Key Points
Question
Was earlier introduction of peanut to infants associated with a decrease in the population prevalence of peanut allergy?
Findings
This study that included 7209 infants compared 2 Australian cross-sectional samples before and after guidelines that recommended early peanut introduction. The prevalence of peanut allergy in the later vs earlier cohort was 2.6% vs 3.1%, a difference that was not statistically significant.
Meaning
Introduction of a guideline recommending early peanut introduction was not associated with a statistically significant change in prevalence of peanut allergy across the population.
Abstract
Importance
Randomized clinical trials showed that earlier peanut introduction can prevent peanut allergy in select high-risk populations. This led to changes in infant feeding guidelines in 2016 to recommend early peanut introduction for all infants to reduce the risk of peanut allergy.
Objective
To measure the change in population prevalence of peanut allergy in infants after the introduction of these new guidelines and evaluate the association between early peanut introduction and peanut allergy.
Design
Two population-based cross-sectional samples of infants aged 12 months were recruited 10 years apart using the same sampling frame and methods to allow comparison of changes over time. Infants were recruited from immunization centers around Melbourne, Australia. Infants attending their 12-month immunization visit were eligible to participate (eligible age range, 11-15 months), regardless of history of peanut exposure or allergy history.
Exposures
Questionnaires collected data on demographics, food allergy risk factors, peanut introduction, and reactions.
Main Outcome and Measures
All infants underwent skin prick tests to peanut and those with positive results underwent oral food challenges. Prevalence estimates were standardized to account for changes in population demographics over time.
Results
This study included 7209 infants (1933 in 2018-2019 and 5276 in 2007-2011). Of the participants in the older vs more recent cohort, 51.8% vs 50.8% were male; median (IQR) ages were 12.5 (12.2-13.0) months vs 12.4 (12.2-12.9) months. There was an increase in infants of East Asian ancestry over time (16.5% in 2018-2019 vs 10.5% in 2007-2011), which is a food allergy risk factor. After standardizing for infant ancestry and other demographics changes, peanut allergy prevalence was 2.6% (95% CI, 1.8%-3.4%) in 2018-2019, compared with 3.1% in 2007-2011 (difference, −0.5% [95% CI, −1.4% to 0.4%]; P = .26). Earlier age of peanut introduction was significantly associated with a lower risk of peanut allergy among infants of Australian ancestry in 2018-2019 (age 12 months compared with age 6 months or younger: adjusted odds ratio, 0.08 [05% CI, 0.02-0.36]; age 12 months compared with 7 to less than 10 months: adjusted odds ratio, 0.09 [95% CI, 0.02-0.53]), but not significant among infants of East Asian ancestry (P for interaction = .002).
Conclusions and Relevance
In cross-sectional analyses, introduction of a guideline recommending early peanut introduction in Australia was not associated with a statistically significant lower or higher prevalence of peanut allergy across the population.
This cross-sectional study examines the change in prevalence of peanut allergy after the introduction of guidelines recommending early peanut introduction for all infants, in both the general population and in predetermined subgroups of high-risk infants, in Australia.
Introduction
Peanut allergy is one of the most common childhood food allergies, and children rarely grow out of it.1 The only evidence-based prevention strategy currently available is timely introduction of peanut in the diet. Introduction of peanut by age 11 months was associated with a lower risk of peanut allergy (risk ratio, 0.29 [95% CI, 0.11-0.74]) in a meta-analysis of 2 randomized clinical trials conducted in the UK.2 One was conducted in infants at high risk of developing peanut allergy.3
These results led to infant feeding guidelines changing in 2016 in Australia to recommend introduction of peanut before age 12 months, although US guidelines initially restricted recommendations to high-risk infants only.4,5,6,7 This represented a major change from 1990s guidelines that recommended avoiding allergenic foods until age 1 to 3 years, leading to their widespread avoidance in infancy.8 By 2008, there was increasing evidence that delaying allergenic foods was associated with increased food allergy risk and avoidance was not recommended.9 Even so, most Australian parents from 2007 to 2011 still avoided feeding their infants peanut products before 1 year.8 There was an increase in infant feeding of peanut products in Australia after introduction of the 2016 guidelines. In 2 population-based studies conducted in 2007 to 2011 and 2018 to 2019, introduction of peanut in the first year of life increased from 28% to 89%.10
It was not known to what extent early peanut introduction would reduce peanut allergy prevalence in the general population.11 The aim of the current study was to measure the change in prevalence of peanut allergy using these 2 cohorts, both in the general population and in predetermined subgroups of high-risk infants. It was hypothesized that a decrease in the prevalence of peanut allergy would be observed due to the shift toward early peanut introduction.
Method
Study Design
Ethical approval for both studies was obtained from the Royal Children’s Hospital Research Ethics Committee (2007-2011: number 27047; 2018-2019: number 36160A). The 2018-2019 study was registered with the Australian New Zealand Clinical Trials Registry (registration number ACTRN12618001990213) as an observational study. Parents or guardians provided written informed consent before participation.
These 2 population-based studies used the same methodology, in Melbourne, Australia, to recruit participants 10 years apart. The baseline study, HealthNuts, was initially conceived as a cross-sectional study that recruited 5276 infants from September 2007 to August 2011. The 2018-2019 comparator study, EarlyNuts, was specifically designed to replicate the 2007-2011 study methodology and is the main sample of interest within these analyses. The study recruited from January 2018 to December 2019 (“Vanguard” study using the same protocol: November 2016 to December 2017).
The studies have been described in detail.10,12 Briefly, both studies recruited infants from council-run immunization sessions at the time of their 12-month immunization visit. Eligible infants were aged between 11 and 15 months, regardless of exposure or allergy history. Infants underwent a skin prick test and open oral food challenges if the wheal size was 1 mm or larger (see eMethods in the Supplement). Parents completed questionnaires in the 15-minute wait time before the skin prick test results were read. Age of peanut introduction was evaluated with a researcher-administered questionnaire. Participants were excluded if the parent or guardian were unable to complete the questionnaires in English. Eligible parents who declined participation were asked to complete a nonparticipation survey (eBox in the Supplement).
All electronic data were collected and managed using REDCap software hosted at Murdoch Children’s Research Institute in Melbourne.13,14
Exposures
The key measures are defined below, with additional definitions provided in the eMethods in the Supplement.
Early peanut introduction was defined as introduction of any peanut-containing product (eg, peanut butter) before 12 months (0-11 months) or as a categorical variable: 6 months or earlier, 7 to 9 months, and 10 to 11 months compared with greater than or equal to 12 months.
Early eczema (early-onset eczema) was defined as parent-reported eczema diagnosis with onset in first 6 months of life that was managed with topical steroids (over-the-counter or prescribed).15
Infant ancestry was parent-reported maternal and paternal country of birth categorized into 10 country/regions: Australia, UK/Britain, Europe, Middle East, Africa, South America, North America, Oceania, South Asia, or East Asia (eMethods in the Supplement). Infant ancestry was grouped as Australian (both parents born in Australia), East Asian (1 parent born in East Asia 1 born in Australia or both born in East Asia), or other (all other combinations).
Sex was defined as parent-reported sex of the infant.
Outcome
The primary outcome was peanut allergy, defined as positive oral food challenge result or a clear history of recent reaction (within the past 2 months) in infants with a skin prick test wheal of at least 1 mm (eMethods in the Supplement).3,15
Statistical Analysis
Sample Size
The planned sample size of 2000 infants was estimated to have 81% power to detect a 40% relative decrease in peanut allergy prevalence in the study compared with the 2007-2011 study (3.1%), based on modeling showing an expected decrease of at least 40% when applying randomized clinical trial findings to the general population.15
Descriptive Statistics
Each analysis was conducted as a complete case analysis, using all records with no missing data on the relevant variables. Participant characteristics were summarized throughout using percentages for binary variables and means for continuous variables, with the number of participants without missing data provided. To assess the representativeness of the recruited sample with respect to the eligible sample, characteristics between nonparticipants, partial participants, and complete participants were compared (eMethods in the Supplement).
Age of Peanut Introduction and Peanut Allergy
The association between age of peanut introduction and peanut allergy within studies was assessed using multivariable logistic regression models. Models were adjusted for risk factors of both peanut introduction and peanut allergy (socioeconomic status, infant ancestry, number of siblings, and family history of food allergy), as guided a priori using a directed acyclic graph (eFigure 1 in the Supplement) using DAGitty16 (version 3.0). Interactions between age at peanut introduction and the aforementioned risk factors were examined within each study using likelihood ratio testing. Stratified results were presented when P values were less than .05. A sensitivity analysis was performed adding early-onset eczema to the model.
Peanut Allergy Prevalence Estimates
The 2018-2019 prevalence of peanut allergy was estimated as the observed percentage and compared with 2007-2011 prevalence by calculating the absolute difference. We conducted a sensitivity analysis using inverse probability weighting to assess whether this estimate was affected by potential selection bias due to partial participation (eMethods in the Supplement). We then used direct regression standardization bootstrapped 1000 times to weight prevalence estimates of peanut allergy in the study to the population structure at baseline (2007-2011). Standardization models were adjusted for factors that changed substantially between the studies and that may alter the prevalence of peanut allergy (potential food allergy risk factors), as well as pet dog ownership and number of siblings because of strong evidence that these factors were associated with food allergy in 2007-2011,17,18 as guided by a directed acyclic graph (eFigure 2 in the Supplement). In addition, interaction terms between a covariate and study indicator (2018-2019 study or 2007-2011 study) were assessed using likelihood ratio testing and added into the model if P < .05.19 The primary multivariable logistic regression model used for standardization included study, infant ancestry, number of siblings, family history of food allergy, family history of hay fever, and dog ownership, plus an interaction term between study and family history of food allergy. Adjusted 2018-2019 prevalence was compared with 2007-2011 prevalence by calculating the absolute difference. Sensitivity analyses included stratifying the primary standardization analysis by age of peanut introduction categories (≤6 months, 7-9 months, 10-11 months, and ≥12 months). An additional post hoc analysis examined the prevalence of peanut allergy among those with and without early peanut introduction in the 2018-2019 study, both as the observed proportion and the standardized estimate.
Subgroup Analyses
The standardization analysis described above was also stratified by prespecified high-risk subgroups of interest: presence or absence of early-onset eczema and infant ancestry (eMethods in the Supplement). Interactions between the study indicator (2018-2019 study or 2007-2011 study) and infant eczema and ancestry were assessed using likelihood ratio testing, but are presented as stratified irrespective of the P values to comply with predetermined analysis plans.
Significance level was decided a priori as P < .05. All analyses were conducted with the statistical software package Stata, release 17.0 (StataCorp).
Results
Study Population
Researchers approached parents of 9677 infants born between 2006-2010 and 2016-2018 (Figure 1). One study recruited 1933 infants from January 2018 to December 2019 (Vanguard study: November 2016 to December 2017; n = 90). The participation rate in 2018-2019 was 76.0%. Of the 1933 participants, 1420 (73.5%) completed skin prick tests and the remainder completed the questionnaires but declined skin prick tests (26.5%) (Figure 1). Most parents reported declining a skin prick test because their child was already tolerating the study foods (n = 248 [48.3%]) or previously underwent an allergy assessment after reacting (n = 61 [11.9%]) (Figure 1). Of the 513 participants missing skin prick tests, 28 were probable or possible allergic, 369 had a history of tolerating peanut, and 116 had unknown outcomes. Overall, 1405 participants had peanut allergy outcomes, of whom 1311 had complete data for all the required variables and could be included in the analysis.
Figure 1. Flow of Participants in a Comparison of 2 Studies Evaluating Exposure and Development of Peanut Allergy in Australia.
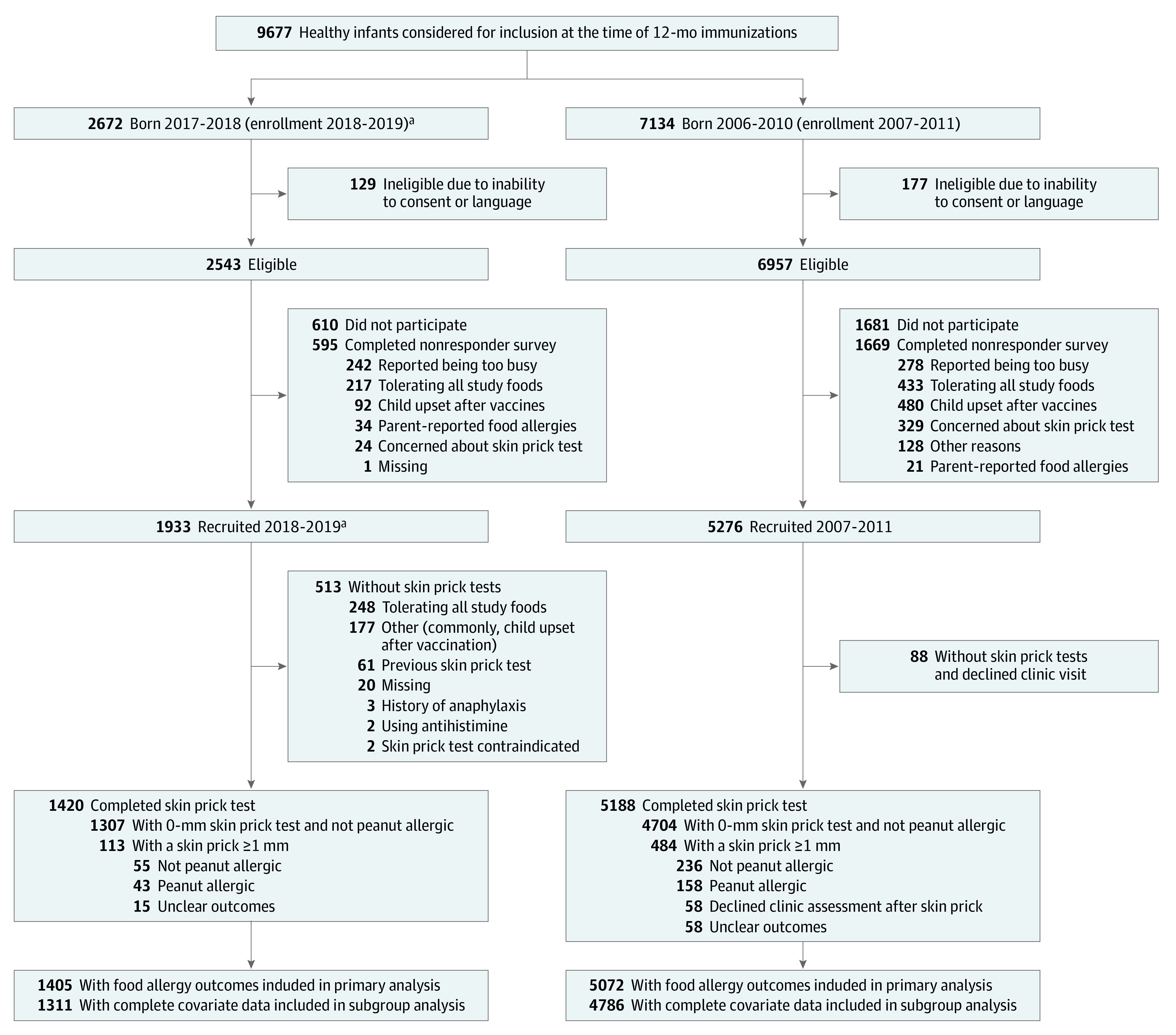
aIncludes 90 participants enrolled in the Vanguard study from November 2016 to December 2017 (born 2015-2016).
The participation rate from 2007-2011 was 74.0% and 2.8% of participants declined skin prick tests.12 In 2007-2011, a total of 5072 participants had peanut allergy outcomes, of whom 4786 participants had complete data for all the necessary variables and could be included in the analysis.
Participants were a median (IQR) age of 12.5 (12.2-13.0) months at recruitment in 2018-2019 and 12.4 (12.2-12.9) months in 2007-2011. Some differences in demographics between the 2 studies were observed (Table 1). There was a significant increase in the percentage of infants of East Asian ancestry, a previously documented risk factor for food allergy in Australia, from 10.5% to 16.5% (P < .001).20 The geographic origins of parents in the “other” infant ancestry category are reported in eTable 2 in the Supplement. Infants in 2018-2019 were also more likely to have parent-reported eczema; however, there were only small differences after stratifying by infant ancestry (eTable 3 in the Supplement). Infants in 2018-2019 were more likely to have a family history of food allergy compared with those in 2007-2011. Participant characteristics in 2018-2019 were similar to the 2016 population demographics from the Victorian Perinatal Data Collection (eTable 4 in the Supplement).21
Table 1. Participant Demographics and History in the 2018-2019 Study and 2007-2011 Study.
| Characteristic | No. (%)a | |
|---|---|---|
| 2018-2019 study (n = 1933) |
2007-2011 study (n = 5276) | |
| Infant sex | n = 1932 | n = 5244 |
| Male | 1001 (51.8) | 2665 (50.8) |
| Female | 931 (48.2) | 2579 (49.2) |
| Mode of birth (vaginal) | 1177/1849 (63.7) | 3502/5244 (66.8) |
| Preterm (<37 weeks) | 136/1824 (7.5) | 305/5006 (6.1) |
| Parent-reported eczema diagnosis | 524/1794 (29.2) | 1325/4982 (26.6) |
| Socioeconomic status by postcodeb | n = 1913 | n = 5261 |
| Median (IQR) | 9 (7-10) | 9 (7-10) |
| Mother age >34 y | 647/1758 (36.8) | 1772/5225 (33.9) |
| Mother age, mean (SD), y | 33.6 (4.4) | 33.0 (4.8) |
| No. of siblings | n = 1752 | n = 5215 |
| 0 | 850 (48.5) | 2582 (49.5) |
| 1 | 652 (37.2) | 1734 (33.3) |
| 2 | 196 (11.2) | 667 (12.8) |
| 3 or more | 54 (3.1) | 232 (4.4) |
| Parent's country of birth (infant ancestry)c | n = 1754 | n = 5098 |
| Both Australia | 864 (49.3) | 3023 (59.3) |
| One or both East Asia | 289 (16.5) | 535 (10.5) |
| Other | 601 (34.3) | 1540 (30.2) |
| History of allergy in the infant’s familyd | 1186/1797 (66.0) | 3661/5276 (69.4) |
| Asthma | 527/1786 (29.5) | 1619/5276 (30.7) |
| Eczema | 538/1786 (30.1) | 1611/5276 (30.5) |
| Hay fever | 825/1786 (46.2) | 2640/5276 (50.0) |
| Food allergy | 342/1786 (19.2) | 683/5276 (12.9) |
| Mother with history of allergy | 712/1797 (39.6) | 2331/5276 (44.2) |
| Father with history of allergy | 707/1797 (39.3) | 2001/5276 (37.9) |
| Siblings with history of allergy | 356/914 (38.9)e | 1066/2633 (40.5)f |
| Childcare attendance | n = 1769 | n = 5012 |
| No daycare | 1157 (65.4) | 3718 (74.2) |
| Yes, 1-6 mo | 105 (5.9) | 353 (7.0) |
| Yes, 7-12 mo | 507 (28.7) | 941 (18.8) |
| Pet dog ownership | n = 1779 | n = 5274 |
| No | 1224 (68.8) | 3665 (69.5) |
| Yes, allowed inside | 358 (20.1) | 1072 (20.3) |
| Yes, outdoors only or undisclosed location | 197 (11.1) | 537 (10.2) |
| Infant lives on a farm | 16/1750 (0.9) | 40/5012 (0.8) |
Study years refer to years of enrollment.
Socioeconomic status decile as measured by the Australian Bureau of Statistics, in the IRSAD statistical area 1, 2006 and 2016 based on family postcodes.
Self-reported country of birth. One or both East Asia comprised of both parents born in East Asia or 1 parent born in East Asia and the other born in Australia. Other comprised of all other combinations of country/regions from Australia, UK/Britain, Europe, Middle East, Africa, South America, North America, Oceania, and East Asia.
Family comprised of the infant’s mother, father, and siblings. Allergy history includes asthma, eczema, hay fever, or food allergies.
This excludes 850 participants without siblings.
This excludes 2582 participants without siblings.
Nonparticipants in 2018-2019 were less likely to report reactions to peanut, food allergy, and introduction of peanut compared with participants who underwent the skin prick test (eTable 5 in the Supplement). Participants without skin prick tests were more likely to have introduced peanut than participants with skin prick tests (94.6% vs 86.9%), but the prevalence of parent-reported eczema and other characteristics was similar between all groups (eTable 6 in the Supplement).
Changes in Timing of Peanut Introduction
Consistent with an interim analysis of the first half of the study sample,10 infants in the whole 2018-2019 sample were introduced to peanut earlier than the 2007-2011 sample (85.6% vs 21.6% of participants introduced before 12 months; eTable 6 in the Supplement). Fewer infants of East Asian ancestry consumed peanut before 12 months than infants of Australian ancestry in both studies (71.8% vs 92.9% in 2018-2019 and 16.7% vs 22.5% in 2007-2011). Peanut introduction before 12 months was less common among infants with early-onset eczema in the 2007-2011 study, but not in the 2018-2019 study (eTable 6 in the Supplement).
Association Between Age of Introduction and Peanut Allergy
There was no significant association between age of peanut introduction and peanut allergy in either of the total samples (Figure 2). There was a significant association between earlier peanut introduction and peanut allergy among infants of Australian ancestry. In the 2018-2019 study, compared with those aged 12 months or older, the adjusted odds ratio was 0.08 (95% CI, 0.02-0.36) for those aged 6 months or younger and 0.09 (95% CI, 0.02-0.53) for those aged 7 months to younger than 10 months (P value for interaction in the 2018-2019 study between ancestry and earlier peanut introduction = .002; eTable 1 in the Supplement). There was no significant association between earlier peanut introduction and peanut allergy among infants of East Asian ancestry. Results were similar when early-onset eczema was added into the model as a sensitivity analysis (eFigure 3 in the Supplement) and in unadjusted results (eTable 7 in the Supplement).
Figure 2. Association Between Age of Peanut Introduction and Peanut Allergy at 1 Year.
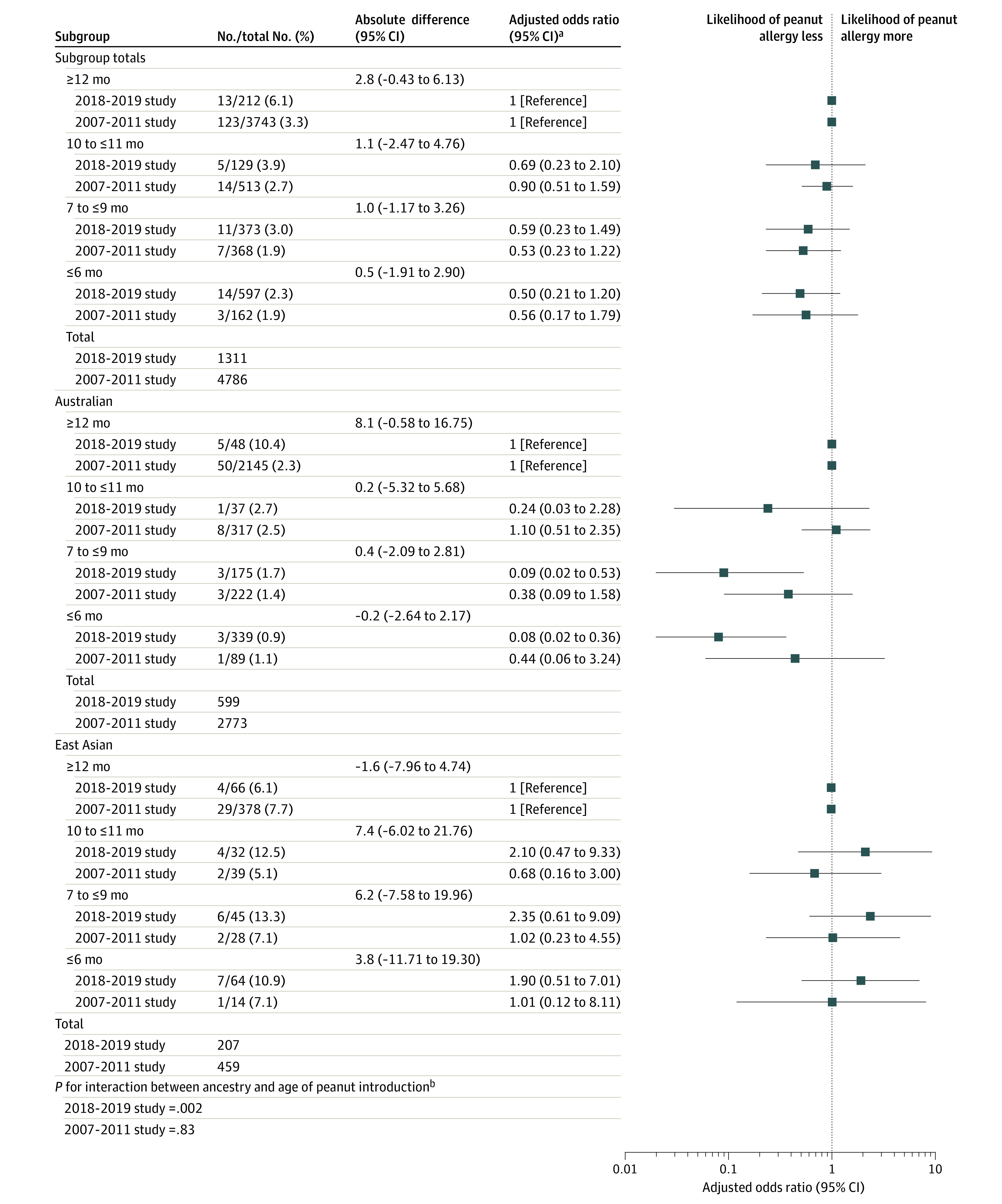
Adjusted odds ratio (aOR) was adjusted for socioeconomic status, number of siblings, family history of food allergy, and infant ancestry (unless stratified by the factor). Firth penalized likelihood logistic regression was used when sample sizes were too small to use a conventional maximum likelihood logistic regression. Error bars indicate 95% CIs.
aStudy years refer to years of enrollment.
bInteraction between the age of peanut introduction and infant ancestry when included in the logistic regression model and assessed using likelihood ratio testing.
Prevalence of Peanut Allergy in 2018-2019 vs 2007-2011
The observed prevalence of peanut allergy in 2018-2019 was 3.1% ([95% CI, 2.3%-4.1%]; 43 of 1405 infants; Table 2). This estimate was similar after using inverse probability weighting to correct for potential selection bias due to incomplete participation (3.0% [95% CI, 2.1%-4.1%]).
Table 2. Prevalence of Peanut Allergy in 2018-2019 vs 2007-2011 in Infants in Australia.
| Outcome | 2018-2019 studya | 2007-2011 study, No./No. (%)a | Difference (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| No./No. (%) | Adjusted % (95% CI) | Unadjusted | Adjustedb,c | |||
| Peanut allergyd | 43/1405 (3.1) | 2.6 (1.9-3.4) | 158/5072 (3.1) | −0.05 (−1.0 to 1.0)e | −0.5 (−1.4 to 0.4) | .26 |
Study years refer to years of enrollment.
The 2018-2019 study prevalence is standardized to 2007-2011 study distribution of infant ancestry, family history of food allergy, family history of hay fever, dog ownership, number of siblings, and interactions between family history of food allergy and study.
Absolute difference between the adjusted 2018-2019 study prevalence (2.6%) and 2007-2011 prevalence. Absolute differences were calculated from values before rounding.
Positive oral food challenge result or a clear history of recent reaction (within the past 2 months) in infants with a skin prick test wheal of more than or equal to 1 mm. A total of 15 participants in the 2018-2019 study and 204 in the 2007-2011 study were missing peanut allergy outcomes.
P = .92.
After standardizing to the 2007-2011 baseline distribution for the select factors, the adjusted prevalence of peanut allergy in 2018-2019 was 2.6% (95% CI, 1.9-3.4) (Table 2). There was no significant decrease in peanut allergy compared with the observed prevalence in 2007-2011 (158 of 5072 [3.1% (95% CI, 2.7%-3.6%)]; P = .24).
Peanut allergy prevalence was then further stratified by the risk factors infant ancestry and early-onset eczema. There was no significant decrease in standardized peanut allergy prevalence among either East Asian infants or among Australian infants (eTable 8 in the Supplement). There was also no significant interaction with infant ancestry (P = .07).
Among infants with early-onset eczema, 20 of 165 in 2018-2019 and 73 of 516 in 2007-2011 had peanut allergies. The standardized peanut allergy prevalence among children with early-onset eczema in 2018-2019 was 9.3% (95% CI, 5.2%-13.9%), compared with 14.1% (95% CI, 11.4%-17.4%) in 2007-2011 (difference, −4.9% [95% CI, −10.4% to 0.4%]; P = .08; eTable 9 in the Supplement). There was no significant interaction with early-onset eczema (P = .21).
The unadjusted prevalence of peanut allergy in 2018-2019 was 2.7% (95% CI, 1.9%-3.9%) among infants with early peanut introduction and 6.1% (95% CI, 3.6%-10.3%) among infants without early peanut introduction (Table 3). Within strata of age of peanut introduction categories, the standardized prevalences of peanut allergy within every category from 2007-2011 to 2018-2019 were not significantly different (Table 3 and eTable 10 in the Supplement). A sensitivity analysis adding early-onset eczema to the models gave similar results (eTable 11 in the Supplement).
Table 3. Prevalence of Peanut Allergy by Age of Peanut Introduction.
| Early peanut introduction | 2018-2019 studya | 2007-2011 study peanut allergy, No./total No. (%)a,b | Difference (95% CI) | P valuee | ||
|---|---|---|---|---|---|---|
| Peanut allergy, No./total No. (%)b | Adjusted % (95% CI) | Unadjusted | Adjustedc,d | |||
| <12 mo | 30/1099 (2.7) | 1.9 (1.2 to 2.7) | 24/1043 (2.3) | −0.3 (−1.7 to 1.0) | 0.4 (−0.6 to 1.7) | .46 |
| ≥12 mo | 13/212 (6.1) | 4.8 (2.3 to 7.9) | 123/3743 (3.3) | −3.0 (−6.8 to 0.2) | −1.5 (−4.6 to 1.1) | .35 |
Study years refer to years of enrollment.
Positive oral food challenge or a clear history of recent reaction (within the past 2 months) in infants with a skin prick test wheal of more than or equal to 1 mm.
The 2018-2019 study prevalence is adjusted to 2007-2011 study distribution of infant ancestry, family history of food allergy, family history of hay fever, dog ownership, number of siblings, and interactions between family history of food allergy and study.
Absolute difference between the adjusted 2018-2019 prevalence and 2007-2011 prevalence.
P value for the adjusted difference. Absolute differences were calculated from values before rounding.
Discussion
The introduction of a guideline recommending early peanut introduction in Australia was not associated with a statistically significant lower or higher prevalence of peanut allergy across the population in analyses of 2 population-based samples of infants aged 1 year.
This study found an increase in infants of East Asian ancestry (parents born in East Asia), concordant with population data from the 2016 Victorian Perinatal Data Collection, compared with the 2007-2011 study.22 This increase was relevant because a previous study showed higher likelihood of peanut allergy (adjusted odds ratio, 3.3 [95% CI, 2.5-4.5]) among infants born in Australia to East Asian parents.18 Despite the higher prevalence of this risk factor in the 2018-2019 study, peanut allergy prevalence did not increase in 2018-2019. It is possible that widespread early introduction of peanut products in infancy blunted the rise in peanut allergy that could have otherwise occurred.
Earlier peanut introduction was significantly associated with lower risk of peanut allergy among infants of Australian-born parents, but not among infants of East Asian ancestry, in the 2018-2019 cohort. These results were contrary to findings from the LEAP randomized clinical trial in UK infants, which showed earlier peanut introduction prevented peanut allergy among those of the “Indian subcontinent” (n = 24) but not in “Asian” infants (n = 8) from “China, Middle East, and other.”3 Different introduction patterns may have contributed to the findings. Infants of East Asian ancestry in this study were introduced to peanut later, which may have attenuated the association with early peanut introduction in East Asian infants. There is also some evidence from other studies conducted in Asia that the timing of introduction of allergenic foods may be less important among infants of Asian ancestry.23,24 More trials are needed to look into the optimal frequency and quantity of peanut consumption to prevent peanut allergy, because the study design and data structure in this study were not optimal to explore this question.
The evidence that early introduction of peanut could prevent peanut allergy was largely derived from a UK trial conducted in infants with early-onset eczema, who were therefore at high-risk of developing peanut allergy.3 A second trial in infants recruited from the general population did not show a significant decrease in peanut allergy in the early introduction group in the intention-to-treat analysis.25 Similarly, the prevalence of peanut allergy in this study did not decrease significantly in the general population over time.
The high prevalence of peanut allergy in Melbourne, Australia, despite early peanut introduction, suggests an important contribution of other early life environmental factors. An increase in less-researched environmental risk factors, potentially interacting with genetic susceptibility, could have masked the protective association with earlier peanut introduction.26,27,28 Maternal gut microbiome, vitamin D deficiency, and early life microbial exposure are 3 potential food allergy risk factors that were not measured in this study.29,30,31 Early introduction of peanut alone is also unlikely to prevent other food allergies and therefore may not change overall food allergy prevalence. Overall, the findings of this study were consistent with early peanut introduction as an important preventative strategy; however, early peanut introduction alone was not likely to be sufficient to eliminate peanut allergy.
This study has several strengths. First, these large population-based studies were specifically designed to answer this research question and used objective, challenge-proven peanut allergy outcomes. They allowed a comparison of peanut allergy prevalence before and after the introduction of new infant feeding guidelines. Second, both studies recruited participants from the same geographical location using the same recruitment methods and sampling frame. Third, the demographic characteristics of both studies were broadly representative of the general population.10 A large range of food allergy risk factors and temporal changes in population demographics were measured and adjusted for over time using inverse probability weighting and standardization, thereby reducing bias.
Limitations
This study has several limitations. First, there was a lower rate of participation in skin prick tests in 2018-2019 compared with 2007-2011. Families mainly declined a skin prick test when their infants were already eating and tolerating peanut or already seeing a physician after earlier allergic reactions after eating peanut. Second, there were minor differences in peanut allergy risk factors between those with and without skin prick tests as well as nonparticipants; however, the prevalence estimates did not substantially change when weighting to correct for potential bias due to partial participation. Moreover, the prevalence of “probable/possible peanut allergy” among partial participants was similar to peanut allergy among complete participants, suggesting that the lower rate of skin prick tests in 2018-2019 vs 2007-2011 is not likely to explain the small difference in peanut allergy between the studies. Third, because data were collected retrospectively at 12 months, any nondifferential misclassification of age at first peanut introduction (parents misremembering age) would bias the association results toward the null, rather than explain the observed association. Furthermore, cross-sectional data collection avoids possible changes in participant feeding behavior caused by the awareness of being studied.32
Conclusion
In this cross-sectional analyses, introduction of a guideline recommending early peanut introduction in Australia was not associated with a statistically significant lower or higher prevalence in peanut allergy across the population.
eBox 1. Short, anonymous non-responder survey
eFigure 1. Directed acyclic graph of the association between peanut introduction and peanut allergy
eFigure 2. Directed acyclic graph of temporal changes between studies that may alter the prevalence of peanut allergy
eMethods
eTable 1. Interactions assessed using likelihood ratio testing
eTable 3. Participant demographics in 2018-2019 and 2007-2011 studies, stratified by parent country of birth
eTable 4. Demographic characteristics of both studies compared to population data from the Victorian Perinatal Data Collection
eTable 5. Characteristics of the 2018-2019 study participants compared to non-participant survey
eTable 6. Observed peanut introduction before 12 months, stratified by infant ancestry and early eczema
eFigure 3. Association between age of peanut introduction and peanut allergy at 1 year with early eczema as an additional potential confounding variable
eTable 7. Unadjusted association between age of peanut introduction and peanut allergy at 1 year
eTable 8. Peanut allergy prevalence in 2018-2019 compared to 2007-2011, stratified by infant ancestry
eTable 9. Peanut allergy prevalence by presence of early-onset eczema
eTable 10. Peanut allergy prevalence by age of introduction of peanut in 2018-2019 compared to 2007-2011
eTable 11. Peanut allergy prevalence in 2018-2019 by age of introduction of peanut, adjusted adding early eczema into the model compared to the observed 2007-2011 prevalence as a sensitivity analysis
eReferences
References
- 1.Peters RL, Allen KJ, Dharmage SC, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population-based assessment. J Allergy Clin Immunol. 2015;135(5):1257-1266. doi: 10.1016/j.jaci.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316(11):1181-1192. doi: 10.1001/jama.2016.12623 [DOI] [PubMed] [Google Scholar]
- 3.Du Toit G, Roberts G, Sayre PH, et al. ; LEAP Study Team . Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-813. doi: 10.1056/NEJMoa1414850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ASCIA Guidelines: Infant Feeding and Allergy Prevention. Australasian Society of Clinical Immunology and Allergy ; 2016. [DOI] [PubMed]
- 5.Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and prevention of peanut allergy in high-risk infants. Pediatr Dermatol. 2016;33(1):103-106. doi: 10.1111/pde.12685 [DOI] [PubMed] [Google Scholar]
- 6.Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. World Allergy Organ J. 2017;10(1):1. doi: 10.1186/s40413-016-0137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119-132. doi: 10.1097/MPG.0000000000001454 [DOI] [PubMed] [Google Scholar]
- 8.Tey D, Allen KJ, Peters RL, et al. ; HealthNuts study investigators . Population response to change in infant feeding guidelines for allergy prevention. J Allergy Clin Immunol. 2014;133(2):476-484. doi: 10.1016/j.jaci.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 9.Netting MJ, Allen KJ. Advice about infant feeding for allergy prevention: a confusing picture for Australian consumers? J Paediatr Child Health. 2017;53(9):870-875. doi: 10.1111/jpc.13594 [DOI] [PubMed] [Google Scholar]
- 10.Soriano VX, Peters RL, Ponsonby A-L, et al. Earlier ingestion of peanut after changes to infant feeding guidelines: the EarlyNuts study. J Allergy Clin Immunol. 2019;144(5):1327-1335.e5. doi: 10.1016/j.jaci.2019.07.032 [DOI] [PubMed] [Google Scholar]
- 11.Allen KJ, Koplin JJ. Does LEAP change the screening paradigm for food allergy in infants with eczema? Clin Exp Allergy. 2016;46(1):42-47. doi: 10.1111/cea.12685 [DOI] [PubMed] [Google Scholar]
- 12.Koplin JJ, Wake M, Dharmage SC, et al. ; HealthNuts study group . Cohort profile: the HealthNuts study: population prevalence and environmental/genetic predictors of food allergy. Int J Epidemiol. 2015;44(4):1161-1171. doi: 10.1093/ije/dyu261 [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koplin JJ, Peters RL, Dharmage SC, et al. ; HealthNuts study investigators . Understanding the feasibility and implications of implementing early peanut introduction for prevention of peanut allergy. J Allergy Clin Immunol. 2016;138(4):1131-1141.e2. doi: 10.1016/j.jaci.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 16.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887-1894. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Betancur M, Koplin JJ, Ponsonby AL, Lynch J, Carlin JB. Measuring the impact of differences in risk factor distributions on cross-population differences in disease occurrence: a causal approach. Int J Epidemiol. 2018;47(1):217-225. doi: 10.1093/ije/dyx194 [DOI] [PubMed] [Google Scholar]
- 18.Koplin JJ, Dharmage SC, Ponsonby AL, et al. ; HealthNuts Investigators . Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. 2012;67(11):1415-1422. doi: 10.1111/all.12015 [DOI] [PubMed] [Google Scholar]
- 19.Peters RL, Koplin JJ, Allen KJ, et al. The prevalence of food sensitization appears not to have changed between 2 Melbourne cohorts of high-risk infants recruited 15 years apart. J Allergy Clin Immunol Pract. 2018;6(2):440-448.e2. doi: 10.1016/j.jaip.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 20.Koplin JJ, Peters RL, Ponsonby AL, et al. ; HealthNuts Study Group . Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014;69(12):1639-1647. doi: 10.1111/all.12487 [DOI] [PubMed] [Google Scholar]
- 21.Australia’s Mothers, Babies and Children 2016: In Brief. Australian Institute of Health and Welfare; 2018. [Google Scholar]
- 22.Victoria's Mothers, Babies and Children 2016. Department of Health & Human Services ; 2016. [Google Scholar]
- 23.Suaini NHA, Loo EXL, Peters RL, et al. Children of Asian ethnicity in Australia have higher risk of food allergy and early-onset eczema than those in Singapore. Allergy. 2021;76(10):3171-3182. doi: 10.1111/all.14823 [DOI] [PubMed] [Google Scholar]
- 24.Tham EH, Lee BW, Chan YH, et al. Low food allergy prevalence despite delayed introduction of allergenic foods-data from the GUSTO cohort. J Allergy Clin Immunol Pract. 2018;6(2):466-475.e1. doi: 10.1016/j.jaip.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkin MR, Logan K, Tseng A, et al. ; EAT Study Team . Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374(18):1733-1743. doi: 10.1056/NEJMoa1514210 [DOI] [PubMed] [Google Scholar]
- 26.Tham EH, Shek LP, Van Bever HP, et al. ; Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) . Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective: an Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr Allergy Immunol. 2018;29(1):18-27. doi: 10.1111/pai.12820 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Zhang S, Tsai HJ, et al. Genetic and environmental contributions to allergen sensitization in a Chinese twin study. Clin Exp Allergy. 2009;39(7):991-998. doi: 10.1111/j.1365-2222.2009.03228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis JA, Kemp AS, Ponsonby AL. Gene-environment interaction in autoimmune disease. Expert Rev Mol Med. 2014;16:e4. doi: 10.1017/erm.2014.5 [DOI] [PubMed] [Google Scholar]
- 29.Allen KJ, Panjari M, Koplin JJ, et al. VITALITY trial: protocol for a randomised controlled trial to establish the role of postnatal vitamin D supplementation in infant immune health. BMJ Open. 2015;5(12):e009377. doi: 10.1136/bmjopen-2015-009377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuillermin P, O’Hely M, Collier F, et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat Commun. 2020;11(1):1452. doi: 10.1038/s41467-020-14552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano VX, Koplin JJ, Forrester M, et al. Infant pacifier sanitization and risk of challenge-proven food allergy: a cohort study. J Allergy Clin Immunol. 2021;147(5):1823-1829. doi: 10.1016/j.jaci.2021.01.032 [DOI] [PubMed] [Google Scholar]
- 32.Soriano VX, Peters R, Dharmage SC, et al. Earlier ingestion of peanuts following change to infant feeding guidelines in Australia. J Allergy Clin Immunol. 2019;143(2):AB421. doi: 10.1016/j.jaci.2018.12.947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBox 1. Short, anonymous non-responder survey
eFigure 1. Directed acyclic graph of the association between peanut introduction and peanut allergy
eFigure 2. Directed acyclic graph of temporal changes between studies that may alter the prevalence of peanut allergy
eMethods
eTable 1. Interactions assessed using likelihood ratio testing
eTable 3. Participant demographics in 2018-2019 and 2007-2011 studies, stratified by parent country of birth
eTable 4. Demographic characteristics of both studies compared to population data from the Victorian Perinatal Data Collection
eTable 5. Characteristics of the 2018-2019 study participants compared to non-participant survey
eTable 6. Observed peanut introduction before 12 months, stratified by infant ancestry and early eczema
eFigure 3. Association between age of peanut introduction and peanut allergy at 1 year with early eczema as an additional potential confounding variable
eTable 7. Unadjusted association between age of peanut introduction and peanut allergy at 1 year
eTable 8. Peanut allergy prevalence in 2018-2019 compared to 2007-2011, stratified by infant ancestry
eTable 9. Peanut allergy prevalence by presence of early-onset eczema
eTable 10. Peanut allergy prevalence by age of introduction of peanut in 2018-2019 compared to 2007-2011
eTable 11. Peanut allergy prevalence in 2018-2019 by age of introduction of peanut, adjusted adding early eczema into the model compared to the observed 2007-2011 prevalence as a sensitivity analysis
eReferences


