Take Home Message
Aggressiveness of prostate cancer evaluated before treatment by incorporating magnetic resonance imaging (MRI) and MRI-guided biopsy results gives a better estimate of the risk of metastatic and biochemical recurrences than previous parameters not based on MRI.
Keywords: Prostate cancer, Recurrence risk, Tumor volume, Gleason pattern 4/5
Abstract
Background
The risk of prostate cancer metastatic is correlated with its volume and grade. These parameters are now best estimated preoperatively with magnetic resonance imaging (MRI) and MRI-guided biopsy.
Objective
To estimate the risk of metastatic recurrence after radical prostatectomy (RP) in our model versus conventional clinical European Association of Urology (EAU) classification. The secondary objective is biochemical recurrence (BCR).
Design, setting, and participants
A retrospective study was conducted of a cohort of 713 patients having undergone MRI-guided biopsies and RP between 2009 and 2018. The preoperative variables included prostate-specific antigen, cT stage, tumor volume (TV) based on the lesion’s largest diameter at MRI, percentage of Gleason pattern 4/5 (%GP4/5) at MRI-guided biopsy, and volume of GP4/5 (VolGP4/5) calculated as TV × %GP4/5.
Outcome measurements and statistical analysis
The variables’ ability to predict recurrence was determined in univariable and multivariable Fine-and-Gray models, according to the Akaike information criterion (AIC) and Harrell’s C-index.
Results and limitations
Overall, 176 (25%), 430 (60%), and 107 (15%) patients had low, intermediate, and high-risk disease, respectively, according to the EAU classification. During a median follow-up period of 57 mo, metastatic recurrence was observed in 48 patients with a 5-yr probability of 5.6% (95% confidence interval [CI] 3.9–7.7). VolGP4/5 (categories: <0.5, 0.5–1.0, 1.01–3.2, and >3.2 ml) was the parameter with the lowest AIC and the highest C-index for metastatic recurrence of 0.82 (95% CI 0.76–0.88), and for BCR it was 0.73 (95% CI 0.68–0.78). In a multivariable model that included %GP4/5 and TV, C-index values were 0.86 (95% CI 0.79–0.91) for metastatic recurrence and 0.77 (0.72–0.82) for BCR. The same results for EAU classification were 0.74 (0.67–0.80) and 0.67 (0.63–0.72), respectively. Limitations are related to short follow-up and expertise of radiologists and urologists.
Conclusions
We developed a preoperative risk tool integrating the VolGP4/5 based on MRI and MRI-guided biopsies to predict metastatic recurrence after RP. Our model showed higher accuracy than conventional clinical risk models. These findings might enable physicians to provide more personalized patient care.
Patient summary
Aggressiveness of prostate cancer evaluated before treatment by incorporating magnetic resonance imaging (MRI) and MRI-guided biopsy results gives a better estimate of the risk of metastatic recurrence than previous parameters not based on MRI.
1. Introduction
Before the treatment of localized prostate cancer (PCa), the risk of recurrence is assessed by reference to the European Association of Urology (EAU) risk group classification, which is based on the cT stage, serum prostate-specific antigen (PSA) level, and tumor’s Gleason grade group (GG) [1]. Most men with PCa are unlikely to develop local or metastatic recurrence after definitive treatment. To improve the predictive accuracy of conventional clinical risk models, new classification parameters such as prostate magnetic resonance imaging (MRI) findings and MRI-targeted biopsy (TB) results are now available. Prostate MRI is an accurate method for detecting PCa and determining the number, volume, and location of clinically significant lesions. Mazzone et al [2] have developed and validated a novel preoperative classification integrating, in addition to PSA, biopsy GG, MRI stage, and the maximum diameter of lesion at MRI. This novel classification exhibited higher accuracy than the available tools for biochemical recurrence (BCR) risk. Improved predictive classification could modify care decision by adding potential neoadjuvant or adjuvant treatment. Furthermore, MRI-guided biopsy improves tissue sampling, that is, the maximum cancer core length (MCCL) and grade assessment [3], relative to systematic biopsies (SBs). For men with an elevated PSA level, MRI is now recommended before the initial biopsy [1]. The inclusion of tumor volume (TV) at MRI and the percentage of Gleason pattern 4 or 5 (%GP4/5) in risk models may give a better estimate of metastatic or local recurrence. Hence, it has been shown that quantification of the %GP4/5 provides clinically relevant information in addition to the prognostic GG both in biopsies and in radical prostatectomy (RP) specimens [4]. Rubin et al [5] showed that GG 4 or 5 exhibit genomic similarities. According to the published histological data on RPs, %GP4/5 is an independent predictor of 5-yr BCR-free [6] and metastasis-free survival [7]. The same observation was made for prostate biopsy samples [8]. Even though %GP4/5 and TV are among the strongest predictors of BCR reported in the literature on RP analyses, the absolute volume of the GP4/5 (VolGP4/5) cancer (ie, cancer volume × %GP4/5, corresponding to the volume of poorly differentiated tumor) was demonstrated to be an even better predictor [9], [10].
After more than a decade of the MRI era, midterm follow-up is now possible for series of RPs with MRI-based preoperative measurements of TV and %GP4/5. The objective of the study was to estimate the risk of metastatic recurrence and BCR after RP by incorporating %GP4/5 and MRI parameters into a predictive model.
2. Patients and methods
2.1. Study design and population
We retrospectively analyzed a prospectively maintained database on patients having undergone RP in our tertiary referral center. The study was registered with the French National Data Protection Commission (DEC20-123), and the patients had given their consent for the use of their personal data for research purposes. We assessed all 1864 consecutive patients included in the database between January 2009 and December 2018 (Supplementary Fig. 1). The main inclusion criteria were nonmetastatic (pelvic nodes or any other metastasis), treatment-naïve PCa diagnosed and treated in our center, and available data for prebiopsy MRI, TBs, and SBs performed in our center. No patients received preoperative hormonal therapy. We excluded patients for whom the time interval between biopsy and RP was >12 mo, and those with postbiopsy MRI data only. Other exclusion criteria are listed in Supplementary Fig. 1. The following parameters were recorded at diagnosis: age, family history of PCa, PSA level, prostate volume, and cT stage.
2.2. Magnetic resonance imaging
MRI was performed using a 1.5 or 3 T system and a pelvic phased-array coil. The MRI included T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences. Images were interpreted by uroradiologists with >10 yr of experience in prostate MRI reading. In ten patients, the only available MRI results were from outside of our institution. Their reviews by our radiologists were judged as of good quality. Areas with suspected cancer, scored according to the Prostate Imaging Reporting and Data System (PI-RADS) [11], were defined to have a PI-RADS score of ≥3. We recorded the number of distinct lesions and the TV (0.52 × the greatest dimension [3], regardless of the sequence and the plane) [11]. In 74/728 cases (10%), MRI was negative; these cases were not excluded, but the tumor size was noted as 0 mm.
2.3. Biopsy techniques
All patients underwent 12-core transrectal ultrasound (TRUS)-guided SB and two TBs for each suspicious MRI lesion. For suspected lesions (according to MRI) located in the posterior part of peripheral zone, SBs were considered as TBs if the largest anteroposterior axis of the tumor was on the route of the SB. In these cases (n = 164), no additional TBs were taken. These SBs were then analyzed in the same way as TBs. Software-based three-dimensional TRUS/MRI fusion (GE Logiq E9, General Electric Healthcare, Wauwatosa, WI, USA ) was performed for all anterior lesions or lesions with the greatest dimension of <10 mm. Cognitive fusion was performed for the remaining cases.
2.4. Pathology assessments
Biopsies were evaluated according to the EAU guidelines and the International Society of Urological Pathology (ISUP) 2014 classification [12], [13]. A separate ISUP GG was assessed and reported for each individual biopsy core. Discontinuous tumor foci that were ≥2 mm apart were given a composite measurement as the sum of the lengths of each focus. The MCCL that should be ≤17 mm (needle notch) was reported as 20 mm in one case, due to core fragmentation. Semicontinuous estimation of %GP4/5 (1–5%, 6–10%, and by deciles [11–20%, 21%–30%, and 31–40%]) according to the 2014 ISUP modified Gleason grading was recorded on biopsy.
VolGP4/5 was calculated as TV × %GP4/5 in cubic centimeters, and the MCCL of GP4/5 tissue (MCCLGP4/5) was calculated as MCCL × %GP4/5 in millimeters. For the evaluation of the RP specimen, 4-mm sections were prepared from the base to the apex, and placed on whole-mount slides. The GG, periprostatic extension (T3a or T3b), margin status (focal [<3 mm] vs extended [≥3 mm]), and presence of lymph node metastases were reported. Lymph nodes were sampled at surgery according to the EAU guidelines.
2.5. Characteristics of the index tumor
Clinically significant PCa was defined as tumors with GG ≥2, an MCCL of ≥6 mm, more than three positive SBs, and/or a visible tumor on MRI that was positive on TB. Cancer foci were considered to be separate when they were at least 10 mm apart in all three dimensions on MRI or at least one sextant apart with a negative SB. When several tumor foci had the same GG, the one with the largest diameter on MRI was designated as the index tumor. However, if one focus was GG ≥2 and all the others were GG1, the former was selected as the index tumor. In 23 cases, there were more than one GG ≥2 foci; here, the focus with the highest VolGP4/5 was designated as the index tumor. In 73 cases, no lesion was visible on MRI. In 31 out of 73, GG was >1 and Vol%GP4/5 was 0 cc (as the diameter at MRI was 0 mm), but MCCLGP4/5 was not null.
2.6. Follow-up
Follow-up was based on PSA at 3, 6, and 12 mo after RP, then every 6 mo until 5 yr, and then annually. BCR after RP was defined as a rising PSA level of >0.2 ng/ml. MRI and choline positron emission tomography (PET)/computed tomography (CT) were performed in case of BCR to detect local recurrence in the prostatic bed and/or pelvic nodes, or distant metastases within 3 mo of BCR and then every 6 mo if negative for distant metastasis. Metastatic recurrence was defined as the presence of at least one suspicious node (>1 cm) or bone lesion on MRI or PET/CT. Nonmetastatic BCR was defined as the presence of a lesion in the prostatic bed on imaging or the absence of a lesion on imaging. membrane antigen PET/CT imaging was not available during the study period. In case of negative imaging, patients received only salvage radiotherapy (RT) in the prostatic bed. RT in the pelvic node area was performed only in cases of positive imaging for node metastasis.
2.7. Statistical analysis
Quantitative variables are expressed as medians (interquartile range [IQR]), and categorical variables are expressed as frequencies and percentages. The median (IQR) follow-up time was calculated using the reverse Kaplan-Meier method [14]. The cumulative incidences of BCR and metastatic recurrence were calculated using the method of Prentice et al [15], with non-PCa death treated as a competing event. Using univariable Fine-Gray models, we assessed the ability of the preoperative parameters and the well-established prognostic EAU risk groups to predict BCR and metastatic recurrence [16], with death again treated as a competing event. Quantitative preoperative parameters were treated as continuous variables (after applying a log transformation for PSA density and MRI volume to reduce the skewness) as well as categorical variables according to prespecified thresholds. For each parameter, the proportional subhazard assumption was checked by examining the Schoenfeld [17] residual plot. Several combinations of preoperative parameters were tested in multivariable Fine-Gray models. Using Fine-Gray models, we calculated the subhazard ratios (SHRs) and the corresponding 95% confidence intervals (CIs) associated with the preoperative parameters and the established prognostic classifications, as a guide to the effect size. To compare the performance of individual preoperative variables and combinations thereof, we calculated the Akaike information criterion and Harrell’s C-index (adjusted for competing risks) [18]. We also assessed the issue of calibration (ie, the predicted-to-observed incidence function agreement) for multivariable Fine-Gray models by comparing the predicted mean cumulative incidences (predicted from multivariable models) with the Prentice et al [15] cumulative incidences (observed) in four risk groups determined as the 16th, 50th, and 84th percentiles of the prognostic index’s distribution [19]. We also reported the decision curve analysis (DCA) for 5-yr prediction to compare the clinical net benefit of preoperative parameters versus the reference EAU risk classification systems [20]. All statistical tests were two tailed. The threshold for statistical significance was set at p < 0.05. The data were analyzed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
Between January 2009 and December 2018, 713 patients were included (Supplementary Fig. 1). Clinical, biological, MRI, and pathological data are summarized in Table 1. Pathological results according to biopsy targeting are detailed in Supplementary Table 1, and causes of death are provided in Supplementary Table 2.
Table 1.
Clinical, laboratory, MRI, and biopsy results at inclusion
| Baseline characteristics | Values |
|---|---|
| Age (yr) | 64 (60–69) |
| Family history of PCa | 142/713 (19.9) |
| PSA (ng/ml) | 6.79 (5.30–9.80) |
| Prostate volume (ml) | 43 (33–56) |
| PSA density (ng/ml/ml) | 0.16 (0.12–0.25) |
| cT stage | |
| Tx | 2/713 (0.3) |
| T1c | 450/713 (63.1) |
| T2a | 210/713 (29.5) |
| T2b | 21/713 (2.9) |
| T2c | 21/713 (2.9) |
| T3/T4 | 9/713 (1.3) |
| EAU risk group | |
| Low | 176/713 (24.7) |
| Intermediate | 430/713 (60.3) |
| High | 107/713 (15.0) |
| Number of lesions on MRI | |
| 0 | 73/713 (10.2) |
| 1 | 493/713 (69.1) |
| ≥2 | 147/713 (20.6) |
| MRI lesion site | |
| MRI index lesion | |
| PZ vs TZ/AFMS | 510/639 (79.8) vs 129/639 (20.2) |
| Posterior vs anterior | 448/637 (70.3) vs 189/637 (29.7) |
| MRI secondary lesion | |
| PZ vs TZ/AFMS | 104/147 (70.7) vs 43/147 (29.3) |
| Posterior vs anterior | 81/146 (55.5) vs 65 /146 (44.5) |
| Diameter on MRI (mm) | 13 (8–17) |
| Gleason grade group on biopsy | |
| GG1 | 217/713 (30.4) |
| GG2 | 292/713 (41.0) |
| GG3 | 154/713 (21.2) |
| GG4 | 34/713 (4.8) |
| GG5 | 16/713 (2.2) |
| Percentage of Gleason grade 4 or 5 | |
| 0 | 217/713 (30.4) |
| 1–9 | 79/713 (11) |
| 10 | 74/713 (10.4) |
| 20 | 48/713 (6.7) |
| 30 | 42/713 (5.9) |
| 40 | 49/713 (6.9) |
| 75 | 154/713 (21.6) |
| 100 | 50/713 (7.0) |
| MCCL (mm) | 7 (4–9) |
| Volume of grade 4 or 5 (ml) | 0.09 (0.00–0.67) |
| MCCL of grade 4 or 5 (mm) | 0.80 (0.00–3.81) |
AFMS = anterior fibromuscular stroma; EAU = European Association of Urology; GG = grade group; MCCL = maximum cancer core length; MRI = magnetic resonance imaging; PCa = prostate cancer; PSA = prostate-specific antigen; PZ = peripheral zone; TZ = transition zone.
Data are presented as n/N (%) or median (interquartile range).
3.1. Pathology assessments at the time of RP
The GG at the time of the RP was the same as the GG on biopsy in 62.6% of cases and higher in 31.2%. In the low-risk group, upgrading concerned 52% (92/176) of cases at final pathology. Positive margins were noted in 237 cases (33.2%); pT3a and pT3b stages were observed in 29% and 6.7% of the cases, respectively. Of the 288 patients having undergone limited lymph node dissection, 15 (2.1%) had positive nodes (Supplementary Table 3).
3.2. Recurrence
During a median follow-up period of 57 (32–91) mo, we observed 132 (18.5%) cases of BCR including 48 (7%) cases of metastatic recurrence among the 713 patients. BCR occurred at a median time of 18 (5–47) mo and metastatic recurrence at a median time of 23 (2–62) mo. The 5- and 9-yr cumulative incidences were, respectively, 16.3% (13.4–19.3%) and 28.6% (23.5–33.8%) for BCR and 5.6% (3.9–7.7%) and 10.2% (7.7–14.8%) for metastatic recurrence (Supplementary Fig. 2). Out of the 73 patients with no lesion at MRI before surgery, nine had BCR and one had metastatic recurrence. Among the 84 patients with nonmetastatic recurrence, 16 had imaging-confirmed recurrence in the prostatic bed and 68 had normal imaging results. Of these 68 cases, 39 received salvage RT to the prostatic bed, and 29 were left untreated (based on age or life expectancy) without androgen deprivation therapy (ADT). All the 48 patients with metastatic recurrence at imaging received RT and/or ADT.
3.3. Performance of preoperative parameters in univariate analyses
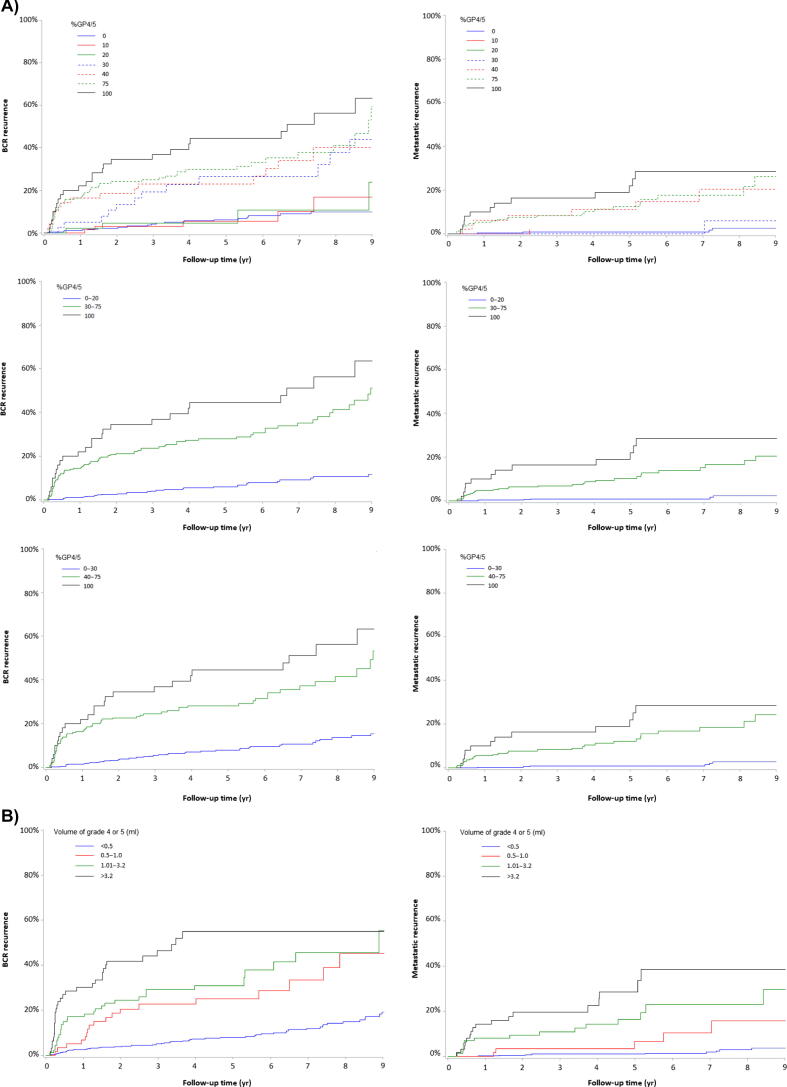
PSA density (PSAD), %GP4/5, MRI diameter, MCCL, VolGP4/5, and MCCLGP4/5 were significantly associated with BCR and metastatic recurrence (Table 2). Different thresholds were tested for %GP4/5 as a noncontinuous variable. The best prediction was obtained with three-level classifications: <30% versus 30–75% versus 100% for BCR and <40% versus 40–75% versus 100% for metastatic recurrence (Supplementary Table 4). The cumulative incidence curves with different threshold values of %GP4/5 are shown in Fig. 1A. The risk of metastatic recurrence increased with %GP4/5, giving an SHR of 13.36 (5.59–31.92) for 40–75% and an SHR of 19.57 (7.21–53.09) for 100% versus <40%. The risk of BCR also increased with %GP4/5, giving an SHR of 5.46 (3.59–8.29) for 30–75% and 8.93 (5.24–15.19) for 100% versus <30% (Table 2).
Table 2.
The ability of preoperative variables of interest to predict BCR and metastatic recurrence, in a univariate analysisa
| Preoperative parameters | BCR | Metastatic recurrence | ||||||
| SHR (95% CI) | p value | AIC | C-index (95% CI) | SHR (95% CI) | p value | AIC | C-index (95% CI) | |
| EAU risk groups components | ||||||||
| EAU risk groups | ||||||||
| 1 | 1.00 (Ref.) | <0.0001 | 1560 | 0.67 (0.63–0.72) | 1.00 (Ref.) | <0.0001 | 554 | 0.74 (0.67–0.80) |
| 2 | 3.04 (1.69–5.48) | 4.05 (1.23–13.28) | ||||||
| 3 | 8.70 (4.67–16.19) | 16.22 (4.93–53.35) | ||||||
| cT stage | ||||||||
| T1c-T2a | 1.00 (Ref.) | 0.056 | 1614 | 0.53 (0.49–0.56) | 1.00 (Ref.) | 0.014 | 584 | 0.55 (0.49–0.60) |
| T2b | 2.10 (1.03–4.26) | 1.33 (0.32–5.38) | ||||||
| ≥T2c | 1.64 (0.81–3.35) | 3.53 (1.50–8.27) | ||||||
| PSA (ng/ml) | ||||||||
| <10 | 1.00 (Ref.) | <0.0001 | 1593 | 0.61 (0.56–0.66) | 1.00 (Ref.) | 0.0002 | 575 | 0.65 (0.56–0.73) |
| 10–20 | 1.97 (1.33–2.92) | 2.69 (1.44–4.99) | ||||||
| >20 | 4.38 (2.34–8.17) | 4.94 (1.99–12.23) | ||||||
| Gleason score | ||||||||
| ≤6 | 1.00 (Ref.) | <0.0001 | 1566 | 0.65 (0.61–0.70) | 1.00 (Ref.) | <0.0001 | 562 | 0.70 (0.63–0.76) |
| 7 | 3.39 (2.01–5.70) | 5.06 (1.80–14.21) | ||||||
| ≥8 | 9.01 (4.85–15.7) | 15.62 (4.99–48.89) | ||||||
| Grade groups | ||||||||
| 1 | 1.00 (Ref.) | <0.0001 | 1543 | 0.71 (0.66–0.75) | 1.00 (Ref.) | <0.0001 | 544 | 0.77 (0.70–0.84) |
| 2 | 2.32 (1.29–4.14) | 2.08 (0.64–6.73) | ||||||
| 3 | 6.50 (3.69–11.45) | 10.99 (3.82–31.64) | ||||||
| 4–5 | 9.52 (5.06–17.91) | 15.49 (4.94–48.56) | ||||||
| PSA density (ng/ml/ml) log | 2.16 (1.71–2.71) | <0.0001 | 1578 | 0.66 (0.60–0.71) | 2.12 (1.53–2.91) | <0.0001 | 573 | 0.68 (0.59–0.78) |
| %GP4/5b | ||||||||
| 0–20 | 1.00 (Ref.) | <0.0001 | 1522 | 0.73 (0.69–0.77) | 1.00 (Ref.) | <0.0001 | 533 | 0.78 (0.72–0.84) |
| 30–75 | 5.46 (3.59–8.29) | 11.93 (4.65–30.56) | ||||||
| 100 | 8.93 (5.24–15.19) | 21.25 (7.38–61.20) | ||||||
| %GP4/5b | ||||||||
| 0–30 | 1.00 (Ref.) | <0.0001 | 1535 | 0.72 (0.67–0.76) | 1.00 (Ref.) | <0.0001 | 526 | 0.80 (0.74–0.85) |
| 40–75 | 4.49 (3.05–6.60) | 13.36 (5.59–31.92) | ||||||
| 100 | 6.98 (4.24–11.46) | 19.57 (7.21–53.09) | ||||||
| MRI diameter (mm) | 1.05 (1.03–1.07) | <0.0001 | 1585 | 0.68 (0.63–0.73) | 1.08 (1.05–1.11) | <0.0001 | 554 | 0.78 (0.70–0.84) |
| 0–9 | 1.00 (Ref.) | <0.0001 | 1586 | 0.66 (0.61–0.70) | 1.00 (Ref.) | <0.0001 | 551 | 0.74(0.68–0.81) |
| 10–19 | 2.15 (1.36–3.40) | 9.27 (2.20–39.06) | ||||||
| 20–25 | 3.55 (1.96–6.42) | 19.16 (4.15–88.34) | ||||||
| >25 | 5.07 (2.80–9.18) | 32.37 (7.41–141.40) | ||||||
| Biopsy MCCL (mm) | 1.19 (1.13–1.25) | <0.0001 | 1566 | 0.69 (0.64–0.74) | 1.27 (1.18–1.37) | <0.0001 | 554 | 0.76 (0.68–0.83) |
| <6 | 1.00 (Ref.) | <0.0001 | 1578 | 0.66 (0.62–0.71) | 1.00 (Ref.) | <0.0001 | 564 | 0.72 (0.65–0.79) |
| 6–8 | 2.31 (1.45–3.68) | 3.23 (1.33–7.84) | ||||||
| ≥9 | 4.00 (2.56–6.24) | 6.94 (3.06–15.71) | ||||||
| Volume of grade 4 or 5 (ml) log | 1.02 (1.01–1.04) | 0.013 | 1610 | 0.74 (0.68–0.79) | 1.03 (1.01–1.05) | 0.0001 | 581 | 0.83 (0.76–0.89) |
| Volume of grade 4 or 5 (ml) | ||||||||
| <0.5 | 1.00 (Ref.) | <0.0001 | 1528 | 0.73 (0.68–0.78) | 1.00 (Ref.) | <0.0001 | 524 | 0.82 (0.76–0.88) |
| 0.5–1.0 | 3.34 (1.96–5.68) | 4.17 (1.47–11.77) | ||||||
| 1.01–3.2 | 4.59 (2.93–7.20) | 10.40 (4.86–22.23) | ||||||
| >3.2 | 8.79 (5.49–14.06) | 19.64 (9.32–41.37) | ||||||
| MCCL of grade 4 or 5 (mm) | 1.22 (1.17–1.28) | <0.0001 | 1529 | 0.73 (0.68–0.78) | 1.29 (1.21–1.36) | <0.0001 | 532 | 0.82 (0.75–0.88) |
| <6 | 1.00 (Ref.) | <0.0001 | 1556 | 0.66 (0.61–0.71) | 1.00 (Ref.) | <0.0001 | 554 | 0.72 (0.64–0.80) |
| 6–8 | 3.32 (2.18–5.03) | 4.49 (2.29–8.79) | ||||||
| ≥9 | 6.51 (4.06–10.44) | 8.85 (4.32–18.09) | ||||||
AIC = Akaike information criterion; BCR = biochemical recurrence; CI = confidence interval; EAU = European Association of Urology; %GP4/5 = percentage of Gleason pattern 4 or 5; MCCL = maximum cancer core length; PSA = prostate-specific antigen; Ref. = reference; SHR = subhazard ratio.
The AIC and Harrell's C-index are presented as a guide to each variable’s performance: the lower the AIC and the higher the C-index, the better the prediction.
As a noncontinuous variable; %GP4/5 threshold groups were tested and compared.
Fig. 1.
The cumulative incidence of BCR and metastatic recurrence, as a function of the (A) %GP4/5 and (B) VolGP4/5 threshold values (p < 0.0001; Gray’s test) for all comparisons. BCR = biochemical recurrence; %GP4/5 = percentage of Gleason patterns 4 or 5; VolGP4/5 = volume of Gleason patterns 4 or 5.
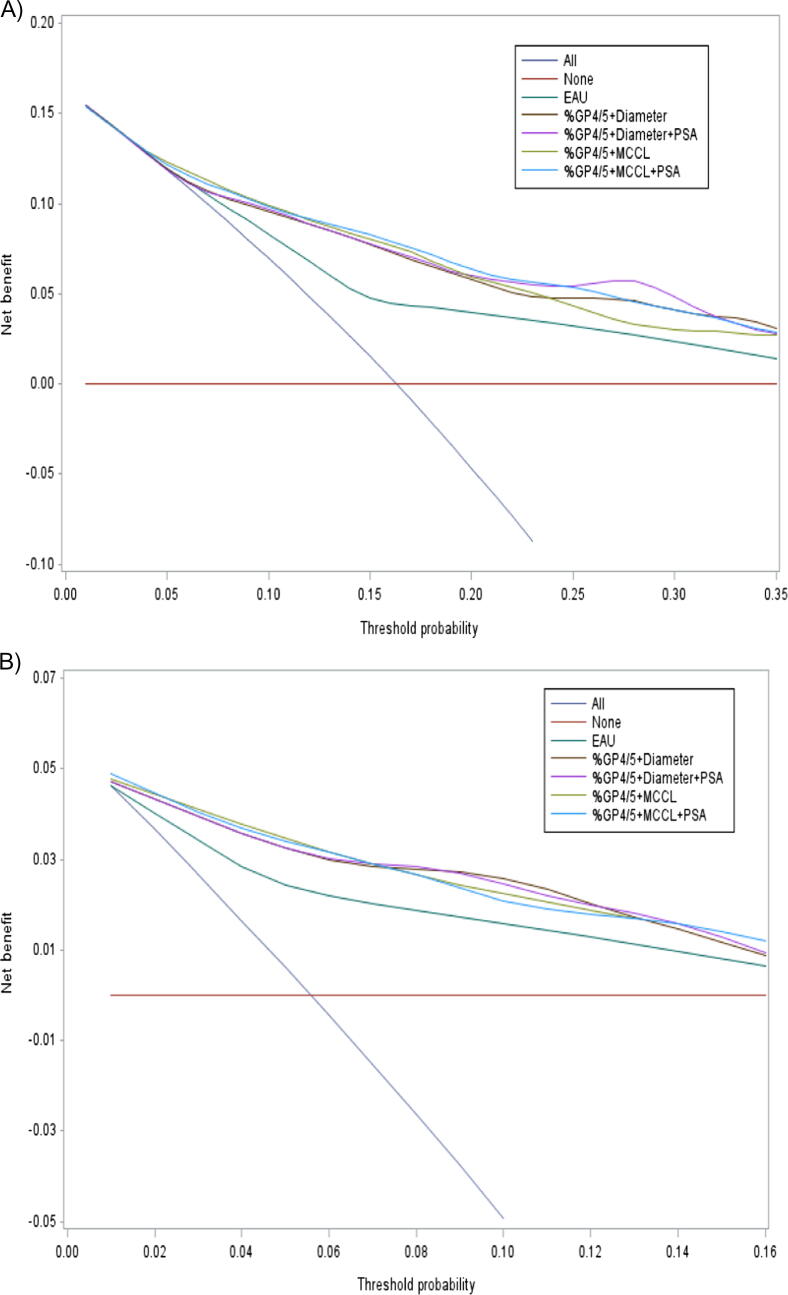
VolGP4/5 classified as a four-level categorical variable (<0.5, 0.5–1.0, 1.01–3.2, >3.2 ml) was the preoperative parameter with best performance for predicting BCR and metastatic recurrence (Table 2). The C-index was 0.73 (0.68–0.78) for BCR and 0.82 (0.76–0.88) for metastatic recurrence. The cumulative incidence curves for the four-level VolGP4/5 variable are shown in Fig. 1B. Relative to VolGP4/5, the EAU risk groups were less predictive for BCR (0.67 [0.63–0.72]) and metastatic recurrence (0.74 [0.67–0.80]). Similarly, DCA revealed that the different models improved clinical risk prediction of BCR and metastatic recurrence compared with the EAU risk tools over all threshold probabilities (Fig. 2).
Fig. 2.
Decision curve analysis comparing the net benefit associated with the use of our combinations of preoperative parameters of interest versus the EAU risk classification systems in predicting the (A) risk of BCR and (B) metastatic recurrence at 5 yr. BCR = biochemical recurrence; EAU = European Association of Urology; %GP4/5 = percentage of Gleason patterns 4 or 5; MCCL = maximum cancer core length; PSA = prostate-specific antigen.
3.4. Performance of preoperative parameters in multivariate analyses
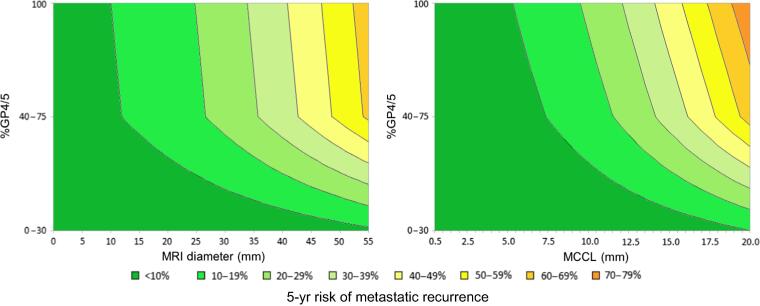
The ability of combinations of EAU components to predict BCR and metastatic recurrence is shown in Supplementary Table 5. The ability of combinations of relevant parameters and %GP4/5 to predict the risk of recurrence risk is shown in Table 3. PSAD was never an independent predictor of metastatic recurrence. The combinations of %GP4/5 + MCCL and %GP4/5 + MRI diameter were used to generate contour plots for the 5-yr risk of metastatic recurrence (Fig. 3) and BCR (Supplementary Fig. 3). Calibration plots showing the observed and predicted probabilities are shown in Supplementary Fig. 4.
Table 3.
The ability of various combinations of preoperative parameters of interest to predict BCR and metastatic recurrence, in a multivariable analysisa
| Variable | SHR (95% CI) | p value | AIC | C-index (95% CI) | ||
|---|---|---|---|---|---|---|
| BCR | Model 1 | %GP4/5 | <0.0001 | 1512 | 0.77 (0.72–0.82) | |
| 0–20 | 1.00 (Ref.) | – | ||||
| 30–75 | 5.03 (3.31–7.63) | <0.0001 | ||||
| 100 | 7.11 (4.08–12.37) | <0.0001 | ||||
| Diameter (mm) | 1.03 (1.01–1.05) | 0.003 | ||||
| Model 2 | %GP4/5 | <0.0001 | 1505 | 0.78 (0.73–0.82) | ||
| 0–20 | 1.00 (Ref.) | – | ||||
| 30–75 | 4.66 (3.05–7.10) | <0.0001 | ||||
| 100 | 6.02 (3.34–10.85) | <0.0001 | ||||
| Diameter (mm) | 1.02 (1.00–1.05) | 0.031 | ||||
| PSA density (ng/ml/ml) log | 1.45 (1.11–1.89) | 0.006 | ||||
| Model 3 | %GP4/5 | <0.0001 | 1500 | 0.77 (0.73–0.82) | ||
| 0–20 | 1.00 (Ref.) | – | ||||
| 30–75 | 4.39 (2.88–6.69) | <0.0001 | ||||
| 100 | 7.01 (4.87–11.79) | <0.0001 | ||||
| MCCL (mm) | 1.14 (1.08–1.20) | <0.0001 | ||||
| Model 4 | %GP4/5 | <0.0001 | 1493 | 0.78 (0.74–0.83) | ||
| 0–20 | 1.00 (Ref.) | – | ||||
| 30–75 | 4.05 (2.64–6.19) | <0.0001 | ||||
| 100 | 5.83 (3.34–10.18) | <0.0001 | ||||
| MCCL (mm) | 1.12 (1.06–1.19) | <0.0001 | ||||
| PSA density (ng/ml/ml) log | 1.46 (1.12–1.89) | 0.004 | ||||
| Metastatic recurrence | Model 5 | %GP4/5 | <0.0001 | 512 | 0.86 (0.79–0.91) | |
| 0–30 | 1.00 (Ref.) | – | ||||
| 40–75 | 11.10 (4.64–26.56) | <0.0001 | ||||
| 100 | 12.20 (4.16–35.72) | <0.0001 | ||||
| Diameter (mm) | 1.05 (1.02–1.08) | <0.0001 | ||||
| Model 6 | %GP4/5 | <0.0001 | 513 | 0.86 (0.80–0.92) | ||
| 0–30 | 1.00 (Ref.) | – | ||||
| 40–75 | 10.68 (4.44–25.62) | <0.0001 | ||||
| 100 | 11.42 (3.80–34.32) | <0.0001 | ||||
| Diameter (mm) | 1.05 (1.02–1.08) | 0.0004 | ||||
| PSA density (ng/ml/ml) log | 1.18 (0.81–1.71) | 0.37 | ||||
| Model 7 | %GP4/5 | <0.0001 | 511 | 0.85 (0.78–0.91) | ||
| 0–30 | 1.00 (Ref.) | – | ||||
| 40–75 | 9.70 (4.08–23.06) | <0.0001 | ||||
| 100 | 14.02 (5.22–37.63) | <0.0001 | ||||
| MCCL (mm) | 1.20 (1.10–1.30) | <0.0001 | ||||
| Model 8 | %GP4/5 | <0.0001 | 512 | 0.85 (0.79–0.92) | ||
| 0–30 | 1.00 (Ref.) | – | ||||
| 40–75 | 9.06 (3.78–21.66) | <0.0001 | ||||
| 100 | 12.63 (4.59–34.74) | <0.0001 | ||||
| MCCL (mm) | 1.19 (1.09–1.29) | <0.0001 | ||||
| PSA density (ng/ml/ml) log | 1.29 (0.92–1.80) | 0.13 |
AIC = Akaike information criterion; BCR = biochemical recurrence; CI = confidence interval; %GP4/5 = percentage of Gleason pattern 4 or 5; MCCL = maximum cancer core length; PSAD = prostate-specific antigen density; Ref. = reference; SHR = subhazard ratio.
Several multivariable models were tested, including PSAD (as a continuous variable, after log transformation), a tumor size variable (either diameter or, after log transformation, the MCCL), and/or %GP4/5 (using the best threshold value from the univariate analysis). The AIC and Harrell's C-index are reported as a guide to the models’ respective performances: the lower the AIC and the higher the C-index, the better the prediction.
Fig. 3.
The %GP4/5 + MCCL combination and the %GP4/5 + greatest dimension combination were used to predict the 5-yr risk of metastatic recurrence, using contour plots. %GP4/5 = percentage of Gleason patterns 4 or 5; MCCL = maximum cancer core length; MRI = magnetic resonance imaging.
4. Discussion
A multivariable model including %GP4/5 and MRI diameter gave a C-index of 0.86 (0.79–0.91) for predicting metastatic recurrence after RP, which is more accurate than the EAU risk group classification C-index of 0.74 (0.67–0.80). The better sampling of the index tumor explains this better prediction of metastatic recurrence. Hence, metastatic recurrence is based on pre-existing lymph node or distant metastases at the time of surgery, linked to the aggressive GP4/5 part of the tumor. Was tumor sampling quality as good as it should be? At final pathology, GG was higher in 31.2% of cases, which resulted in an underestimation of the preoperative %GP4/5 and thus the risk of recurrence. However, it was previously shown that GG, MCCL for TB cores, and tumor index characteristics on MRI were correlated with the grade and volume of clinically significant index tumors at RP on the final pathology [21]. Among clinical questions before local treatment are the choice of curative techniques (RT or surgery), the extent of lymph node dissection, and the use of neoadjuvant drugs (ADT and homologous recombination gene deficiency inhibitors) to prevent metastatic recurrence. These experimental approach rationales will take advantage of prediction models by selecting cases with elevated (>50%, >60%, and >70%) risk of metastatic recurrence, independently of current classifications that estimate all types of recurrences. Outcomes may include, for example, DNA-damage response defects after prostatectomy or median of residual cancer burden [22].
Many classifications have been proposed to predict the outcomes after RP. These classifications are mainly based on historical cohorts of patients, and do not take into account MRI parameters or new histological parameters that may have an important impact on the capacity of staging PCa patients accurately. Recently, Mazzone et al [2] developed a new tool based on clinical and radiological parameters to predict early BCR with a C-index of 70%. Chen et al [23] recently published a study showing that the long diameter of the largest MRI lesion and targeted fusion biopsy ISUP GGs were significantly predictive of BCR after RP. The risk stratification integrating these parameters could better predict the BCR than the traditional model. None of them studied the metastatic risk, which is a validated surrogate for PCa mortality.
The risk of stage migration associated with targeting the aggressive component of the visible lesion has been highlighted by some researchers, and has potential implications for the choice of treatment [24]. However, the risk of downgrading (ie, overestimation of the tumor grade in a TB) was only 6% in our cohort, and similar studies tended to achieve a lower downgrading rate by combining SB and TB [25].
VolGP4/5 treated as a categorical variable (categories: <0.5, 0.5–1.0, 1.01–3.2, and >3.2 ml) was derived from McNeal et al’s [9] research, in which there was a strong correlation between cancer volume, %GP4/5, and nodal metastasis at the time of the RP: 22 of the 38 patients with >3.2 cc of GP4/5 cancer had positive nodes, compared with one of the 171 with <3.2 cc of GP4/5 cancer. In a univariable analysis, we tested several category ranges for %GP4/5: <30% or 40%, between 30% and 40–75%, and 100%. These category ranges differ from the values typically recommended (<5%, 5%, 10%, 20%, and 10% increments thereafter) for GP4 [26].
Models with %GP4/5 + MCCL and %GP4/5 + MRI diameters had similar predictive performances. However, this result should be considered with caution. In fact, the MCCL was based on a single TRUS-guided biopsy on the anteroposterior axis and could not exceed 17 mm (the needle notch); However, MCCL might still be valid for other biopsy routes (eg, the transperineal route) and should be evaluated in these settings.
Our results challenge the conventional EAU risk group classification, and underlined the limitations of cT stage at digital rectal examination (which is not applicable to the one in three cancers that are located anteriorly) and the PSA (which is not PCa specific enough). Only the new prognostic GG classification gave better estimates of the risk of BCR or metastatic recurrence on the basis of the biopsy and the RP specimen [4]. It is noteworthy that the currently used risk classifications were developed and validated in patient cohorts undergoing SBs alone, and the models’ validity in the prebiopsy MRI era remains subject to debate [27]. Given that TV data were available, we did not test the number or percentage of positive SBs in our model. However, the latter parameters might improve the predictions; Gandaglia et al [28] recommended keeping SB results in the new prognostic models. It has also been shown that the number of TBs is not important [29].
4.1. Study limitations
Our model was not corrected for the type of salvage treatment according to PSA doubling time. There were limitations due to inter-reader and intercenter variability of MRI interpretation, and due to interurologist variability of TB hit rate.
Our series might not reflect the whole spectrum of indications for curative treatment. The definition of clinically significant PCa used in the cohort for curative treatment indication is not consensual and was based on previous work. It may not be the same today. In our center, under-representation of the EAU low-risk group reflects the current guidelines. Among cancers diagnosed at a local stage, 299 (16%) were referred to active surveillance, and 147 (8%) patients with a visible tumor on MRI and GG1–2 were referred for high-intensity focused ultrasound in an observational study. Patients referred for RT were not included, since almost half had also undergone ADT for a duration of 1–3 yr, which modifies the risk of metastatic recurrence. However, no differences between surgery and RT were observed in a study based on MRI staging [30]. Lymph node removal was positive for cancer in 2.1%. It reflects a low risk of the operated cohort and a low number of lymph nodes retrieved (median number of two to four per site).
The rationale of this study was based on volume of GP4/5 based on the best biopsy only (TB or SB as a surrogate of TB if sampling the center of the index lesion), evaluating the index tumor only [16], [17], [18]. Other parameters such as % of SB, total cancer length, genomic tests, or the presence of extracapsular extension or seminal vesicle invasion at MRI were not studied. This represents a limitation to our results. In addition to the sampling bias (biopsy guidance), TV based on the greatest dimension of the index tumor on MRI is correlated with (but underestimated by) the lesion volume at RP [31]. However, a validated correction for this underestimation is not available. The inclusion of patients with negative MRI may represent a bias since the diagnosis was obtained at SB and not at TB. However, we decided to include them as they were considered to have significant cancers. Other MRI findings (such as PI-RADS score and staging parameters) were associated with post-treatment cancer recurrence [30]. In a study by Wibmer et al [30], patients with an MRI-visible lesion (PI-RADS score ≥4) had an almost 20% chance of developing metastases and a 10% probability of cancer-specific death. Cancer-specific survival was not studied. Four deaths due to PCa were observed during the study period. However, metastasis-free survival has been proved to be a strong surrogate for overall survival in cases of localized PCa [32]. Lastly, the analysis is biased by the lack of an external validation cohort. Therefore, definitive conclusion on the predictive value of the variables and models under testing cannot be drawn until multicentric validation.
5. Conclusions
Preoperative MRI and TB findings predicted metastatic recurrence after RP accurately. The overall extent of the poorly differentiated component can be evaluated accurately by prebiopsy MRI and TBs of visible lesions that are related to the expertise of the radiologist and the urologist. These findings might enable physicians to provide more personalized patient care.
Author contributions: Jonathan Olivier had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bommelaere, Villers, Olivier.
Acquisition of data: Bommelaere.
Analysis and interpretation of data: Labreuche, Drumez.
Drafting of the manuscript: Bommelaere, Olivier.
Critical revision of the manuscript for important intellectual content: Villers, Ploussard, Puech, Leroy.
Statistical analysis: Labreuche, Drumez.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Olivier.
Other: None.
Financial disclosures: Jonathan Olivier certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Ethics statement: All patients gave their consent to the use of their personal medical data. The study was registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés [Paris, France]; reference: DEC20-123).
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.04.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.EAU. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. https://uroweb.org/guideline/prostate-cancer/#4.
- 2.Mazzone E., Gandaglia G., Ploussard G., et al. Risk stratification of patients candidate to radical prostatectomy based on clinical and multiparametric magnetic resonance imaging parameters: development and external validation of novel risk groups. Eur Urol. 2022;81:193–203. doi: 10.1016/j.eururo.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Kasivisvanathan V., Stabile A., Neves J.B., et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol. 2019;76:284–303. doi: 10.1016/j.eururo.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 4.Sauter G., Steurer S., Clauditz T.S., et al. Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol. 2016;69:592–598. doi: 10.1016/j.eururo.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Rubin M.A., Girelli G., Demichelis F. Genomic correlates to the newly proposed grading prognostic groups for prostate cancer. Eur Urol. 2016;69:557–560. doi: 10.1016/j.eururo.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Choy B., Pearce S.M., Anderson B.B., et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am J Surg Pathol. 2016;40:1400–1406. doi: 10.1097/PAS.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L., Koch M.O., Juliar B.E., et al. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol. 2005;23:2911–2917. doi: 10.1200/JCO.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Cole A.I., Morgan T.M., Spratt D.E., et al. Prognostic value of percent Gleason grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J Urol. 2016;196:405–411. doi: 10.1016/j.juro.2016.01.120. [DOI] [PubMed] [Google Scholar]
- 9.McNeal J.E., Villers A.A., Redwine E.A., Freiha F.S., Stamey T.A. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–1233. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Villers A., Rubin M.A. Re: Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Eur Urol. 2017;71:301. doi: 10.1016/j.eururo.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb J.C., Barentsz J.O., Choyke P.L., et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein J.I., Egevad L., Srigley J.R., Humphrey P.A. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 13.Epstein J.I., Zelefsky M.J., Sjoberg D.D., et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 15.Prentice R.L., Kalbfleisch J.D., Peterson A.V., Flournoy N., Farewell V.T., Breslow N.E. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541. [PubMed] [Google Scholar]
- 16.Fine J.P., Gray R.J. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 18.Wolbers M., Koller M.T., Witteman J.C.M., Steyerberg E.W. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 19.Royston P., Moons K.G.M., Altman D.G., Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 20.Vickers A.J., Elkin E.B. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simopoulos D.N., Sisk A.E., Priester A., et al. Cancer core length from targeted biopsy: an index of prostate cancer volume and pathological stage. BJU Int. 2019;124:275–281. doi: 10.1111/bju.14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay R.R., Xie W., Ye H., et al. Results of a randomized phase II trial of intense androgen deprivation therapy prior to radical prostatectomy in men with high-risk localized prostate cancer. J Urol. 2021;206:80–87. doi: 10.1097/JU.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z., Wu J., Sun K., et al. Risk model based on MRI fusion biopsy characteristics predicts biochemical recurrence after radical prostatectomy. Prostate. 2022;82:566–575. doi: 10.1002/pros.24303. [DOI] [PubMed] [Google Scholar]
- 24.Vickers A., Bennette C., Steineck G., et al. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. Eur Urol. 2012;62:204–209. doi: 10.1016/j.eururo.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploussard G., Beauval J.B., Lesourd M., et al. Added value of concomitant systematic and fusion targeted biopsies for grade group prediction based on radical prostatectomy final pathology on positive magnetic resonance imaging. J Urol. 2019;202:1182–1187. doi: 10.1097/JU.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 26.Epstein J.I., Kryvenko O.N. A comparison of genitourinary society pathology and International Society of Urological Pathology prostate cancer guidelines. Eur Urol. 2021;79:3–5. doi: 10.1016/j.eururo.2020.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Ploussard G., Manceau C., Beauval J.B., et al. Decreased accuracy of the prostate cancer EAU risk group classification in the era of imaging-guided diagnostic pathway: proposal for a new classification based on MRI-targeted biopsies and early oncologic outcomes after surgery. World J Urol. 2020;38:2493–2500. doi: 10.1007/s00345-019-03053-6. [DOI] [PubMed] [Google Scholar]
- 28.Gandaglia G., Ploussard G., Valerio M., et al. Prognostic implications of multiparametric magnetic resonance imaging and concomitant systematic biopsy in predicting biochemical recurrence after radical prostatectomy in prostate cancer patients diagnosed with magnetic resonance imaging–targeted biopsy. Eur Urol Oncol. 2020;3:739–747. doi: 10.1016/j.euo.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Gandaglia G., Martini A., Ploussard G., et al. External validation of the 2019 Briganti nomogram for the identification of prostate cancer patients who should be considered for an extended pelvic lymph node dissection. Eur Urol. 2020;78:138–142. doi: 10.1016/j.eururo.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Wibmer A.G., Chaim J., Lakhman Y., et al. Oncologic outcomes after localized prostate cancer treatment: associations with pretreatment prostate magnetic resonance imaging findings. J Urol. 2021;205:1055–1062. doi: 10.1097/JU.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pooli A., Johnson D.C., Shirk J., et al. Predicting pathologic tumor size in prostate cancer based on multiparametric prostate MRI and preoperative findings. J Urol. 2021;205:444–451. doi: 10.1097/JU.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 32.Xie W., Regan M.M., Buyse M., et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.