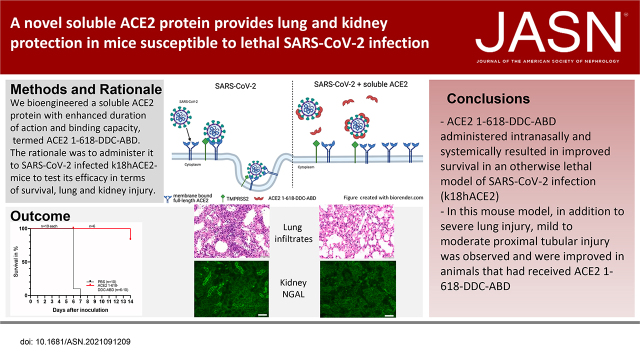
Significance Statement
A novel soluble angiotensin converting enzyme 2 (ACE2) protein with increased binding to the spike protein of the coronavirus that causes coronavirus disease 2019 (COVID-19) is protective in an animal model of severe COVID-19 , providing a proof of concept of efficacy. Administration of soluble ACE2 protein resulted in conversion of a lethal disease into a mild one that is reversible, with improved lung and kidney injury.
Keywords: virology, kidney disease, renin angiotensin system, acute renal failure, renal protection, mice, COVID-19, SARS-CoV-2, angiotensin-converting enzyme 2, disease susceptibility
Visual Abstract
Abstract
Background
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) uses full-length angiotensin converting enzyme 2 (ACE2) as a main receptor to enter target cells. The goal of this study was to demonstrate the preclinical efficacy of a novel soluble ACE2 protein with increased duration of action and binding capacity in a lethal mouse model of COVID-19.
Methods
A human soluble ACE2 variant fused with an albumin binding domain (ABD) was linked via a dimerization motif hinge-like 4-cysteine dodecapeptide (DDC) to improve binding capacity to SARS-CoV-2. This novel soluble ACE2 protein (ACE2–1-618-DDC-ABD) was then administered intranasally and intraperitoneally to mice before intranasal inoculation of SARS-CoV-2 and then for two additional days post viral inoculation.
Results
Untreated animals became severely ill, and all had to be humanely euthanized by day 6 or 7 and had pulmonary alveolar hemorrhage with mononuclear infiltrates. In contrast, all but one mouse infected with a lethal dose of SARS-CoV-2 that received ACE2–1-618-DDC-ABD survived. In the animals inoculated with SARS-CoV-2 that were untreated, viral titers were high in the lungs and brain, but viral titers were absent in the kidneys. Some untreated animals, however, had variable degrees of kidney proximal tubular injury as shown by attenuation of the proximal tubular brush border and increased NGAL and TUNEL staining. Viral titers in the lung and brain were reduced or nondetectable in mice that received ACE2–1-618-DDC-ABD, and the animals developed only moderate disease as assessed by a near-normal clinical score, minimal weight loss, and improved lung and kidney injury.
Conclusions
This study demonstrates the preclinical efficacy of a novel soluble ACE2 protein, termed ACE2–1-618-DDC-ABD, in a lethal mouse model of SARS-CoV-2 infection that develops severe lung injury and variable degrees of moderate kidney proximal tubular injury.
Coronavirus disease 2019 (COVID-19) is primarily a respiratory disease, but kidney involvement is frequently seen as well. The incidence of AKI in hospitalized patients ranges from 22% to 57% and is associated with high mortality.1–5 Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), like SARS-CoV, uses angiotensin converting enzyme 2 (ACE2) as its main receptor.6–19 After binding of the SARS-CoV-2 S spike to cell membrane–bound ACE2, there is priming by proteases, namely TMPRSS2, which are needed for the fusion and internalization of the ACE2–viral spike complex.7,15–17 As early as 2003, when ACE2 was discovered to be the host receptor for SARS-CoV, in vitro experiments with native soluble ACE2 showed that this protein can neutralize the S spike protein of SARS-CoV.12,19 In keeping with this concept, early in 2020, we hypothesized that by acting as a decoy, soluble ACE2 could be used to combat SARS-CoV-2 infection.13 Monteil et al. showed that native soluble ACE2 can neutralize SARS-CoV-2 infectivity in human kidney and vascular organoids.18 We confirmed these findings in kidney organoids using the native soluble ACE2 and extended them to a novel ACE2 protein fused with a small (5-kD) albumin binding domain (ABD) that prolongs in vivo duration of action.17 Plasma enzymatic activity of this protein, termed ACE2–1-618-ABD, remains relatively high for 48–96 hours, whereas the duration of action of native soluble ACE2 and naked ACE2–1-618 is only 4–8 hours.17,20
A key property of an optimal soluble ACE2 protein to act as a decoy for SARS-CoV-2, however, should include not only extended duration of action, but also strong binding for the receptor binding domain (RBD) of SARS-CoV-2. Viral spike trimers may be more amenable to simultaneous binding to dimeric ACE2 forms analogously to that found with enhanced binding of dimeric diabodies to trimeric fusion protein in respiratory syncytial virus.21 ACE2–1-618 and ACE2–1-618-ABD, however, are monomers and therefore not likely to have very strong binding to SARS-CoV-2. We reasoned that a dimerized ACE2–1-618-ABD might display increased affinity to the S1 spike of SARS-CoV-2. To accomplish dimerization, ACE2–1-618-ABD was bridged by fusion with a hinge-like 13-mer KCHWECRGCRLVC (DDC). Here, we report the generation of this new chimera that we termed ACE2–1-618-DDC-ABD and demonstrate three key characteristics: (1) enhanced duration of action, (2) increased binding to SARS-CoV-2, and (3) full enzymatic activity. Moreover, we tested the preclinical efficacy of this protein in vivo using a transgenic mouse model that is susceptible to infection: the k18-hACE2 mouse.22 In this model, inoculation with SARS-CoV-2 causes a lethal disease with extensive lung injury.22–28 Kidney proximal tubular injury in this model, however, has not been previously reported. Here, we report variable degrees of moderate proximal tubular injury in this model of lethal SARS-CoV-2 infection. In animals that received ACE2–1-618-DDC-ABD, lethality was prevented, converting a lethal disease to a milder one with improved lung and kidney injury.
Methods
Generation of Human ACE2–1-618-DDC-ABD Protein
A recombinant human ACE2 protein chimera was generated using an approach similar to that described recently.17,29 Briefly, a cDNA coding for the C-terminal portion of the 618 amino acid fragment of human ACE2 protein (termed ace2–1-618) was fused with the abd cDNA encoding for a small ABD protein (5 kD) using a flexible linker placed between the N terminal end of the abd cDNA (IDT) and the C-terminal end of ace2–1-618. This resulted in prolonged in vivo duration of action of ACE2 as described previously.17 To achieve dimerization of the ACE2-ABD chimeric protein, we inserted cDNA coding for a hinge-like region containing a dodecapeptide motif30 termed DDC between the c-terminus of ACE2–1-618 and the n-terminus for the linker (GGSSRSSSSGGGGSGGGG) followed by the cDNA for ABD. The cDNA of the fusion chimera termed ace2–1-618-ddc-abd was then inserted into pcDNA3–4 plasmid (Invitrogen) using custom synthesized complementary primers (IDT) and the Gibson assembly kit (NEB). After verifying the DNA sequence of the pcDNA3–4 fused with the cDNA for ace2–1-618-ddc-abd, the protein was produced in 293 cells and purified using fast protein liquid chromatography on Q Sepharose followed by size exclusion chromatography on Superdex to around 95% purity in the Recombinant Protein Core at Northwestern University.
Binding Affinity Studies
Binding affinity of different soluble ACE2 variants was examined using an assay that relies on concentration dependence to capture ACE2 enzymatic activity by immobilized SARS-CoV-2 RBD.17 Briefly, purified his-tagged SARS-CoV-2 S1-RBD protein (1 µg/ml) dissolved in Tris-buffered saline (TBS; pH 7.4) was loaded onto a 96-well Ni-coated black plate and incubated with shaking for 1 hour at room temperature. After five washes with 200 µl TBS supplemented with 0.05% Tween (wash buffer) each, 100 µl soluble ACE2 variants (ACE2–1-618-DDC-ABD, ACE2–1-618-ABD, and ACE2–1-740) dissolved in TBS in concentrations ranging from 0.01 ng/ml to 100 µg/ml was added and incubated with shaking for 1 hour at room temperature. Wells were then washed five times with 200 µl wash buffer again, before 90 µl TBS and 10 µl Mca-APK-Dnp substrate were added, and fluorescence formation was measured in a microplate fluorescence reader (FLX800, at 320 nm using excitation and 400 nm emission filters).
In vitro Infectivity Studies
All work with live SARS-CoV-2 was performed in the BSL-3 facility of the Ricketts Regional Biocontainment Laboratory. Five hundred plaque forming units (PFU) of each SARS-CoV-2 strain (nCoV/Washington/1/2020 isolate, or Gamma or Delta variant of SARS-CoV-2) was incubated with various concentrations (0, 45, 90, and 180 µg/ml) of the different soluble ACE2 proteins (human ACE2–1-618-DDC-ABD, human ACE2–1-618-ABD, human ACE2–1-740, or mouse ACE2–1-740) for 1 hour at 37°C. These mixtures were then used to infect Vero E6 cells. Cells were allowed to grow for 3–4 days until a noticeable cytopathic effect was observed in control wells (0 µg/ml soluble ACE2 proteins). Cell numbers were assessed by staining cells with crystal violet and reading absorbance of each well at 595 nm. Values were then normalized to the 0 µg/ml control and expressed as a percentage of the mock (no virus) control wells.
ACE2 Activity and Staining after Administration of ACE2–1-618-ABD-DDC to Mice
Pharmacokinetics of ACE2–1-618-DDC-ABD in plasma was examined in wild-type mice (C57/B6) as previously described.17 To demonstrate that intranasal administration of ACE2–1-618-DDC-ABD can increase lung ACE2 activity using a dose comparable with that planned in the in vivo studies in k18hACE2 mice, we performed experiments in male wild-type mice (CD1; see Supplemental Material). For lung studies, ACE2–1-618-DDC-ABD was administered intranasally at two different doses (1 and 6–7 µg/g body wt, both in 40 µl PBS). Twenty-four hours later, lungs were harvested. One portion of the lung tissue was snap frozen at –80°C, and the other one was fixed in 10% formalin for subsequent staining studies. Parts of the frozen lung tissues were homogenized in RIPA buffer, centrifuged at 6400 g for 10 minutes at 4°C, and the supernatant saved. In the resulting lung lysates, total ACE2 enzyme activity was measured using Mca-APK-Dnp substrate (Bachem).
In vivo Infectivity Studies
All work with live SARS-CoV-2 was performed in the BSL-3 facility of the Ricketts Regional Biocontainment Laboratory, according to a protocol approved by the Institutional Animal Care and Use Committees of Northwestern University and University of Chicago. We used k18-hACE2 mice that express human ACE2 and are susceptible for SARS-CoV-2 infection,23–28 purchased from The Jackson Laboratory (8 weeks old), which are estimated to have a standardized hACE2 gene copy number of 8. Animals were infected with 2 × 104 PFU SARS-CoV-2 in 20 µl (strain: nCoV/Washington/1/2020) by intranasal inoculation. Animals infected with this viral invariably succumb to disease by days 5–9.23–26 ACE2–1-618-DDC-ABD (30 µl, around 13 µg/g body wt) was administered intranasally 1 hour before SARS-CoV-2 followed by the same dose 24 and 48 hours later for a total of three doses. Additionally, at the same time points (1 hour before and 24 and 48 hours after inoculation), an intraperitoneal (ip) injection of ACE2–1-618-DDC-ABD (200 µl, around 1 µg/g body wt) was administered. Control animals were treated at the same time points with the same volumes of PBS intranasally and ip. Animals were weighed once daily and monitored twice daily for health using a clinical scoring system (Supplemental Table 1). Animals that lost more than 20% of their baseline body weight or had a clinical score of 3 were euthanized for humane reasons. This was considered a fatal event. Otherwise, animals that did not meet these criteria were monitored for up to 14 days in the BSL-3 facility. One part of the lungs, kidneys, and brains removed from all euthanized animals were used for viral load measurement by plaque assays, whereas the other part was fixed in paraffin-embedded blocks for histopathology. Formalin-fixed lung and kidney sections were released from the BSL-3 facility after verifying the absence of infectious virus, embedded in paraffin blocks, and used to generate slides for staining studies by the Mouse Histology and Phenotyping Laboratory Center, Northwestern University. Lung histopathology was evaluated by two expert lung pathologists. Two blinded lung pathologists evaluated the severity and presence of lung injury using a scoring system recently described in k18-hACE2 mice infected with SARS-CoV-2.23 The alterations scored were mononuclear infiltrates, alveolar hemorrhage, edema, cellular necrosis, hyaline membranes, and thrombosis. The scale was as follows: 0, no detection; 1, uncommon detection in <5% lung fields (200 Å); 2, detectable in up to 30% of lung fields; 3, detectable in 33%–66% of lung fields; and 4, detectable in >66% of lung fields. Neutrophil infiltration was evaluated on a scale of 0–3 as follows: 0, within normal range; 1, scattered PMNs sequestered in septa; 2, score 1 and solitary PMNs extravasated in airspaces; 3, score 2 plus and aggregates in vessel and airspaces. Kidney histology was evaluated in periodic acid–Schiff-stained slides by two blinded pathologists independently of each other. Proximal tubular injury was assessed using a score of 0–3 on the basis of brush border loss and cytolysis. Tubular dilatation was rarely seen in our nonperfused kidneys and therefore was not part of the score.
Plaque Assay for Infectious Virus
Tissue samples were collected in DMEM with 2% FBS and were homogenized using 1.4 mm ceramic beads in a tissue homogenizer using two 30-second pulses. Samples were then centrifuged at 1000 g for 5 minutes, and the supernatant was serially diluted 10-fold and used to infect Vero E6 cells for 1 hour. Inoculum was removed, and 1.25% methylcellulose DMEM solution was added to the cells and incubated for 3 days. Plates were fixed in 1:10 formalin for 1 hour, stained with crystal violet for 1 hour, and counted to determine PFU per milliliter.
Immunofluorescence Studies
For staining studies of ACE2 in the lung and kidney, an ACE2 antibody (AF933; R&D Systems) was used17 as previously described.31 Kidney slides were stained with anti-lipocain-2/NGAL antibody (Abcam; ab216462), anti-megalin antibody (Santa Cruz Biotechnology; sc-515750), and anti-aquaporin-2 antibody (Novus Biologicals; NBP170378). The intensity of NGAL staining in kidney sections was scored in four fields per animal by two independent, blinded observers by a score of 0–3 using the following criteria: 0, none; 1, mild; 2, moderate; and 3, intense.32 To evaluate apoptosis, kidneys were stained with the TUNEL kit (Roche; 11684795910). In addition, nuclei were stained with TO-PRO-3 iodide (Invitrogen; T3605)33 to demonstrate nuclear staining.
Statistical Analyses
Statistics were calculated using GraphPad Prism v8.4.3 (GraphPad Software). The Shapiro–Wilk test was used to test normality. Differences between more than two groups with normally distributed data were analyzed by ANOVA followed by post hoc Dunnett’s multiple comparisons test. Differences between more than two groups with non-normally distributed data were analyzed by the Kruskal–Wallis test followed by the post hoc Dunn’s multiple comparisons test. Data from contingency tables were analyzed by Fisher’s exact test.
Results
Generation of a Soluble ACE2 Variant with Enhanced Binding to SARS-CoV-2
A cDNA coding for the C-terminal portion of a 618 amino acid fragment of human ACE2 protein (termed ace2–1-618) was fused with the abd cDNA encoding for a small ABD protein (5 kD) as recently described by us.17 Here, the dimerization of the ACE2–1-618-ABD chimeric protein was achieved by inserting a cDNA coding for a hinge-like region containing a dodecapeptide (DDC) just after the coding sequence for the c-terminus of ace2–1-618 and between the n-terminus of a spacer (GGSSRSSSSGGGGSGGGG) followed by cDNA for ABD.
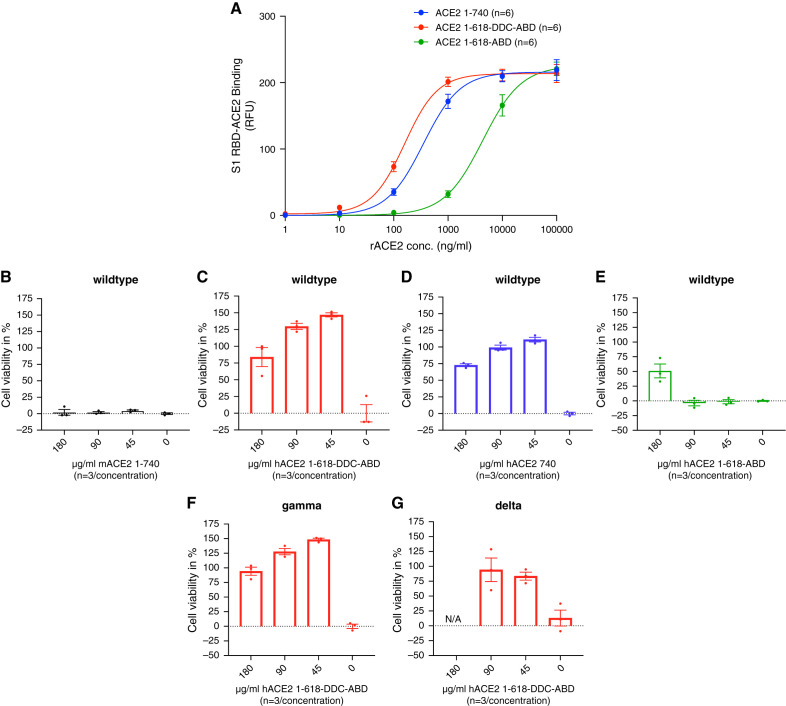
The binding of ACE2–1-618-DDC-ABD to the SARS-CoV-2 S1-RBD was compared with two other soluble ACE2 proteins: ACE2–1-618-ABD and the native ACE2–1-740. The binding affinity of ACE2–1-618-DDC-ABD was higher than that of native ACE2–1-740, also a dimer, and much higher than ACE2–1-618-ABD, a monomer (Figure 1A). This is evident from the half maximal effective concentration (EC50), which was significantly lower for ACE2–1-618-DDC-ABD (158 ng/ml) than for ACE2–1-740 (352 ng/ml; P=0.02) and much lower than for ACE2–1-618-ABD (4359 ng/ml; P=0.004), calculated by repeated-measures analysis.
Figure 1.
Binding affinity of soluble ACE2 proteins to the RBD of the viral S1 glycoprotein and neutralization of live SARS-CoV-2 in Vero E6 cells. (A) The binding of ACE2 variants 1-618-DDC-ABD (red), 1-618-ABD (green), and the native ACE2–1-740 protein (blue) to the S1-RBD of SARS-CoV-2 was studied using a recently published assay.17 The binding affinity of ACE2–1-618-DDC-ABD is markedly higher compared with that of ACE2–1-618-ABD, and also slightly higher than that of native ACE2–1-740 soluble protein. The EC50 values are given in the Results section. (B–E) The efficacy of these three proteins (0–180 µg/ml, n=3/concentration) to neutralize live wild-type SARS-CoV-2 infection of Vero E6 cells was measured by a cell death assay. (B) Mouse ACE2–1-740 used as negative control, as expected, had no protective effect on cell viability at any concentration tested (black). (C) ACE2–1-618-DDC-ABD (red) resulted in slightly higher percentages of cell viability, although (D) native ACE2–1-740 (blue) also prevented cell death almost completely at all of the high concentrations tested (but not at 0 µg/ml). By contrast, (E) ACE2–1-618-ABD (green) provided only partial protection at its highest concentration (180 µg/ml) and had no neutralizing effect at lower concentrations (90 and 45 µg/ml). (F) and (G) ACE2–1-618-DDC-ABD also neutralizes infection by the SARS-CoV-2 (F) Gamma and (G) Delta variants at the concentrations tested (45, 90, and 180 µg/ml, but not at 0 µg/ml). Please note that there are no data at 180 µg/ml for the Delta variant for technical reasons (overgrown cells did not attach well to the plate).
ACE2–1-618-DDC-ABD Neutralizes Several SARS-CoV-2 Variants in Vero E6 cells
The in vitro neutralization of SARS-CoV-2 by ACE2–1-618-DDC-ABD, ACE2–1-618-ABD and native ACE2–1-740 (45–180 µg/ml) was tested in Vero E6 cells, a permissive cell line, using the percentage of cell viability as the end point (Figure 1, C–E). Mouse ACE2–1-740 used as a negative control, as expected, had no effect on cell viability at any concentration tested (Figure 1B). ACE2–1-618-DDC-ABD and ACE2–1-740 both protected the Vero E6 cells from cell death at the concentrations tested (Figure 1, C and D). ACE2–1-618-ABD, by contrast, provided only partial protection from cell death at the highest concentration (180 µg/ml) and had no effect at the lower concentrations (45 and 90 µg/ml) that were fully effective with the other two proteins (Figure 1E).
Additionally, we tested the effect of ACE2–1-618-DDC-ABD to neutralize the Gamma and Delta variants of SARS-CoV-2. Similar to the neutralizing effect on SARS-CoV-2, ACE2–1-618-DDC-ABD neutralized the Gamma and Delta variants of SARS-CoV-2 at all three concentrations tested (Figure 1, F and G).
Prolonged Duration of Plasma ACE2 Enzymatic Activity after Injection of ACE2–1-618-DDC-ABD
We then examined the in vivo plasma enzymatic activity of the novel ACE2–1-618-DDC-ABD variant as a measure of protein duration of action and compared it with the native ACE2–1-740, injected ip (Supplemental Figure 1). By measuring plasma enzymatic activity, one cannot discriminate between exogenous human plasma activity (from the infused protein) and endogenous mouse ACE2 activity. Baseline plasma ACE2 activity in mice, however, is usually extremely low.20,34 Therefore, the observed large increase in activity after injection of soluble ACE2 proteins essentially reflects exogenous activity from the injected soluble ACE2 protein.35,36 After injection of ACE2–1-618-DDC-ABD, plasma ACE2 activity peaked at 8 hours, with high levels remaining at 24, 36, and 48 hours (Supplemental Figure 1). By contrast, after injection of the native ACE2–1-740, plasma activity had decreased markedly by 8 hours and was reduced to very low levels thereafter (Supplemental Figure 1).
ACE2–1-618-DDC-ABD Administration to k18-hACE2 Mice Infected with SARS-CoV-2
Because wild-type mice are resistant to SARS-CoV-2 infection, we used the transgenic k18-hACE2 mouse as a model that expresses human ACE2 and therefore is infectable.22–28 After SARS-CoV-2 inoculation with 2 × 104–2 × 105 PFU, these animals have essentially 100% mortality by days 5–9.23–26 In total, 20 animals (ten male, ten female) were infected with SARS-CoV-2. Either ACE2–1-618-DDC-ABD or PBS (n=10 per group, 5 male and 5 female each) was administered intranasally and by ip injection under light anesthesia 1 hour before virus inoculation and 24 and 48 hours later.
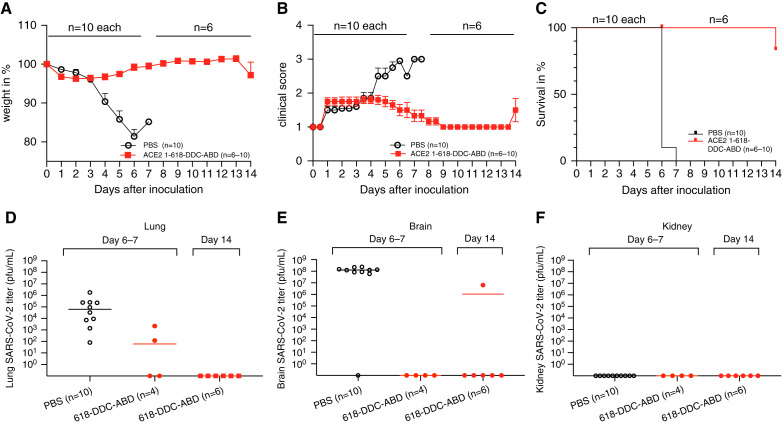
The ACE2–1-618-DDC-ABD-treated mice had only minimal weight loss by day 6 (Figure 2A), and the clinical score was ≤2, and moreover, all returned to a normal score of 1 when followed after day 6 (Figure 2B). By contrast, seven out of ten PBS-treated mice lost >20% of their body weight by day 6 (Figure 2A). The remaining three, all male, also had marked weight loss but <20% of their body weight (11%, 15%, and 16%). The clinical score, also by day 6, was the worst (level 3) in all PBS-treated mice except for one male mouse that was scored 2.5 (Figure 2B). By study protocol, the animals that lost >20% of their body weight were considered to have reached the mortality end point and were euthanized for humane reasons. The three male control mice with body weight losses of 11%, 15%, and 16% also had to be euthanized by protocol on day 6 (for two of them) and day 7 (for the remaining one) because they were very sick, reaching a clinical score of 3. Therefore, by day 6 or 7, the mortality rate was 100% in PBS-treated mice and 0% in ACE2–1-618-DDC-ABD-treated mice (Figure 2C).
Figure 2.
Prevention of mortality and improvement of clinical score, weight loss, and SARS-CoV-2 titers (by plaque assay) after ACE2–1-618-DDC-ABD administration to female and male k18-hACE2 mice (n=10, five male and five female) compared with animals that received PBS (n=10, five male and five female). All 20 animals were infected with SARS-CoV-2. (A–C) The administration of ACE2–1-618-DDC-ABD (red) largely (A) prevented body weight loss, (B) improved clinical scores, and (C) prevented mortality in ten k18-hACE2 transgenic mice compared with 10 vehicle-treated mice (PBS, black), all inoculated with SARS-CoV-2. (D) and (E) At 6 or 7 days post infection, titers were high in all untreated animals (PBS, black) that had to be humanely euthanized by study design. (D) In contrast, at the same time point (day 6) in two male and two female ACE2 618-DDC-ABD-treated mice (red) that were healthy but were euthanized, lung viral titers were lower or undetectable. No lung viral titers were detectable in ACE2 618-DDC-ABD-treated animals on day 6. (E) No SARS-CoV-2 virus was detectable in the lungs of remaining k18-hACE2 mice that received ACE2 618-DDC-ABD and were euthanized 14 days after viral inoculation. Except for the brain viral titer in one male (case 20) that was treated with ACE2–1-618-DDC-ABD and was high on day 14, titers were undetectable in all other mice from this group lung. This was the only animal that lost 20% of its body weight on day 14 (see A) and therefore had to be euthanized as a treatment failure. (F) Kidney viral titers were not detectable in any animals from the treated and untreated group whether they were examined on day 6 or 7 or on day 14 post infection.
To be able to have viral titers at the same time as the untreated animals, two males and two females from the group that received ACE2–1-618-DDC-ABD were euthanized on day 6. The remaining (three male and three female mice) were monitored for an additional week during which they had a completely normal clinical score (Figure 2B) and stable weight (Figure 2A). One male mouse that had received ACE2–1-618-DDC-ABD, however, that was deemed to be healthy until then, by day 14, abruptly lost 20% of its body weight and became sick. This male mouse (case 20) was euthanized together with all of the remaining mice that were healthy at this time point. This male was considered a single mortality event that occurred late on day 14 (Figure 2C).
Viral Titers in the Lungs, Brains, and Kidneys of Mice Treated with ACE2–1-618-DDC-ABD
Administration of ACE2–1-618-DDC-ABD resulted in a marked reduction of SARS-CoV-2 lung and brain titers measured by plaque assay. At 6 days post infection (except for a male mouse that was not euthanized until day 7 per study protocol), brain and lung titers were high, ranging from 102 to 107 PFU/ml in all control animals. By contrast, on day 6, in two males and two females treated with ACE2–1-618-DDC-ABD, lung viral titers were in the low range in two animals and undetectable in the other two (Figure 2D). Brain viral titers on day 6 were nondetectable in any of these four animals from the ACE2–1-618-DDC-ABD group. In the six remaining mice that had received ACE2–1-618-DDC-ABD euthanized 14 days after viral inoculation, no viral titers were detectable in the lungs in any of them (Figure 2D). Brain viral titers on day 14 were nondetectable in five of six animals (Figure 2E). The one male mouse that lost 20% of its body weight abruptly by day 14 had high brain viral titers (case 20; Figure 2E). This is the only animal in the group that received ACE2–1-618-DDC-ABD that had to be euthanized and therefore can be considered a treatment failure, albeit delayed.
Kidney viral titers, also assessed by plaque assay, were not detectable in any untreated animal euthanized on day 6 or 7 or in any animal that received ACE2–1-618-DDC-ABD, euthanized on day 6 or 14 (Figure 2F).
Lung Histopathology
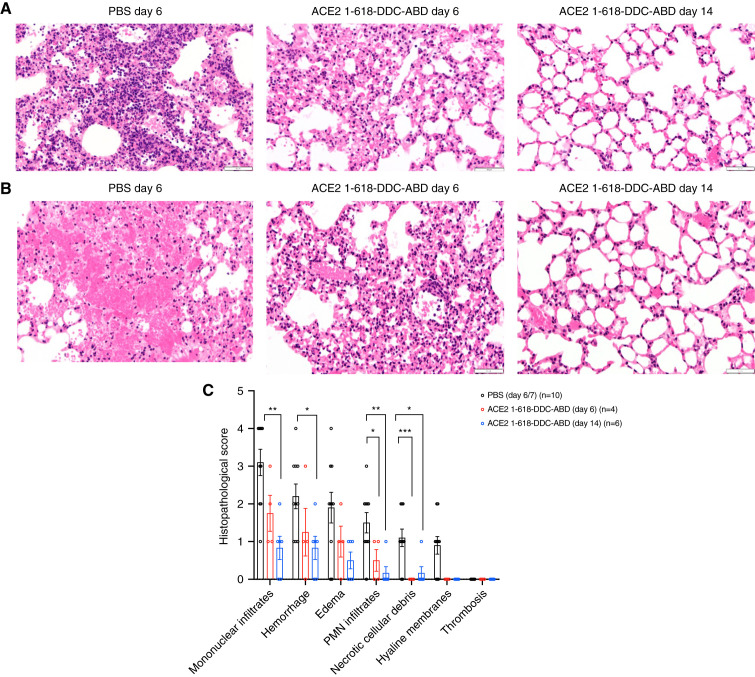
Examination of a lung from each one of the ten untreated animals showed extensive cellular infiltrates evident at low magnification (Supplemental Figure 2, left panels). The lesions consistently seen in controls were dense perivascular mononuclear infiltrates and scattered neutrophils (Figure 3A) with alveolar edema and hemorrhage (Figure 3B). In contrast, the lungs of animals that had received ACE2–1-618-DDC-ABD euthanized at the same time as controls (day 6) showed less cell infiltration and only mild to moderate alveolar hemorrhage (Figure 3, A and B, Supplemental Figures 2 and 3, right panels). In animals that had received ACE2–1-618-DDC-ABD euthanized on day 14, the lesions were much improved, and in some cases the lungs appeared normal (Figure 3, A and B, Supplemental Figures 2 and 3, right panels).
Figure 3.
Representative examples of lung histopathology and lung injury scores. (A) Representative lung histopathology in k18-hACE2 mice infected with SARS-CoV-2 on day 6 (left and middle) and day 14 (right) post infection. Untreated animals (PBS vehicle) show dense perivascular mononuclear infiltrates and scattered neutrophils (left). (B) Untreated animals (PBS vehicle) show more extensive alveolar hemorrhage (left) in contrast to animals treated with ACE2–1-618-DDC-ABD on days 6 and 14. Hematoxylin-eosin, original magnification ×400. (C) The histopathologic lung injury scores are lower in animals treated with ACE2–1-618-DDC-ABD on both day 6 (red) and day 14 (blue) than vehicle-treated controls on day 6 or 7 (black). Significance is indicated in the figure (***P<0.001; **P<0.01; *P<0.05) and was calculated using ANOVA followed by post hoc Dunnett’s multiple comparisons test.
To semi-quantitate the findings, we used a histologic scoring system (score 0–4) recently used in the same mouse transgenic model infected with SARS-CoV-223 (Figure 3C). All untreated animals (euthanized on day 6 or 7) had higher scores than animals that had received ACE2–1-618-DDC-ABD (Figure 3C). At this time point, the differences, however, were statistically significant only for PMN infiltrates and necrotic cellular debris (see Figure 3). The scores of lungs from animals that received ACE2–1-618-DDC-ABD, euthanized on day 14, were much lower than in the other two groups (Figure 3C). At this time point, the scores for mononuclear infiltrates, hemorrhage, edema, and PMN infiltrates were significantly lower than in PBS-treated animals (see Figure 4). Hyaline membranes were also seen in a few untreated animals but none of the treated animals. Thrombosis was not seen in any of the groups (Figure 3C).
Figure 4.
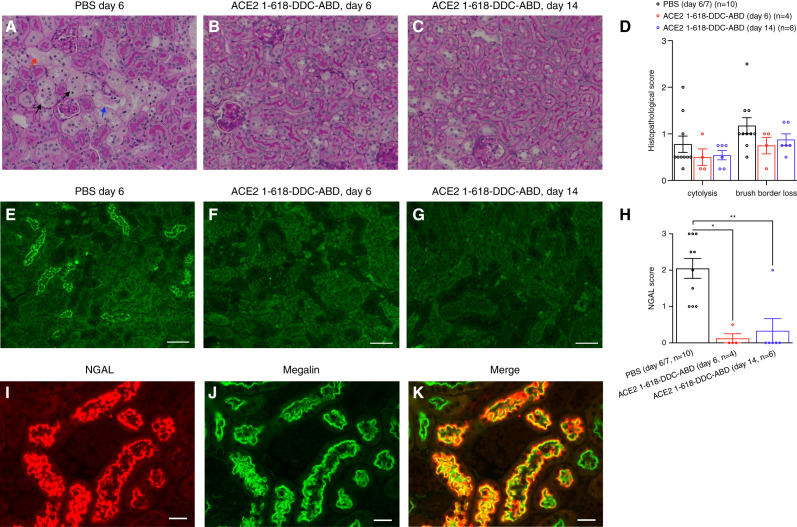
Kidney histopathology examined by PAS-staining and NGAL staining. (A–C) PAS staining in k18hACE2 mice studied on day 6 or 14 (magnification, ×200). Kidneys displayed variable degrees of proximal tubular injury. (A) An example of an untreated animal with the more severe alterations: proximal tubule brush border loss (black arrows) and cytolysis (red arrow) and tubular basement membrane disruption (blue arrow). (B) and (C) Examples of attenuated tubular injury in mice that had received ACE2–1-618-DDC-ABD and were euthanized on days 6 or 14, respectively. (D) The histopathologic injury score, on the basis of the criteria cytolysis and brush border loss, was slightly higher in the PBS group compared with the ACE2–1-618-DDC-ABD group, but this did not reach statistical significance (calculated using ANOVA followed by post hoc Dunnett’s multiple comparisons test). (E–G) NGAL staining on day 6 or 14. (E) Kidney from the untreated animal at day 6 (PBS vehicle) shows NGAL expression in the proximal tubules, whereas it is markedly reduced or non-detectable in animals that received ACE2–1-618-DDC-ABD euthanized on (F) the same day or (G) day 14 (magnification, ×200, scale bar=100 μm. (H) NGAL staining intensity is significantly higher in the PBS group (n=10, 2.05±0.273) compared with the ACE2–1-618-DDC-ABD group at day 6 (0.125±0.125, P=0.01) or day 14 (0.333±0.333, P=0.005, calculated using Kruskal–Wallis test followed by post hoc Dunn’s multiple comparisons test). (I–K) Example of the kidney of an infected k18hACE2 mouse that was untreated. NGAL immunofluorescence is strong, and there is co-localization with megalin (yellow; scale bar=20 μm, magnification, ×400).
Kidney Histopathology
Examination of the kidneys from k18hACE2 mice euthanized on days 6 and 14 post viral inoculation showed variable degrees of proximal tubular injury assessed on periodic acid–Schiff-stained sections both in treated and untreated animals (Figure 4, A–C). The following features were seen: loss of the proximal tubule brush border (black arrows, Figure 4A), cytolysis (red arrow, Figure 4A), and tubular basement membrane attenuation (blue arrow, Figure 4E). When all of the infected animals were evaluated using a score of 0–3 for the criteria brush border loss and cytolysis, the average scores from two independent, blinded pathologists were lower in the treated animals for each criterion, but this difference did not achieve statistical significance on day 6 or 14 (Figure 4D).
Kidneys were also stained with a neutrophil-gelatinase associated lipocalin (NGAL) antibody (Figure 4, E–G).37 NGAL staining was rated on a score of 0–3.32 NGAL staining was significantly lower in mice that received ACE2–1-618-DDC-ABD than in PBS-treated mice (Figure 4H). A score of ≥1 was found in all the kidneys from untreated mice (Figure 4E, Supplemental Figure 4, left panels) but only in two of the ten kidneys from the ACE2–1-618-DDC-ABD-group (Figure 4, F and G, Supplemental Figure 4, right panels). That is, all of the untreated animals had positive staining for NGAL, but only two of the animals that had received ACE2–1-618-DDC-ABD had positive staining—a difference that was found to be statistically significant (P<0.001, Fisher’s exact test).
To localize tubular NGAL expression, co-staining with megalin and ACE2 as markers of the proximal tubule was performed.31 Consistent with previous studies,38,39 control mice of the same background (C57bl/6J) as the infected animals had no NGAL staining (Supplemental Figure 5A). In SARS-CoV-2-infected k18hACE2 mice that received ACE2–1-618-DDC-ABD, similar to the uninfected control, NGAL expression was not detectable (Supplemental Figure 5B). In untreated k18hACE2 mice, by contrast, NGAL staining was present, and there was clear co-localization with megalin (Figure 4I). Using ACE2 as another proximal tubular marker, likewise, in untreated k18hACE2 mice, there was co-localization with NGAL in the proximal tubule (Supplemental Figure 5C), whereas there was no co-localization with aquaporin 2, a marker of the collecting tubule (Supplemental Figure 5D).
Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining was performed to search for apoptosis in kidneys of k18hACE2 mice from both groups. TUNEL staining in the proximal tubules can be seen in the kidneys of untreated mice (Supplemental Figure 6A, left panels), whereas in kidneys of the ACE2–1-618-DDC-ABD-group, TUNEL staining was rarely seen (Supplemental Figure 6A, right panels). TUNEL positive dots per field quantified with ImageJ was significantly higher in kidneys from the PBS group (n=10, 138.2±52) compared with the ACE2–1-618-DDC-ABD group on both days 6 (n=4, 10.2±2.7, P=0.002) and 14 (n=6, 26.8±12.9, P=0.04; Supplemental Figure 6B). However, much of the positivity depicted in Supplemental Figure 6A reflected small dots along the basement membrane rather than nuclear staining. Therefore, to evaluate the nuclear localization of TUNEL-positive staining, we performed co-localization studies using TUNEL-TO-PRO-3 staining.33 In kidneys from infected k18hACE2 mice that received ACE2–1-618-DDC-ABD, there was no co-localization of TUNEL-TO-PRO-3 staining detectable because TUNEL staining was absent (Supplemental Figure 7, G–I). In kidneys of infected, untreated k18hACE2 mice, positive TUNEL staining was observed in the cortical region (Supplemental Figure 7D). Thus, the co-localization of TUNEL-staining with TO-PRO-3 iodide counterstained nuclei was clearly present (Supplemental Figure 7F). In addition, co-staining with TO-PRO-3 iodide with the small TUNEL-positive bodies along the basement membrane was also seen (Supplemental Figure 8C). This co-localization pattern was seen in the four untreated animals examined and may possibly represent DNA fragments after nuclear disintegration.40
Lung ACE2 Activity and Immunostaining for ACE2
To demonstrate that intranasal injection of ACE2–1-618-DDC-ABD results in lung uptake, presumably in the alveolar space, we measured ACE2 activity in the lungs of uninfected mice. ACE2 protein is very low in the lungs overall,41–44 which facilitates the detection of intranasally administered ACE2 proteins. Twenty-four hours after intranasal administration of ACE2–1-618-DDC-ABD (either 1 or 6–8 µg/g body wt), variable but substantial ACE2 activity was detected in the lungs of five mice that received ACE2–1-618-DDC-ABD but not in control animals (Supplemental Figure 9A). Staining of the lung with an ACE2 antibody confirmed the presence of increased ACE2 in the lung of animals that received ACE2–1-618-DDC-ABD (Supplemental Figure 9C) compared with control animals (Supplemental Figure 9B).
In the lungs of infected, PBS-treated k18hACE2 mice that were euthanized 6 days post viral inoculation, similar to uninfected controls, ACE2 staining was also very weak (Supplemental Figure 9D). In the lungs from infected k18hACE2 mice also euthanized on day 6 that had received their final dose of ACE2–1-618-DDC-ABD 96 hours earlier, ACE2 staining was still detectable (Supplemental Figure 9E) but less evident than in the uninfected control mouse that received its dose of ACE2–1-618-DDC-ABD 24 hours earlier (Supplemental Figure 9C).
Discussion
We recently reported a truncated human soluble ACE2 protein with increased duration of action, capable of neutralizing SARS-CoV-2 infection in human kidney organoids.17 To increase the binding affinity to SARS-CoV-2, we generated a new construct, with the idea of developing an improved ACE2 protein in terms of its decoy effect to neutralize SARS-CoV-2. The new soluble ACE2 protein is a dimer that we termed ACE2–1-618-DDC-ABD and displays an almost 30-fold enhanced affinity for SARS-CoV-2 S1-RBD compared with its monomeric version, ACE2–1-618-ABD (Figure 1). When tested in Vero E6 cells, a monkey cell line, to examine the ability to neutralize SARS-CoV-2 infectivity using a cell viability assay as readout, this novel form of soluble ACE2 was much more potent than ACE2–1-618-ABD to neutralize SARS-CoV-2. Of interest, in two variants of SARS-CoV-2 (Beta and Gamma), ACE2–1-618-DCC-ABD also was found to be very effective in Vero E6 cells. When tested in a permissive mouse model of lethal SARS-CoV-2 infection, ACE2–1-618-DDC-ABD administration resulted in prevention of severe weight loss, a marked improvement in clinical scores, and reduced mortality. Specifically, only one of the ten animals that received ACE2–1-618-DDC-ABD had to be euthanized as opposed to the uniform mortality in the infected mice that served as controls. A marked reduction in lung and brain viral titers of SARS-CoV-2 measured by plaque assay was found in animals that received ACE2–1-618-DDC-ABD compared with the high titers in untreated infected controls.
We found that k18hACE2 mice infected with SARS-CoV-2 develop severe lung injury as previously reported.23–25 Examination of the lungs from untreated, infected mice showed extensive damage, mainly consisting of alveolar hemorrhage, perivascular infiltration, and interstitial edema. The infiltrates consisted mainly of mononuclear cells, but neutrophils were also frequently seen. The lungs of treated animals showed reduced damage 6 days post infection and near normalization of histopathology 14 days post infection.
Evidence of kidney proximal tubular injury was found in some infected animals as shown by variable degrees of attenuation of the proximal tubular brush border and cytolysis mainly in untreated animals, but the difference did not achieve statistical significance between treated and untreated kidney sections, possibly because of the low number of animals (Figure 4). Unfortunately, functional studies in terms of BUN or creatinine could not be obtained because of limitations in releasing samples from the BSL-3 facility. We also used kidney NGAL staining because in patients with COVID-19, urinary NGAL levels are quantitatively associated with AKI and tubular injury.45 In experimentally induced AKI, moreover, NGAL expression is increased and located mainly in the proximal tubule.38,39,46–48 Kidney NGAL staining was positive in 12 out of 20 mice. Of those 12, ten were untreated, whereas only two had received ACE2–1-618-DDC-ABD—a difference that was highly significant (Figure 4). We showed co-localization of NGAL with two different markers of the proximal tubule: megalin and ACE2.31 NGAL, however, is actually produced by the distal nephron and extrarenal sites.49–51 It has been hypothesized that in AKI, NGAL from the circulation is being filtered and then taken up in the proximal tubule via the megalin pathway.48,52 Because there is also extrarenal production of NGAL as for example in ventilator-associated lung injury,53 alternative to kidney injury being the source of kidney proximal tubule NGAL expression, it may also originate from the lungs because there was extensive lung injury in our model. Therefore, we cannot conclude that the observed increase in NGAL in untreated k18hACE2 mice is mainly the result of tubular injury, and additional studies are needed to characterize tubular injury in the k18hACE2 mouse. We also found some evidence of apoptosis in untreated animals (Supplemental Figure 7) and other TUNEL-positive bodies along the tubular membrane that need to be further characterized. The improvement of kidney injury in animals that received ACE2–1-618-DDC-ABD stems most likely from overall disease prevention.
Of note, SARS-CoV-2 titers in the kidney were nondetectable, despite high lung and brain titers, which is also consistent with several studies showing no evidence of SARS-CoV-2 kidney invasion in patients with COVID-19.54–57. There is evidence of kidney and urine SARS-CoV-2 presence, however, from other studies such that the issue of direct viral invasion has not been completely settled.56,58 The present study adds to the evidence that proximal tubular injury can occur in the absence of detectable kidney viral titers of SARS-CoV-2. A more comprehensive analysis to search for viral kidney invasion should include various approaches such as ELISA or quantitative RT-PCR evaluation of urines collected sequentially, but this was just not possible, given the limitations of what can be done within a BSL-3 facility.
The design of this study was at least in part preventative because the soluble ACE2 protein was given before viral inoculation and then continued daily for 2 days. We chose this approach as proof of concept that our soluble human ACE2 protein can obliterate SARS-CoV-2 infectivity at least when given early before viral exposure and continued after. The hypothesis for the use of soluble ACE2 protein for treatment and prevention of SARS-CoV-2 infection was first outlined by us13 and Zhang et al. in March 2020.59 That is, provision of soluble ACE2 protein could act as a decoy to bind to the spike of SARS-CoV-2 and thereby limit the binding to membrane-bound full-length ACE2 (Figure 5). Consequently, SARS-CoV-2 entry into the cells and viral replication should be prevented as a result of this decoy effect. This neutralizing effect of native soluble ACE2 proteins was demonstrated in human organoids by Monteil et al.18 and Wysocki et al.17 More recently, others have reported studies examining different soluble ACE2 proteins in cell models that generally showed neutralization of SARS-CoV-2.14,17,18,60–63 There have also been recent reports of soluble ACE2 proteins or nanobodies showing benefit against SARS-CoV-2 infection in vivo, but none of these reported kidney data.64,65(preprint),66,67
Figure 5.
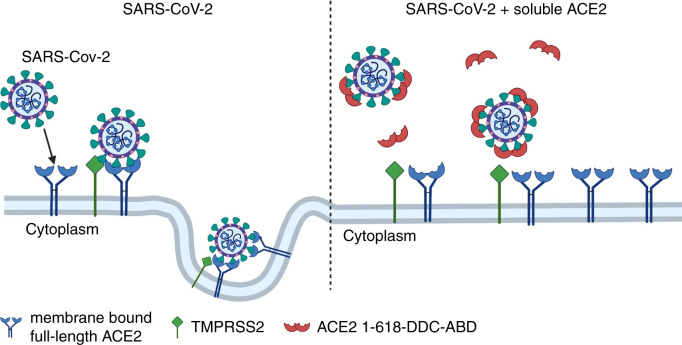
Postulated mechanism of action of ACE2–1-618-DDC-ABD. Administered ACE2–1-618-DDC-ABD (red semi-circles) binds to SARS-CoV-2, acting as a decoy to prevent the binding of SARS-CoV-2 to membrane-bound full-length ACE2 receptors (blue). This prevents the internalization of the ACE2-SARS-CoV-2 complex activated by TMPRSS2 (green). It has been also postulated that there is significant loss of full-length membrane-bound ACE2 (left panel), which is prevented by the use of soluble ACE2 proteins (right panel). Created with biorender.com.
There is one case report of a patient that was given native soluble ACE2 for compassionate use.68 This patient survived, but one cannot be sure that the therapy was directly responsible for the good outcome. In a clinical trial, not published to our knowledge, native soluble ACE2 was given twice a day intravenously to seriously ill COVID-19 patients but failed to meet the primary outcome, a composite of all cause-death or invasive mechanical ventilation (NCT04335136). One possibility for these results is that this study may have been underpowered and that the administration of a short-acting soluble ACE2 protein such as native ACE2–1-740 may leave intervals where the protection is not complete. More importantly, the systemic route is likely not as effective as the intranasal route to neutralize SARS-CoV-2 (see below). Considering that the nasal and lung epithelia are the entry sites for SARS-CoV-2, it is likely that the intranasal route is essential for the protective effect of soluble ACE2. Susceptibility to severe COVID-19 is often characterized by enhanced ACE2 in pulmonary macrophages and type 2 alveolar cells.69 ACE2 expression in the lungs is low and restricted to type 2 alveolar cells,41–44 whereas it is abundant in the kidney proximal tubule.31,70–72 After intranasal administration to uninfected control mice, our studies revealed detectable ACE2 activity and protein staining. Although the focus of our approach is the intranasal route to protect the lungs directly, we also wanted to cover the possibility of systemic effects and brain invasion that may occur in some k18-hACE2 mice infected with SARS-CoV-2.23–26 Because we administered ACE2–1-618-DDC-ABD both intranasally and systemically, we cannot distinguish with certainty which route of administration was more effective in neutralizing SARS-CoV-2. Future studies using the soluble ACE2 protein only intranasally or systemically will address the issue of dual versus intranasal only route of administration, and administration after established SARS-CoV-2 infection.
It should be noted that as part of SARS-CoV-2 infection and internalization of the ACE2-SARS-CoV-2 complex, there may be depletion of cell membrane–bound full-length ACE2.15,73,74 This, in turn, can contribute to lung injury because of the accumulation of angiotensin II (Ang II) and des-Arg9 bradykinin.15,59,74–77 If this is the case, further therapeutic benefit of administering soluble ACE2 protein ought to result from providing ACE2 activity. With organ ACE2 depletion, the balance of Ang II and bradykinins is altered, with the accumulation of these peptides normally metabolized by ACE2.78–80 By providing exogenous soluble ACE2 and intercepting the virus from fully accessing the membrane bound ACE2 to prevent internalization, there should be preservation of full-length, membrane-bound ACE2. This likely would minimize the development of Ang II/Ang1–7 imbalance at the tissue level—conceivably a pivotal factor in the pathogenesis of COVID-19 disease as previously suggested.15,81,82 Studies are needed to confirm this hypothesis, and we think k18hACE2 mice may be a good model. However, this would require the availability of the lungs for measurements other than staining to assess full-length ACE2 and RAS components, which we could not obtain from the BSL-3 facility.
ACE2 proteins tagged with Fc, like our novel soluble ACE2–1-618-DDC-ABD, have the advantage of extended duration of action.61,63,83 It has been noted, however, that Fc-receptor activation could potentially lead to complement-dependent cytotoxicity, antibody-dependent cytotoxicity, and infection via the CD16-Fc-receptor.84 To avoid these uncertain effects, our approach was to fuse a shorter ACE2 variant with an ABD tag and to improve the binding capacity to the SARS-CoV-2 RBD by fusing it with a hinge-like region termed DDC.30 This feature renders the ACE2–1-618-DDC-ABD variant much improved in that the dosing required to intercept SARS-CoV-2 could be reduced because of the enhanced binding characteristics while retaining the prolonged duration of action conferred by the ABD fusion.
Although convalescent plasma and monoclonal antibodies are therapeutically important approaches, there is clearly a distinctive role of a soluble ACE2 protein, particularly if it can be administered directly to the respiratory tract by inhalation. Further studies are needed comparing different soluble ACE2 proteins with each other and with monoclonal antibodies that should also include tissue levels of biologic inflammatory markers. Moreover, an efficient soluble ACE2 protein could be given in combination with human monoclonal antibodies to achieve a more complete effect in severely ill patients. In addition, mutations of SARS-CoV-2, including the most recent Omicron variant,85–89 make antibodies from vaccinated and convalescent patient sera and antibody-based therapies less effective.89–91 Neutralizing antibody therapies, moreover, can lead to the occurrence of escape mutations in the SARS-CoV-2 sequence. Multiple passaging of SARS-CoV-2 in the presence of soluble ACE2 protein, by contrast, should not induce escape mutations.64 A soluble ACE2 protein with enhanced binding capacity such as the one described here therefore ought to be effective in neutralizing mutated variants of SARS-CoV-2 as our data shows for the Gamma and Delta variants in Vero E6 cells.
In summary, the administration of a novel soluble human ACE2 variant with extended duration of action and increased binding efficacy for the RBD of SARS-CoV-2 prevented the universal lethality observed in transgenic k18-hACE2 mice infected with SARS-CoV-2. Administration of ACE2–1-618-DDC-ABD converted a lethal disease into a milder one, demonstrating its preclinical efficacy in an animal model of COVID-19 with severe lung and moderate degrees of proximal tubular kidney injury.
Data Sharing Statement
All data is included in the manuscript and/or supporting materials.
Disclosures
D. Batlle and J. Wysocki are coinventors of patents entitled “Active low molecular weight variants of angiotensin converting enzyme 2 (ACE2),” “Active low molecular weight variants of angiotensin converting enzyme 2 (ACE2) for the treatment of diseases and conditions of the eye,” and “Soluble ACE2 variants and uses therefor.” D. Batlle is founder of Angiotensin Therapeutics, Inc. D. Batlle received consulting fees from AstraZeneca, Relypsa, and Tricida, all unrelated to this work. During the conduct of these studies, D. Batlle received unrelated support from the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01DK104785) and from a grant from AstraZeneca. D. Batlle also reports research funding from the Feinberg Foundation; honoraria from Astra Zeneca, Relypsa, and Tricida; and is a scientific advisor for Relypsa and Tricida. L. Hassler reports other interests/relationships with Stipend from the BMEP in support of research in Chicago. Y. Kanwar reports an advisory or leadership role for the American Journal of Physiology, JASN, Kidney International, and the Journal of Biological Chemistry. S. Pshenychnyi is employed by and consults for COUR Pharma. G. Randall reports consultancy agreements with Optikira. J. Wysocki reports being a scientific advisor for Angiotensin Therapeutics, Inc. All remaining authors have nothing to disclose.
Funding
We acknowledge the support of a gift from the Joseph and Bessie Feinberg Foundation and a National Institutes of Health grant (1R21 AI166940-01; to D. Batlle) and a stipend (to L. Hassler) from the BMEP.
Supplementary Material
Acknowledgments
We acknowledge Dr. Benjamin Singer, from the pulmonary and critical care division of Northwestern University, Feinberg School of Medicine, for advice and critical reading of the manuscript. We also acknowledge the George M. O’Brien Kidney Research Core Center (NU GoKidney) supported by the award P30 DK114857 (National Institute of Diabetes and Digestive and Kidney Disease) and the Northwestern University Clinical and Translational Sciences Institute (NUCATS) COVID-19 Collaborative Innovation Award.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
D. Batlle was responsible for conceptualization, funding acquisition, and project administration and wrote the original draft of the manuscript; D. Batlle, L. Hassler, and J. Wysocki were responsible for the formal analysis; D. Batlle, J. Henkin, L. Hassler, and J. Wysocki reviewed and editing the manuscript; D. Batlle, D. Missiakas, and G. Randall were responsible for supervision; I. Gelarden, H. Gula, L. Hassler, Y. Kanwar, N. Khurram, V. Nicolaescu, S. Pshenychnyi, G. Randall, I. Sharma, A. Tomatsidou, J. Wysocki, M. Ye, and A. Yeldandi were responsible for the investigation; L. Hassler and J. Wysocki were responsible for data curation; and J. Wysocki was responsible for the methodology.
Supplementary Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021091209/-/DCSupplemental.
Supplemental Figure 1. Pharmacokinetics of ACE2–1-618-DDC-ABD and ACE2–1-740.
Supplemental Figure 2. Histopathology of lungs stained with hematoxylin and eosin, presented are low-power views ×20.
Supplemental Figure 3. Histopathology in lungs stained with hematoxylin and eosin, presented are high-power views ×400.
Supplemental Figure 4. Kidneys stained for NGAL, presented are low-power views ×100.
Supplemental Figure 5. Representative patterns of NGAL co-staining with megalin, ACE2, or aquaporin 2.
Supplemental Figure 6. Kidneys stained with the TUNEL assay kit, presented are low-power views ×200.
Supplemental Figure 7. TUNEL-TO-PRO-3 iodide co-staining in kidneys of k18hACE2-mice infected with SARS-CoV-2.
Supplemental Figure 8. TUNEL-TO-PRO-3 iodide co-staining in kidneys of k18hACE2-mice infected with SARS-CoV-2, presented are high-power views ×400.
Supplemental Figure 9. Lung ACE2 activity and staining after intranasal administration of ACE2–1-618-DDC-ABD in controls and infected k18hACE2 mice.
Supplemental Table 1. Scoring system in BSL-3 facility for health evaluation of mice infected with SARS-CoV-2.
References
- 1.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. ; COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group : Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. ; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. ; Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. : Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. : AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y, Shang J, Graham R, Baric RS, Li F: Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol 94: e00127-20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. : SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D: Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292.e6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. : Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181: 894–904.e9, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. : Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. : Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey GH, et al. : Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun 319: 1216–1221, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlle D, Wysocki J, Satchell K: Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin Sci (Lond) 134: 543–545, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Chan KK, Dorosky D, Sharma P, Abbasi SA, Dye JM, Kranz DM, et al. : Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 369: 1261–1265, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson AM, Wysocki J, Batlle D: Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: Therapeutic implications. Hypertension 76: 1339–1349, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, et al. : Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 117: 7001–7003, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wysocki J, Ye M, Hassler L, Gupta AK, Wang Y, Nicoleascu V, et al. : A novel soluble ACE2 Variant with prolonged duration of action neutralizes SARS-CoV-2 infection in human kidney organoids. J Am Soc Nephrol 32: 795–803, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. : Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913.e7, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. : Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, et al. : Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin II-dependent hypertension. Hypertension 55: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steeland S, Vandenbroucke RE, Libert C: Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov Today 21: 1076–1113, 2016 [DOI] [PubMed] [Google Scholar]
- 22.McCray PB Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, et al. : Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81: 813–821, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, et al. : COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589: 603–607, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, et al. : SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21: 1327–1335, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden JW, Cline CR, Zeng X, Garrison AR, Carey BD, Mucker EM, et al. : Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight 5: e142032, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A, et al. : Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 11: 6122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau GB, Burgess SL, Sturek JM, Donlan AN, Petri WA Jr, Mann BJ: Evaluation of K18-hACE2 mice as a model of SARS-CoV-2 infection. Am J Trop Med Hyg 103: 1215–1219, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, García-Sastre A, et al. : Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 9: 2433–2445, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wysocki J, Schulze A, Batlle D: Novel variants of angiotensin converting enzyme-2 of shorter molecular size to target the kidney renin angiotensin system. Biomolecules 9: 886, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrimpf A, Hempel F, Li A, Linne U, Maier UG, Reetz MT, et al. : Hinge-type dimerization of proteins by a tetracysteine peptide of high pairing specificity. Biochemistry 57: 3658–3664, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. : Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21: 856–863, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Tominaga T, Dutta RK, Joladarashi D, Doi T, Reddy JK, Kanwar YS: Transcriptional and translational modulation of myo-inositol oxygenase (Miox) by fatty acids: Implications in renal tubular injury induced in obesity and diabetes. J Biol Chem 291: 1348–1367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wysocki J, Garcia-Halpin L, Ye M, Maier C, Sowers K, Burns KD, et al. : Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol 305: F600–F611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysocki J, Ye M, Khattab AM, Fogo A, Martin A, David NV, et al. : Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int 91: 1336–1346, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, et al. : Murine recombinant angiotensin-converting enzyme 2: Effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension 60: 730–740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abassi Z, Sagi O, Armaly Z, Bishara B: [Neutrophil gelatinase-associated lipocalin (NAGL): A novel biomarker for acute kidney injury]. Harefuah 150: 111–116, 207, 206, 2011 [PubMed] [Google Scholar]
- 38.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. : Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath KA, Singh RD, Grande JP, Garovic VD, Croatt AJ, Ackerman AW, et al. : Expression of ACE2 in the intact and acutely injured kidney. Kidney360 2: 1095–1106, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CL, Savenka AV, Basnakian AG: TUNEL assay: A powerful tool for kidney injury evaluation. Int J Mol Sci 22: 412, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serfozo P, Wysocki J, Gulua G, Schulze A, Ye M, Liu P, et al. : Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 75: 173–182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D: Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: Implications for COVID-19. J Am Soc Nephrol 31: 1941–1943, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss H-P, et al. : Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides 26: 1270–1277, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. ; HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org; HCA Lung Biological Network : SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu K, Shang N, Levitman A, Corker A, Kudose S, Yaeh A, et al. : Elevated neutrophil gelatinase-associated lipocalin is associated with the severity of kidney injury and poor prognosis of patients with COVID-19. Kidney Int Rep 6: 2979–2992, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bank JR, van der Pol P, Vreeken D, Monge-Chaubo C, Bajema IM, Schlagwein N, et al. : Kidney injury molecule-1 staining in renal allograft biopsies 10 days after transplantation is inversely correlated with functioning proximal tubular epithelial cells. Nephrol Dial Transplant 32: 2132–2141, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. : Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. : Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Paragas N, Qiu A, Zhang Q, Samstein B, Deng S-X, Schmidt-Ott KM, et al. : The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skrypnyk NI, Gist KM, Okamura K, Montford JR, You Z, Yang H, et al. : IL-6-mediated hepatocyte production is the primary source of plasma and urine neutrophil gelatinase-associated lipocalin during acute kidney injury. Kidney Int 97: 966–979, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cowland JB, Borregaard N: Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 45: 17–23, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N: The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 579: 773–777, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Xiao R, Chen R: Neutrophil gelatinase-associated lipocalin as a potential novel biomarker for ventilator-associated lung injury. Mol Med Rep 15: 3535–3540, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al. : AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 31: 1688–1695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, et al. : Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 77: 82–93.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassler L, Reyes F, Sparks MA, Welling P, Batlle D: Evidence for and against direct kidney infection by SARS-CoV-2 in patients with COVID-19. Clin J Am Soc Nephrol 16: 1755–1765, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. ; Northwell Nephrology COVID-19 Research Consortium : COVID-19-associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caceres PS, Savickas G, Murray SL, Umanath K, Uduman J, Yee J, et al. : High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J Am Soc Nephrol 32: 2517–2528, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med 46: 586–590, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan KK, Tan TJ, Narayanan KK, Procko E: An engineered decoy receptor for SARS-CoV-2 broadly binds protein S sequence variants. Sci Adv 7: eabf1738, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glasgow A, Glasgow J, Limonta D, Solomon P, Lui I, Zhang Y, et al. : Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. Proc Natl Acad Sci U S A 117: 28046–28055, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linsky TW, Vergara R, Codina N, Nelson JW, Walker MJ, Su W, et al. : De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2. Science 370: 1208–1214, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Wang H, Tang X, Fang S, Ma D, Du C, et al. : SARS-CoV-2 and three related coronaviruses utilize multiple ACE2 orthologs and are potently blocked by an improved ACE2-Ig. J Virol 94: e01283-20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Higuchi Y, Suzuki T, Arimori T, Ikemura N, Mihara E, Kirita Y, et al. : Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat Commun 12: 3802, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwanaga N, Cooper L, Rong L, Beddingfield B, Crabtree J, Tripp RA, et al. : Novel ACE2-IgG1 fusions with improved in vitro and in vivo activity against SARS-CoV2. bioRxiv. 10.1101/2020.06.15.152157 (Preprint posted July 24, 2020) [DOI] [Google Scholar]
- 66.Li Z, Wang Z, Dinh PC, Zhu D, Popowski KD, Lutz H, et al. : Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol 16: 942–951, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tada T, Fan C, Chen JS, Kaur R, Stapleford KA, Gristick H, et al. : An ACE2 microbody containing a single immunoglobulin Fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep 33: 108528, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, et al. : Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med 8: 1154–1158, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abassi Z, Knaney Y, Karram T, Heyman SN: The lung macrophage in SARS-CoV-2 infection: A friend or a foe? Front Immunol 11: 1312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D: Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension 43: 1120–1125, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Zou X, Chen K, Zou J, Han P, Hao J, Han Z: Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khoury EE, Knaney Y, Fokra A, Kinaneh S, Azzam Z, Heyman SN, et al. : Pulmonary, cardiac and renal distribution of ACE2, furin, TMPRSS2 and ADAM17 in rats with heart failure: Potential implication for COVID-19 disease. J Cell Mol Med 25: 3840–3855, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minato T, Hoshizaki M, Yamaguchi T, An J, Niiyama M, Nirasawa S, et al. : ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. Nat Commun 12: 6691, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. : A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, et al. : Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verdecchia P, Cavallini C, Spanevello A, Angeli F: The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gurwitz D: Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 81: 537–540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burrell LM, Johnston CI, Tikellis C, Cooper ME: ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 15: 166–169, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imai Y, Kuba K, Penninger JM: Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci 64: 2006–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, et al. : Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol 314: L17–L31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abassi Z, Higazi AAR, Kinaneh S, Armaly Z, Skorecki K, Heyman SN: ACE2, COVID-19 infection, inflammation, and coagulopathy: missing pieces in the puzzle. Front Physiol 11: 574753, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abassi ZA, Skorecki K, Heyman SN, Kinaneh S, Armaly Z: Covid-19 infection and mortality: A physiologist’s perspective enlightening clinical features and plausible interventional strategies. Am J Physiol Lung Cell Mol Physiol 318: L1020–L1022, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu P, Wysocki J, Souma T, Ye M, Ramirez V, Zhou B, et al. : Novel ACE2-Fc chimeric fusion provides long-lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney Int 94: 114–125, 2018 [DOI] [PubMed] [Google Scholar]
- 84.de Taeye SW, Bentlage AEH, Mebius MM, Meesters JI, Lissenberg-Thunnissen S, Falck D, et al. : FcγR binding and ADCC activity of human IgG allotypes. Front Immunol 11: 740, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang JW, Tambyah PA, Hui DS: Emergence of a new SARS-CoV-2 variant in the UK. J Infect 82: e27–e28, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, et al. : Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol 83: 104351, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tegally H, Wilkinson E, Lessells RJ, Giandhari J, Pillay S, Msomi N, et al. : Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat Med 27: 440–446, 2021 [DOI] [PubMed] [Google Scholar]
- 88.Baric RS: Emergence of a highly fit SARS-CoV-2 variant. N Engl J Med 383: 2684–2686, 2020 [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. : The Omicron variant is highly resistant against antibody-mediated neutralization—Implications for control of the COVID-19 pandemic. Cell 185: 447–456.e11, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jangra S, Ye C, Rathnasinghe R, Stadlbauer D, Krammer F, Simon V, et al. ; Personalized Virology Initiative Study Group : SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2: e283–e284, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mannar D, Saville JW, Zhu X, Srivastava SS, Berezuk AM, Tuttle KS, et al. : SARS-CoV-2 Omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science 375: 760–764, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.