Abstract
Context.—
It is unclear whether HER2+ tumors expressing both estrogen receptor (ER) and progesterone receptor (PR), that is, triple-positive breast carcinomas (TPBCs), show unique morphologic and clinical features and response to neoadjuvant chemotherapy (NAC).
Objective.—
To study the morphologic and immunohistochemical features of TPBCs from patients who underwent NAC.
Design.—
We retrospectively reviewed core biopsy and post-NAC slides of 85 TPBCs. H-scores were calculated for ER and PR. HER2 slides and fluorescence in situ hybridization (FISH) reports were reviewed. Residual cancer burden was calculated for post-NAC specimens.
Results.—
Eighty-one of the 85 tumors (95.3%) showed ductal histology, 3 (3.5%) were invasive lobular carcinomas, and 1 (1.2%) showed mixed ductal and lobular features. A subset showed mucinous (n=7, 8.2%), apocrine (n = 5, 5.9%), and/or micropapillary (n = 4, 4.7%) differentiation. Fifty-four TPBCs (63.5%) showed high ER expression (H-score >200), including 27 (31.8%) with high expression of ER and PR. Fifty-two tumors (61.1%) showed HER2 3+staining. Mean HER2/CEP17 ratio by FISH was 3.6 (range, 2–12.2) and mean HER2 signals per cell was 8 (range, 3.7–30.4). Pathologic complete response (pCR) rate was 35.3% (30 of 85). HER2 3+ staining was the only significant predictor of pCR on multivariate analysis (odds ratio=9.215; 95% CI, 2.401–35.371; P < .001). The ER/PR expression did not correlate with response to therapy.
Conclusions.—
TPBCs are heterogeneous with some showing mucinous, lobular, or micropapillary differentiation. The pCR rate of TPBCs is similar to that reported for ER+/PR−/HER2+ tumors. HER2 overexpression by IHC was associated with significantly better response to therapy and may help select patients for treatment in the neoadjuvant setting.
HER2 protein overexpression/gene amplification is seen in approximately 15% to 25% of invasive breast carcinomas and has historically been associated with aggressive histopathologic features and poor clinical outcome.1,2 HER2-targeted therapy has been shown to improve disease-free and overall survival in patients with HER2+ tumors and is administered in the adjuvant, neoadjuvant, and metastatic settings.3,4 Patients treated with HER2-targeted therapy show high rates of pathologic complete response (pCR) and improved survival rates.5,6 Currently, neoadjuvant chemotherapy in combination with HER2-targeted therapy is increasingly offered to patients presenting with stage II-III HER2+ breast carcinomas.
Approximately half of HER2+ breast carcinomas express hormone receptors (HRs), namely, estrogen receptor (ER) and/or progesterone receptor (PR).7 The degree of HR expression in HR+/HER2+ invasive carcinomas is variable; however, most HR+/HER2+ tumors tend to express ER at a low level and are negative for PR. It is well established that HR−/HER2+ and HR+/HER2+ breast carcinomas differ with respect to prognosis and response to systemic treatment, specifically HER2-targeted therapies (trastuzumab, pertuzumab) and endocrine therapy (tamoxifen, aromatase inhibitors). In the neoadjuvant setting, significantly greater rates of pCR are seen in HR−/HER2+ tumors than in HR+/HER2+ tumors, both with and without the use of neoadjuvant HER2-targeted therapies.5,8,9
Triple-positive breast carcinoma (TPBC) is a subset of HR+/HER2+ tumors expressing both ER and PR. To date, only few published reports have specifically studied TPBC and have focused on clinical outcomes in the adjuvant setting.10–12 In these studies, it appears that TPBCs behave more akin to “luminal” tumors than “HER2-enriched” tumors and show lesser degrees of response to trastuzumab.
No published studies have specifically addressed the histopathology of TPBC and it is unclear whether this subset of tumors displays unique morphologic features. Further, a detailed assessment of ER and PR expression in TPBC and the relationship with HER2 expression and response to neoadjuvant chemotherapy have not been reported. We studied a series of TPBCs treated with neoadjuvant chemotherapy plus HER2-targeted therapy to further characterize this group of tumors with respect to morphologic features, HR and HER2 staining patterns, and fluorescence in situ hybridization (FISH) results, and response to neoadjuvant chemotherapy.
MATERIALS AND METHODS
Patient Selection
This study was conducted under an institutional review board–approved protocol. An electronic search was performed for patients who underwent definitive surgery following neoadjuvant chemotherapy for invasive breast carcinoma during an 11-year period (2008–2018). We identified patients with tumors in pretreatment core biopsies positive by immunohistochemistry (IHC) for ER (6F11, Leica, Buffalo Grove, Illinois) and PR (16, Leica) and showed HER2 overexpression (3+ staining) by IHC (4B5,Ventana, Tucson, Arizona) or amplification by FISH (HER2 IQFISH pharmDx, Dako, Carpinteria, California; PathVysion HER-2 DNA Probe Kit, Vysis, Downers Grove, Illinois) according to current guidelines.13–15 Cases with IHC and/or FISH performed at our institution and with IHC slides available for review were included. Clinical and imaging data were retrieved from electronic medical records.
Slide Review
Hematoxylin-eosin slides from core biopsy and excision samples were reviewed by 3 pathologists. Each tumor was graded according to the Nottingham System. Histologic type and any notable morphologic features such as mucinous, micropapillary, apocrine, or lobular differentiation were recorded. The ER, PR, and HER2 IHC-stained slides were reviewed for all cases. ER and PR were assessed by using a semiquantitative approach to generate an H-score for each case. The H-score, which produces a value ranging from 0 (no staining) to 300 (diffuse strong staining), represents the sum of percentage of tumor nuclei staining at each intensity level multiplied by the staining intensity (0=staining, 1=weak staining, 2=moderate staining, and 3=strong staining).16 H-scores for HRs were grouped into 3 categories: low positive (1–100), moderate positive (101–200), and high positive (201–300).17 HER2 IHC stains were scored: 0, 1+, 2+, or 3+.14 For cases in which FISH was performed, the number of HER2 signals per cell and HER2/CEP17 ratio were recorded from the original FISH reports.
Slides were reviewed from postneoadjuvant surgical excision specimens and residual cancer burden (RCB) was calculated for each case to assess the extent of residual disease.18 The ER, PR, and HER2 IHC slides as well as FISH results, when available, were reviewed for postneoadjuvant surgical excision specimens.
Statistical Analyses
All statistical analyses were performed with the SPSS software 22.0 (IBM Corporation, New York, New York). The pathologic features, results of ER, PR, HER2, and clinical parameters were compared by using appropriate statistical tests, that is, Fisher exact test for nonparametric variables, 2-tailed Student t test or 1-way analysis of variance for continuous variables, and Pearson correlation for correlation between continuous variables. Univariate and multivariate logistic regression model was performed to study the impacts of biopsy characteristics to predict pCR. P values less than .05 were considered to be statistically significant.
RESULTS
Clinical Features and Treatment
The study included 85 patients (84 women, 1 man) with a median age of 47 years at the time of diagnosis (range, 26–70 years) (Table 1). The mean clinical tumor size at presentation was 3 cm (range, 1.1–6.5 cm), with 83.5% (71 of 85) of patients having at least clinical T2 (>2 cm) tumors. Four patients (4.7%) presented with inflammatory carcinoma. Fifty patients (58.9%) were clinically node-positive and/or had biopsy-proven nodal metastasis before chemotherapy. Most patients (72 of 85, 84.7%) received AC-THP (doxorubicin, cyclophos-phamide, paclitaxel, trastuzumab, pertuzumab) as the neoadjuvant regimen. Fifty-two patients (61.2%) underwent mastectomy and 33 (38.8%) had breast-conserving surgery.
Table 1.
Clinical and Histomorphologic Features of Studied Patients and Hormone Receptor and HER2 Results Determined in Pretreatment Core Biopsies
| Characteristic | No. | % |
|---|---|---|
| Age, median (range) | 47 (26–70) | |
| Clinical T stage | ||
| cT1 | 14 | 16.5 |
| cT2 | 58 | 68.2 |
| cT3 | 9 | 10.6 |
| cT4 | 4 | 4.7 |
| Nodal status | ||
| Positive | 50 | 58.9 |
| Negative | 35 | 41.1 |
| Histologic type | ||
| Ductal | 81 | 95.3 |
| Lobular | 3 | 3.5 |
| Mixed ductal and lobular | 1 | 1.2 |
| Histologic grade | ||
| 2 | 43 | 50.6 |
| 3 | 42 | 49.4 |
| ER IHC (H-score) | ||
| 0–100 | 12 | 14.1 |
| 101–200 | 19 | 22.4 |
| 201–300 | 54 | 63.5 |
| PR IHC (H-score) | ||
| 0–100 | 35 | 41.2 |
| 101–200 | 17 | 20 |
| 201–300 | 33 | 38.8 |
| HER2 IHC/FISH | ||
| IHC 0/1 + | 5 | 5.9 |
| HER2/CEP17 ratio, mean (range) | 2.46 (2.3–2.9) | |
| HER2 signals/cell, mean (range) | 6.32 (4.6–9.3) | |
| IHC 2+ | 28 | 33 |
| FISH ratio, mean (range) | 3.41 (2–9.6) | |
| HER2 signals/cell, mean (range) | 7.28 (4.1–18.4) | |
| IHC 3+ | 52 | 61.1 |
| FISH ratio, mean (range) | 5.93 (3–12.2) | |
| HER2 signals/cell, mean (range) | 15 (7.1–30.4) |
Abbreviations: ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor type 2; IHC, immunohistochemistry; PR, progesterone receptor.
Morphologic Features of Triple-Positive Breast Carcinomas in Pretreatment Biopsies
Eighty-one of the 85 tumors (95.3%) were invasive ductal carcinomas and 3 (3.5%) were invasive lobular carcinomas, including 2 with pleomorphic morphology and 1 with classic morphology. One patient (1.2%) had invasive carcinoma with mixed ductal and lobular features. Seven tumors (8.2%) showed mucinous differentiation, 5 (5.9%) showed apocrine morphologic features, and 4 (4.7%) had micropapillary features (Figure 1, A through D). One case had both mucinous and micropapillary features (Figure 2, A through F), and another single case had mucinous and apocrine features. The tumors were Nottingham histologic grade 2 in 43 cases (50.6%) and grade 3 in 42 cases (49.4%).
Figure 1.
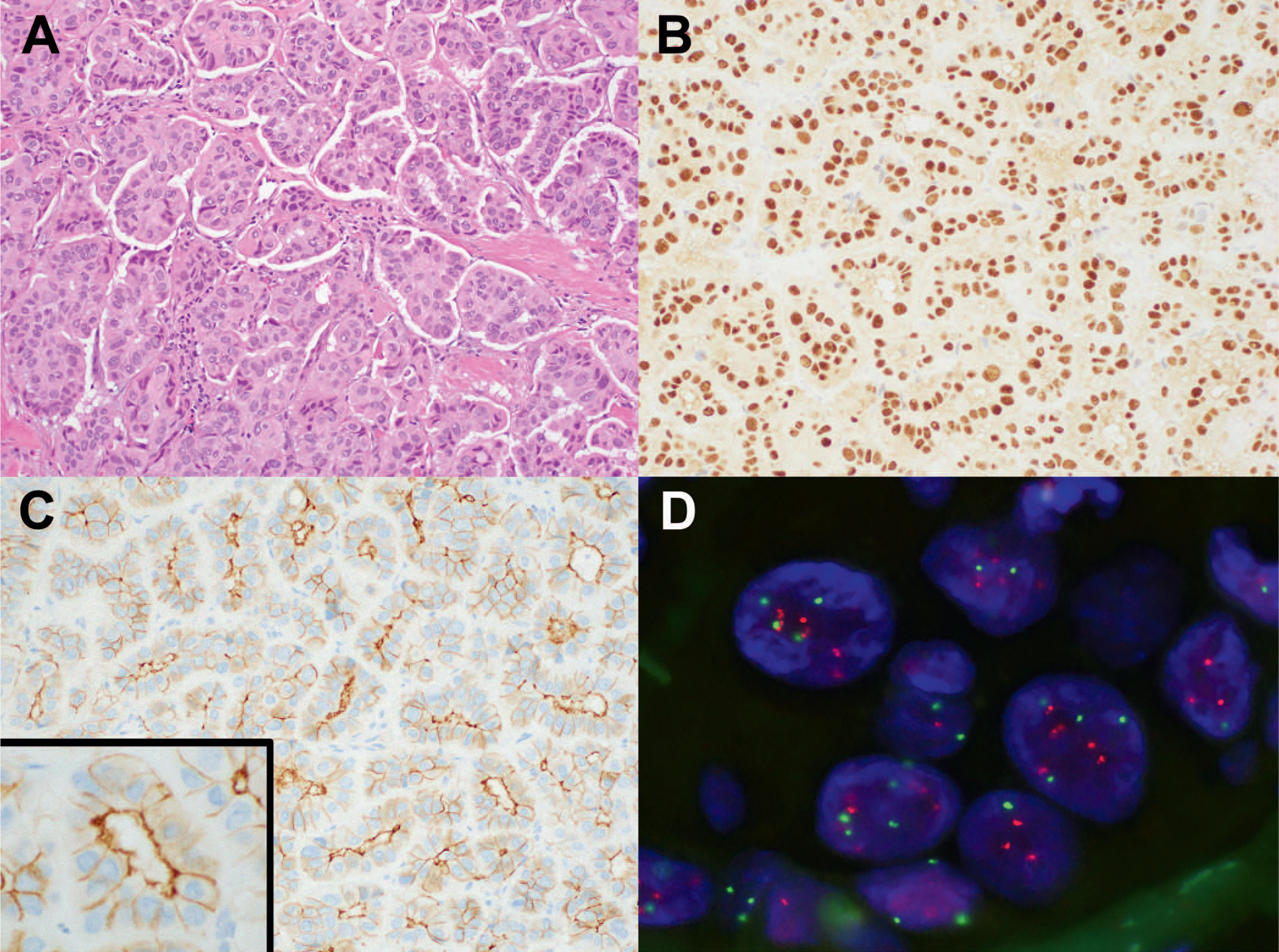
A, Invasive ductal carcinoma with micropapillary features in a core biopsy. B, ER is diffusely positive with strong nuclear staining. PR (not shown) stained approximately 20% of tumor cells with moderate intensity. C, HER2 immunohistochemistry shows 2+ staining with a “basolateral” pattern. D, HER2 amplification determined by FISH with HER2/CEP17 ratio of 2.2 and 5.2 HER2 signals per cell (hematoxylin-eosin, original magnification ×20 [A]); original magnification ×20 [B]; HER2 immunohistochemistry, original magnifications ×20 [C] and ×40 [inset C]). Abbreviations: ER, estrogen receptor; FISH, fluorescence in situ hybridization; PR, progesterone receptor.
Figure 2.
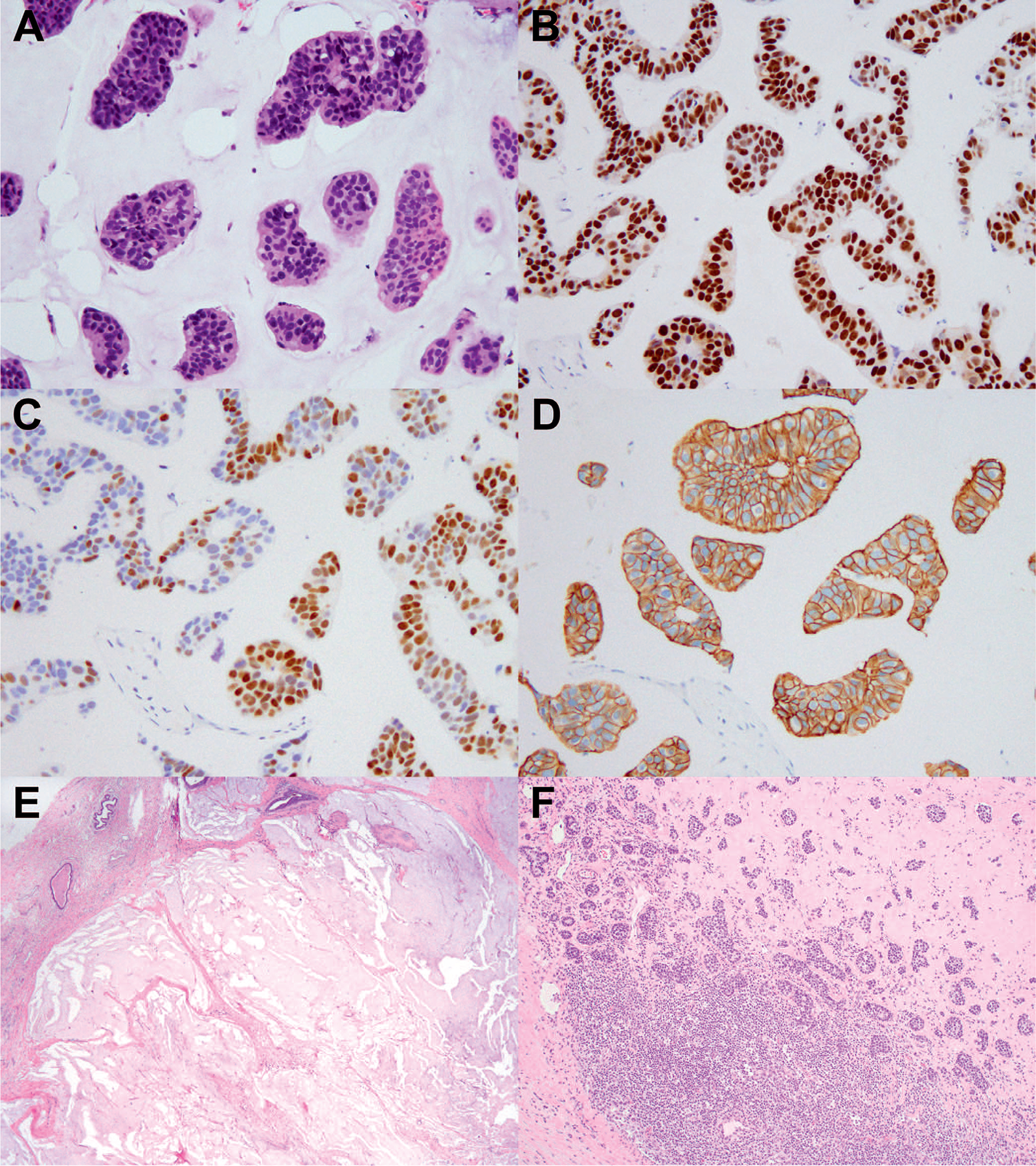
A, Triple-positive breast carcinoma in pretreatment core biopsy showing mucinous and micropapillary features. B, Invasive carcinoma shows diffuse and strong nuclear staining with ER. C, Variable staining with PR. D, HER2 showed 3+ staining. E, Postneoadjuvant excision specimen showing a complete response in the breast, characterized by a tumor bed composed of mucin pools only. F, Residual carcinoma was identified in an axillary lymph node associated with fibrosis, indicative of partial response (hematoxylin-eosin, original magnifications ×20 [A], ×2 [E], and ×4 [F]); original magnification ×20 [B through D]). Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
ER and PR Immunohistochemical Staining Results Before Neoadjuvant Chemotherapy
TPBCs showed high expression of ER in 63.5% (54 of 85) of cases as assessed by H-score (high expression: 201–300) (Table 1). High expression of PR was observed in 38.8% (33 of 85) of cases. Twenty-seven of the 85 tumors (31.8%) showed high expression of both ER and PR (Figure 3, A through C), while 8 (9.4%) showed low expression (H-score: 1–100) of both hormone receptors. The median H-scores for ER and PR were 270 (mean, 216.8; range, 1–300) and 150 (mean, 147.0; range, 1–297), respectively.
Figure 3.
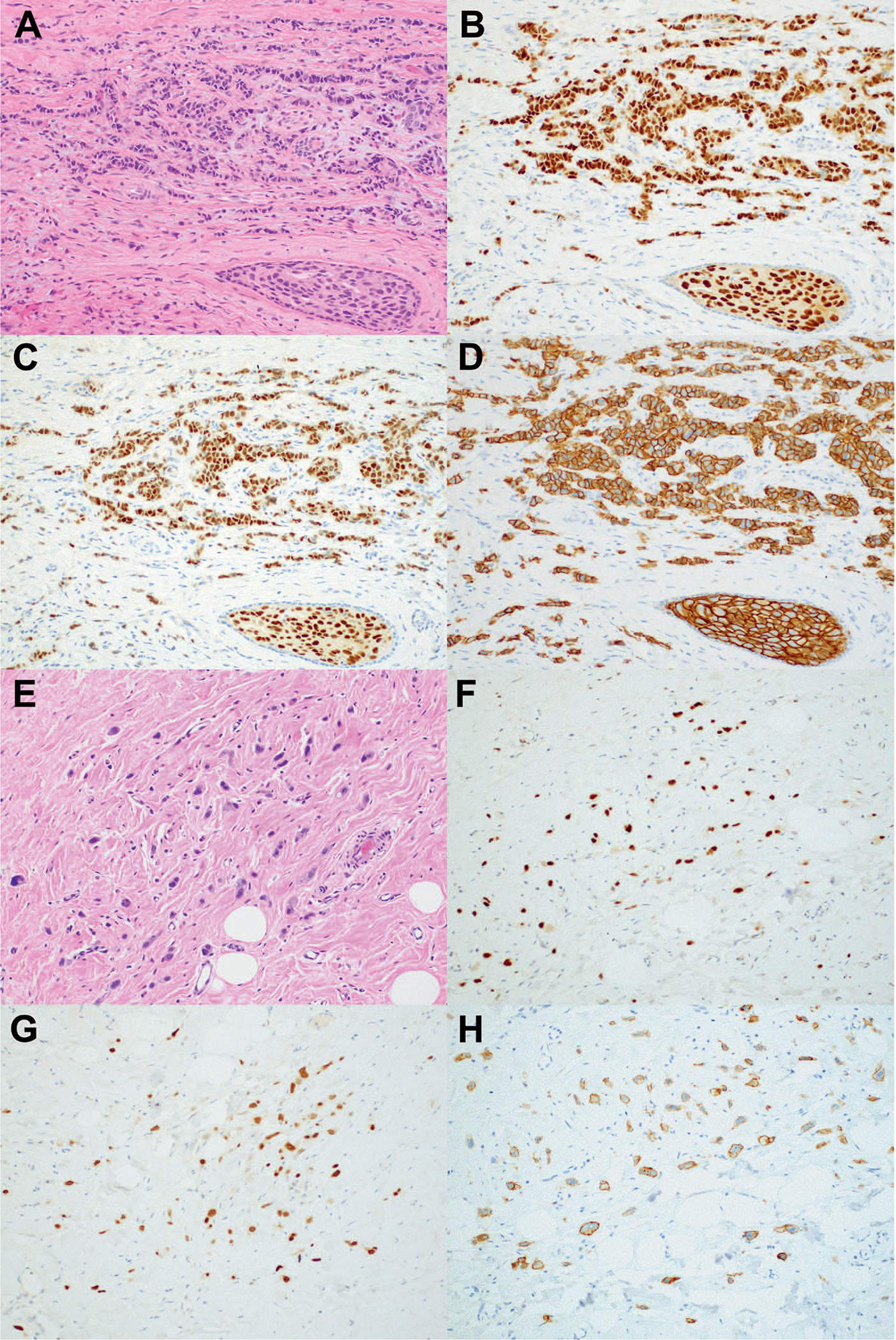
Triple-positive breast carcinoma and associated DCIS in pretreatment core biopsy (A) shows strong and diffuse staining for (B) ER and (C) PR, and (D) 3+ HER2 staining. E, Postneoadjuvant excision shows residual invasive carcinoma with treatment effect and positive staining with (F) ER, (G) PR, and (H) HER2 (hematoxylin-eosin, original magnification×10 [A and E]; original magnification ×10 [B, C, D, F, G, and H]). Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; PR, progesterone receptor.
HER2 Immunohistochemical Staining and HER2 FISH Results Before Neoadjuvant Chemotherapy
Fifty-two tumors (61.1%) showed 3+ staining, 28 (33%) showed 2+staining, and 5 (5.9%) showed 0/1+staining (Table 1). FISH was performed in 36 cases including 4 cases that showed 3+ HER2 IHC staining. The mean FISH HER2/CEP17 ratio for all tumors studied was 3.6 (range, 2–12.2) and the mean HER2 signals per cell was 8 (range, 3.7–30.4). Sixteen tumors (44.4%) showed “low amplification” defined as HER2/CEP17 ratio of 2 or greater and 4 to 6 HER2 signals per cell,14 which included 14 tumors with 2+ staining and 2 with 0/1+ staining. No tumors showed other “nonclassical” FISH results such as “monosomy-like” or “coamplification/polysomy.”15
The mean HER2 FISH ratio was significantly greater in IHC 3+ tumors than in those with 0 to 2+ staining (5.93 versus 3.27, P = .03), as was the mean number of HER2 signals per cell (15.03 versus 7.06, P=.002). Grade 3 tumors showed significantly more frequent HER2 3+ staining than grade 2 tumors (76.2% versus 46.5%, P = .007). One tumor with micropapillary features showed a basolateral HER2 staining pattern,19 characterized by relatively weak membranous staining that is noncircumferential and more pronounced in the basolateral aspect of the gland not facing the stroma (Figure 1, C). Per American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines this tumor was scored as 2+and reflexed to FISH, which showed gene amplification (HER2/CEP17: 2.2; HER2 signals per cell: 5.2) (Figure 1, D). Of the 3 lobular carcinomas in this series, the 2 with pleomorphic morphology showed 3+ staining. The classic lobular carcinoma reportedly yielded 2+ staining at an outside hospital, but repeated staining in our laboratory showed 1+ staining. Nonetheless, FISH was performed in this case and showed gene amplification with HER2/CEP17 ratio of 2.4.
Relationship Between Hormone Receptor and HER2 Results Before Neoadjuvant Chemotherapy
The results of hormone receptor expression compared with HER2 IHC results are summarized in Table 2. Neither the expression of ER nor that of PR by H-score correlated with HER2 IHC expression (P = .35, P = .19, respectively), HER2 FISH ratio (P = .08, P = .47, respectively), or HER2 signals per cell (P=.11, P=.17, respectively). Further, cases that showed either high expression or low expression of both ER and PR also did not show a significant correlation with HER2 staining, FISH ratio, or HER2 signals per cell.
Table 2.
ER and PR Results With Corresponding HER2 Immunohistochemical Staining Results
| HER2 Immunohistochemistry, n (%) | Total | P Value | ||
|---|---|---|---|---|
| 0–2+ | 3+ | |||
| ER IHC (H-score) | .35 | |||
| 0–100 | 3 (25) | 9 (75) | 12 | |
| 101–200 | 10 (52.6) | 9 (47.4) | 19 | |
| 201–300 | 20 (37) | 34 (63) | 54 | |
| PR IHC (H-score) | .19 | |||
| 0–100 | 9 (25.7) | 26 (74.3) | 35 | |
| 101–200 | 10 (58.8) | 7 (41.2) | 17 | |
| 201–300 | 14 (42.4) | 19 (57.6) | 33 | |
| ER/PR combined (H-score) | ||||
| ER low/PR low | 1 (12.5) | 7 (87.5) | 8 | .14 |
| ER high/PR high | 11 (40.7) | 16 (59.3) | 27 | .82 |
| All others | 21 (42) | 29 (58) | 50 | |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; IHC, immunohistochemistry; PR, progesterone receptor.
Pathologic Response to Neoadjuvant Chemotherapy
Overall, 30 of 85 patients’ tumors (35.3%) showed pCR following neoadjuvant chemotherapy. In the group of patients who were node pretreatment node-positive, 28 of 50 (56%) were downstaged to N0 following chemotherapy. pCR was significantly associated with Nottingham grade: 50% (21 of 42) of grade 3 tumors showed complete response versus 21% (9 of 43) of grade 2 tumors (P = .007). pCR was significantly more frequent for patients whose tumors showed 3+ IHC staining than for those with tumors with 0 to 2+ IHC staining and HER2 amplification by FISH (52% versus 9%, P < .001). Among the 36 tumors with pretreatment HER2 FISH assessments, neither the mean HER2/CEP17 ratio nor the mean HER2 signals per cell were significantly different between cases that achieved pCR and those that did not (ratio: 3.58 versus 3.55, respectively; P = .97) (HER2 signals per cell: 8.54 versus 7.63, P = .6). The degree of ER and PR expression was not significantly associated with response to therapy. The mean ER H-score for tumors with pCR was 212.93, versus 219.02 for those that did not achieve pCR (P=.76), and the mean PR H-score was also not significantly different between the groups (148.3 versus 146.33, P = .94). These values were also not significant when comparing ER and PR H-scores with RCB continuous values.
HER2 IHC (3+ versus 0–2+) was the only significant predictor for pCR by both univariate and multivariate logistic regression analyses (univariate analysis: odds ratio [OR] = 10.8, 95% CI, 2.927–39.849, P < .001; multivariate analysis: OR = 9.215, 95% CI, 2.401–35.371, P < .001). Histologic grade was associated with improved pCR on univariate (P = .007) but not multivariate (P = .07) analysis, whereas ER H-score and PR H-score did not correlate with pCR.
Change in Hormone Receptor Results and HER2 Results After Neoadjuvant Chemotherapy
ER, PR, and HER2 assessments were repeated in 30 cases with residual invasive carcinoma in the postneoadjuvant surgical specimen (Figure 3, A through H). The residual invasive carcinoma was ER-negative in 3 cases (10%) and PR-negative in 4 cases (13.3%). HER2 was negative by IHC (0/1+) in 11 (36.7%) residual carcinomas. FISH was performed in 2 cases negative by IHC, and 1 was HER2 amplified. HER2 was 2+ in 11 cases (36.7%), and FISH was positive in 6 of 7 of these 2+ cases. The remaining cases tested showed 3+ HER2 staining. The overall rate of change from HER2+ to HER2− was 36.7% (11 of 30 cases).
Follow-up Information
During a median follow-time period of 33.0 months (mean, 39.9; range; 5.0–117.0), 12 patients (14.1%) developed distant metastasis and 5 (5.9%) died of disease. The median time to distant metastasis was 26 months (mean, 19; range, 1–66). RCB was significantly associated with distant metastasis by RCB classes (P = .03) and by RCB as a continuous variable (mean RCB value in nonmetastatic cases, 1.24; mean RCB value in metastatic cases, 2.93, P < .001). Among patients with distant metastasis, 9 of 12 had extensive residual disease (RCB 3), 1 patient had moderate residual disease (RCB 2), and 2 patients had pCR.
DISCUSSION
We performed a detailed morphologic and immunohistochemical study to better understand the histopathologic features of TPBC and the correlation between ER and PR expression with HER2 expression in these tumors. To address these questions, our study focused on patients treated in the neoadjuvant setting and we examined which features in pretreatment core biopsies were associated with response to neoadjuvant chemotherapy.
In our series, TPBCs showed a pCR rate of 35.3% after neoadjuvant chemotherapy. This pCR rate is similar to what is observed in HR+/HER2+ tumors in other series5,20 and significantly lower than the pCR rate seen in HR−/HER2+ tumors. In contemporary studies with similar regimens, patients with HER2+ tumors treated with dual HER2-targeted therapies experience rates of pCR between 50% and 65%.9,21,22 The pCR rate of tumors showing 3+ HER2 IHC staining was significantly greater than for tumors lacking protein overexpression by IHC and showing amplification by FISH (52% versus 9%), and 3+ IHC staining was the only factor associated with pCR on multivariate analysis. This finding is consistent with that of a larger study that highlighted greater rates of response among IHC 3+ HER2+ tumors (both HR+ and HR−) than among those that were IHC 1 to 2+ and were amplified by FISH.23 Greater HER2 overexpression on the cell surface correlating with a better response to HER2-targeted therapy is consistent with the mechanism of action of trastuzumab and pertuzumab, which target HER2 protein on the cell surface. This finding can be explained by the direct correlation between greater HER2 gene copy numbers in tumors with 3+ staining versus those without protein overexpression by FISH, as was seen with the few 3+ cases tested with FISH in our study. We hypothesize that if all IHC 3+ cases had been tested by FISH, we would have observed a significant association between HER2 ratio and HER2 copy number with response to NAC, as has been reported in other studies.24–26 Regardless, our observations are most relevant to the routine clinical setting where FISH analysis is performed only in equivocal cases according to ASCO/CAP guidelines. Of the cases studied by FISH, we found that 44% of tumors displayed “low HER2 amplification” defined as HER2/CEP17 ratio of 2 or greater and 4 to 6 HER2 signals per cell. In comparison, this was observed in 6.3% of more than 6000 cases from the HERA (HERceptin Adjuvant) trial screening population and only 2.1% of a large multi-institutional study of HER2 FISH analyses.27 Tumors with low HER2 amplification tend to show high expression of ER, which is likely the reason for the high proportion of low HER2-amplified cases in our series.
In general, the pCR rate of tumors with high levels of ER expression is lower than for those that are ER-negative or show low ER expression.17 Among HER2+ tumors, significant differences in response are observed based on levels of ER expression, where response to therapy is typically inversely related to ER expression. We assessed ER and PR by using H-scores to quantify expression levels for each case to determine how ER and PR correlated with response specifically in TPBCs. By using this semiquantitative approach, we studied these variables in a continuous fashion, while also using predetermined cutoff points for high and low expression.17 Most TPBCs in our study (63.5%) showed high expression of ER, or H-score greater than 200, which roughly equates to 67% of cells staining with strong nuclear intensity. Only 12 TPBCs (14.1%) showed low ER expression. We found no correlation between ER and PR expression and HER2 results, either by FISH or by IHC. This was unexpected as most studies show an inverse relationship between the levels of HER2 expression and ER/PR expression.7,28 We believe that this lack of correlation is due to the few number of cases with low ER expression in our study, whereas most large studies of HER2+ tumors show a greater proportion of tumors with low levels of HR expression and also include HR-negative tumors. Most likely this observation accounts for the lack of correlation between the levels of ER and PR and response to NAC. In a study examining how hormone receptor expression (measured by H-score) influenced the response to NAC plus trastuzumab, Bhargava et al29 reported a significant difference of ER and PR levels in tumors achieving a pCR versus those that did not. In their analysis, mean H-scores for ER and PR were 47 and 13, respectively, for tumors with pCR versus H-scores of 122 and 64, respectively, for tumors that did not have pCR. The highest rate of response, 52%, was seen in the group with ER and PR H-scores of 10% or less, the so-called ERBB2 group. Notably, only 8 of the 85 tumors in our study (9.4%) would be classified as “ER-low/PR-low” when using H-score of 100 as a cutoff, and only 3 (3.5%) would be classified as such when using H-score of 10 as a cutoff.
From a morphologic standpoint, most (>90%) TPBCs studied were invasive carcinomas of no special type. A minority of tumors showed apocrine, mucinous, and/or micropapillary features. Apocrine morphology is a common feature among HER2+ breast carcinomas, particularly those that are negative for hormone receptors. In a study from South Korea comparing morphologic and clinical characteristics between HR+/HER2+ and HR−/HER2+ breast carcinomas, apocrine differentiation was seen in 79.3% of HR− tumors, which was significantly more frequent than in HR+ tumors, which displayed apocrine differentiation in 55.8% of cases.28 The study also reported significantly more frequent micropapillary differentiation (6.8% versus 2.5%) and mucinous differentiation (3.9% versus 0%) in HR+ tumors than in HR− tumors. TPBCs were not specifically addressed in this study. The relatively lower frequency of tumors with apocrine morphology in our study, 6%, is likely attributable to higher levels of HR expression seen in our cohort. We also noted that some TPBCs showed mucinous and/or micropapillary features. Pure mucinous carcinoma is classically regarded as a low-grade ER+ tumor with favorable prognosis and less than 5% overexpress HER2. None of the tumors in our series was a “pure mucinous carcinoma.” All cases were mixed tumors with a nonmucinous ductal component, except 1 tumor with features of “micropapillary variant of mucinous carcinoma,” a morphologic subtype with higher histologic grade and greater rates of lympho-vascular invasion and lymph node positivity than pure mucinous carcinoma.30–33 Published studies of this variant show the majority to be ER and PR positive, with up to 20% showing HER2 overexpression, although the rate of “triple-positivity” is not specifically indicated in these reports. Three tumors in our series displayed lobular differentiation, confirmed by lack of E-cadherin expression, including 2 pleomorphic invasive lobular carcinomas and 1 classic-type invasive lobular carcinoma. Pleomorphic lobular carcinoma is a high-grade variant of lobular carcinoma that shows HER2 amplification in about 15% to 25% of cases,34 in contrast to classic-type invasive lobular carcinoma, in which HER2 amplification is rare. The 1 case in our study with classic lobular features showed low-level HER2 amplification (HER2/CEP17 ratio: 2.4; HER2 signals per cell: 4.8) by FISH. This tumor was strongly positive for ER and PR. One of the 2 pleomorphic carcinomas showed pCR, while the remaining 2 lobular carcinomas had extensive residual disease (RCB III) following NAC.
A strength of our study is that all cases were reviewed by the study pathologists, including all IHC-stained slides. All IHC stains were performed in the same laboratory by using validated protocols and scored according to ASCO/CAP guidelines. This allowed for a uniform assessment and quantification of ER, PR, and HER2. This is in contrast to many large studies or clinical trials that collect ER, PR, and HER2 data from pathology reports from different laboratories, which accounts for variation in staining as well as differences in interpretation between laboratories. A weakness of our study is the relatively small overall sample size. Our institution is a large referral cancer center in which most patients have core biopsies performed outside of our system and typically only hematoxylin-eosin slides are reviewed. Thus, the strict inclusion criteria of our study limited the number of cases included. Additionally, the length of follow-up was relatively short for a study of HR+ carcinomas, which tend to recur after 5 years; however, long-term outcome was not the focus of this study. As expected, RCB correlated with distant recurrence, as 75% of patients with recurrence had extensive residual disease (RCB III) after NAC. Longer follow-up would be necessary to determine what factors other than RCB correlate with outcome in TPBCs with residual disease.
CONCLUSIONS
In summary, TPBCs are somewhat heterogeneous morphologically with a minority showing mucinous, lobular, or micropapillary differentiation. TPBCs showed a pCR rate that is similar to that reported for ER+/PR−/HER2+ carcinomas and is roughly half the rate seen with HR−/HER2+ tumors. TPBCs showed high levels of HR expression and lower levels of HER2 amplification by FISH compared with other HER2+ tumors. While HR expression did not correlate with pCR, HER2 overexpression determined by IHC staining was associated with a significantly better response to therapy and may help select patients for treatment in the neoadjuvant setting.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 2.Kallioniemi OP, Holli K, Visakorpi T, et al. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991;49(5): 650–655. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 4.Pegram MD. Treating the HER2 pathway in early and advanced breast cancer. Hematol Oncol Clin North Am. 2013;27(4):751–765, viii. [DOI] [PubMed] [Google Scholar]
- 5.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. [DOI] [PubMed] [Google Scholar]
- 7.Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol. 2005;123(4):541–546. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. [DOI] [PubMed] [Google Scholar]
- 10.Vici P, Pizzuti L, Sperduti I, et al. “Triple positive” early breast cancer: an observational multicenter retrospective analysis of outcome. Oncotarget. 2016; 7(14):17932–17944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vici P, Pizzuti L, Natoli C, et al. Outcomes of HER2-positive early breast cancer patients in the pre-trastuzumab and trastuzumab eras: a real-world multicenter observational analysis: the RETROHER study. Breast Cancer Res Treat. 2014;147(3):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You SH, Chae BJ, Eom YH, et al. Clinical differences in triple-positive operable breast cancer subtypes in Korean patients: an analysis of Korean Breast Cancer Registry data. J Breast Cancer. 2018;21(4):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134(6):907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–1382. [DOI] [PubMed] [Google Scholar]
- 16.McCarty KS Jr, Miller LS, Cox EB, et al. Estrogen receptor analyses: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 17.Landmann A, Farrugia DJ, Zhu L, et al. Low estrogen receptor (ER)-positive breast cancer and neoadjuvant systemic chemotherapy: is response similar to typical ER-positive or ER-negative disease? Am J Clin Pathol. 2018;150(1):34–42. [DOI] [PubMed] [Google Scholar]
- 18.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. [DOI] [PubMed] [Google Scholar]
- 19.Stewart RL, Caron JE, Gulbahce EH, et al. HER2 immunohistochemical and fluorescence in situ hybridization discordances in invasive breast carcinoma with micropapillary features. Mod Pathol. 2017;30(11):1561–1566. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 21.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284. [DOI] [PubMed] [Google Scholar]
- 22.Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018; 29(3):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krystel-Whittemore M, Xu J, Brogi E, et al. Pathologic complete response rate according to HER2 detection methods in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Breast Cancer Res Treat. 2019;177(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kogawa T, Fouad TM, Liu DD, et al. High HER2/centromeric probe for chromosome 17 fluorescence in situ hybridization ratio predicts pathologic complete response and survival outcome in patients receiving neoadjuvant systemic therapy with trastuzumab for HER2-overexpressing locally advanced breast cancer. Oncologist. 2016;21(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer CF, Tan YY, Fitzal F, et al. Pathological complete response to neoadjuvant trastuzumab is dependent on HER2/CEP17 ratio in HER2-amplified early breast cancer. Clin Cancer Res. 2017;23(14):3676–3683. [DOI] [PubMed] [Google Scholar]
- 26.Fumagalli D, Venet D, Ignatiadis M, et al. RNA sequencing to predict response to neoadjuvant anti-HER2 therapy: a secondary analysis of the NeoALTTO Randomized Clinical Trial. JAMA Oncol. 2017;3(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard M, Jalikis F, Krings G, et al. ‘Non-classical’ HER2 FISH results in breast cancer: a multi-institutional study. Mod Pathol. 2017;30(2):227–235. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Park IA, Park SY, et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res Treat. 2014;145(3):615–623. [DOI] [PubMed] [Google Scholar]
- 29.Bhargava R, Dabbs DJ, Beriwal S, et al. Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod Pathol. 2011;24(3):367–374. [DOI] [PubMed] [Google Scholar]
- 30.Ranade A, Batra R, Sandhu G, et al. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010;63(12):1043–1047. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Park K, Kim JY, et al. Prognostic significance of a micropapillary pattern in pure mucinous carcinoma of the breast: comparative analysis with micropapillary carcinoma. J Pathol Transl Med. 2017;51(4):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbashina V, Corben AD, Akram M, et al. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013;44(8):1577–1585. [DOI] [PubMed] [Google Scholar]
- 33.Pareja F, Selenica P, Brown DN, et al. Micropapillary variant of mucinous carcinoma of the breast shows genetic alterations intermediate between those of mucinous carcinoma and micropapillary carcinoma. Histopathology. 2019;75(1): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Baimani K, Bazzarelli A, Clemons M, et al. Invasive pleomorphic lobular carcinoma of the breast: pathologic, clinical, and therapeutic considerations. Clin Breast Cancer. 2015;15(6):421–425. [DOI] [PubMed] [Google Scholar]


