Abstract
OBJECTIVES
Cardiac tamponade is a life-threatening complication after cardiac surgery. Echocardiography, both transthoracic (TTE) and transesophageal (TEE), may help to identify cardiac tamponade after surgery, but its diagnostic value remains unverified after cardiac surgery.
METHODS
This retrospective single-centre cohort study used the electronic medical record and echocardiography database of the Catharina Hospital Eindhoven, a tertiary referral cardiothoracic centre, to identify patients who received echocardiography because they were clinically suspected of having cardiac tamponade within the 4 weeks after cardiac surgery. Overall diagnostic accuracy of both TTE and TEE was calculated (sensitivity, specificity, positive predictive value, negative predictive value, and receiver operation characteristics curves). Subgroup analyses were performed based on the timing of the echocardiography after primary surgery (<24, 24–72, >72 h).
RESULTS
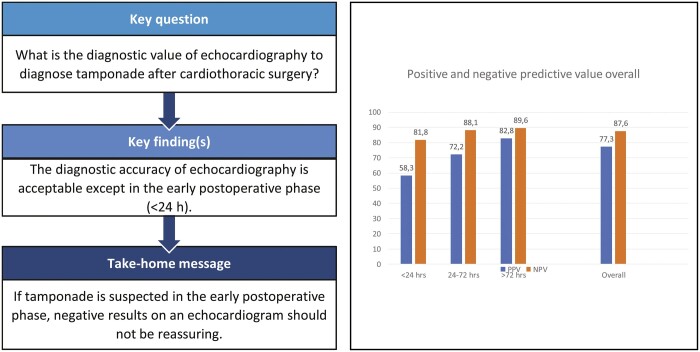
The query identified 427 echocardiographs, 373 TTEs and 54 TEEs, being performed in 414 patients (65% males, mean age 67 years). Of them, 116 patients underwent surgical re-exploration in which a cardiac tamponade was determined in 105 patients with a 30-day mortality of 8.6%. The area under the receiver operation characteristics curve for echocardiography in the 4 weeks after cardiac surgery was 0.78 [95% confidence interval (CI): 0.72–0.84, P < 0.001]. In the first 24 h after surgery was the positive predictive value of echocardiography 58.3% (95% CI: 28.6–83.5) with an area under the curve of 0.64 (95% CI: 0.49–0.80, P = 0.06). The diagnostic accuracy improved over time for both TTE and TEE.
CONCLUSIONS
Diagnostic accuracy of echocardiography in the 4 weeks after cardiac surgery for cardiac tamponade is acceptable and improves over time. However, in the early postoperative phase (<24 h), the diagnostic accuracy of echocardiography is poor.
Keywords: Tamponade, Cardiac surgery, Echocardiography, Ultrasound, Sensitivity, Specificity
Haemodynamic impairment in patients after cardiac surgery is a common challenge with a wide differential.
INTRODUCTION
Haemodynamic impairment in patients after cardiac surgery is a common challenge with a wide differential. Cardiac tamponade is one of the most feared life-threatening complications with mortality rates up to ∼30% [1, 2]. The incidence of cardiac tamponade after cardiothoracic surgery ranges from 0.5% to 8.8% [1, 3]. Although there is no clear consensus, literature differentiates between early (<72 h) and late (>72 h) tamponade depending on the timing after surgery [3, 8]. Early diagnosis and re-exploration are important to prevent further deterioration, serious complications and death [2, 8]. However, re-explorations also increase the risk of acute-kidney injury, atrial fibrillation, sternal wound infections, pulmonary infections, increased number of days on mechanical ventilation and an increased ICU length of stay [2, 9]. Therefore, the threshold to perform additional diagnostic evaluations should be low, while the diagnostic accuracy of these evaluations must be high suitable to the severity of the complication, the lack of specific clinical symptoms, and the need to avoid unnecessary re-explorations [3, 5, 10–12].
In the overall medical population, transthoracic echocardiography (TTE) and transoesophageal echocardiography (TEE) are the cornerstone for the diagnosis of cardiac tamponade with excellent diagnostic properties [13]. However, in patients after cardiothoracic surgery, the diagnostic accuracy of echocardiography is limited by several barriers. In these patients, loculated clots and pericardial effusion without a significant haemodynamic compromise are common but can complicate the interpretation of the echocardiography [1, 4, 10, 14]. In addition, image acquisition can be difficult because of a poor echogenicity due to the inability to position the patient, wounds, dressings, tubing and mechanical ventilation. TEE may overcome these practical drawbacks of TTE. However, evidence on the diagnostic accuracy of TTE and TEE remains scarce in this postoperative setting with broad ranges of sensitivity (34–93% for TTE) and specificity (30–90% for TTE) [1, 3, 5, 8, 10, 11, 15–19]. These broad ranges hamper the involved multidisciplinary team of surgeons, cardiologists and anaesthesiologists or intensivists to determine the value of the TTE or TEE within their decision-making process in patients with a suspected tamponade after cardiac surgery. More insight into the diagnostic accuracy of TTE and TEE will therefore help cardiologists, cardiothoracic surgeons, anaesthesiologists and intensive care specialists to interpret the echocardiographic results for the daily decision-making process at the bedside in case of haemodynamic deterioration after cardiac surgery in the early postoperative period on the ICU or later on the surgical ward.
The aim of this study is to assess the diagnostic accuracy of TTE and TEE in a large cohort of patients who are clinically suspected of having an early or late cardiac tamponade in the first 4 weeks after cardiac surgery.
PATIENTS AND METHODS
Study design
This single-centre retrospective analysis of 427 echocardiographic evaluations was carried out between January 2011 and March 2017 in the Catharina Hospital Eindhoven, a tertiary referral hospital specialized in cardiothoracic surgery. In this time period, 8974 cardiothoracic surgeries were performed. All patients were documented in a fully operational electronic health record with established and stable documentation processes (CS-EZIS test; Chipsoft BV, Amsterdam, Netherlands). Clinical protocols remained unchanged during the period of the study, but alterations in the senior consultant staffs have occurred since new staff members were added to the teams. Approval of the institutional review board was obtained prior to beginning of the study (nWMO-2017.59). Requirement of individual patient consent was waived due to the retrospective design of the study in accordance with the rules of the institutional review board of the Catharina Hospital Eindhoven. The Standards for Quality Improvement Reporting Excellence guidelines were used in preparing this article [20].
Data acquisition and included patients
The electronic medical record (EMR) and the databases of the ultrasound systems were queried to identify patients who were suspected to have cardiac tamponade or had an echocardiographic evaluation in the first 4 weeks after cardiothoracic surgery. Patients were identified for eligibility by querying the EMR for ‘resternotomy for tamponade or bleeding’, ‘tamponade’ or ‘pericardial effusion’ and by gathering all patients who had echocardiographic evaluations within 4 weeks after surgery. Patients were excluded if they had emergency surgery without echocardiography, if the echocardiography did not have a written report in the EMR or ultrasound database. In addition, patients who had pericardiocentesis were also excluded since perioperative confirmation was impossible due to insufficient anaesthesiological registrations to confirm the final diagnosis of a cardiac tamponade. The preoperative intake records, surgery and echocardiography reports and clinical charts from the ICU and cardiothoracic surgery ward of all eligible patients were reviewed.
Diagnosis of cardiac tamponade
Echocardiography
Echocardiographic evaluation, either transthoracic or transoesophageal, was performed by a senior cardiologist or a cardiology resident under supervision of a senior cardiologist (Philips CX50 CompactXtreme or a Philips iE33 xMatrix echo system; Royal Philips Electronics N.V., Amsterdam, Netherlands). The evaluation performed (transthoracic or transoesophageal) was at the discretion of the staff on duty and also depended on the clinical situation. Echocardiography was considered positive for tamponade if pericardial effusion or a clot was seen combined with one of the following: atrial or ventricular collapse, swinging heart, non-pulsatile dilatation of the inferior vena cava and increased respiratory variation in mitral or tricuspid valve flow velocities. These definitions have been used in previous comparable studies [3, 8, 16, 19].
Surgery
Surgical confirmation of tamponade was considered as the standard. Diagnosis of tamponade was confirmed if the surgical report included a documented clinical suspicion by the surgeon, an evacuation of blood or fluid under pressure or an improvement of haemodynamics at incision. If the surgical report was inconclusive, anaesthesiological registrations were reviewed for haemodynamic improvement at incision, defined as a mean arterial pressure increase of >25%, a jugular vein pressure decrease of >5 mmHg or an end tidal CO2 increase of >5%. An increase in mean arterial pressure with a significant decrease of vasopressive or inotropic medication also accounted as a mean arterial pressure increase of >25%. Patients without re-exploration were considered negative for tamponade if they survived 24 h and became haemodynamically stable without surgical intervention.
Outcomes
The primary outcome of this study was to establish the overall diagnostic accuracy of both TTE and TEE assessed as the sensitivity, specificity and positive and negative predicted values of these diagnostic evaluations.
Secondary outcomes included analyses of the diagnostic accuracy of TTE and TEE separately, either for early tamponade or for late tamponade. Subgroups were therefore created based on the time of echocardiography after the first primary surgery (<24, 24–72 and ≥72 h). For each of these subgroups and the overall group, the predictive value of TTE and TEE based on receiver operator characteristic (ROC) curves was established.
Statistics
Statistical analysis was performed with SPSS Statistics 24. Descriptive data were reported in mean with standard deviation or median with interquartile ranges depending on the distribution of the continuous variables by assessing the histograms with Q–Q plots and the Shapiro–Wilk test, or as frequencies with percentages for categorical data (%). Sensitivity, specificity and positive and negative predictive values were calculated as dichotomous variables for predicting cardiac tamponade by echocardiography. ROC curve analysis of sensitivity versus 1 – specificity were performed yielding an area under the curve (AUC) to evaluate the predictive values of the echocardiography for cardiac tamponade after cardiac surgery. An AUC value of <0.7 is considered as poor discrimination, 0.7–0.8 is considered acceptable, 0.8–0.9 is considered excellent and >0.9 is considered outstanding [21, 22]. All the reported P-values are two-sided with a P-value of 0.05 or lower being considered as statistically significant.
Ethical considerations
Approval of the institutional review board was obtained prior to beginning of the study (nWMO-2017.59). Requirement of individual patient consent was waived due to the retrospective design of the study in accordance with the rules of the institutional review board of the Catharina Hospital Eindhoven.
RESULTS
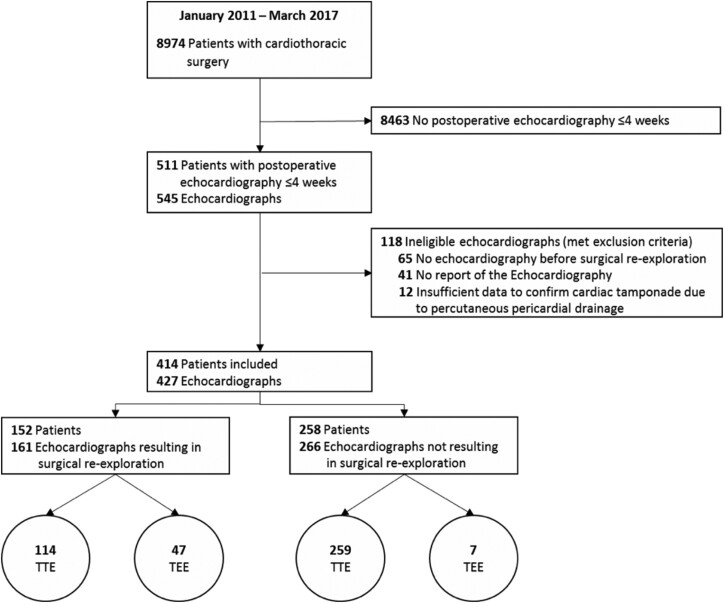
During the study period, cardiac surgery was performed in 8974 patients of whom 174 (1.9%) developed cardiac tamponade determined during surgical exploration. In total, 511 patients were identified of whom 414 patients were eligible for further analysis. In these 414 patients, 427 echocardiographs were performed and 116 surgical explorations within the first 4 weeks after the primary surgery, to rule out or treat a cardiac tamponade. Baseline characteristics are shown in Table 1 and the perioperative findings are reported in Table 2. Of the 414 included patients, a cardiac tamponade was determined in 105 patients. In these patients, the 30-day mortality rate was 8.6% (n = 9/105).
Table 1:
Baseline characteristics
| Patient characteristics | n = 414 |
|---|---|
| Male, n (%) | 279 (65.5) |
| Age (years) | |
| Mean (SD) | 66.6 (10.9) |
| Range | 18–87 |
| BMI (kg/m2) | |
| Mean (SD) | 27.5 (4.8) |
| Range | 16.7–49.8 |
| Emergency surgery, n (%) | 63 (15.2) |
| Surgery type, n (%) | |
| OPCAB | 26 (6.3) |
| CABG | 123 (29.7) |
| Valve | 121 (29.2) |
| CABG + valve | 86 (20.8) |
| Aortic surgery | 47 (11.4) |
| Others | 11 (2.7) |
| Second sternotomy, n (%)a | 25 (6.0) |
| EuroSCORE | |
| Median (IQR) | 4.9 (2.7–11.4) |
| Range | 0.82–84.3 |
| ECC time, min | |
| Mean (95% CI)b | 114.6 (108.7–120.8) |
| Aorta occlusion, min | |
| Mean (95% CI)b | 72.9 (68.8–77.2) |
| Echocardiography | n = 427 |
| TTE, n (%) | 373 (87.4) |
| TEE, n (%) | 54 (12.6) |
| Type of pericardial effusion on echo (%) | |
| Localized | 38 |
| Diffuse | 35 |
| Missing in reports | 27 |
| Time to echocardiography (h) | |
| Median (IQR) | 91.0 (44.0–168.0) |
| Range | 1–701 |
BMI: body mass index; CABG: coronary artery bypass graft; CI: confidence interval; ECC: extracorporeal circulation; IQR: interquartile range; OPCAB: off pump coronary artery bypass; SD: standard deviation; TEE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
For example, re-CABG or re-AVR (aortic valve replacement).
Back-transformed from log-transformation.
Table 2:
Perioperative findings
| Perioperative findings | n = 161 |
|---|---|
| Pericardial effusion (ml), median (IQR) | 500 (137.5–762.5) |
| Improved Mean Arterial Pressure (>25%) (%)a | 68 |
| Central Venous Pressure reduction >5 mmHg (%) | 57 |
| EtCO2 increase >5% (%) | 65 |
| Subjective haemodynamic improvement (%) | 57 |
IQR: interquartile range.
Or reduction of vasopressive or inotropic agents.
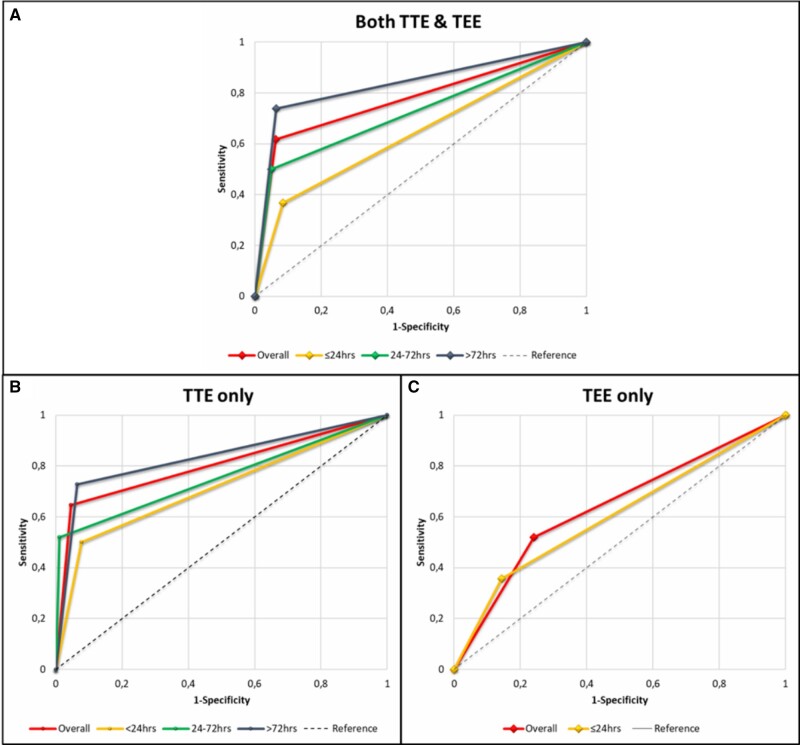
Of 427 echocardiographs, 373 were TTE and 54 were TEE. Table 3 shows the diagnostic accuracies of all echocardiographs together and of the TTE and TEE separately. During the first 72 h after surgery, 163 TTEs and 42 TEEs were performed. In 9.2% (15/163) of the TTEs and 26.1% (11/42) of the TEEs, the echocardiographer was unsuccessful to indicate the presence of a confirmed cardiac tamponade (false negatives). After the first 72 postoperative hours, the percentage of false negatives of TTE was 7.1 (15/210). The ROC curves and their AUC for both TTE and TEE, and for all the subgroups are demonstrated in Fig. 2. The area under the ROC curve for echocardiography, both TTE and TEE, in the first 4 weeks after cardiac surgery was acceptable [AUC of 0.78; 95% confidence interval (CI): 0.72–0.84, P < 0.001]. The AUC for the TTE increased over time, but the AUC did not significantly differ for TTEs that were performed within the first 24 postoperative hours (Table 3). There was an 83% (95% CI: 0.76–0.91, P < 0.001) chance that the cardiologist could correctly distinguish a late cardiac tamponade from another diagnose or normal situation based on a TTE at the bedside.
Table 3:
Diagnostic accuracy of echocardiography for a cardiac tamponade after cardiac surgery
| Overall | TTE and TEE |
TTE |
TEE |
|||
|---|---|---|---|---|---|---|
| n = 410 | n = 361 | n = 54 | ||||
| Sensitivity | 61.8% | (52.0–70.8) | 64.7% | (53.5–74.6) | 52.0% | (31.8–71.7) |
| Specificity | 93.7% | (90.3–96.0) | 95.5% | (92.2–97.5) | 75.9% | (56.1–89.0) |
| PPV | 77.3% | (66.9–85.2) | 80.8% | (69.2–89.0) | 65.0% | (40.9–83.7) |
| NPV | 87.6% | (83.5–90.8) | 90.2% | (86.1–93.2) | 62.9% | (44.9–78.0) |
| AUC | 0.778 | (0.719–0.836) P < 0.001 | 0.801 | (0.737–0.865) P < 0.001 | 0.639 | (0.489–0.790) P = 0.08 |
| ≤24 h | n = 78 | n = 43 | n = 35 | |||
| Sensitivity | 36.8% | (17.2–61.4) | 40.0% | (7.3–83.0) | 35.7% | (14.0–64.4) |
| Specificity | 91.5% | (80.6–96.8) | 94.7% | (80.9–99.1) | 85.7% | (62.6–96.2) |
| PPV | 58.3% | (28.6–83.5) | 40.0% | (9.2–90.8) | 62.5% | (25.9–89.8) |
| NPV | 81.8% | (70.0–89.9) | 92.3% | (78.3–98.0) | 66.7% | (46.0–82.8) |
| AUC | 0.642 | (0.486–0.798) P = 0.06 | 0.674 | (0.380–0.968) P = 0.21 | 0.607 | (0.410–0.805) P = 0.29 |
| 24–72 h | n = 127 | n = 120 | n = 7a | |||
| Sensitivity | 50.0% | (30.4–69.6) | 52.0% | (31.7–71.7) | – | |
| Specificity | 95.0% | (88.3–98.2) | 98.9% | (93.4–99.9) | – | |
| PPV | 72.2% | (46.4–89.3) | 92.9% | (64.2–99.6) | – | |
| NPV | 88.1% | (80.1–93.2) | 88.7% | (80.7–93.8) | – | |
| AUC | 0.755 | (0.598–0.852) P < 0.001 | 0.755 | (0.626–0.884) P < 0.001 | – | |
| >72 h | n = 222 | n = 210 | n = 12a | |||
| Sensitivity | 73.8% | (61.2–83.6) | 72.7% | (59.0–83.9) | – | |
| Specificity | 93.6% | (88.3–96.7) | 93.6% | (88.5–96.9) | – | |
| PPV | 82.8% | (70.1–91.0) | 80.0% | (68.3–88.2) | – | |
| NPV | 89.6% | (83.7–97.7) | 90.6% | (86.2–93.7) | – | |
| AUC | 0.837 | (0.770–0.905) P < 0.001 | 0.831 | (0.757–0.905) P < 0.001 | – | |
AUC: area under curve; NPV: negative predictive value; PPV: positive predictive value; TEE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
Insufficient data for analysis.
Figure 2:
Receiver operation characteristics curves of both transthoracic echocardiography and transoesophageal echocardiography (A), transthoracic echocardiography only (B) and transoesophageal echocardiography only (C) for their predictive value of diagnosing a cardiac tamponade after cardiac surgery (area under the curve and P-values are reported in Table 2).
Figure 1:
Flowchart patient inclusion.
DISCUSSION
In this study, we retrospectively analysed the diagnostic accuracy of echocardiography in patients post-cardiac surgery. A cardiac tamponade was determined in 105 from 8974 patients with a 30-day mortality of 8.6%. The overall diagnostic accuracy of echocardiography was acceptable with a 77% chance that the cardiologist was able to confirm the diagnosis of cardiac tamponade and an 88% chance to rule out this diagnosis. However, during the first 24 h after cardiac surgery our data suggested a poor ability of both TTE and TEE to discriminate patients with and without an early cardiac tamponade. For TTEs that were performed during the first 24–72 postoperative hours and 72 h after surgery this ability increased over time with an 76% and 83% chance that the cardiologist could correctly distinguish a cardiac tamponade from another diagnose or normal situation, respectively. The broad 95% CIs for the TEE suggest that the included population was too small to draw any firm conclusions.
To our knowledge, this study reports on the largest cohort on the diagnostic properties of echocardiography after cardiac surgery. In addition, we deliberately chose to include only patients who in whom echocardiography was performed because of a clinical suspicion of tamponade. This is in contrast to other studies in which also patients with routine echocardiography were included [4, 7, 14]. This influences the a priori chance of tamponade and consequently effects the diagnostic properties of echocardiography.
The diagnostic yield in our cohort was comparable to the results of previous studies, though the sensitivity and specificity of echocardiography improved over time after surgery. The improving diagnostic accuracy of TTE over time is in accordance with previous reports [3, 15, 16, 19]. This improvement is most likely the results of an improving echogenicity, the ability of the patient to cooperate during the echocardiography and the absence of other early postoperative features. In addition, the different aetiologies of an early cardiac tamponade or a late cardiac tamponade might also explain the improvement over time [3–6, 18, 23, 24]. Early tamponade is mostly caused by bleeding and coagulation disorders causing a rapid accumulation of small volumes or localized clots in the pericardial space. As a result, the intrapericardial pressure elevates, which impairs the filling and thus the function of the heart, predominantly the low-pressure atria and right ventricle but also the left ventricle can be compressed [5, 25]. For example, Price et al. showed that a relevant quantity of pericardial effusions could not be identified in 60% of the patients who were clinically suspected for a tamponade in the first 72 h after cardiac surgery [3]. Late effusions are present in 64% of patients, often have a multifactorial origin and develop more slowly with often a lacking or stagnation of clinical recovery or therapy resistant atrial fibrillation [4]. In these patients, a more gradual and equal transfer of intrapericardial pressures in a circumferential fashion causes the classical tamponade physiology and matching echocardiographic findings, Price et al. confirmed these classical signs in 68% of patients with late and large effusions (640 ml ± 71) [3].
Although a TEE can overcome many of the barriers causing the poor echogenicity with TTE in patients with an early tamponade such as the presence of mechanical ventilation, chest tubes, retrosternal air and the reduced cooperation, both sensitivity and specificity of TEE in this study appeared rather poor. The reason for this poor diagnostic accuracy of TEE might be a result of patient selection, because in practice TEEs are mostly performed when the TTE is inconclusive due to the limitations such as air, tubes and difficult to visualize localized clots against the right atrium and ventricle. Previous reports have shown conflicting evidence ranging from excellent to poor diagnostic properties, which frequently depended on the varying priori chance of tamponade in these studies [3, 11, 12, 19, 26]. In addition, all these studies had limited sample sizes and all had a retrospective character. The value of TEE in diagnosing an early cardiac tamponade remains therefore uncertain. Prospective research or retrospective analysis of large series is thus warranted since TEE persists the clinically preferred bedside diagnostic tool in haemodynamically unstable patients if the expertise is nearby. These studies should also focus on the promising modality of having an indwelling TEE probe in the early postoperative phase when the patient is still intubated [27, 28].
Contrast-enhanced computerized tomography (CT) is gaining popularity in the clinics, which can show a global effusion or local haematoma not detected by echocardiography [8, 17, 29, 30]. Ay and Kahraman Ay [8] showed in a small retrospective series of late tamponade a positive predictive value of 97.4% and negative predictive value of 36.3% with an accuracy of 89.7% when a cutoff value of 3.1 cm of pericardial fluid thickness was used. However, CT scanning carries the risk of transportation to the radiology suite, radiation, allergy and kidney failure and fails to provide information on cardiac function.
Future prospective studies should thus especially focus and compare the diagnostic value of TTE, TEE with indwelling probe and CT scan in the early postoperative phase (<72 h) since in this period the greatest clinical benefit can be achieved. At present, considering the reasonably high positive predictive value of TTE, a re-exploration is justified considering the detrimental effects of waiting. A negative TTE result however should not be reassuring, especially not in the early (<72 h) postoperative phase. In this phase, additional diagnostic evaluations with indwelling TEE or a CT scan, or even immediate re-exploration must be considered when the clinical suspicion remains high despite the negative echocardiographic findings.
Limitations
Our study has a few limitations of which the retrospective design is the most important one. We were unable to extract in retrospect the presenting complaints and clinical parameters from the EMR and paper-based records since the digital recording of these factors has changed substantially over the study period. Therefore, subjective interpretations of the clinicians, such as the cardiologists and surgeons, might have influenced the results and the reported presence of a cardiac tamponade on echocardiography or during surgery. We were also unable to report specific echocardiographic parameters and measurements because these findings were not recorded sufficiently when the diagnosis of tamponade was evident or easy to reject at first sight. Patients might also be missed at inclusion if ultrasound results were not reported or when patients did not fit our query. The exclusion of patients with a pericardiocentesis might underestimate the diagnostic value of echocardiography, but the excluded number of patients was limited. Although this is the largest dataset in medical literature describing the diagnostic properties of echocardiography for diagnosing a postoperative tamponade, the number of included echocardiographs in certain subgroups, especially for TEE, is underpowered to draw clear conclusions. Finally, the study included patients until 2017 because the hospital updated the EMR and patient data management system in 2017 which made it impossible to run the same query. However, no new diagnostic tools nor new protocolized work-ups for diagnosing a tamponade have emerged since 2017, so that the study period almost certainly represents current practice.
CONCLUSION
In patients suspected to have cardiac tamponade within the first 4 weeks after cardiac surgery, the overall diagnostic accuracy of echocardiography is acceptable. However, during the first 24 h after surgery the diagnostic accuracy is poor with a positive predictive value of only 58%. Therefore, if tamponade is suspected in the early postoperative phase, a high clinical suspicion may prompt immediate re-exploration. Prospective studies on the diagnostic accuracy of echocardiography, both transthoracic and transoesophageal, in patients after cardiac surgery in larger cohorts are still needed.
ACKNOWLEDGEMENTS
The authors thank Th.W.O. Elenbaas, MD, cardiothoracic surgeon, Catharina Hospital Eindhoven, the Netherlands, and S. van Dort, MD, cardiology department, Catharina Hospital Eindhoven, the Netherlands.
Conflict of interest: A. Bouwman has been clinical consultant with Philips Research since 2016, but this work did not affect this study. Regarding this manuscript, none of the authors have something to disclose.
Author contributions
Dennis F.J. Ellenbroek: Formal analysis; Project administration; Validation; Writing—original draft; Writing—review & editing. Luc van Kessel: Conceptualization; Data curation; Investigation. Wilma Compagner: Formal analysis; Investigation; Software. Tim Brouwer: Data curation; Methodology. R. Arthur Bouwman: Methodology; Resources. Bart A.H.M. van Straten: Resources; Software. Luuk C. Otterspoor: Conceptualization; Supervision. Ashley J.R. De Bie: Conceptualization; Formal analysis; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Cornelius Keyl, Staffan Svenmarker, Praveen K. Varma and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Glossary
ABBREVIATIONS
- AUC
Area under the curve
- CI
Confidence interval
- CT
Computerized tomography
- EMR
Electronic medical record
- ROC
Receiver operation characteristics
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
REFERENCES
- 1. Kuvin JT, Harati NA, Pandian NG, Bojar RM, Khabbaz KR.. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg 2002;74:1148–53. [DOI] [PubMed] [Google Scholar]
- 2. Haneya A, Diez C, Kolat P, Suesskind-Schwendi M. V, Ried M, Schmid C. et al. Re-exploration for bleeding or tamponade after cardiac surgery: impact of timing and indication on outcome. Thorac Cardiovasc Surg 2015;63:51–7. [DOI] [PubMed] [Google Scholar]
- 3. Price S, Prout J, Jaggar SI, Gibson DG, Pepper JR.. ‘Tamponade’ following cardiac surgery: terminology and echocardiography may both mislead. Eur J Cardiothorac Surg 2004;26:1156–60. [DOI] [PubMed] [Google Scholar]
- 4. Pepi M, Muratori M, Barbier P, Doria E, Arena V, Berti M. et al. Pericardial effusion after cardiac surgery: incidence, site, size ande haemodynamic consequences. Br Heart J 1994;72:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russo AM, O'Connor WH, Waxman HL.. Atypical presentations and echocardiographic findings in patients with cardiac tamponade occurring early and late after cardiac surgery. Chest 1993;104:71–8. [DOI] [PubMed] [Google Scholar]
- 6. Carmona P, Mateo E, Casanovas I, Peña JJ, Llagunes J, Aguar F. et al. Management of cardiac tamponade after cardiac surgery. J Cardiothorac Vasc Anesth 2012;26:302–11. [DOI] [PubMed] [Google Scholar]
- 7. You SC, Shim CY, Hong G-R, Kim D, Cho IJ, Lee S. et al. Incidence, predictors, and clinical outcomes of postoperative cardiac tamponade in patients undergoing heart valve surgery. PLoS One 2016;11:e0165754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ay Y, Kahraman Ay N.. Diagnostic value of transthoracic echocardiography and computerized tomography for surgically confirmed late tamponade after cardiac surgery. J Card Surg 2019;34:1486–91. [DOI] [PubMed] [Google Scholar]
- 9. Ruel M, Chan V, Boodhwani M, McDonald B, Ni X, Gill G. et al. How detrimental is reexploration for bleeding after cardiac surgery? J Thorac Cardiovas Surg 2017;154:927–35. [DOI] [PubMed] [Google Scholar]
- 10. Chuttani K, Tischler MD, Pandian NG, Lee RT, Mohanty PK.. Diagnosis of cardiac tamponade after cardiac surgery: relative value of clinical, echocardiographic, and hemodynamic signs. Am Heart J 1994;127:913–8. [DOI] [PubMed] [Google Scholar]
- 11. Grumann A, Baretto L, Dugard A, Morera P, Cornu E, Amiel J-B. et al. Localized cardiac tamponade after open-heart surgery. Ann Thorac Cardiovasc Surg 2012;18:524–9. [DOI] [PubMed] [Google Scholar]
- 12. ten Tusscher BL, Groeneveld JA, Kamp O, Jansen EK, Beishuizen A, Girbes AR.. Predicting outcome of rethoracotomy for suspected pericardial tamponade following cardio-thoracic surgery in the intensive care unit. J Cardiothorac Surg 2011;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imazio M, Adler Y.. Management of pericardial effusion. Eur Heart J 2013;34:1186–97. [DOI] [PubMed] [Google Scholar]
- 14. Ikäheimo MJ, Huikuri HV, Airaksinen KE, Korhonen UR, Linnaluoto MK, Tarkka MR. et al. Pericardial effusion after cardiac surgery: incidence, relation to the type of surgery, antithrombotic therapy, and early coronary bypass graft patency. Am Heart J 1988;116:97–102. [DOI] [PubMed] [Google Scholar]
- 15. Sahni J, Ivert T, Herzfeld I, Brodin LA.. Late cardiac tamponade after open-heart surgery. Scand J Thorac Cardiovasc Surg 1991;25:63–8. [DOI] [PubMed] [Google Scholar]
- 16. Büyükbayrak F, Uyar I, Aksoy E. et al. An evaluation of diagnostic sensitivity of transthoracic echocardiography in diagnosis of post-cardiac surgery tamponade. Turk Gogus Kalp Dama 2014;22:35–42. [Google Scholar]
- 17. Floerchinger B, Camboni D, Schopka S, Kolat P, Hilker M, Schmid C.. Delayed cardiac tamponade after open heart surgery—is supplemental CT imaging reasonable? J Cardiothorac Surg 2013;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bommer WJ, Follette D, Pollock M, Arena F, Bognar M, Berkoff H.. Tamponade in patients undergoing cardiac surgery: a clinical-echocardiographic diagnosis. Am Heart J 1995;130:1216–23. [DOI] [PubMed] [Google Scholar]
- 19. Imren Y, Tasoglu I, Oktar GL, Benson A, Naseem T, Cheema FH. et al. The importance of transesophageal echocardiography in diagnosis of pericardial tamponade after cardiac surgery. J Card Surg 2008;23:450–3. [DOI] [PubMed] [Google Scholar]
- 20. Goodman D, Ogrinc G, Davies L, Baker GR, Barnsteiner J, Foster TC. et al. Explanation and elaboration of the squire (standards for quality improvement reporting excellence) guidelines, v.2.0: examples of squire elements in the healthcare improvement literature. BMJ Qual Saf 2016;25:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosmer DSL. Assessing the fit of the model. In: Shewhart WA, Wilks SS. Applied Logistic Regression, 2nd edn. Wiley Online Library: John Wiley and Sons, 2000, 160–164 (Chapter 5). [Google Scholar]
- 22. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5:1315–6. [DOI] [PubMed] [Google Scholar]
- 23. Faehnrich JA, Noone RB, White WD, Leone BJ, Hilton AK, Sreeram GM. et al. Effects of positive-pressure ventilation, pericardial effusion, and cardiac tamponade on respiratory variation in transmitral flow velocities. J Cardiothorac Vas Anesth 2003;17:45–50. [DOI] [PubMed] [Google Scholar]
- 24. Ionescu A, Wilde P, Karsch KR.. Localized pericardial tamponade: difficult echocardiographic diagnosis of a rare complication after cardiac surgery. J Am Soc Echocardiogr 2001;14:1220–3. [DOI] [PubMed] [Google Scholar]
- 25. Yamada E, Zhang Y, Davies R, Coddington W, Kerber RE.. Phased-array intracardiac echocardiographic imaging of acute cardiovascular emergencies: experimental studies in dogs. J Am Soc Echocardiography 2002;15:1309–14. [DOI] [PubMed] [Google Scholar]
- 26. Reichert CL, Visser CA, Koolen JJ, Vd Brink RB, van Wezel HB, Meyne NG. et al. Transesophageal echocardiography in hypotensive patients after cardiac operations. Comparison with hemodynamic parameters. J Thorac Cardiovasc Surg 1992;104:321–6. [PubMed] [Google Scholar]
- 27. Begot E, Clavel M, Piccardo A, Bellier R, François B, Pichon N. et al. Hemodynamic monitoring using a single-use indwelling transesophageal echocardiography probe in an unstable patient after open-heart surgery. BMC Med Imaging 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maltais S, Costello WT, Billings FT, Bick JS, Byrne JG, Ahmad RM. et al. Episodic monoplane transesophageal echocardiography impacts postoperative management of the cardiac surgery patient. J Cardiothorac Vasc Anesth 2013;27:665–9. [DOI] [PubMed] [Google Scholar]
- 29. Solem JO, Kugelberg J, Stahl E, Olin C.. Late cardiac tamponade following open-heart surgery. Scand J Thorac Cardiovasc Surg 1986;20:129–31. [DOI] [PubMed] [Google Scholar]
- 30. Restrepo CS, Lemos DF, Lemos JA, Velasquez E, Diethelm L, Ovella TA. et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics 2007;27:1595–610. [DOI] [PubMed] [Google Scholar]