Key Points
Question
Can a collaborative learning network of pediatric cardiac intensive care teams implement a low-technology cardiac arrest prevention practice bundle and decrease cardiac arrest rates?
Findings
In this quality improvement study including 31 hospitals, 15 cardiac intensive care units implementing the cardiac arrest prevention bundle realized a significant 30% relative reduction in aggregate cardiac arrest rate after implementation compared with control hospitals.
Meaning
Some cardiac arrests are avoidable, but future studies are necessary to determine which bundle elements are essential for cardiac arrest prevention.
This quality improvement study evaluates whether a low-technology cardiac arrest prevention practice bundle decreases in-hospital cardiac arrest rate.
Abstract
Importance
Preventing in-hospital cardiac arrest (IHCA) likely represents an effective strategy to improve outcomes for critically ill patients, but feasibility of IHCA prevention remains unclear.
Objective
To determine whether a low-technology cardiac arrest prevention (CAP) practice bundle decreases IHCA rate.
Design, Setting, and Participants
Pediatric cardiac intensive care unit (CICU) teams from the Pediatric Cardiac Critical Care Consortium (PC4) formed a collaborative learning network to implement the CAP bundle consistent with the Institute for Healthcare Improvement framework; 15 hospitals implemented the bundle voluntarily. Risk-adjusted IHCA incidence rates were analyzed across 2 time periods, 12 months (baseline) and 18 months after CAP implementation (intervention) using difference-in-differences (DID) regression to compare 15 CAP and 16 control PC4 hospitals that chose not to participate in CAP but had IHCA rates tracked in the PC4 registry. Patients deemed at high risk for IHCA, based on a priori evidence-based criteria and empirical hospital-specific criteria, were selected to receive the CAP bundle. Data were collected from July 2018 to December 2019, and data were analyzed from March to August 2020.
Interventions
CAP bundle included 5 elements developed to promote increased situational awareness and communication among bedside clinicians to recognize and mitigate deterioration in high-risk patients.
Main Outcomes and Measures
Risk-adjusted IHCA incidence rate across all CICU admissions (IHCA events divided by all admissions).
Results
The bundle was activated in 2664 of 10 510 CAP hospital admissions (25.3%); admission characteristics were similar across study periods. There was a 30% relative reduction in risk-adjusted IHCA incidence rate at CAP hospitals (intervention period: 2.6%; 95% CI, 2.2-2.9; baseline: 3.7%; 95% CI, 3.1-4.0), but no change at control hospitals (intervention period: 2.7%; 95% CI, 2.3-2.9; baseline: 2.7%; 95% CI, 2.2-3.0). DID analysis confirmed significantly reduced odds of IHCA among all admissions at CAP hospitals compared with control hospitals during the intervention period vs baseline (odds ratio, 0.72; 95% CI, 0.56-0.91; P = .01). DID odds ratios were 0.72 (95% CI, 0.53-0.98) for the surgical subgroup, 0.74 (95% CI, 0.48-1.14) for the medical subgroup, and 0.72 (95% CI, 0.50-1.03) for the high-risk admission subgroup at CAP hospitals after intervention. All-cause risk-adjusted mortality rate did not change after intervention.
Conclusions and Relevance
Implementation of this CAP bundle led to significant IHCA reduction across multiple pediatric CICUs. Future studies may determine if this bundle can be effective in other critically ill populations.
Introduction
Morbidity and mortality from in-hospital cardiac arrest (IHCA) remain high despite extensive investment in resources to improve cardiopulmonary resuscitation (CPR) and postresuscitation care, suggesting the need for new strategies to improve outcomes.1,2,3,4,5 Implementing specific practices to prevent IHCA represents a novel but unproven approach to limit harm. Pediatric IHCA events occur more frequently in patients with cardiac disease5,6,7 and in the intensive care unit (ICU) rather than ward settings.8,9 Thus, exploring IHCA prevention initiatives within pediatric cardiac ICUs (CICUs) represents a tangible opportunity to test the theory that IHCA prevention is possible.
In this context, we developed a multicenter cardiac arrest prevention (CAP) quality improvement (QI) project for hospitals participating in the Pediatric Cardiac Critical Care Consortium (PC4). We hypothesized that implementing a minimal-cost, low-technology practice bundle within a collaborative learning network would reduce IHCA incidence across participating hospitals within 1 year.
Methods
Infrastructure
PC4 is a QI collaborative currently comprising more than 60 hospitals aiming to improve outcomes in patients with critical pediatric and congenital cardiovascular disease. We previously described the QI data infrastructure of PC4.10 Hospitals enter data on every CICU admission into a clinical registry, including IHCA details. Cardiac Networks United (CNU) provides infrastructure to multiple pediatric heart disease networks (including PC4) to accelerate improvement science.11 CNU’s improvement team led this project in alignment with the Institute for Healthcare Improvement Breakthrough Series QI framework.12 The University of Michigan Institutional Review Board approved the project with a waiver of informed consent. We followed the Standards for Quality Improvement Reporting Excellence (SQUIRE) reporting guideline for design, analysis, and presentation of this project.13
Bundle
Informed by PC4 IHCA epidemiologic data,5 a team of resuscitation experts, QI leaders, and PC4 clinician champions developed a CAP bundle aimed at preventing IHCA (Table 1) modeled after one PC4 hospital’s successful initiative (eFigure 1 in Supplement 1).14 The 5-element bundle was minimal cost, technology independent, and lightly prescriptive, allowing each CICU to adapt to their local system, QI resources, and clinical workflow. The only mandatory bundle element was twice-daily CAP safety huddles that provided bedside just-in-time training (Table 1).15 This multidisciplinary huddle was designed to facilitate communication by creating situational awareness for high-risk patients, developing plans to recognize and reverse early deterioration and, if necessary, rescue patients from imminent arrest. Key points were posted bedside, along with CAP visual identifiers (CAP Toolkit in Supplement 2). The other 4 elements were optional but strongly recommended.
Table 1. Cardiac Arrest Prevention (CAP) Bundle Elements.
| Element | Rationale | Definition |
|---|---|---|
| CAP safety huddle | Entire team shares the same mental model for recognition or prevention of early deterioration and agree on mitigation plan(s) for reversal and/or rescue | A formal multidisciplinary bedside discussion to create IHCA prevention situation awareness among the entire care team for high-risk patients
|
| Vital sign discussion | Shared mental model for target vital sign goals and defining vital sign changes that may represent early deterioration | Patient-specific vital sign goals/targets are discussed and alarm parameters established
|
| Discussion of presedation | Patients with tenuous physiologies may have hemodynamic collapse related to agitation or pain | A discussion regarding the use of presedation for noxious stimuli (eg, endotracheal tube suctioning/venipuncture)
|
| Emergency medication | Many IHCA episodes are preceded by acute hypotension; rapid epinephrine may rescue patient prior to IHCA | A patient-specific dose of epinephrine is available and drawn up at the bedside for rapid administration
|
| Formal code review | Identification of modifiable etiologies and preventable IHCA such that clinical team targets improvement efforts toward related interventions | Each cardiac arrest should be reviewed within 2 weeks of the event
|
Abbreviation: IHCA, in-hospital cardiac arrest.
Inclusion Criteria
This project prescribed bundle application for patients previously identified as being high risk for IHCA during specified time periods5: (1) neonates admitted after cardiac surgery with cardiopulmonary bypass; (2) infants (younger than 4 months) admitted after non–cardiopulmonary bypass single ventricle surgery; and (3) patients with acute medical conditions receiving invasive mechanical ventilation within 4 hours of admission. We recommended initiating CAP bundle processes less than 1 hour after meeting inclusion criteria and continuing for a minimum of 7 days for surgical patients and of 3 days for medical patients or until 24 hours postextubation, if this occurred sooner. Any patient considered at risk for IHCA by local teams exclusive of these 3 criteria could also receive the bundle but were not included in high-risk subgroup analyses.
Collaborative Learning Network
Monthly webinars led by CAP project leaders and CNU QI experts featured QI education, data review, and collaborative learning to empower teams toward successful CAP implementation. Teams were guided through the Model for Improvement and coached to use Plan-Do-Study-Act cycles during implementation.16 Key driver diagrams were established at onset; the Smart Aim was 25% relative aggregate IHCA incidence reduction among all admissions within 1 year (eFigure 2 in Supplement 1). Statistical process control (SPC) U charts, displaying real-time IHCA rates, were shared on webinars; the end-of-study SPC chart is shown in eFigure 3 in Supplement 1. CNU experts engaged each hospital on demand, mitigating institution-specific implementation barriers. Hospitals audited 10 or more CAP initiations per month, assessing reliability. All audit-related and bundle-related data were collected from July 2018 to October 2019 by each CAP hospital within REDCap version 9.0 (Vanderbilt University). Data collection tools are in the CAP Toolkit (Supplement 2).
Hospital Recruitment and Time Line
All PC4 hospitals were exposed to preliminary IHCA prevention data and this CAP QI proposal at annual PC4 meetings and follow-up webinars. Any PC4 hospital could join the CAP collaborative; participation was voluntary and not randomized. A priori criteria were established for full project participation, and 15 CAP hospitals implemented the intervention accordingly: (1) 80% webinar participation; (2) aggregate 70% safety huddle completion for eligible patients; and (3) 100% monthly submission of process and outcome data. A total of 11 of 15 CAP hospitals adopted all 5 bundle elements. One hospital excluded bedside epinephrine, 2 excluded presedation discussion, and 1 excluded patient-specific vital sign communication and presedation discussion. There were 16 control hospitals that submitted the same IHCA data to PC4 during the study period; CAP and control data were audited equally per standard PC4 processes. Most control hospitals elected to not participate at onset of CAP intervention; however, some control hospitals were so defined when they did not meet full participation criteria.
PC4 hospitals with 1 or more years of baseline data at project initiation (n = 31) were eligible for inclusion in final analysis. Two hospitals had full participation but did not have baseline data. We defined 2 distinct study periods to compare hospital groups: at 12 months (baseline; May 2017 to April 2018) and at 18 months (intervention; July 2018 to December 2019). Admissions during the transition between study periods (May to June 2018) were excluded.
Statistical Analysis
IHCA was defined according to the American Heart Association (AHA) Get With the Guidelines—Resuscitation: any hypoperfusion episode requiring any chest compressions and/or defibrillation, eliciting a resuscitation response and documentation.17 The primary outcome was risk-adjusted IHCA incidence rate (CICU admissions with 1 or more IHCA events divided by all admissions) compared across study periods at 15 CAP hospitals and 16 control hospitals. Secondary analyses similarly compared IHCA rates within surgical, medical, and high-risk admission subgroups and compared all-cause CICU mortality at CAP hospitals.
To calculate risk-adjusted IHCA incidence rate, we fit multivariable logistic regression models based on patient-level IHCA predictors identified in our previous work5,10; models were fit for all admissions and surgical or medical subgroups. We performed difference-in-differences (DID) analysis, a quasi-experimental technique, evaluating whether changes in IHCA incidence rate at CAP hospitals could be attributed to the intervention, independent of secular trends at similar hospitals (eMethods in Supplement 1). The DID association was estimated based on the interaction term between study period (baseline vs intervention) and hospital groups (CAP hospitals vs controls) in our multivariable models, while controlling for case mix. The parallel trends assumption during the baseline period for DID was met (eMethods in Supplement 1).18 We accounted for patient clustering within hospitals by including hospital-specific random-effects term in models. Bias-corrected 95% CI for DID odds ratios (ORs) were obtained using bootstrap resampling (1000 samples). All analyses used SAS version 9.4 (SAS Institute) or Stata version 14 (StataCorp).
We complemented this analysis by creating SPC P charts on monthly case mix–adjusted IHCA incidence rates for CAP and control hospitals. Standard special cause variation rules identified statistically significant changes in the system during the intervention.19 P values were calculated using χ2 tests. Significance was set at P < .05, and all P values were 2-tailed.
Results
The analysis included 41 204 admissions, including 10 510 admissions at 15 CAP hospitals during the intervention period. Admission characteristics were similar between study periods for both CAP and control hospitals (Table 2), including high-risk admission rate (approximately 15% of total admissions in both hospital groups across eras). Compared with CAP hospitals, control hospitals had higher average admission volume (approximately 47 per month vs approximately 39 per month) and lower medical admission proportion (9131 vs 23 720 [38.5%] vs 7373 of 17 484 [42.2%]). The proportion of neonates, CAP high-risk patients and high-risk surgical patients was similar between CAP and control hospitals, Table 2.
Table 2. Pediatric Cardiac Intensive Care Unit (CICU) Volume and Admission Characteristics.
| Characteristic | No. (%) | P valuea | |||
|---|---|---|---|---|---|
| CAP hospitals (n = 15) | Control hospitals (n = 16) | ||||
| Baseline | Intervention | Baseline | Intervention | ||
| All CICU admissions, No. | 6974 | 10 510 | 10 030 | 136,90 | NA |
| Age at admission | |||||
| Neonate (0-30 d) | 1378 (19.8) | 1927 (18.3) | 1811 (18.1) | 2501 (18.3) | <.001 |
| Infant (31-365 d) | 2263 (32.5) | 3353 (31.9) | 3144 (31.4) | 4380 (32.0) | |
| Child (1-18 y) | 2831 (40.6) | 4448 (42.3) | 4487 (44.7) | 5960 (43.5) | |
| Adult (>18 y) | 502 (7.2) | 782 (7.4) | 588 (5.9) | 849 (6.2) | |
| Sex | |||||
| Female | 3138 (45.0) | 4578 (43.6) | 4482 (44.7) | 6294 (46.0) | .69 |
| Male | 3836 (55.0) | 5932 (56.4) | 5548 (55.3) | 7396 (54.0) | |
| Raceb | |||||
| Asian | 240 (3.4) | 337 (3.2) | 390 (3.9) | 544 (4.0) | <.001 |
| Black | 1133 (16.3) | 1722 (16.4) | 1418 (14.1) | 2024 (14.8) | |
| White | 4574 (65.6) | 6852 (65.2) | 5428 (54.1) | 7777 (56.8) | |
| Other | 824 (11.8) | 1166 (11.1) | 1252 (12.5) | 1520 (11.1) | |
| Unknown | 203 (2.9) | 433 (4.1) | 1542 (15.4) | 1825 (13.3) | |
| Ethnicityb | |||||
| Hispanic | 1202 (17.2) | 1870 (17.8) | 1796 (17.9) | 2450 (17.9) | <.001 |
| Non-Hispanic | 5701 (81.8) | 8447 (80.4) | 7331 (73.1) | 9750 (71.2) | |
| Unknown | 71 (1.0) | 193 (1.8) | 903 (9.0) | 1490 (10.9) | |
| Surgical admissions | 4086 (58.6) | 6026 (57.3) | 6182 (61.6) | 8407 (61.4) | <.001 |
| Surgical complexityc | |||||
| Low and moderate complexity | 2938 (42.1) | 4386 (41.7) | 4440 (44.3) | 6021 (44.0) | .93 |
| High complexity | 1148 (16.5) | 1640 (15.6) | 1742 (17.4) | 2386 (17.4) | |
| Medical admissions | 2888 (41.4) | 4484 (42.7) | 3848 (38.4) | 5283 (38.6) | <.001 |
| High-risk admissions | 1149 (16.5) | 1646 (15.7) | 1553 (15.5) | 2032 (14.8) | .17 |
| Neonatal surgery with CPB | 638 (9.2) | 908 (8.6) | 824 (8.2) | 1091 (8.0) | NA |
| Single ventricle palliation surgery | 58 (0.8) | 77 (0.7) | 91 (0.9) | 119 (0.9) | NA |
| Acute medical condition with mechanical ventilation | 453 (6.5) | 661 (6.3) | 638 (6.4) | 822 (6.0) | NA |
Abbreviations: CAP, cardiac arrest prevention; CPB, cardiopulmonary bypass; NA, not applicable.
Comparisons between CAP vs control hospitals at baseline.
Race and ethnicity were selected from a drop-down menu as identified from the medical record by data extractors, which comprised clinician-determined and self/caregiver assignments. The other category includes American Indian, Native Hawaiian or Pacific Islander, multiracial, and any child not meeting these or the other reported race categories.
Surgical complexity defined by the Society of Thoracic Surgeons–European Association for Cardiothoracic Surgery (STAT) score mortality categories. Categories 1 to 3 indicated low and moderate complexity and categories 4 and 5 indicated high complexity.
Bundle Initiations
CAP hospitals initiated the bundle in 2664 of 10 510 admissions (25.3%) for a mean (SD) of 4.4 (1.9) days per CAP patient. eFigure 4 in Supplement 1 shows proportions of specific bundle indications. Of the eligible predefined high-risk admissions, 1420 of 1646 (86.3%) received the bundle. Additionally, 821 of 2664 (30.8%) bundle initiations were performed in patients on the discretion of the treating clinicians outside these a priori criteria. Individual bundle elements were audited 1097 times; the mandatory CAP safety huddle was performed correctly in 842 (76.8%). The eTable in Supplement 1 shows aggregate compliance rate for all bundle elements.
IHCA Incidence Rate in CAP vs Control Hospitals
Table 3 shows IHCA incidence rates across time periods and results of our DID analysis. The baseline aggregate risk-adjusted IHCA incidence rate was higher at CAP hospitals compared with control hospitals but varied across hospitals in both categories (eFigure 5 in Supplement 1). For all admissions at CAP hospitals, there was a 30% relative reduction in risk-adjusted IHCA incidence rate at CAP hospitals (intervention period: 2.6%; 95% CI, 2.2-2.9; baseline: 3.7%; 95% CI, 3.1-4.0; P < .001), but no change at control hospitals (intervention period: 2.7%; 95% CI, 2.3-2.9; baseline: 2.7%; 95% CI, 2.2-3.0). This aggregate reduction translated to approximately 11 fewer CA per month in the intervention period vs baseline at CAP hospitals (Table 2). Hospital-specific IHCA incidence rate change from baseline to intervention for both CAP and control hospitals is shown in Figure 1.
Table 3. Comparison of In-Hospital Cardiac Arrest (IHCA) Metrics Between Cardiac Arrest Prevention (CAP) and Control Hospitals.
| Measure | Cardiac arrest prevention hospitals (n = 15) | Control hospitals (n = 16) | IHCA odds after intervention in CAP vs control hospitals, DID OR (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Baseline | Intervention | OR (95% CI)a | Baseline | Intervention | OR (95% CI)a | ||
| All patients | |||||||
| Total, No. | 6974 | 10 510 | 0.70 (0.58-0.84) | 10 030 | 13 690 | 0.97 (0.82-1.15) | 0.72 (0.56-0.91) |
| Total IHCA episodes, No. | 336 | 314 | NA | 347 | 457 | NA | NA |
| IHCA episodes/mo | 28.0 | 17.4 | NA | 28.9 | 25.4 | NA | NA |
| Patients with ≥1 IHCA episode, No. | 252 | 259 | NA | 262 | 363 | NA | NA |
| Risk-adjusted IHCA incidence rate, % (95% CI) | 3.7 (3.1-4.0) | 2.6 (2.2-2.9) | NA | 2.7 (2.2-3.0) | 2.7 (2.3-2.9) | NA | NA |
| High-risk patients | |||||||
| Total, No. | 1149 | 1646 | 0.67 (0.52-0.87) | 1553 | 2032 | 0.97 (0.76-1.25) | 0.72 (0.50-1.03) |
| Total IHCA episodes, No. | 191 | 164 | NA | 150 | 191 | NA | NA |
| IHCA episodes/mo | 15.9 | 9.1 | NA | 12.5 | 10.6 | NA | NA |
| Patients with ≥1 IHCA episode, No. | 129 | 129 | NA | 116 | 148 | NA | NA |
| Observed IHCA incidence rate, %b | 11.2 | 7.8 | NA | 7.5 | 7.3 | NA | NA |
| Surgical patients | |||||||
| Total, No. | 4086 | 6026 | 0.67 (0.54-0.84) | 6182 | 8407 | 0.93 (0.76-1.15) | 0.72 (0.53-0.98) |
| Total IHCA episodes, No. | 239 | 207 | NA | 208 | 274 | NA | NA |
| IHCA episodes/mo | 19.9 | 11.5 | NA | 17.3 | 15.2 | NA | NA |
| Patients with ≥1 IHCA episode, No. | 179 | 171 | NA | 172 | 228 | NA | NA |
| Risk-adjusted IHCA incidence rate, % (95% CI) | 4.3 (3.6-4.8) | 2.9 (2.4-3.3) | NA | 2.9 (2.2-3.0) | 2.8 (2.4-3.1) | NA | NA |
| Medical patients | |||||||
| Total, No. | 2888 | 4484 | NA | 3848 | 5283 | NA | 0.74 (0.48-1.14) |
| Total IHCA episodes, No. | 97 | 107 | NA | 139 | 183 | NA | NA |
| IHCA episodes/mo | 8.1 | 5.9 | NA | 11.6 | 10.2 | NA | NA |
| Patients with ≥1 IHCA episode, No. | 73 | 88 | NA | 90 | 135 | NA | NA |
| Risk-adjusted IHCA incidence rate, % (95% CI) | 2.8 (2.2-3.5) | 2.2 (1.7-2.7) | 0.78 (0.57-1.08) | 2.3 (1.8-2.9) | 2.6 (2.0-3.1) | 1.05 (0.79-1.39) | NA |
Abbreviations: DID, difference-in-differences; NA, not applicable; OR, odds ratio.
Baseline vs intervention adjusted OR within hospital group.
High-risk cohort not risk adjusted.
Figure 1. Individual Hospital In-Hospital Cardiac Arrest (IHCA) Incidence Rate Change From Baseline to Intervention Among Cardiac Arrest Prevention (CAP) vs Control Hospitals.
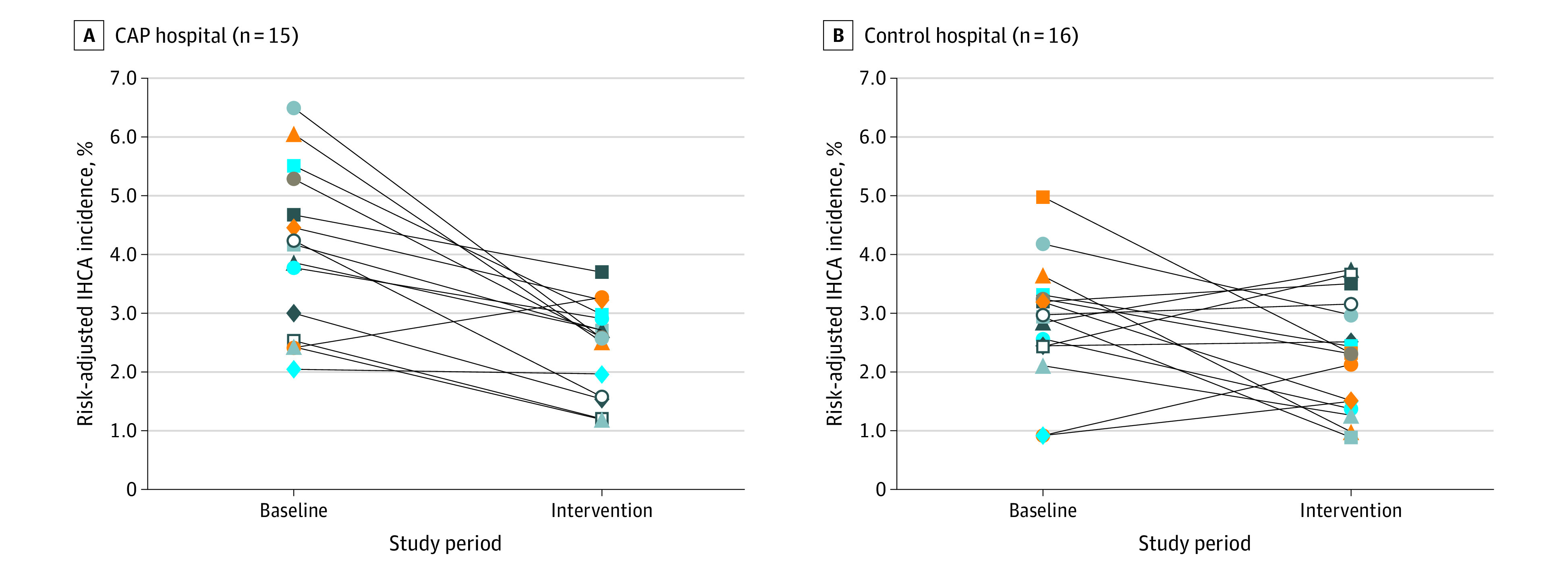
Each point indicates an individual hospital. There was significant variability among hospitals with respect to baseline and intervention IHCA incidence as well as direction and degree of change. CAP hospitals tended to have higher IHCA incidence rate during the baseline period but similar IHCA rates to control during the intervention period. A total of 14 of 15 CAP hospitals had lower rates of IHCA compared with 9 of 16 control centers.
DID analysis demonstrated a 28% relative reduction in odds of IHCA at CAP hospitals compared with control hospitals in the intervention period vs baseline period (DID OR, 0.72; 95% CI, 0.56-0.91; P = .01) There was also significant decrease in odds of IHCA at CAP hospitals compared with control hospitals in the intervention period for surgical admissions (33% relative reduction; DID OR, 0.72; 95% CI, 0.53-0.98). Odds of IHCA after intervention at CAP hospitals was not statistically different from control hospitals for high-risk admissions (DID OR, 0.72; 95% CI, 0.50-1.03) or medical admissions (DID OR, 0.74; 95% CI, 0.48-1.14).
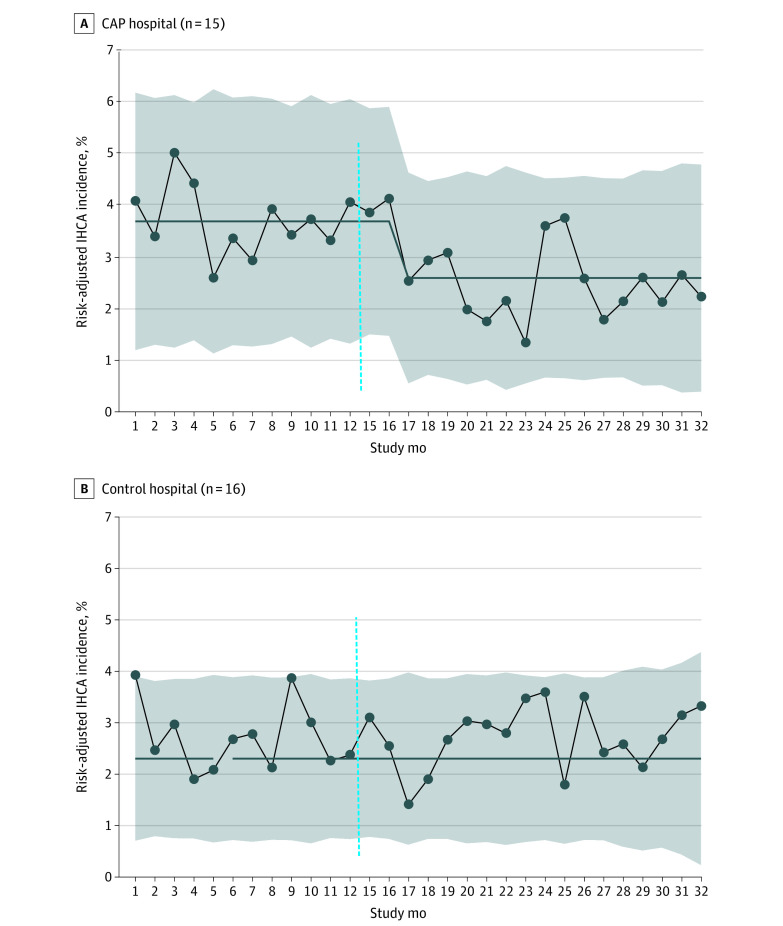
Figure 2 shows our complementary SPC analysis, which confirms a significant 30% reduction in monthly risk-adjusted IHCA incidence rate from 3.7% to 2.6% for all admissions at CAP hospitals but not control hospitals. Risk-adjusted all-cause CICU mortality was unchanged after intervention at the 15 CAP hospitals (baseline: 3.2%; 95% CI, 2.6-3.6; intervention: 3.1%; 95% CI, 2.6-3.4; P = .75).
Figure 2. Aggregate Monthly Risk-Adjusted In-Hospital Cardiac Arrest (IHCA) Incidence in Cardiac Arrest Prevention (CAP) Hospitals Compared With Control Hospitals.
Comparative statistical process control P charts showing aggregate monthly risk-adjusted IHCA incidence at 15 CAP hospitals and 16 control hospitals. At CAP hospitals, starting in study month 18 during the intervention period, there was significant IHCA reduction, with special cause variation as determined by 8 consecutive months below the baseline rate. There was centerline shift, representing a 30% reduction in mean aggregate IHCA incidence from 3.7% to 2.6%. At control hospitals, there was no change in baseline risk-adjusted IHCA incidence (2.7%). The baseline period included study months 1 to 12, and the intervention period included study months 15 to 32. There was a 2-month transition period (dotted vertical line).
Discussion
We report a successful QI intervention to prevent IHCA in pediatric CICU patients. Our low-cost, technology-independent CAP bundle facilitated significant reduction in risk-adjusted IHCA incidence rates at hospitals that engaged in our collaborative learning network and implemented the CAP bundle elements, while IHCA incidence rates at control PC4 hospitals remained unchanged. Our DID analysis suggests this IHCA improvement was independent of secular trends. These data demonstrate that some IHCA events are preventable and translates to 198 IHCA events avoided across 15 CAP hospitals during the 18-month intervention period.
Changing the Paradigm—IHCA Is Preventable
We believe this is the first multicenter project of its kind, representing an important paradigm shift in critical care to prioritize IHCA prevention. The American Heart Association recently added surveillance and prevention as the first link to the IHCA chain of survival20 in response to growing sentiment that prevention may be additive to high-quality CPR for improving outcomes.21 IHCA prevention efforts to date include adoption of early warning scores and medical emergency response teams in non-ICU settings.22,23,24,25 As most IHCAs now occur in ICUs,8 there has also been work in this setting to identify risk factors for IHCA26,27,28; however, there is sparse literature regarding interventions to prevent IHCA in high-risk ICU cohorts.29
Despite evidence that recognizable periods of deterioration often precede IHCA30 and that many IHCAs are classified as avoidable during formal postarrest debriefings,31,32 belief still persists that IHCA is an inevitability of critical illness. Our results question that dogma and demonstrate the effectiveness of a breakthrough series learning network12: temporal SPC analyses confirmed significant interval improvement, and prevention successes and learnings from CAP early adopters were shared on webinars, steadily increasing confidence among once-reluctant participants that IHCA is preventable. Proving feasibility of IHCA prevention represents the most essential message of this project and hopefully will encourage spread of these IHCA prevention practices at other hospitals.
Spreading CAP
Progressive voluntary spread of CAP to other patients not meeting high-risk criteria ultimately comprised nearly one-third of all CAP indications, reflecting growing confidence in the bundle’s effectiveness and clinicians’ desire to apply it more widely. Spillover of CAP concepts and practices to these additional patients likely contributed to the consistent reduction of IHCA odds during the intervention period across all CICU admissions and within the subgroups analyzed. This supports our hypothesis that our CAP bundle may facilitate similar IHCA reduction if applied to other critical care units.
Focused IHCA Improvement Efforts Within a Quality Collaborative
We recently published data showing hospital participation for 2 or more years in PC4 was associated with aggregate improvement in outcomes for cardiac surgical patients, including mortality, yet IHCA rate did not improve through PC4 participation alone.10 Consequently, this CAP QI intervention focused directly on IHCA prevention within the PC4 infrastructure, and with a mean (SD) bundle exposure of only 4.4 (1.9) days per patient, we achieved IHCA reduction. Control hospitals did not improve despite having exposure to the principles of CAP and, in some instances, limited engagement in CAP QI work, supporting our previous finding that PC4 participation alone may not be sufficient for IHCA reduction. One possibility for the lack of improvement at control hospitals is that intense, focused collaborative QI work and intentional implementation of the CAP bundle may be necessary for improvement. While time and effort necessary to implement and perform the bundle were not trivial, we quickly achieved an aggregate decrease in IHCA within 3 months (Figure 2), emphasizing the high value of investment in the CAP processes.
Impact of Baseline IHCA Rate on Improvement
PC4 enables CICUs to transparently compare their outcomes with others. Although all hospitals were invited to join the CAP collaborative, we presume hospitals with high IHCA rates were more motivated to participate fully in this project and that hospitals with lower IHCA rates were less likely to expend extra effort on IHCA prevention. Our data demonstrated higher aggregate baseline IHCA rates at CAP hospitals compared with control hospitals, perhaps confirming this hypothesis. Thus, although we present clear evidence of IHCA reduction at CAP hospitals, we cannot conclude CAP will further reduce IHCA at hospitals with already low IHCA rates. It is possible there is a limit to the degree of improvement attainable through this particular bundle, after which significant change becomes more difficult because of the rarity of IHCA and/or IHCA etiologies that are not impacted by current CAP processes. At the end of the intervention period, CAP and control hospitals had similar IHCA incidence rates. Follow-up studies may elucidate whether IHCA rates can get even lower with continued CAP processes in place. Interhospital variation in IHCA rate is expected,21,33,34 and reducing IHCA at hospitals with higher-than-expected rates represents an important accomplishment to prevent the morbidity and mortality attributable to IHCA. We presume our multicenter model for improvement and CAP principles will be translatable to other cohorts in hospitals with high IHCA rates, at minimum.
Safety Huddle Situational Awareness
The mandatory CAP safety huddle was initiated in almost all eligible high-risk patients. We believe this element was essential for IHCA reduction. CAP safety huddles create team situational awareness regarding high-risk clinical status and defines patient-specific and diagnosis-specific mitigation strategies while IHCA is still preventable. Situational awareness is associated with decreased risk for other hospital safety events.35 A single pediatric CICU recently detailed significant IHCA reduction from a high baseline rate, using a situational awareness care bundle featuring bedside epinephrine availability to treat acute hypotension.29 Our project confirms and extends these findings across multiple hospitals with variable clinical environments and baseline IHCA rates. However, as many of the control (and CAP) hospitals used bedside rescue epinephrine for IHCA prevention before this project, our results suggest epinephrine availability without establishment of CAP safety huddle situational awareness may be inadequate for IHCA prevention.
Mortality
IHCA prevention was not associated with lower CICU mortality, despite previous literature suggesting 50% mortality after IHCA.5,36 Stable mortality might suggest some IHCA episodes prevented were in patients who would have most likely been rescued after CPR, with reversible conditions and/or with mode(s) of deterioration that can be targeted by focused IHCA prevention processes. For example, cardiac surgical patients are known to have significantly better survival after IHCA because of reversible causes and more rapid deployment of extracorporeal membrane oxygenation during CPR.5,37,38 Additionally, surgical patients typically have predictable deterioration modes in the early postoperative period (ie, progressive low cardiac output), which are amenable to standard just-in-time training and situational awareness concepts to identify and prevent worsening toward IHCA. Alternatively, an indirect benefit of focus on IHCA reduction may be more elegant end-of-life decision-making and avoidance of IHCA in patients whose outcome is destined for hospital mortality.
Even without clear CICU mortality benefit, IHCA prevention may improve important IHCA-related morbidity, such as brain injury, length of stay, and resource utilization. Furthermore, there may be indirect benefits of CAP on CPR quality metrics, such as shorter time to epinephrine and chest compressions and reducing time to spontaneous circulation. All these hypotheses warrant follow-up investigation.
Limitations
This project has important limitations. We observed variability in engagement, implementation, and start date across CAP hospitals; and all control hospitals were, at minimum, exposed to CAP principles through regular PC4 activities. These factors bias our analysis toward the null hypothesis. We cannot assess which bundle element(s) most affected IHCA prevention; factorial design would help identify individual and/or synergistic effects of each bundle element during the spread phase of this project. Some CAP hospitals did not improve; therefore, we cannot conclude our bundle is universally effective, especially in hospitals with low IHCA rates. Our lightly prescriptive bundle may have enabled important nuances in implementation and execution across hospitals that influenced success, which are as yet not defined or measured. We did not formally evaluate balancing measures; future study can determine if increased resource utilization was necessary to achieve these results. Additionally, we do not know whether this intervention has sustainable effects.
Conclusions
The PC4 CAP project demonstrated that some IHCA is preventable in the pediatric CICU. Collaborative learning and reliable implementation of our bundle were necessary and sufficient for improvement, and this bundle can be implemented at other ICUs almost immediately if high-risk patient selection is adapted to noncardiac units. In future study, we hope to determine key IHCA prevention practices and hospital organizational factors that best facilitate successful CAP implementation and IHCA reduction.
eMethods. Detailed Description of Difference-in-Differences Analysis and Parallel Trends Assumption
eFigure 1. High-Risk Surgical Patient IHCA, Children’s of Alabama, August 2014 to March 2017
eFigure 2. Project Key Driver Diagram
eFigure 3. End of CAP Project Statistical Process Control Chart: IHCA Episodes per 1000 CICU Days, All CICU Admissions at CAP Hospitals
eFigure 4. Indication for CAP Bundle Initiation
eTable. Aggregate Bundle Element Compliance From Audits (n = 1097)
eFigure 5. Hospital-Specific Baseline Period Risk-Adjusted IHCA Incidence Rate for All CICU Admissions
PC4 Cardiac Arrest Prevention Quality Improvement Project Toolkit
PC4 CAP Collaborators
References
- 1.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators . Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med. 2017;376(4):318-329. doi: 10.1056/NEJMoa1610493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson JD, Neish SR, Cabrera AG, et al. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids’ Inpatient Database. Crit Care Med. 2012;40(11):2940-2944. doi: 10.1097/CCM.0b013e31825feb3f [DOI] [PubMed] [Google Scholar]
- 3.Merchant RM, Yang L, Becker LB, et al. ; American Heart Association Get With The Guidelines-Resuscitation Investigators . Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401-2406. doi: 10.1097/CCM.0b013e3182257459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe HA, Sutton RM, Reeder RW, et al. ; Eunice Kennedy Shriver National Institute of Child Health; Human Development Collaborative Pediatric Critical Care Research Network; Pediatric Intensive Care Quality of Cardiopulmonary Resuscitation Investigators . Functional outcomes among survivors of pediatric in-hospital cardiac arrest are associated with baseline neurologic and functional status, but not with diastolic blood pressure during CPR. Resuscitation. 2019;143:57-65. doi: 10.1016/j.resuscitation.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alten JA, Klugman D, Raymond TT, et al. Epidemiology and outcomes of cardiac arrest in pediatric cardiac ICUs. Pediatr Crit Care Med. 2017;18(10):935-943. doi: 10.1097/PCC.0000000000001273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg RA, Nadkarni VM, Clark AE, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network . Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med. 2016;44(4):798-808. doi: 10.1097/CCM.0000000000001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowry AW, Knudson JD, Cabrera AG, Graves DE, Morales DL, Rossano JW. Cardiopulmonary resuscitation in hospitalized children with cardiovascular disease: estimated prevalence and outcomes from the kids’ inpatient database. Pediatr Crit Care Med. 2013;14(3):248-255. doi: 10.1097/PCC.0b013e3182713329 [DOI] [PubMed] [Google Scholar]
- 8.Berg RA, Sutton RM, Holubkov R, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network and for the American Heart Association’s Get With the Guidelines-Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators . Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41(10):2292-2297. doi: 10.1097/CCM.0b013e31828cf0c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perman SM, Stanton E, Soar J, et al. ; American Heart Association’s Get With the Guidelines®—Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators . Location of in-hospital cardiac arrest in the United States—variability in event rate and outcomes. J Am Heart Assoc. 2016;5(10):e003638. doi: 10.1161/JAHA.116.003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaies M, Pasquali SK, Banerjee M, et al. ; Improvement in Pediatric Cardiac Surgical Outcomes Through Interhospital Collaboration . Improvement in pediatric cardiac surgical outcomes through interhospital collaboration. J Am Coll Cardiol. 2019;74(22):2786-2795. doi: 10.1016/j.jacc.2019.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaies M, Anderson J, Kipps A, et al. ; Cardiac Networks United Executive Committee and Advisory Board . Cardiac Networks United: an integrated paediatric and congenital cardiovascular research and improvement network. Cardiol Young. 2019;29(2):111-118. doi: 10.1017/S1047951118001683 [DOI] [PubMed] [Google Scholar]
- 12.Institute for Healthcare Improvement . The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. Institute for Healthcare Improvement; 2003. [Google Scholar]
- 13.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi: 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith NZH, Campbell K, Borasino S, Alten J, Jackson K. Select abstracts from Cardiology 2016: the 19th Annual Update on Pediatric and Congenital Cardiovascular Disease. implementation of a resuscitation action plan in the cardiovascular intensive care unit. World J Pediatr Congenit Heart Surg. 2016;7(2):245-289. doi: 10.1177/2150135115626922 [DOI] [Google Scholar]
- 15.Peebles RC, Nicholson IK, Schlieff J, Peat A, Brewster DJ. Nurses’ just-in-time training for clinical deterioration: development, implementation and evaluation. Nurse Educ Today. 2020;84:104265. doi: 10.1016/j.nedt.2019.104265 [DOI] [PubMed] [Google Scholar]
- 16.Berwick DM. Developing and testing changes in delivery of care. Ann Intern Med. 1998;128(8):651-656. doi: 10.7326/0003-4819-128-8-199804150-00009 [DOI] [PubMed] [Google Scholar]
- 17.Nolan JP, Berg RA, Andersen LW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: a consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation. 2019;140(18):e746-e757. doi: 10.1161/CIR.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 18.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401-2402. doi: 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 19.Provost LP, Murray SK. The Health Care Data Guide: Learning From Data for Improvement. John Wiley & Sons; 2011. [Google Scholar]
- 20.Kronick SL, Kurz MC, Lin S, et al. Part 4: systems of care and continuous quality improvement: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl 2):S397-S413. doi: 10.1161/CIR.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 21.Chen LM, Nallamothu BK, Spertus JA, Li Y, Chan PS; American Heart Association’s Get With the Guidelines-Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators . Association between a hospital’s rate of cardiac arrest incidence and cardiac arrest survival. JAMA Intern Med. 2013;173(13):1186-1195. doi: 10.1001/jamainternmed.2013.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campello G, Granja C, Carvalho F, Dias C, Azevedo LF, Costa-Pereira A. Immediate and long-term impact of medical emergency teams on cardiac arrest prevalence and mortality: a plea for periodic basic life-support training programs. Crit Care Med. 2009;37(12):3054-3061. doi: 10.1097/CCM.0b013e3181b02183 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Ou L, Hillman K, et al. The impact of implementing a rapid response system: a comparison of cardiopulmonary arrests and mortality among four teaching hospitals in Australia. Resuscitation. 2014;85(9):1275-1281. doi: 10.1016/j.resuscitation.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. doi: 10.1016/j.jcrc.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 25.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306-312. doi: 10.1097/PCC.0b013e318198b02c [DOI] [PubMed] [Google Scholar]
- 26.Dewan M, Muthu N, Shelov E, et al. Performance of a clinical decision support tool to identify PICU patients at high risk for clinical deterioration. Pediatr Crit Care Med. 2020;21(2):129-135. doi: 10.1097/PCC.0000000000002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futterman C, Salvin JW, McManus M, et al. Inadequate oxygen delivery index dose is associated with cardiac arrest risk in neonates following cardiopulmonary bypass surgery. Resuscitation. 2019;142:74-80. doi: 10.1016/j.resuscitation.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niles DE, Dewan M, Zebuhr C, et al. A pragmatic checklist to identify pediatric ICU patients at risk for cardiac arrest or code bell activation. Resuscitation. 2016;99:33-37. doi: 10.1016/j.resuscitation.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 29.Ferguson LP, Thiru Y, Staffa SJ, Guillén Ortega M. Reducing cardiac arrests in the PICU: initiative to improve time to administration of prearrest bolus epinephrine in patients with cardiac disease. Crit Care Med. 2020;48(7):e542-e549. doi: 10.1097/CCM.0000000000004349 [DOI] [PubMed] [Google Scholar]
- 30.Andersen LW, Kim WY, Chase M, et al. ; American Heart Association’s Get With the Guidelines—Resuscitation Investigators . The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation. 2016;98:112-117. doi: 10.1016/j.resuscitation.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewan M, O’Halloran A, Kleinman M, et al. Establish and Formalize Expert Criteria for Avoidable Resuscitation Review (SAFECARR) electronic Delphi: development of a consensus framework for classifying and reviewing cardiac arrests within the PICU. Pediatr Crit Care Med. 2020;21(11):992-999. doi: 10.1097/PCC.0000000000002488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galhotra S, DeVita MA, Simmons RL, Dew MA; Members of the Medical Emergency Response Improvement Team (MERIT) Committee . Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Qual Saf Health Care. 2007;16(4):260-265. doi: 10.1136/qshc.2007.022210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong RA, Kane C, Oglesby F, Barnard K, Soar J, Thomas M. The incidence of cardiac arrest in the intensive care unit: a systematic review and meta-analysis. J Intensive Care Soc. 2019;20(2):144-154. doi: 10.1177/1751143718774713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolte D, Khera S, Aronow WS, et al. Regional variation in the incidence and outcomes of in-hospital cardiac arrest in the United States. Circulation. 2015;131(16):1415-1425. doi: 10.1161/CIRCULATIONAHA.114.014542 [DOI] [PubMed] [Google Scholar]
- 35.Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. doi: 10.1542/peds.2012-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta P, Pasquali SK, Jacobs JP, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators . Outcomes following single and recurrent in-hospital cardiac arrests in children with heart disease: a report from American Heart Association’s Get With the Guidelines Registry—Resuscitation. Pediatr Crit Care Med. 2016;17(6):531-539. doi: 10.1097/PCC.0000000000000678 [DOI] [PubMed] [Google Scholar]
- 37.Lasa JJ, Rogers RS, Localio R, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: a report from the American Heart Association’s Get With The Guidelines—Resuscitation (GWTG-R) Registry. Circulation. 2016;133(2):165-176. doi: 10.1161/CIRCULATIONAHA.115.016082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortmann L, Prodhan P, Gossett J, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators . Outcomes after in-hospital cardiac arrest in children with cardiac disease: a report from Get With the Guidelines—Resuscitation. Circulation. 2011;124(21):2329-2337. doi: 10.1161/CIRCULATIONAHA.110.013466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Description of Difference-in-Differences Analysis and Parallel Trends Assumption
eFigure 1. High-Risk Surgical Patient IHCA, Children’s of Alabama, August 2014 to March 2017
eFigure 2. Project Key Driver Diagram
eFigure 3. End of CAP Project Statistical Process Control Chart: IHCA Episodes per 1000 CICU Days, All CICU Admissions at CAP Hospitals
eFigure 4. Indication for CAP Bundle Initiation
eTable. Aggregate Bundle Element Compliance From Audits (n = 1097)
eFigure 5. Hospital-Specific Baseline Period Risk-Adjusted IHCA Incidence Rate for All CICU Admissions
PC4 Cardiac Arrest Prevention Quality Improvement Project Toolkit
PC4 CAP Collaborators