidin concentrations were associated with those of creatinine because of renal excretion of hepcidin. In general, several patients with severe COVID-19 have hypoferremia and showed inflammation-induced hepcidin upregulation [37]. Also, considering serious patients in ICU, elevated hepcidin levels can predict mortality, independent of lung function, age, inflammation, and tissue destruction. In coronavirus disease, hepcidin can be regarded as a sign of morbidity and severe unhealthy in patients admitted to ICU [37]. More studies are needed for verifying whether modulating hepcidin level can affect the disease consequences.
Iron and COVID-19 pathogenesis
Changed iron homeostasis followed by inflammation and oxidative stress is associated with COVID-19 pathogenesis [38], which indicates the possible role of changed iron homeostasis (characterized by hyperferritinemia) in the pathogenesis and management of COVID-19. Iron, as an essential trace element, is involved in oxygen transference and plays the role of an acceptor or electron giver in several biological functions. Ferritin is the main site of iron storage in the cell primarily in its ferric state (Fe3+). Ferritin is able to carry a maximum of 4500 iron molecules in its core [39]. In general, systemic inflammations are linked to elevated blood ferritin amounts. In a high-inflammation condition cytokines especially IL6 induces ferritin and hepcidin generation [40].
Lots of iron in the cell can interact with molecular oxygen, producing ROS [39], which causes oxidative damage to cellular components of many organs, like the liver, lungs, kidney, and heart. Increased ferritin concentrations are associated with different inflammatory pathologies, like cardiovascular events [41]. Also, a complex interaction between iron metabolism and reactive sulfur species (RSS) and reactive nitrogen species (RNS) besides ROS indicates the interplay between iron metabolism and reactive species interactome (RSI) [42]. Interestingly, in inflammatory diseases, ferroptosis as a type of programmed cell death is promoted by excess iron-dependent lipid peroxidation [43], affecting many organs including the liver, kidney, heart and lung [43]. Ferroptosis is involved in neurological disturbances, like cognitive impairment, anosmia and ageusia (taste and smell loss) as regular manifestations of COVID-19 [44]. Iron chelators as well as ferroptosis inhibitors showed protective effects through the inhibition of intracellular iron-related lipid peroxidation [38]. Iron overload is strongly involved in mitochondrial dysfunction [45] and microbiota dysbiosis (gut and lungs) [46]. Also, serum coagulability is highly considered in COVID-19 and is accredited as the main health threat in prone cases [47]. Regarding cellular iron overload, coagulopathy is a marker of iron poisoning. Oxidized iron can accelerate blood coagulation through interaction with proteins of the coagulation cascade. Coagulation and increased cardiac biomarkers in COVID-19 patients indicate high systemic inflammation with coagulation activation and vascular endothelial dysfunction which are determinants of mortality [48, 49]. Acute generation of large vessel strokes occurs in young adults with COVID-19 [50]. The inflammation in the blood vessel walls as well as changes in platelets mitochondria cab be driving thrombosis generation [47].
Acute Respiratory Distress Syndrome (ARDS) of COVID-19 and iron
ARDS is a hazardous lung disorder and a common complication of severe viral pneumonia, such as pneumonia due to SARS-CoV-2 [51]. This condition prevents adequate oxygen supply to the lungs leading to high mortality because of acute lung injury and respiratory disorders [52]. In fatal cases of human MERS-CoV, SARS-CoV-2 and SARS-CoV infections, patients show severe respiratory distress and need mechanical ventilation, and their histopathology findings also support ARDS[4]. Genetic susceptibility and inflammatory cytokines are associated with ARDS[53]. More than 40 candidate genes, such as IL-10, TNF, Angiotensin-converting enzyme 2 (ACE2) and vascular endothelial growth factor (VEGF) are linked to the outcome or development of ARDS[52]. High levels of serum IL6 and IL8 are associated with adverse outcomes of ARDS. These biomarkers propose a molecular elucidation for the severe ARDS as well as a potential treatment for ARDS after coronavirus infection [54].
High levels of iron in the lung are linked to a high risk of pulmonary injury [55]. Both acute and chronic lung injury disrupts the iron regulatory state in the lungs [56]. Alterations in oxygen saturation levels (hypoxia and hyperoxia) change the iron metabolic process and damage the lungs because the organs are very vulnerable to metal-related oxidative stress [55]. In lung injury, serious hypoxemia, elevated endothelial and epithelial permeability, high cytokine concentrations in the lungs, and alveolar neutrophil infiltration are observed [57]. ARDS is characterized by lung damage through endothelial activation as well as capillary membrane damage leading to a leak of proteins [58]. Iron can intensify this inflammatory lung injury through the combination of hydrogen peroxide and superoxide from ARDS with the ability of iron for catalyzing more toxic ROS [55]. Elevated serum ferritin is linked to ARDS progression[59]. After an increase in iron levels, ferritin enhances isolating reactive iron. Also, the production of ferritin (an acute reactive protein) is increased in the inflammatory response, which is owing to high tissue destruction and lysis [59]. Lavage fluid from ARDS patients has exhibited a high concentration of total and non-heme iron and also cellular content of transferrin and ferritin [60], indicating disrupted pulmonary iron homeostasis in ARDS [55].
COVID-19 and hyperferritinemia
Several COVID-19 patients with high plasma ferritin levels (> 300 µg/l) had a nine-fold further chance of death prior to discharge [7]. Hyperferritinemia is an index of the “hyperferritinemia syndromes” and occurred in severe COVID-19. Hyperferritinemia syndromes characterize many autoimmune diseases [61] and because of its immunomodulatory effects possibly play a pathogenic role. High circulating ferritin displays an acute phase response and is crucial to inflammation [56]. Ferritin is a crucial iron storage protein in the cell that has two subunits of H and L and their ratio are different by tissue type and cell physiological status. [62]. The origin of ferritin in the inflammatory state is unknown but in-vitro studies indicated that it is secreted by hepatocytes[63] and macrophages through the non-conventional pathway [64] which indicates the possible effect of ferritin elevation via macrophage activation in “hyperferritinemic syndromes” [3]. Also, in ARDS ferritin serum levels are associated with disease activity and also macrophage activation. a cohort study on 39 COVID-19 patients with elevated blood ferritin concentration showed a significant correlation with disease severity [51]. The death of hepatic cells is another main trait of a high level of serum ferritin in addition to its secretion during an inflammatory response. Following secretion, serum ferritin loses part of its iron content, leading to increased amounts of free iron[65]. The excess free iron in severe inflammatory conditions is deteriorative of the inflammatory reaction and especially induces a marked pro-coagulant state [65]. Coronavirus can display effects similar to hepcidin, resulting in high ferritin levels independent of the inflammatory reaction and increased risk of coagulation [66].
Iron and thrombosis
Lipinski and Pretorius reported that the free iron levels in the serum are associated with the nonenzymatic production of parafibrin [67]. Parafibrin is similar to fibrin and is an insoluble biomaterial that can promote inflammatory reactions in the arteries following deposition. Free iron in the blood can produce hydroxyl radicals for converting fibrinogen into fibrin clots; thus, iron is a key factor in different facets of pathological thrombosis, such as oxidative stress, thrombocytosis, and high erythrocytes viscosity [13]. A prior study reported excess iron is linked to a high risk of venous thromboembolism [68]. According to these studies, it is suggested that overburdened iron triggers hypercoagulation in patients with severe COVID-19.
Coronavirus proteins, iron dysregulation and hepcidin
The coronaviruses envelope commonly consists of three proteins including the membrane protein (M), the envelope protein (E), and the spike protein (S). M and E proteins are involved in virus assembly, while the S protein that composed of two subunits (S1 and S2) plays a key role in penetrating host cells and initiating infection via binding to host ACE2 receptors (S1) and membrane fusion (S2) [69, 70]. In addition to these structural proteins, at least eleven viral accessory proteins in coronavirus have roles during infection which aren’t still completely known. Some accessory proteins like ORF3b, ORF6, ORF7a, and ORF8 have been indicated to be important IFN-I inhibitors causing a disturbance in the patient immune response. Moreover, ORF3a is engaged in apoptosis while others like ORF9b and ORF9c affect cellular organelles resulting in the prevention of the antiviral response in infected cells [71].
So far, limited studies have been performed on the role of coronavirus proteins in iron disturbances. A proposed theory expresses that coronavirus proteins have direct interaction with hemoglobin (Hb) and simplify iron separation from the heme prosthetic group resulting in the loss of functional hemoglobin, and iron accumulation. This opinion suggests ORF1ab, ORF10, and ORF3a are evolved from infected blood cells and work in harmony to eliminate heme from the beta chain of hemoglobin, dissociate iron from heme, and detach the resulting iron-free protoporphyrin IX (PPIX) [72].
Besides the above-mentioned roles of excess iron in oxidative damage and acute inflammation, iron is a key cofactor for multiple proteins and enzymes which have a role in the basic functions of viruses in the cells such as energy production, DNA replication and transcription. Coronavirus needs iron as regards it needs the patient metabolic systems to replicate its genome and produces mRNAs for their translation into functional viral proteins. So, while cellular iron repletion can enhance viral replication and propagation, the deficit of iron can disturbs the viral life cycle [73, 74].
Ehsani has indicated a unique sequence similarity between SARS-CoV-2 S protein and mature hepcidin and suggested that this similarity can be a potential indication for the probable iron disturbance in COVID-19 patients [17]. Coronavirus spike protein has hepcidin-like action, namely, the virus can directly increase ferritin amounts independent of the inflammatory effect. Hepcidin-mimetic action of SARS-CoV-2 may induce ferroportin blockade, which leads to progressive anemia and hyperferritinemia [75].
On the other hand, primary findings on hepcidin have shown its inhibitory effect on the binding affinity of the S protein toward the ACE2 protein [76]. Therefore, in addition to its role in regulating iron levels (which through this reduces inflammatory reactions), hepcidin can directly reduce coronavirus infectivity by inhibiting its entry into the cell and so ameliorate the complications of infection with the virus.
Although these findings are in preliminary stages can provide a perspective for studies in the coronavirus research field with respect to prior-confirmed investigations on the collaboration of coronavirus proteins, iron regulation, inflammatory processes, pulmonary infections and the hepcidin protein.
Hepcidin levels and diabetes mellitus
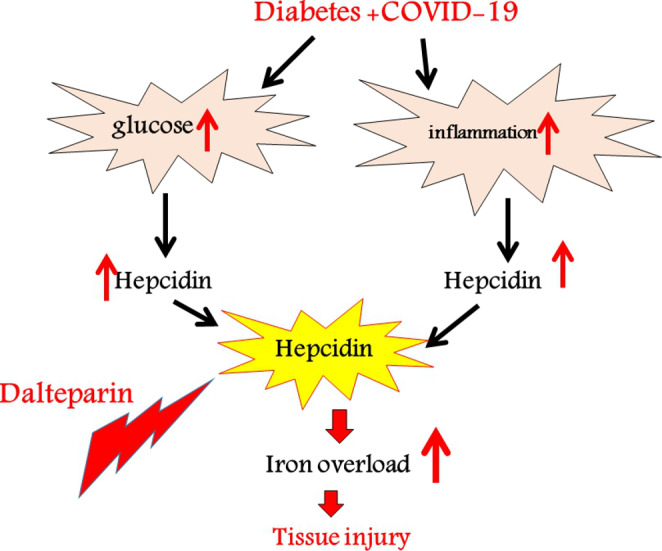
The association between inflammation and Fe stores in diabetics was evaluated. High levels of hsCRP and ferritin in diabetics were found. Ferritin is a protein reflecting body iron stores and is an acute-phase protein with high levels in inflammatory conditions [77]. Increased level of ferritin is a component of the IR syndrome and are associated with the onset of diabetes [78]. High ferritin concentrations can reflect high circulating Fe levels. High levels of Fe can change hepatic insulin clearance, leading to hyperinsulinemia which in turn induces a decrease in insulin secretion of the pancreas [79]. β cells’ hyperactivity finally leads to their apoptosis [80]. Disturbed Fe status besides increased Oxidative stress (OS) levels is a risk factor for T2DM development and is involved in the onset of its complications. High ferritin and TBARS concentrations, and increased hepcidin expression can increase the risk of diabetes development. However, inflammation in diabetics is independent of adiposity, thus, the origin of inflammation can be owing to high levels of glucose and/or OS in diabetics [78]. In addition, high mortality due to COVID-19 infection is linked to diabetes, high BMI, age and hypertension. Iron and hepcidin play an important role in these conditions indicating their potential as targets for therapy [81]. Also, Battaille et al. have declared elevated blood ferritin associated with the infection, even in patients with no symptoms and negative CRP, suggesting this factor as an appropriate marker for coronavirus disease [82]. In the same direction, Connelly et al. reported elevated blood ferritin concentrations as a predictor of ARDS as one of the major complications of COVID-19 [83]. Dixon in 2012 described ARDS as “Ferroptosis happens” [84], and recently Abbas et al. assessed this phenomenon in COVID-19 patients and as a result, they suggested iron chelation as an appropriate adjunctive therapy for treating these patients [85]. This can be considered a new type of cell death that varies from necrosis, apoptosis, or autophagy and results from iron-dependent lipid peroxidation. It is determined by mitochondrial shrinkage due to excess iron in cells, which is associated with hepcidin expression.
Also, Huang et al. declared very high ischemia-related redox-active iron mobilization in lung injury is of accumulation of the intracellular iron in the vascular space [86]. Excess iron in the vascular space can increase the production of devastating extracellular ROS. Cao (2018) announced the suppression of the ferroptosis process by regulating TLR4 as the main factor linked to hepcidin generation. Also, Sauler reported that ferroptosis is associated with iron presence [87]. Iron affects pulmonary injury in lung transplantation, and using iron-depleting therapy is a potential preventive measure for lung allograft [88]. Lagan proposed a distinction in genetics in ferritin light-chain gene genotype, which confers vulnerability to ARDS, whereas introducing the hemeoxygenase-2 (HO-2) haplotype as a preventive one [89]. Accordingly, we can consider the opinion suggesting that free iron can bind to oxygen, in an oxidoreductase reaction leading to a decrease in the arterial oxygen pressure. Some studies introduced deferoxamine as a possible therapy for coronavirus disease [90] because of the evidence regarding its effect on enterovirus-71 (EV-71) infection [91] through enhancing β-cell concentrations and fatality in infected mice. Therefore, COVID-19 affects iron through the hepcidin pathway. Hepcidin overexpression and iron overload are important in COVID-19 and can be possible targets for treatment. Hepcidin can be regarded as a biomarker for measuring the efficacy of treatment and modulating iron homeostasis as the final goal. Thus, in diabetes, many factors operate indirectly or directly on hepcidin levels and can affect the effectiveness of present therapies.
Low molecular weight heparin (LMWHs)
Heparin and low molecular weight heparins (LMWHs) have been applied to prevent and treat thromboembolic disorders [92]. Besides their anticoagulant properties, during the last ten years, the non-anticoagulant application of heparins has been considered for treating disorders that are outside thrombolytic diseases [93]. Their generic mechanism can be their capability of regulating inflammations [94]. The non-anticoagulant impacts of heparins offer opportunities to develop new heparin-based agents for treating several disorders. The main disadvantage of heparins is their pleiotropic effects. Also, LMWHs are characterized by a longer half-life, more favorable bio-availability, and a more pharmacokinetic response compared to Ultra Fractionated Heparin (UFH) [95]. LMWHs are able to reduce problems caused by full-length heparin and can be used in non-anticoagulant clinical settings. Both animal and human investigations have indicated that commercially existing LMWHs show remarkable antimetastatic [96], anti-inflammatory [97], and anti-fibrotic effects [98]. Clinical trials propose LMWHs as an appropriate candidate for different inflammatory disorders. For instance, pediatric and diabetic patients subjected to cataract surgery were given LMWHs to decrease the risk of inflammatory syndrome [99]. In cases with stable chronic obstructive pulmonary disease (COPD), LMWHs as an add-on therapy could improve dyspnea and blood gas tension, and also decreased salbutamol dosage [100]. Low levels of cytokines were found in cases treated with LMWHs. In clinical trials, LMWHs were effective in disorders with a complex pathology, such as lichen planus and diabetic foot ulcers [95, 101].
Antifibrotic effects of LMWHs
Fibrogenesis as a progressive and dynamic process involves complex cellular and molecular mechanisms related to inflammation, such as inflammatory damage, matrix deposition, iterative injury, and excessive tissue repair [102]. LMWHs play different roles in chronic liver fibrosis. They interact with profibrogenic and pro-inflammatory mediators and develop fibrosis [103]. LMWHs interact with platelet-derived growth factor (PDGF) that is released from aggregated platelets resulting in more tissue damage. They also interfere in the interaction between VEGF receptor 2 (VEGFR-2) and vascular endothelial growth factor (VEGF) in the angiogenesis process. They also prevent fibrosis by inhibiting the production of inflammatory cytokines, like HIF-1, TGF-1, and VEGF [104]. Through targeting such mechanisms involved in the development of fibrosis, LMWHs inhibit or delay the activation/proliferation of fibroblasts [105]. In contrast to other fibrogenesis diseases, liver fibrosis has an intrinsic capacity for remodeling and recovery. LMWHs can upregulate the hepatocyte growth factor leading to increasing hepatic repair, as well as reducing hepatic fibrogenesis [95, 106].
Dalteparin as anti –hepcidin
Dalteparin (Fragmin) is heparin with a low molecular weight that has several biological activities in addition to its anticoagulant effects, such as anti-inflammatory, antitumor and anti-hypertensive, effects [107, 108]. Heparin suppresses in-vitro and in-vivo hepcidin expression [109]. Heparin and its derivates (UFH and LMWH) have anti-inflammatory effects and reduce pro-inflammatory cytokines such as IL1 and IL6. IL6 regulates hepcidin synthesis, and dalteparin is able to suppress IL-6 concentrations in inflammatory conditions leading to a decrease in the elevated hepcidin. There is an increase in both local and/or systemic hepcidin during inflammation that results in iron overload [110]. Hepcidin can act as a double-edged sword by reducing intercellular iron overload via iron transportation into intracellular space to combat inflammation[111].
Conclusions
Diabetes is linked to the severity and mortality of COVID- 19. The effects of diabetes on viral entry into cells and inflammatory reaction against the infection have been reported. Similar to most viral infections, COVID-19 can act differently in the iron-hepcidin pathway. The complex interplay among hepcidin, iron, and other related molecules should be evaluated to clarify the relationship between the coronavirus and this process. Excess iron due to hepcidin overproduction is involved in COVID-19 disease, and such overexpression is harmful instead of protective and can be the etiology of many coronavirus symptoms. Blood hepcidin and ferritin measures can be regarded as an indicator of the intensity of infection, and iron modulation via hepcidin reduction can be a key path for improving complications of diabetics with coronavirus disease.
Abbreviations
- COVID-19
Coronavirus Disease 2019.
- ROS
Reactive oxygen species.
- TfR1
Transferrin-bound iron and transferrin receptor 1.
- BMP6 and BMP2
Bone morphogenetic proteins 6 and 2.
- IP10
Interferon-inducible protein 10.
- MCP1
Monocyte chemotactic protein 1.
- HCMV
Human cytomegalovirus.
- HIR
Homeostatic Iron Regulator.
- Nef
Negative Regulatory Factor.
- HIV-1
Human Immunodeficiency Viruses.
- IRP1 and IRP2
Iron regulatory proteins 1 and 2.
- CRP
C-reactive protein.
- IFNγ
Interferon-gamma.
- RSS
Reactive sulfur species.
- RNS
Reactive nitrogen species.
- RSI
Reactive species interactome.
- ARDS
Acute Respiratory Distress Syndrome.
- VEGF
Avascular endothelial growth factor.
- VEGFR-2
VEGF receptor 2.
- OS
Oxidative stress.
- ALI
Acute lung injury.
- LMWHs
Low molecular weight heparins.
- UFH
Ultra-Fractionated Heparin.
- COPD
Chronic obstructive pulmonary disease.
- PDGF
Platelet-derived growth factor.
Author contributions
zeinivand contributed to conceptualization and data collection, Jamali-raeufy and Zavari contributed by proofreading, arranging data, and reference.
Funding
This work did not have any financial support.
Availability of data and materials
Not applicable (data transparency).
Code availability
Not applicable.
Declarations
Conflict of interest
Authors declare they have no competing interests.
Ethical approval
Not applicable. This work is a review study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69(9):1857–63. doi: 10.2337/dbi19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsarou A, Pantopoulos K. Hepcidin therapeutics. Pharmaceuticals. 2018;11(4):127. doi: 10.3390/ph11040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colafrancesco S, Priori R, Alessandri C, Astorri E, Perricone C, Blank M, et al. sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res. 2014;60(2–3):177–83. doi: 10.1007/s12026-014-8563-7. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published online ahead of print February 18, 2020]. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed]
- 5.Lansiaux E, Pébaÿ PP, Picard J-L, Son-Forget J. COVID-19: beta-thalassemia subjects immunised? Med Hypotheses. 2020;142(109827):1–5. doi: 10.1016/j.mehy.2020.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Geng M, Peng Y, Meng L. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–8. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–62. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Zhang X, Ju Z, He W. Research progress on the mechanism of cytokine storm induced by new coronavirus pneumonia and related immunotherapy [J/OL] Chin j burns. 2020;36(0):E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg MF, Goldberg MF, Cerejo R, Tayal A. Cerebrovascular disease in COVID-19. Am J Neuroradiol. 2020;41(7):1170–2. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira AC, Mesquita G, Gomes MS. Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms. 2020;8(4):589. doi: 10.3390/microorganisms8040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–19. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021:111228. [DOI] [PMC free article] [PubMed]
- 14.Gardenghi G. Pathophysiology of worsening lung function in COVID-19. Revista Brasileira de Fisiologia do Exercí cio. 2020;19(2):40–6. [Google Scholar]
- 15.Rawat M, Chandrasekharan P, Hicar MD, Lakshminrusimha S. COVID-19 in newborns and infants—low risk of severe disease: silver lining or dark cloud? Am J Perinatol. 2020;37(08):845–9. doi: 10.1055/s-0040-1710512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao G, Li J, Zhang Y, Chang Y-Z. Cellular iron metabolism and regulation. Brain Iron Metabolism and CNS Diseases. 2019:21–32. [DOI] [PubMed]
- 17.Ehsani S. Distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein: a potential hint at the possibility of local iron dysregulation in COVID-19. arXiv preprint arXiv:200312191. 2020. [DOI] [PMC free article] [PubMed]
- 18.Yilmaz N, Eren E. COVID-19 and iron homeostasis: from hypothesis to evidence: Castleman disease anemia of inflammation and hepcidin. 2020.
- 19.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18(2):394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Sun B, Yin H, Liu S. Hepcidin: a promising therapeutic target for iron disorders: a systematic review. Medicine. 2016;95(14). [DOI] [PMC free article] [PubMed]
- 21.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Paniagua M, Cartmel D, Dominguez C. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS. 2020. [DOI] [PMC free article] [PubMed]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arieh SV-B, Laham N, Schechter C, Yewdell JW, Coligan JE, Ehrlich R. A single viral protein HCMV US2 affects antigen presentation and intracellular iron homeostasis by degradation of classical HLA class I and HFE molecules. Blood. J Am Soc Hematol. 2003;101(7):2858–64. doi: 10.1182/blood-2002-07-2158. [DOI] [PubMed] [Google Scholar]
- 26.Drakesmith H, Chen N, Ledermann H, Screaton G, Townsend A, Xu X-N. HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proceedings of the National Academy of Sciences. 2005;102(31):11017-22. [DOI] [PMC free article] [PubMed]
- 27.Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446(7131):92–6. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz N, Eren E. Covid-19 and Iron Gate: The role of transferrin, transferrin receptor and hepcidin. 2020. Presentation: https://www.researchgatenet/publication/340860987. 2020.
- 29.Wessling-Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu Rev Nutr. 2018;38:431–58. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eren E, Yilmaz N. Diagnostic benefits of Clinical Laboratory data determinations for severe COVID-19 patients. 2020.
- 31.Eren NYE. Covid-19 and Iron Gate: The Role of Transferrin and Transferrin Receptor. 2020.
- 32.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–98. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah A, Frost JN, Aaron L, Donovan K, Drakesmith H. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia J-j, Wang F, Jiang X-n, Jiang T-t, Shen L-j, Liu Y, et al. Serum iron levels are an independent predictor of in-hospital mortality of critically ill patients: a retrospective, single-institution study. J Int Med Res. 2019;47(1):66–75. doi: 10.1177/0300060518795528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8):2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nai A, Lorè NI, Pagani A, De Lorenzo R, Di Modica S, Saliu F, et al. Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol. 2021;96(1):E32-E5. doi: 10.1002/ajh.26027. [DOI] [PubMed] [Google Scholar]
- 38.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–9. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748–73. doi: 10.1039/c3mt00347g. [DOI] [PubMed] [Google Scholar]
- 40.Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. La Presse Médicale. 2017;46(12):e272-e8. doi: 10.1016/j.lpm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortese-Krott MM, Koning A, Kuhnle GG, Nagy P, Bianco CL, Pasch A, et al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27(10):684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouault TA. Mitochondrial iron overload: causes and consequences. Curr Opin Genet Dev. 2016;38:31–7. doi: 10.1016/j.gde.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yilmaz B, Li H. Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals. 2018;11(4):98. doi: 10.3390/ph11040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun rev. 2020;19(7):102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–72. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 53.Meyer NJ, Christie JD, editors. Genetic heterogeneity and risk of acute respiratory distress syndrome. Semin Respir Crit Care Med; 2013: Thieme Medical Publishers. [DOI] [PubMed]
- 54.Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the coronavirus disease 2019 (COVID-19) susceptibility. Clin Infect Dis. 2021;73(2):328–31. doi: 10.1093/cid/ciaa1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Wessling-Resnick M. The role of iron metabolism in lung inflammation and injury. Journal of allergy & therapy. 2012;3(Suppl 4). [DOI] [PMC free article] [PubMed]
- 56.Vickers NJ. Animal communication: when i’m calling you, will you answer too? Curr Biol. 2017;27(14):R713-R5. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 57.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiology-Lung Cell Mol Physiol. 2008;295(3):L379-L99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 59.Khiroya H, Turner AM. The role of iron in pulmonary pathology. Multidisciplinary respiratory medicine. 2015;10(1):1–7. doi: 10.1186/s40248-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit Care Med. 2003;31(2):395–400. doi: 10.1097/01.CCM.0000050284.35609.97. [DOI] [PubMed] [Google Scholar]
- 61.Zandman-Goddard G, Shoenfeld Y. Hyperferritinemia in autoimmunity. Isr Med Association J. 2008;10(1):83. [PubMed] [Google Scholar]
- 62.Mesquita G, Silva T, Gomes AC, Oliveira PF, Alves MG, Fernandes R, et al. H-Ferritin is essential for macrophages’ capacity to store or detoxify exogenously added iron. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-59898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103(6):2369–76. doi: 10.1182/blood-2003-09-3050. [DOI] [PubMed] [Google Scholar]
- 64.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang D-l, Crooks DR, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood The Journal of the American Society of Hematology. 2010;116(9):1574–84. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 65.Pretorius E, Kell DB. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr biology. 2014;6(5):486–510. doi: 10.1039/c4ib00025k. [DOI] [PubMed] [Google Scholar]
- 66.Cavezzi AE, Troiani, Corrao S. Clin Pract. 2020;10:1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipinski B, Pretorius E. Iron-induced fibrin in cardiovascular disease. Curr Neurovasc Res. 2013;10(3):269–74. doi: 10.2174/15672026113109990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benyamin B, Esko T, Ried JS, Radhakrishnan A, Vermeulen SH, Traglia M, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun. 2014;5(1):1–11. doi: 10.1038/ncomms5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet. 2020;395(10224):565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–20. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redondo N, Zaldívar-López S, Garrido JJ, Montoya M. Sars-cov-2 accessory proteins in viral pathogenesis: Knowns and unknowns. Front Immunol. 2021:2698. [DOI] [PMC free article] [PubMed]
- 72.Liu W. COVID LH. 19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism ChemRxiv 2020. Preimpresión https://doi org/1026434/chemrxiv. 2020;11938173:v7.
- 73.Schmidt SM. The role of iron in viral infections. Front Biosci. 2020;25(4):893–911. doi: 10.2741/4839. [DOI] [PubMed] [Google Scholar]
- 74.Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, et al. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proceedings of the National Academy of Sciences. 2014;111(33):12187-92. [DOI] [PMC free article] [PubMed]
- 75.Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):24–30. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Paola L, Hadi-Alijanvand H, Song X, Hu G, Giuliani A. The discovery of a putative allosteric site in the SARS-CoV-2 spike protein using an integrated structural/dynamic approach. J Proteome Res. 2020;19(11):4576–86. doi: 10.1021/acs.jproteome.0c00273. [DOI] [PubMed] [Google Scholar]
- 77.Lee B-K, Kim Y, Kim Y-I. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011;60(10):1416–24. doi: 10.1016/j.metabol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Andrews M, Soto N, Arredondo-Olguín M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition. 2015;31(1):51–7. doi: 10.1016/j.nut.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 79.Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011. [DOI] [PMC free article] [PubMed]
- 80.Spahis S, Borys J-M, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2017;26(9):445–61. doi: 10.1089/ars.2016.6756. [DOI] [PubMed] [Google Scholar]
- 81.Banchini F, Vallisa D, Maniscalco P, Capelli P. Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Bio Medica: Atenei Parmensis. 2020;91(3):e2020013. doi: 10.23750/abm.v91i3.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bataille S, Pedinielli N, Bergounioux J-P. Could ferritin help the screening for COVID-19 in hemodialysis patients? Kidney Int. 2020;98(1):235–6. doi: 10.1016/j.kint.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Connelly KG, Moss M, Parsons PE, Moore EE, Moore FA, Giclas PC, et al. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155(1):21–5. doi: 10.1164/ajrccm.155.1.9001283. [DOI] [PubMed] [Google Scholar]
- 84.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abbas A, Mostafa A, Yousof E, Ali S. Use of iron chelators to reduce the severity of COVID-19. Thromb Haemost. 2020;4:1042. [Google Scholar]
- 86.Huang Y, Ghio AJ, Nozik-Grayck E, Piantadosi CA. Vascular release of nonheme iron in perfused rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L474-L81. doi: 10.1152/ajplung.2001.280.3.L474. [DOI] [PubMed] [Google Scholar]
- 87.Sauler M, Bazan IS, Lee PJ. Cell death in the lung: the apoptosis–necroptosis axis. Annu Rev Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pugh C, Hathwar V, Richards JH, Stonehuerner J, Ghio AJ. Disruption of iron homeostasis in the lungs of transplant patients. J heart lung transplantation. 2005;24(11):1821–7. doi: 10.1016/j.healun.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 89.Lagan AL, Quinlan GJ, Mumby S, Melley DD, Goldstraw P, Bellingan GJ, et al. Variation in iron homeostasis genes between patients with ARDS and healthy control subjects. Chest. 2008;133(6):1302–11. doi: 10.1378/chest.07-1117. [DOI] [PubMed] [Google Scholar]
- 90.Dalamaga M, Karampela I, Mantzoros CS. Commentary: Could iron chelators prove to be useful as an adjunct to COVID-19 Treatment Regimens? Metabolism. 2020;108:154260. doi: 10.1016/j.metabol.2020.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams A, Meyer D. Desferrioxamine as immunomodulatory agent during microorganism infection. Curr Pharm Des. 2009;15(11):1261–8. doi: 10.2174/138161209787846801. [DOI] [PubMed] [Google Scholar]
- 92.Gray E, Hogwood J, Mulloy B. The anticoagulant and antithrombotic mechanisms of heparin. Heparin-A century of progress. 2012:43–61. [DOI] [PubMed]
- 93.Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discovery. 2002;1(2):140–8. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- 94.Page C. Heparin and related drugs: beyond anticoagulant activity. International Scholarly Research Notices. 2013;2013.
- 95.Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, et al. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr Polym. 2017;160:71–81. doi: 10.1016/j.carbpol.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 96.Sanford D, Naidu A, Alizadeh N, Lazo-Langner A. The effect of low molecular weight heparin on survival in cancer patients: an updated systematic review and meta‐analysis of randomized trials. J Thromb Haemost. 2014;12(7):1076–85. doi: 10.1111/jth.12595. [DOI] [PubMed] [Google Scholar]
- 97.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015. [DOI] [PMC free article] [PubMed]
- 98.Obi AT, Diaz JA, Ballard-Lipka NL, Roelofs KJ, Farris DM, Lawrence DA, et al. Low-molecular-weight heparin modulates vein wall fibrotic response in a plasminogen activator inhibitor 1-dependent manner. J Vascular Surgery: Venous Lymphatic Disorders. 2014;2(4):441–50. doi: 10.1016/j.jvsv.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.İlhan Ö, İlhan N, Coşkun M, Dağlioğlu MC, Tuzcu EA, Ayintap E, et al. The effect of enoxaparin-containing irrigation fluid used during cataract surgery on postoperative inflammation in patients with diabetes. Am J Ophthalmol. 2013;156(6):1120–4. doi: 10.1016/j.ajo.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 100.Brown RA, Allegra L, Matera MG, Page CP, Cazzola M. Additional clinical benefit of enoxaparin in COPD patients receiving salmeterol and fluticasone propionate in combination. Pulm Pharmacol Ther. 2006;19(6):419–24. doi: 10.1016/j.pupt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Kalani M, Apelqvist J, Blombäck M, Brismar K, Eliasson B, Eriksson JW, et al. Effect of dalteparin on healing of chronic foot ulcers in diabetic patients with peripheral arterial occlusive disease: a prospective, randomized, double-blind, placebo-controlled study. Diabetes Care. 2003;26(9):2575–80. doi: 10.2337/diacare.26.9.2575. [DOI] [PubMed] [Google Scholar]
- 102.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kukner A, Tore F, Firat T, Terzi EH, Oner H, Balaban YH, et al. The preventive effect of low molecular weight heparin on CCL4-induced necrosis and apoptosis in rat liver. Ann Hepatol. 2010;9(4):445–54. [PubMed] [Google Scholar]
- 104.Li J, Guo ZY, Gao XH, Bian Q, Jia M, Li Lai X, et al. Low molecular weight heparin (LMWH) improves peritoneal function and inhibits peritoneal fibrosis possibly through suppression of HIF-1α, VEGF and TGF-β1. PLoS ONE. 2015;10(2):e0118481. doi: 10.1371/journal.pone.0118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abe W, Ikejima K, Lang T, Okumura K, Enomoto N, Kitamura T, et al. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol. 2007;46(2):286–94. doi: 10.1016/j.jhep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 106.Lee J-H, Lee H, Joung YK, Jung KH, Choi J-H, Lee D-H, et al. The use of low molecular weight heparin–pluronic nanogels to impede liver fibrosis by inhibition the TGF-β/Smad signaling pathway. Biomaterials. 2011;32(5):1438–45. doi: 10.1016/j.biomaterials.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 107.Elsayed E, Becker RC. The impact of heparin compounds on cellular inflammatory responses: a construct for future investigation and pharmaceutical development. J Thromb Thrombolysis. 2003;15(1):11–8. doi: 10.1023/a:1026184100030. [DOI] [PubMed] [Google Scholar]
- 108.Farajdokht F, Nahavandi A, Soleimani M. Effects of dalteparin on structure of hippocampal neurons of rats in chronic stress. Basic and Clinical Neuroscience. 2012;3(3):32–7. [Google Scholar]
- 109.Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, et al. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood The Journal of the American Society of Hematology. 2011;117(3):997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]
- 110.Zhang F-L, Hou H-M, Yin Z-N, Chang L, Li F-M, Chen Y-J, et al. Impairment of hepcidin upregulation by lipopolysaccharide in the interleukin-6 knockout mouse brain. Front Mol Neurosci. 2017;10:367. doi: 10.3389/fnmol.2017.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Y, Xin Z, Li N, Chang S, Chen Y, Geng L, et al. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic Biol Med. 2018;124:1–11. doi: 10.1016/j.freeradbiomed.2018.05.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable (data transparency).
Not applicable.