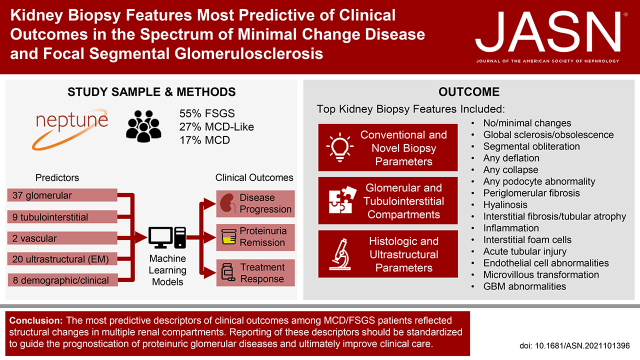
Significance Statement
The classification of podocytopathies, including minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS), has historically been based on limited glomerular features. This study used supervised machine learning methods to identify the most important clinical and histopathologic predictors of disease progression, complete proteinuria remission, and treatment response in MCD/FSGS. The top predictors included conventional and novel glomerular and tubulointerstitial features. Biopsy reporting for podocytopathies should be standardized by including these prognostic morphologic features to inform risk stratification.
Keywords: glomerular disease, nephrotic syndrome, kidney biopsy, renal morphology, outcome prediction
Visual Abstract
Abstract
Background
Heterogeneity in disease course and treatment response among patients with MCD/FSGS necessitates a granular evaluation of kidney tissue features. This study aimed to identify histologic and ultrastructural descriptors of structural changes most predictive of clinical outcomes in the Nephrotic Syndrome Study Network (NEPTUNE).
Methods
Forty-eight histologic (37 glomerular, 9 tubulointerstitial, 2 vascular) and 20 ultrastructural descriptors were quantified by applying the NEPTUNE Digital Pathology Scoring System to NEPTUNE kidney biopsies. Outcomes included time from biopsy to disease progression, first complete remission of proteinuria, and treatment response. Relative importance of pathology and clinical predictors was obtained from random forest models, and predictive discrimination was assessed.
Results
Among 224 participants (34% Black, 24% Hispanic), model performance was excellent, with predictive discrimination of 0.9 for disease progression, 0.85 for complete remission, and 0.81 for treatment response. The most predictive descriptors of outcomes included both conventional—e.g., global sclerosis or segmental sclerosis and interstitial fibrosis/tubular atrophy—and novel features, including adhesion, interstitial foam cells, deflation, periglomerular fibrosis, mononuclear white blood cells, endothelial cell abnormalities, microvillous transformation, and acute tubular injury.
Conclusions
The most predictive descriptors of clinical outcomes among MCD/FSGS patients reflected structural changes in multiple renal compartments. Reporting these descriptors should be standardized to guide prognostication of proteinuric glomerular diseases.
Introduction
Glomerular diseases conventionally diagnosed as minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS) represent manifestations of a continuous spectrum of the “podocytopathies”1–5 with heterogeneous natural histories and responses to therapy. Traditionally, FSGS is subclassified on the basis of presumed etiology to primary and secondary forms6 and by using the presence of five glomerular features to define five or six morphologic variants.7,8 MCD and FSGS patients are also subclassified on the basis of response to therapy. Although recommendations from the Renal Pathology Society highlight the importance of standardizing language, scoring, and reporting of pathologic features in all compartments of the kidney parenchyma,9–11 the majority of current classification systems for glomerular diseases are predominantly based on a limited number of glomerular parameters.7,11–15 A comprehensive morphologic evaluation of the renal biopsy provides information about structural changes throughout the kidney, beyond those observed in glomeruli. This granular characterization of the renal biopsy could elucidate which biopsy features are most useful for predicting disease progression or response to therapy, so that they can be included in standardized reporting in clinical practice. Such standardization of biopsy reports would not only provide evidence-based guidance for patient care, but also improve communication between pathologists and nephrologists and facilitate comparison between institutions and clinical studies.
Cohort studies enriched with detailed pathology and clinical data such as the Nephrotic Syndrome Study Network (NEPTUNE) make it possible to test new approaches for quantitative assessment of structural damage that can ultimately modify pathology reporting and nephrology practice. The NEPTUNE Digital Pathology Scoring System (NDPSS) applied quantitative and semi-quantitative metrics to capture structural changes (“descriptors”) in glomeruli, tubulointerstitium, and vessels on the basis of renal biopsies of nephrotic syndrome patients.16,17 This objectively assessed set of descriptors includes histologic and ultrastructural parameters. Individual descriptors provide specificity, whereas descriptors grouped into classes of pathologic features may have better interobserver reproducibility.18,19 Our previous studies using individual features or renal compartments from the NDPSS uncovered the importance of quantifying both conventional and nonconventional histologic and ultrastructural parameters for subcategorization and prognostication of MCD and FSGS patients.20–23 Although the granularity of the NDPSS can provide a comprehensive description of the biopsy, it is also time-consuming and unfeasible for application in daily practice. Therefore, a limited set of the most effective and informative features is needed. Here, we used the NDPSS descriptor dataset to identify a subset of morphologic parameters that represent those most predictive of clinical outcomes, with a goal of identifying key elements for standardized reporting in biopsied patients with podocytopathies.
Methods
Study Sample
NEPTUNE is a multicenter prospective observational cohort study of children and adults in North America enrolled at the time of the first clinically indicated renal biopsy and with proteinuria >0.5 mg/mg (phase 1, 2009–2014) or >1.5 mg/mg (phase 2, 2014–2019), as described previously.17 Children and adults of all ages are eligible for inclusion. Exclusion criteria include patients with evidence of other renal diseases (e.g., diabetic nephropathy, systemic lupus erythematosus, etc.), prior solid organ transplant, or a life expectancy of <6 months. The current study included NEPTUNE participants enrolled between 2010 and 2017. According to the NEPTUNE protocol, glass slides from kidney biopsies scanned into whole slide images and electron microscopy (EM) images are stored in a digital pathology repository.16 NEPTUNE participants with a conventional diagnosis of MCD or FSGS were included in this study. MCD was further classified into two subsets: MCD (defined as ≥75% effacement with no global sclerosis or global sclerosis expected for age) or MCD-like (defined as <75% effacement with or without presence of global sclerosis exceeding that expected for age or ≥75% effacement with global sclerosis exceeding that expected for age).21,24 Demographics (including race and ethnicity that were self-reported or reported by parents of children), medication history, and laboratory data were collected at each study visit. Reporting race and ethnicity in the NEPTUNE study was mandated by the US National Institutes of Health, consistent with the Inclusion of Women, Minorities, and Children policy. All NEPTUNE participants provided written informed consent (adults) or assent with parental written consent (children).
NDPSS Descriptors
The NDPSS first involves manually annotating (enumerating) glomeruli across all biopsy levels and stains using all of the whole slide images available from each biopsy. Then, study pathologists score 37 glomerular, 9 tubulointerstitial, 2 vascular, and 13 ultrastructural descriptors.16,25 The overall presence of each glomerular descriptor in a participant’s biopsy was quantified by calculating the percentage of glomeruli with each descriptor present. The tubulointerstitial and vascular descriptors were quantified in each biopsy by presence, severity, or extent of the cortex involved with damage. The ultrastructural descriptors were quantified by presence or extent of changes on the basis of a minimum of five EM images for each participant.23
For the current study, each individual morphologic descriptor was used as a separate independent variable. Additionally, individual descriptors were grouped into classes that share similar pathologic features (i.e., all types of segmental sclerosis, all podocyte descriptors, all subtypes of hyalinosis, etc.; Supplemental Table 1) because grouped descriptors often have better reproducibility18,19 and may be more practical for widespread use. Therefore, the set of grouped descriptors—along with any individual descriptors that were not grouped—was also used as independent variables in analyses. The grouped descriptor models were compared with those using only individual descriptors to assess whether prediction was similar or better, in which case grouped descriptors would be preferred because of their higher reproducibility.
Clinical Outcomes
As part of the NEPTUNE protocol, NEPTUNE participants were followed prospectively with study visits every 4 months in the first year and every 6 months thereafter up to 5 years in total. Serum creatinine and urine protein creatinine ratio (UPCR) were measured by a central laboratory from blood and urine samples collected at each study visit. Local serum creatinine and UPCR measurements were also collected at each study visit and in between study visits (phase 2 only). eGFR was estimated using the CKiD-Schwartz equation for children, CKD-Epi for adults >26 years old, and an average of the two equations for adults between 18 and 26 years old,26 all on the basis of serum creatinine levels. Clinical outcomes of interest in the current study included (1) time from biopsy to a composite disease progression outcome of at least 40% decline in eGFR27 with eGFR <90 ml/min per 1.73 m2 or kidney failure (dialysis, transplant, or two consecutive eGFRs <15 ml/min per 1.73 m2), and (2) time from biopsy to complete remission of proteinuria, defined as UPCR <0.3 g/g. For the complete remission outcome, an additional analysis was also conducted only among participants treated with immunosuppression medication (any immunosuppressant use within 1 month before or 1 month after biopsy, where immunosuppressants include any combination of corticosteroids, calcineurin inhibitors, mycophenolate mofetil, cyclophosphamide, rituximab, and others) to capture treatment response. For this analysis, follow-up time began 1 month after biopsy and was therefore also limited to participants with at least 1 month of follow-up time. Participants who did not reach the clinical end points of interest were censored at their last eGFR measurement for disease progression or their last UPCR measurement for complete remission and treatment response outcomes.
Statistical Methods
Demographics, clinical characteristics at the time of biopsy, and follow-up characteristics of the study sample were summarized using mean and SD for normally distributed continuous variables, median and interquartile range (IQR) for non-normally distributed continuous variables, frequency for categorical variables, and number of events per 100 person-years for outcome event rates. Spearman’s correlation coefficient was used to estimate pairwise correlations among demographics, clinical characteristics, and morphologic descriptors (using individual rather than grouped morphologic descriptors). We used machine learning models to identify the most important predictors of each outcome. Because different machine learning algorithms make different assumptions and therefore may be more suitable for different datasets or prediction tasks, we considered several model types. Penalized Cox regressions—including ridge regression, least absolute shrinkage and selection operator (LASSO), and elastic net models with mixing parameters of 0.25, 0.5, and 0.75—and random survival forests were compared. Tuning parameters within each penalized Cox regression model were chosen on the basis of five-fold cross-validation and the final, optimal model type was chosen on the basis of the one with highest integrated area under the time-varying receiver operating characteristic curve (iAUC). Random forest models were repeated 50 times with 1000 trees and variable importance averaged across repetitions to obtain stable variable importance ranks.28
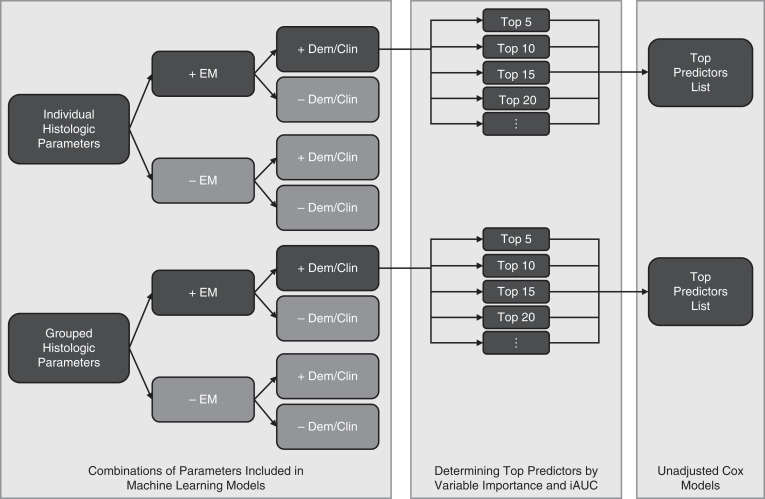
Our primary models included glomerular, tubulointerstitial, vascular, and ultrastructural descriptors—either used individually to capture the most granular information or grouped for better reproducibility and practicality (Figure 1 left panel)—along with several demographic and clinical characteristics used in a clinical assessment setting. We also included the classification of participants into FSGS versus MCD conventional categories because this conventional categorization represents a holistic description of glomerular morphology that is used by nephropathologists and nephrologists. Individual versus grouped descriptor models were compared in terms of iAUC for predictive discrimination. Secondary models were also conducted without ultrastructural descriptors to explore information needed for patient care even when EM may not be available, e.g., in some developing countries, and without demographic and clinical characteristics or the FSGS versus MCD conventional diagnosis. Demographics and biopsy-time clinical characteristics included age, sex, Black or African American race, Hispanic ethnicity, eGFR, UPCR, and immunosuppression use (within 1 month before or at biopsy). Disease progression and complete remission were assessed among all participants in the study sample, and complete remission was also assessed only among participants treated with immunosuppression medication to capture treatment response.
Figure 1.
Analytic strategy. The left panel shows all combinations of parameters included in separate machine learning models, with dark shading denoting the primary models of interest and light shading denoting secondary models; EM, electron microscopy; Dem/Clin, demographics and clinical characteristics. The center panel illustrates that on the basis of the primary models with all predictors, including histologic and ultrastructural (EM) features and Dem/Clin, analyses using only the top 5, 10, 15, 20, etc. most predictive variables were conducted. The right panel shows that the top descriptors list was determined on the basis of variable importance and iAUC and each of these top descriptors were assessed in unadjusted Cox models.
Variable importance from the final model output was used to rank independent variables and determine those that were most predictive of each outcome. For random survival forests, we used permutation importance as the variable importance metric because it performs well with a mix of continuous and categorical variables and even with highly correlated predictors.29 We also conducted analyses using only the top 5, 10, 15, 20, etc. most predictive variables and compared iAUCs across models (Figure 1 center panel). We then determined the minimum number of variables with nontrivial variable importance needed to achieve similar prediction discrimination as the model using all variables. This list was considered the most important variables for predicting outcomes. For each of these top predictors, we used unadjusted Cox models to quantify the direction and relative magnitude of effects using standardized hazard ratios (Figure 1 right panel). Continuous descriptors for which >80% of the sample had zero values were dichotomized to any versus none in these models. For other continuous descriptors, we used clinically meaningful cutoffs or assessed whether its association with outcome was nonlinear by categorizing the descriptor values into quartiles—or categorizing nonzero values dichotomously or into tertiles if there were 20%–80% zero values—and comparing coefficient estimates.
All models were based on complete-case analysis (i.e., participants missing a predictor for a model were excluded from that model). R v3.4 (R Foundation for Statistical Computing, Vienna Austria) was used for machine learning models, whereas all other statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
Results
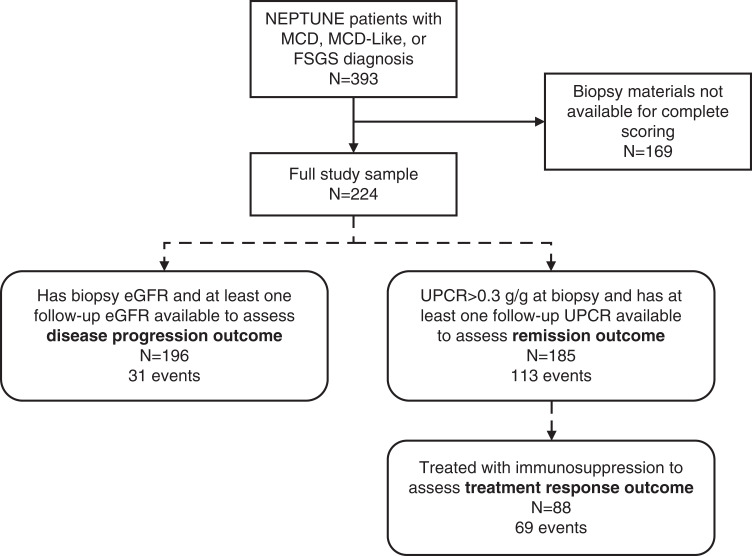
A total of 224 NEPTUNE participants had NDPSS data and a conventional diagnosis of MCD or FSGS (Figure 2, Table 1). More than half (55%) had FSGS, 27% MCD-like, and 17% MCD (classic type). The mean eGFR at biopsy was 85 ml/min per 1.73 m2 (SD=41 ml/min per 1.73 m2) and median UPCR was 2.8 g/g (IQR 1–6.9 g/g). Seventy-one were on an immunosuppressive medication within 30 days before or at the time of biopsy, the majority of whom were treated with corticosteroids alone (n=51) or corticosteroids in combination with other treatments (n=14). Other biopsy time or study enrollment time characteristics are included in Supplemental Table 2. A total of 113 participants experienced complete remission (21 of whom were not on any immunosuppressive medication before remission), and 31 had ≥40% decline in eGFR with an eGFR of <90 ml/min per 1.73 m2 or kidney failure during follow-up (Figure 2).
Figure 2.
Flow diagram of study sample inclusion criteria and selection from NEPTUNE (square corner rectangles) and subsamples included in analyses of each clinical outcome (rounded corner rectangles).
Table 1.
Demographics, clinical characteristics at the time of biopsy, and follow-up characteristics
| Characteristic | Value |
|---|---|
| Age at biopsy (years) | 28.8 (22.3) |
| Pediatric patients | 46% (104) |
| Adult patients | 54% (120) |
| Male | 61% (137) |
| Black or African American racea,c | 34% (75) |
| Hispanica,b | 24% (53) |
| Conventional diagnosis | |
| MCD | 17% (39) |
| MCD-like | 27% (61) |
| FSGS | 55% (124) |
| eGFR at biopsyd | 85.2 (41.2) |
| UPCR at biopsyc | 2.8 (1–6.9) |
| On immunosuppressive medication within 30 days before biopsy or at biopsy | 32% (71) |
| Duration of kidney disease as of biopsy (months)e | 4 (1–24) |
| Follow-up time (months) | 50 (29–55) |
| Change in eGFR during first yeard,f | −1.6 (–11.1 to 11.3) |
| Change in UPCR during first yeard,f | −0.6 (–3.1 to 0.1) |
| On immunosuppressive medications during first yearf | 67% (133) |
| Rate of complete remission during study follow-up (# of events per 100 person-years)g | 37 |
| Rate of ≥40% decline in eGFR with eGFR<90 or kidney failure during study follow-up (# of events per 100 person-years)h | 4.9 |
Data are shown as mean (SD), median (IQR), or % (n).
3% (n=7) participants reported both Black race and Hispanic ethnicity.
Missing: <1%.
Missing: 1%–5%.
Missing: 5%–10%.
Missing: n=42.
Among 200 patients with at least one year of follow-up after biopsy.
n=25 already had complete remission at biopsy; n=8 missing UPCR at biopsy; n=6 had no follow-up UPCR, resulting in n=185.
n=22 missing eGFR at biopsy; n=6 had no follow-up eGFR, resulting in n=196.
All morphology descriptors were observed in at least one study participant (Supplemental Tables 3 and 4). Even some descriptors that are infrequently described in biopsy reports and/or used for diagnostic interpretation—e.g., glomerular foam cells, periglomerular fibrosis, global deflation, adhesion, endothelial cell abnormalities (loss of endothelial cell fenestration or honeycombing), loss of primary processes, and microvillous transformation—were observed in at least several participants. Although a few descriptors had high pairwise correlations (ρ<–0.75 or ρ>0.75), the majority were only weakly or moderately correlated (Supplemental Figure 1).
Random forest models consistently yielded the highest prediction discrimination out of all machine learning models and were therefore used for all subsequent analyses (Supplemental Table 5).
Morphologic Descriptors Most Predictive of Time to Disease Progression
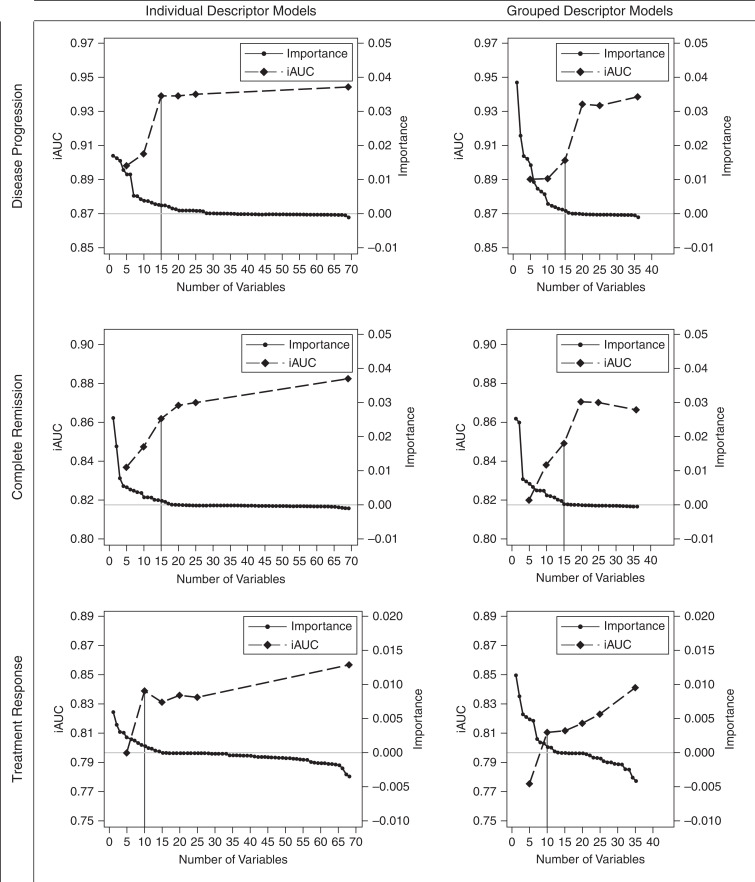
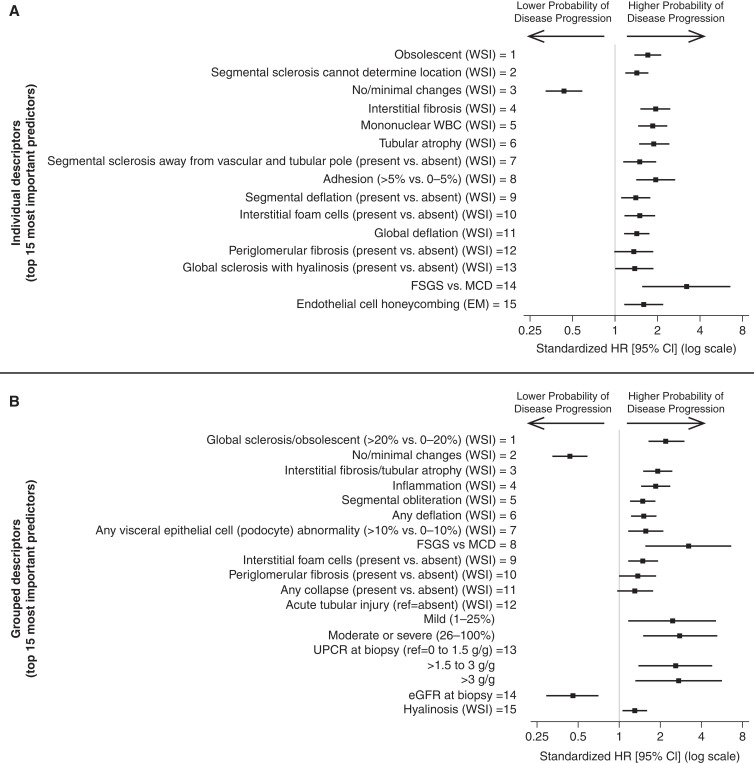
When descriptors were ranked (by variable importance rankings), the order of histologic descriptors was relatively similar regardless of whether ultrastructural descriptors and demographics and clinical characteristics were included in models of time to disease progression (Supplemental Figure 2, A and B). On the basis of the primary model with all predictors, iAUC improved from 0.91 to 0.94 when the top 10 versus top 15 predictors were used. iAUC remained similar after adding lower-ranked descriptors to the model (Figure 3 top left panel). Therefore, the top 15 individual predictors were selected for subsequent analyses. These included glomerular descriptors of obsolescence, segmental sclerosis of unknown location, no/minimal changes, segmental sclerosis away from the vascular and tubular pole, adhesion, segmental deflation, global deflation, periglomerular fibrosis, and global sclerosis with hyalinosis; tubulointerstitial descriptors of interstitial fibrosis, mononuclear white blood cells (WBCs), tubular atrophy, and interstitial foam cells; and only loss of endothelial cell honeycombing from ultrastructural descriptors. FSGS versus MCD was also included in the top 15. Notably, foot process effacement was among the lowest ranked predictors. In unadjusted Cox models, more glomeruli with damage or higher severity/extent of damage were always associated with higher hazards of disease progression (Figure 4 top panel, Supplemental Table 6 column A).
Figure 3.
iAUC values from models with only top 5, 10, 15, 20, etc. predictors and variable importance values from models predicting disease progression (top row), complete remission (middle row), and treatment response (bottom row), using individual descriptors (left column) or grouped descriptors (right column).
Figure 4.
Standardized hazard ratios with 95% confidence intervals showing unadjusted associations from Cox proportional hazards models between top individual (top panel) and grouped (bottom panel) descriptors and time to disease progression (40% decline in eGFR with eGFR <90 ml/min per 1.73 m2 or kidney failure).
When individual descriptors sharing similar pathology features were grouped into descriptor classes, models had similar iAUC (differences between 0.006 and 0.04), and grouped descriptor rankings remained largely consistent, with rankings of at least one individual descriptor included in that descriptor category (Supplemental Figure 3). Although iAUCs for grouped descriptor models increased until 20 predictors were included in the model, only the top 15 had nontrivial variable importance (Figure 3 top right panel) and were selected for subsequent analyses. iAUC was 0.9 in this model. These top predictors included glomerular descriptors of global sclerosis/obsolescent, no/minimal changes, any segmental obliteration, any deflation, visceral epithelial cell (podocyte) abnormalities (hyalin droplets, hypertrophy, hyperplasia, or halo), periglomerular fibrosis, any collapse, and hyalinosis, and tubulointerstitial descriptors of interstitial fibrosis/tubular atrophy, inflammation, interstitial foam cells, and acute tubular injury. Increased prevalence of morphologically abnormal glomeruli was always associated with higher hazards of disease progression (Figure 4 bottom panel, Supplemental Table 6 column B). Having FSGS (versus MCD), higher UPCR at biopsy, and lower eGFR at biopsy were also associated with higher hazards of disease progression.
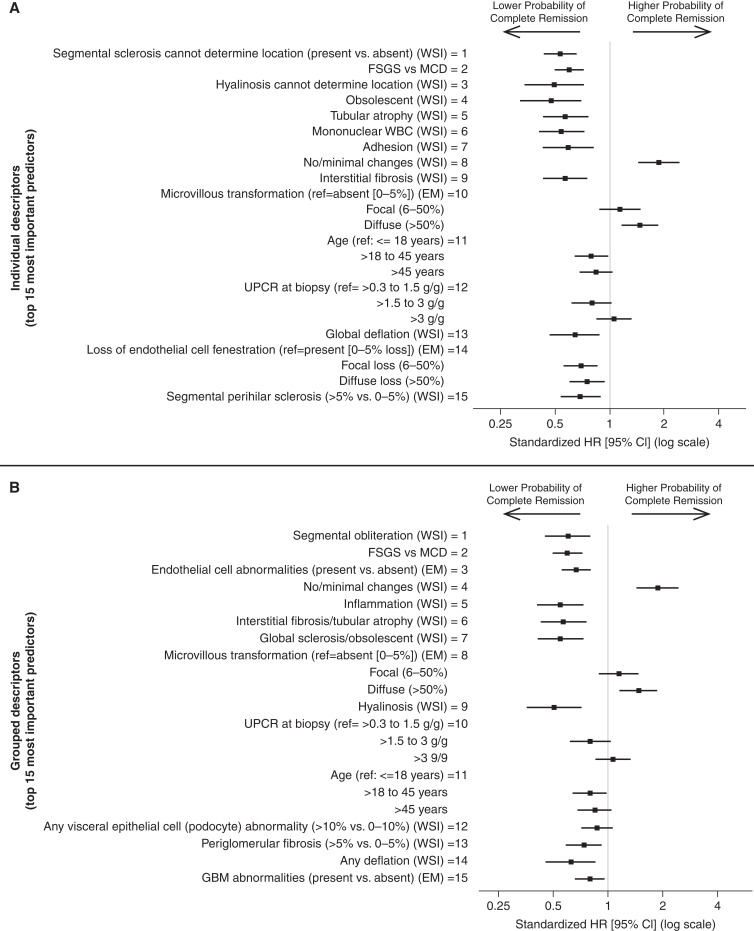
Morphologic Descriptors Most Predictive of Time to Complete Proteinuria Remission
Ordering of top histologic predictors was similar independent of whether ultrastructural descriptors and demographics and clinical characteristics were included in models of time to complete remission among all study participants (Supplemental Figure 4, A and B). Although a few descriptors—e.g., global sclerosis with hyalinosis, arteriosclerosis, any deflation, and any collapse—had different ranks across models, they had trivial variable importance. iAUCs from models using top individual descriptors leveled off after the top 15 predictors, and most descriptors ranked lower than 15 had trivial variable importance (Figure 3 middle left panel). Therefore, the top 15 individual predictors were selected for subsequent analyses; the iAUC from this model was 0.86. These top predictors included glomerular descriptors of segmental sclerosis of unknown location, hyalinosis of unknown location, obsolescence, adhesion, no/minimal changes, global deflation, and segmental perihilar sclerosis; and tubulointerstitial descriptors of tubular atrophy, mononuclear WBCs, and interstitial fibrosis. More glomeruli with damage or higher severity/extent of damage were always associated with lower probability of remission (Figure 5 top panel, Supplemental Table 7 column A). Out of ultrastructural descriptors, diffuse (versus absence of) microvillous transformation was associated with higher probability of remission and both focal and diffuse loss of endothelial cell fenestration were associated with lower probability of remission. Participants with FSGS (versus MCD), age 18–45 years (versus ≤18 years), or UPCR at biopsy between 1.5 and 3 g/g (versus ≤1.5 g/g) had lower probabilities of remission.
Figure 5.
Standardized hazard ratios with 95% confidence intervals showing unadjusted associations from Cox proportional hazards models between top individual (top panel) and grouped (bottom panel) descriptors and time to complete proteinuria remission (UPCR <0.3 g/g).
Grouped descriptor models had similar iAUC (differences between 0.016 and 0.02) and grouped descriptor rankings remained largely consistent, with rankings of at least one individual descriptor included in that group (Supplemental Figure 5). Although iAUCs for grouped descriptor models increased until 20 predictors were included in the model, only the top 15 had nontrivial variable importance (Figure 3 middle right panel) and were selected for subsequent analyses; the iAUC from this model was 0.85. These top 15 predictors included glomerular descriptors of any segmental obliteration, no/minimal changes, global sclerosis/obsolescent, hyalinosis, visceral epithelial cell (podocyte) abnormalities, periglomerular fibrosis, and any deflation; tubulointerstitial descriptors of inflammation and interstitial fibrosis/tubular atrophy; and ultrastructural descriptors of endothelial cell abnormalities, microvillous transformation, and glomerular basement membrane abnormalities (thickening, thinning, or abnormal texture). FSGS versus MCD, UPCR, and age were included in the top 15 predictors (Figure 5 bottom panel, Supplemental Table 7 column B).
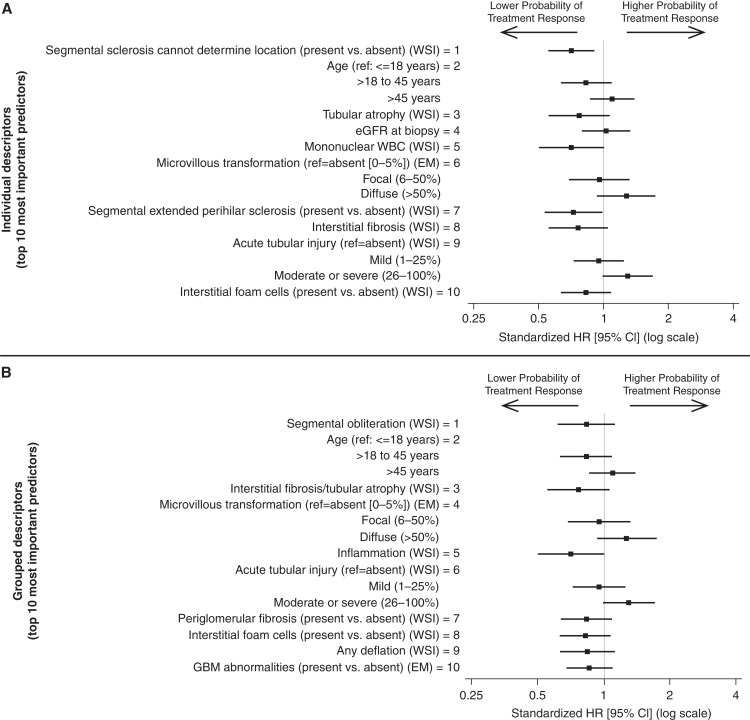
Morphologic Descriptors Most Predictive of Time to Treatment Response
Out of 185 NEPTUNE participants included in the remission model among the full participant cohort, 88 (48%) had at least 1 month of follow-up and were treated with an immunosuppressant medication within 1 month before or after biopsy for prediction of treatment response. Among participants included in time to treatment response models, there were 69 with treatment response events. Variable rankings for individual and grouped descriptor models are shown in Supplemental Figure 6, A and B. iAUCs for individual descriptor models leveled off after including the top 10 predictors (iAUC=0.84; Figure 3 bottom left panel). Within the top 10 were glomerular descriptors of segmental sclerosis of unknown location and segmental extended perihilar sclerosis, with presence of either associated with lower probability of treatment response; and tubulointerstitial descriptors of tubular atrophy, mononuclear WBCs, interstitial fibrosis, acute tubular injury, and interstitial foam cells (Figure 6 top panel, Supplemental Table 8 column A). For all but acute tubular injury, greater extent of damage was associated with lower probability of treatment response. Those with moderate or severe acute tubular injury had a higher probability of treatment response, whereas mild acute tubular injury had similar treatment response compared with those without acute tubular injury. Diffuse microvillous transformation was associated with higher probability of treatment response, and participants aged between 18 and 45 years had a lower probability of treatment response.
Figure 6.
Standardized hazard ratios with 95% confidence intervals showing unadjusted associations from Cox proportional hazards models between top individual (top panel) and grouped (bottom panel) descriptors and treatment response (complete proteinuria remission among participants treated with immunosuppression medication [ISM]).
Grouped descriptor models had iAUC within 0.02–0.04 of individual descriptor models and grouped descriptor rankings were somewhat similar to individual descriptor rankings of at least one descriptor within the group (Supplemental Figure 7). The grouped descriptor model on the basis of the top 10 predictors had an iAUC of 0.81. The top 10 grouped descriptors were also related to the top 10 individual descriptors, with the addition of periglomerular fibrosis, any deflation, and GBM abnormalities, all of which were associated with lower probability of treatment response with more damage (Figure 6 bottom panel, Supplemental Table 8 column B).
Most Predictive Morphologic Descriptors of Any Outcomes
Table 2 provides a summary of the most prognostic grouped morphologic descriptors for at least one of the three outcomes from this study, along with the metrics used to quantify the descriptors in this study. Grouped descriptors were selected for this summary table due to the similar prediction between grouped and individual descriptor models but higher reproducibility of grouped descriptors.
Table 2.
Top prognostic morphologic descriptors as suggested parameters for reporting
| Compartment | Individual Descriptor or Category | Suggested Metric | |
|---|---|---|---|
| Histology | Glomerular | No/minimal changes | % glomeruli (per total number of glomeruli) |
| Global sclerosis/obsolescencea | % glomeruli (per total number of glomeruli) | ||
| Segmental obliterationb (any type of segmental sclerosis and/or intraglomerular foam cells or adhesion of the tuft to the Bowman’s capsule) |
% glomeruli (per total number of glomeruli) | ||
| Deflation (global+segmental) |
% glomeruli (per total number of glomeruli) | ||
| Collapse (global+segmental) |
% glomeruli (per total number of glomeruli) | ||
| Podocyte abnormalities (hypertrophy, hyperplasia, halo, protein droplets) |
% glomeruli (per total number of glomeruli) | ||
| Periglomerular fibrosis | % glomeruli (per total number of glomeruli) | ||
| Glomerular hyalinosisc (any location) |
% glomeruli (per total number of glomeruli) | ||
| Tubulointerstitial | Interstitial fibrosis+tubular atrophyd | % cortex involved | |
| Interstitial inflammatione | % cortex involved | ||
| Interstitial foam cells | % cortex involved | ||
| Acute tubular injury | % cortex involved | ||
| EM | Glomerular | Microvillous transformation | 0, 0 (none) 1+, ≤50% (focal) 2+, >50% (diffuse) |
| Endothelial cell abnormalities (fenestration+honeycombing) |
Present/absent | ||
| GBM abnormalities | 0, 0 (none) 1+, ≤50% (focal) 2+, >50% (diffuse) |
||
Suggest additionally reporting if exceeding that expected for age (ref).
Suggest additionally reporting % of glomeruli with segmental sclerosis: cannot determine location, tip lesion, cellular lesion, perihilar lesion.
Hyalinosis can be present in the absence of segmental sclerosis or associated with any type of segmental sclerosis (cannot determine location, tip lesion, cellular lesion, perihilar lesion).
Suggest reporting % IF and TA separately to capture decoupling that may infer different disease process.
Suggest also reporting if inflammation involves nonfibrotic areas separately or by adding an asterisk.
Discussion
This study leveraged data from the NEPTUNE consortium to identify morphologic features that best predict clinical outcomes, providing a comprehensive and data-driven set of histologic parameters to be considered for inclusion in renal biopsy assessments and reporting for kidney biopsies with a conventional diagnosis of MCD and FSGS. The broadly inclusive NEPTUNE enrollment protocol ensured the capture of the full MCD/FSGS clinical spectrum. As an observational cohort study that enrolled patients at the time of biopsy, NEPTUNE’s prospective data collection also facilitated a methodical evaluation of associations between biopsy features and clinical outcomes under routine clinical care.
The traditional approach to diagnosing podocytopathies such as FSGS and MCD is holistic. Existing podocytopathy-specific guidelines for morphologic evaluation and classification are limited to a few histologic glomerular features (perihilar, tip, cellular collapsing, and not otherwise specified segmental sclerosis)7 and ultrastructural13 glomerular parameters that do not fully take into consideration the entirety of the kidney parenchyma and its pathologic changes. This historical approach carried some useful information as documented in previous studies,30–32 and maintained relevance in this study as the diagnosis of FSGS versus MCD ranked among top predictors of disease progression and complete remission. However, this study also demonstrates the value of detailed quantification of biopsy features that goes beyond those conventionally utilized. For example, we demonstrated that the percentage of glomeruli with no or minimal histologic changes was predictive of lower risk of disease progression and higher probability of remission in both FSGS and MCD, beyond that of the classic MCD designation. Interstitial fibrosis and tubular atrophy are not used in most glomerular disease classification schemes and are frequently reported on a semiquantitative scale. Percentage of cortex involved by interstitial fibrosis and tubular atrophy was previously identified as a predictor of outcomes22 and consistently ranked among the top predictors of worse disease progression, worse proteinuria remission, and worse treatment response in the current study. Similarly, this study is consistent with previous research showing that higher percentages of glomeruli with global sclerosis/obsolescence are predictive of poor outcomes.21
This study identified novel features relevant for the prediction of outcomes, supporting the notion that heterogeneity in this spectrum of diseases cannot be entirely captured by conventional approaches. We observed that presence of interstitial foam cells—an incompletely understood feature33—was predictive of worse disease progression and treatment response. Periglomerular fibrosis also conveys useful information about chronic damage.34 Although description of ultrastructural features in biopsy reports is usually limited to foot process effacement, this feature carried little prognostic value in the current study. Instead, microvillous transformation and endothelial cell abnormalities were both identified as top ultrastructural predictors of clinical outcomes, consistent with a previous study.23 Microvillous transformation was associated with higher probability of remission and treatment response, likely reflecting transience of changes in podocyte shape and associated cellular events. Moderate or severe acute tubular injury was associated with higher probability of treatment response but also higher hazards of disease progression. This may indicate that floridly nephrotic patients with microvillous transformation or acute tubular injury are more likely to respond to immunosuppression in the short term but do worse in the long term.
Systematic, data-driven evaluations of reliability and prognostic ability of histologic parameters have been successfully applied in other contexts. The Oxford Classification of IgA nephropathy (IgAN), for example, was developed on the basis of consensus definitions and a rigorous assessment of reproducibility and outcome prediction.35,36 Subsequent widespread use in clinical practice and numerous validation studies stimulated improvements and updates to the MEST score,15 leading to development of risk prediction models for IgAN.37 Likewise, the Banff classification for kidney transplant pathology continues to evolve on the basis of new evidence,38–42 paving the way for the iBox risk prediction score, which includes both morphologic and clinical factors.43 Our results highlight the importance of reporting the features identified in this study into pathology reports in routine clinical practice. These features can guide design of future large-scale studies and algorithms that predict clinical outcome. Table 2 lists the most prognostic grouped morphologic descriptors for any of this study’s three outcomes. It offers a suggestion for standardization of reporting using prognostic parameters identified in this study along with other parameters (Table 3) that have an uncertain association with outcome but may be relevant for diagnostic purposes or for completeness and are sometimes reported in routine practice (e.g., foot process effacement). Broad implementation of this suggested standardized reporting (e.g., facilitated by the web-based form at https://www.neptunescoring.com) will enable validation of our data across laboratories and populations—including confirmation of feasibility—and serve as a foundation for further refinements. Beyond biopsy-based outcome prediction, risk stratification of patients for individualized clinical management must integrate the most relevant clinical, morphologic, and genetic factors.44 This set of features identified in this study therefore represents one critical component of this multifaceted problem, which can complement studies on mechanistic process discovery and understanding of clinical manifestations of podocytopathies.
Table 3.
Morphologic descriptors with limited or unclear prognostic value that could be reported for completeness on the basis of current clinical practices
| Renal Compartment | Individual Descriptor or Category | Suggested Metric | |
|---|---|---|---|
| Overall assessment for disease category | FSGS versus MCD | FSGS or MCD or MCD-like | |
| Histology | Tubulointerstitial | Interstitial edema | Present/absent |
| Interstitial eosinophils | Present/absent | ||
| Vascular | Arteriosclerosis | 0, none 1+, mild 2+, moderate 3+, severe |
|
| Arteriolar hyalinosis | 0, none 1+, mild 2+, moderate 3+, severe |
||
| EM | Glomerular | Foot process effacement | 0, 0%–10% 1+, 11%–25% 2+, 26%–50% 3+, 51%–75% 4+, >75% |
| Podocyte detachment with newly formed matrix | Present/absent | ||
| Condensation of the actin-based cytoskeleton | 0, 0 (none) 1+, ≤50% (focal) 2+, >50% (diffuse) |
||
| Electron densities/hyaline material | Present/absent | ||
This study used machine learning methods to accommodate high-dimensional data produced by deep phenotyping among a rare disease cohort. Internal validation with multiple machine learning algorithms bolstered the case that final models are optimal. The random forest models can capture nonadditive effects and interactions between predictors through its hierarchical structure. We systematically assessed the shape of the relationship between each top feature and outcome, which identified any deviations from linear associations and offers potential parameterizations to use in future scoring systems. We used different combinations of variables in a series of models to accommodate different practical situations—e.g., those without EM images available, those focusing only on pathology features, or those where grouped descriptor variables would be more feasible. Finally, we evaluated several clinical outcomes—disease progression, complete proteinuria remission, and treatment response—to understand better the influence of morphologic changes on different aspects of MCD/FSGS disease course.
Comparisons between models using individual and grouped descriptors indicated that grouped descriptors contain similar information for outcome prediction compared with individual descriptors. Reassuringly, grouped descriptor rankings were reasonably consistent with at least one component individual descriptor ranking. These results suggest that the grouped descriptors may be at least as useful as individual descriptors if other benefits prevail. For example, the interobserver reproducibility of scoring tends to be higher when descriptors are grouped into classes.18,19 The descriptor classes have higher prevalence compared with individual descriptors and can help with interpretation of prognostic, highly correlated individual descriptors. Therefore, the suggested reporting parameters in Table 2 and Table 3 are based on grouped descriptors.
There are limitations of this study worth noting. First, the NEPTUNE study sample includes some patients, especially children, who have been treated before biopsy. The interpretation of results is thus most appropriate for a population of patients with proteinuria at the time of their first clinically indicated kidney biopsy rather than at disease presentation. Although results may not be generalizable to all treatment-naïve patients, the fact that immunosuppressant use ranked low in models also suggests little effect of immunosuppressant use on effects of morphologic features on outcomes. Generalizability of results to other diseases and/or to patient populations outside of academic centers in North America is also unknown. Second, although the NDPSS is comprehensive of all renal compartments, it was developed with a primary focus on glomerular diseases; thus, the glomerular features are collected in more detail compared with the tubulointerstitial features. The NDPSS did not include features describing podocyte intercellular junction structure or changes in the parietal epithelium and did not differentiate between location of inflammation. Third, the current classification of MCD/FSGS likely represents diverse underlying biologic processes. So, our study identifies features that are predictive across these etiologies. Future studies could use a similar approach to identify predictive features within mechanistically relevant subgroups as they are discovered in the future, including genetic studies and those that investigate the predictive value of clinical or ultrastructural features above and beyond histology. Finally, the number of outcome events observed in this study was limited, partly due to the challenges of collecting such comprehensive and granular data among a large cohort. We were unable to conduct some subgroup analyses of interest, e.g., to identify top predictors of spontaneous remission or to stratify analyses by steroid responsiveness. Future studies using computational tools to identify important morphologic features automatically could facilitate replication in larger studies and improve feasibility for clinical practice.
Our goal has been to contribute to methods that ultimately improve prognostication and focus the use of therapeutics for the heterogeneous diseases that we presently classify as MCD/FSGS. This study contributes to this effort in part by identifying morphologic descriptors that are most predictive of key clinical outcomes. Although these morphologic features include many that are routinely observed and reported by pathologists, the clinical relevance of these features was previously incompletely appreciated. We suggest that, particularly once validated in additional studies, these morphologic features be included in a standardized clinical pathology report that describes MCD/FSGS histopathology. Inclusion of these predictive features in such a standardized report of histopathology—and combining this report with predictive tools that use predictive clinical, environmental, genetic, or molecular biomarkers—should aid clinical nephrologists in estimating the risk of disease progression or treatment response.
Disclosures
L. Barisoni reports personal fees from Moderna, Protalix, Sangamo, and Vertex; consultancy for Protalix, Sangamo, and Vertex; an advisory or leadership role for the Renal Pathology Society, BOA, NephCure Kidney International scientific advisory board, and Nature Review Nephrology; and other interests or relationships with NephCure Kidney International. J.B. Hodgin reports personal fees from AstraZeneca, Gilead, Janssen, Moderna, and Novo Nordisk, and research funding from AstraZeneca, Eli Lilly, Gilead, Janssen, Moderna, and Novo Nordisk. L. Holzman reports consultancy for Arnold & Porter Kaye Scholer LLP; patents or royalties from the University of Michigan; an advisory or leadership role for the ASN, JASN, JCI, and NephCure Kidney International; and other interests or relationships with NephCure Kidney International. L. Mariani reports research funding from the Department of Defense, NephCure Kidney International, the National Institutes of Health/National Institute of Diabetes, Digestive, and Kidney Diseases unrelated to this study, and PCORI; served on the advisory board of Calliditas, Reata Pharmaceuticals, and Travere; received honoraria for lectures at an ASN Board Review Course, Henry Ford Hospital, University of Minnesota, University of North Carolina, and University of Wisconsin; received support from Reata Pharmaceuticals for travel to meetings; reports consultancy for and received honoraria from Calliditas Therapeutics Advisory Board, CKD Advisory Committee, Reata Pharmaceuticals, and Travere Therapeutics Advisory Board. A. Rosenberg reports research funding from the National Institutes of Health and NKF; honoraria from Georgetown University, Ichilov Hospital (Tel Aviv, Israel), and Stony Brook University; and an advisory or leadership role for Escala. J. Zee reports honoraria from Booz Allen Hamilton and grants from National Institutes of Health/National Institute of Diabetes, Digestive, and Kidney Diseases unrelated to this study. All remaining authors have nothing to disclose.
Funding
This study was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) to L. Barisoni, J.B. Hodgin, L.B. Holzman, L.H. Mariani, and J. Zee (R01-DK118431). L.H. Mariani is supported through funding from the NIH/NIDDK (K08-DK115891). Q. Liu and A. Smith were additionally supported through funding from the NIH/NIDDK (U54-DK083912).
The Nephrotic Syndrome Rare Disease Clinical Research Network III (NEPTUNE) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the NIH and led by the National Center for Advancing Translational Sciences (NCATS) through its Office of Rare Diseases Research (ORDR). NEPTUNE is funded under grant number U54DK083912 as a collaboration between NCATS and the NIDDK. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International, and the Halpin Foundation. All RDCRN consortia are supported by the network’s Data Management and Coordinating Center (DMCC; U2CTR002818). Funding support for the DMCC is provided by NCATS and the National Institute of Neurological Disorders and Stroke (NINDS).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Predicting Future Outcomes from Kidney Biopsies with MCD/FSGS Lesions: Opportunities and Limitations” on pages 1233–1235.
Contributor Information
Collaborators: The NEPTUNE Members, S. Adler, G. Alter, A. Athavale, M. Atkinson, C. Avila-Casado, S. Bagnasco, S. Baker, L. Barisoni, C. Bidot, J. Blake, M. Bray, P. Canetta, V. Chernitskiy, A. Cooper, K. Dell, T. Dell, V. Derebail, H. Desmond, S. Eddy, D. Fermin, F. Fervenza, P. Flynn, A. Fornoni, A. Froment, C. Gadegbeku, J. Gaut, K. Gibson, B. Gillespie, D. Gipson, L. Greenbaum, S. Hewitt, S. Hingorani, M. Hladunewich, J. Hodgin, M. Hogan, L. Holzman, M. Itteera, A. Jefferson, K. Kallem, F. Kaskel, C. Klida, M. Kretzler, J. Kopp, M. Kretzler, V. Kurtz, R. Lafayette, J. LaPage, M. Larkina, K. Lemley, J. Lieske, S. Li, S. Li, C.C. Lienczewski, J.J. Lin, P. Ling, J. Liu, T. Mainieri, L. Mariani, K. Meyers, F. Modersitzki, S. Morrison, C. Nast, J. Negrey, J. Ormond-Foster, M. Palmer, E. Pao, M. Pfaiff, A. Pradhan, M. Romano, A. Rosenberg, V. Royal, S. Quinn-Boyle, H. Reich, M. Rogers, M. Ross, K. Sambandam, M. Sampson, M. Schachere, J. Sedor, C. Sethna, T. Srivastava, A. Smith, A. Swenson, S. Tang, D. Thomas, H. Trachtman, K. Tuttle, S. Vento, C. Wang, Z. Wang, A. Williams, B. Yeung, E. Yun, J. Zee, O. Zhdanova, S. Adler, G. Alter, A. Athavale, M. Atkinson, C. Avila-Casado, S. Bagnasco, S. Baker, L. Barisoni, C. Bidot, J. Blake, M. Bray, P. Canetta, C. Cassol, V. Chernitskiy, A. Cooper, K. Dell, T. Dell, D. Demeke, V. Derebail, H. Desmond, S. Eddy, D. Fermin, F. Fervenza, P. Flynn, A. Fornoni, A. Froment, C. Gadegbeku, J. Gaut, K. Gibson, B. Gillespie, D. Gipson, L. Greenbaum, S. Hewitt, S. Hingorani, M. Hladunewich, J. Hodgin, M. Hogan, D. Holanda, L. Holzman, M. Itteera, A. Jefferson, K. Kallem, F. Kaskel, C. Klida, J. Kopp, M. Kretzler, V. Kurtz, R. Lafayette, J. LaPage, M. Larkina, K. Lemley, J. Lieske, S. Li, S. Li, C.C. Lienczewski, J.J. Lin, P. Ling, J. Liu, T. Mainieri, L. Mariani, N. Messias, K. Meyers, A. Michailov, F. Modersitzki, S. Morrison, C. Nast, J. Negrey, J. Ormond-Foster, M. Palmer, E. Pao, M. Pfaiff, A. Pradhan, M. Romano, A. Rosenberg, V. Royal, S. Quinn-Boyle, H. Reich, M. Rogers, M. Ross, K. Sambandam, M. Sampson, M. Schachere, J. Sedor, C. Sethna, T. Srivastava, A. Smith, A. Swenson, S. Tang, D. Thomas, H. Trachtman, K. Tuttle, S. Vento, C. Wang, Z. Wang, A. Williams, M. Yamashita, B. Yeung, E. Yun, J. Zee, O. Zhdanova, and Y. Zuo
Author Contributions
L. Barisoni, B. Gillespie, J.B. Hodgin, L.B. Holzman, L.H. Mariani, and J. Zee were responsible for funding acquisition; L. Barisoni, J.B. Hodgin, L.B. Holzman, L.H. Mariani, A. Rosenberg, and J. Zee were responsible for data curation; L. Barisoni, L.B. Holzman, Q. Liu, and J. Zee were responsible for the project administration; L. Barisoni, L.B. Holzman, L.H. Mariani, A. Smith, and J. Zee were responsible for supervision; L. Barisoni, L.B. Holzman, L.H. Mariani, and J. Zee were responsible for the investigation; L. Barisoni, Q. Liu, L.H. Mariani, A. Smith, and J. Zee were responsible for the methodology; L. Barisoni, Q. Liu, L.H. Mariani, and J. Zee wrote the original draft of the manuscript; L. Barisoni, L.H. Mariani, and J. Zee were responsible for conceptualization; Q. Liu and J. Zee were responsible for visualization; Q. Liu, A. Smith, and J. Zee were responsible for the formal analysis; and all authors reviewed and edited the manuscript.
Data Sharing Statement
There are some restrictions for these data as follows: Data used in this study are from the Nephrotic Syndrome Study Network (NEPTUNE). NEPTUNE data are available upon request and approval of an ancillary study application from the Data Analysis and Coordinating Center (https://www.neptune-study.org/).
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021101396/-/DCSupplemental.
Supplemental Table 1. Groupings of individual morphologic descriptors.
Supplemental Table 2. Other clinical characteristics of the study sample at the time of biopsy or NEPTUNE study enrollment, not included in analyses.
Supplemental Table 3. Descriptive table of individual pathology descriptors, by MCD versus MCD-like versus FSGS.
Supplemental Table 4. Descriptive table of grouped glomerular, tubulointerstitial, vascular, and EM pathology descriptors, by MCD versus MCD-like versus FSGS.
Supplemental Table 5. iAUC values from different machine learning algorithms.
Supplemental Table 6. Unadjusted associations between top descriptors and disease progression.
Supplemental Table 7. Unadjusted associations between top descriptors and complete proteinuria remission.
Supplemental Table 8. Unadjusted associations between top descriptors and treatment response (complete proteinuria remission among participants treated with immunosuppression medication [ISM]).
Supplemental Figure 1. Distribution of pairwise correlation coefficients between predictors (using individual morphologic descriptors).
Supplemental Figure 2. Predictor rankings from models of disease progression with and without demographics and clinical characteristics and with and without ultrastructural descriptors. (A) Models using only individual morphology descriptors. (B) Models using grouped morphology descriptors.
Supplemental Figure 3. Predictor rankings from models of disease progression using individual versus grouped morphology descriptors.
Supplemental Figure 4. Predictor rankings from models of complete proteinuria remission with and without demographics and clinical characteristics and with and without ultrastructural descriptors. (A) Models using only individual morphology descriptors. (B) Models using grouped morphology descriptors.
Supplemental Figure 5. Predictor rankings from models of complete proteinuria remission using individual versus grouped morphology descriptors.
Supplemental Figure 6. Predictor rankings from models of treatment response (complete proteinuria remission only among patients treated with immunosuppression medication) with and without demographics and clinical characteristics and with and without ultrastructural descriptors. (A) Models using only individual morphology descriptors. (B) Models using grouped morphology descriptors.
Supplemental Figure 7. Predictor rankings from models of treatment response (complete proteinuria remission among participants treated with immunosuppression medication) using individual versus grouped morphology descriptors.
References
- 1.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Diamond MJ, Atwater S, Nahman NS: Spectrum of Minimal Change Disease to Focal Segmental Glomerulosclerosis. Encyclopedia of Medical Immunology, New York, Springer, 2014, pp 1093–1099 [Google Scholar]
- 3.Fogo AB: The spectrum of FSGS: Does pathology matter? Nephrol Dial Transplant 25: 1034–1036, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF: Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat Rev Nephrol 12: 768–776, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Stokes MB, Markowitz GS, Lin J, Valeri AM, D’Agati VD: Glomerular tip lesion: A distinct entity within the minimal change disease/focal segmental glomerulosclerosis spectrum. Kidney Int 65: 1690–1702, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group : KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Available at: https://kdigo.org/guidelines/gd/. Accessed April 25, 2022 [Google Scholar]
- 7.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg AZ, Kopp JB: Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 12: 502–517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A, Gibson IW, Cohen AH, Weening JJ, Jennette JC, Fogo AB; Renal Pathology Society : A position paper on standardizing the nonneoplastic kidney biopsy report. Clin J Am Soc Nephrol 7: 1365–1368, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Haas M, Seshan SV, Barisoni L, Amann K, Bajema IM, Becker JU, et al. : Consensus definitions for glomerular lesions by light and electron microscopy: Recommendations from a working group of the Renal Pathology Society. Kidney Int 98: 1120–1134, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, et al. : Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 27: 1278–1287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. : Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93: 789–796, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Fogo AB, Lusco MA, Najafian B, Alpers CE: AJKD atlas of renal pathology: Minimal change disease. Am J Kidney Dis 66: 376–377, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Fervenza FC: Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant 34: 193–199, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. ; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford Classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Barisoni L, Nast CC, Jennette JC, Hodgin JB, Herzenberg AM, Lemley KV, et al. : Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE). Clin J Am Soc Nephrol 8: 1449–1459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PXK, Barisoni L, et al. : Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, et al. : Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 29: 671–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zee J, Hodgin JB, Mariani LH, Gaut JP, Palmer MB, Bagnasco SM, et al. : Reproducibility and feasibility of strategies for morphologic assessment of renal biopsies using the Nephrotic Syndrome Study network digital pathology scoring system. Arch Pathol Lab Med 142: 613–625, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgin JB, Mariani LH, Zee J, Liu Q, Smith AR, Eddy S, et al. : Detailed quantification of glomerular structural lesions associates with clinical outcomes and transcriptomic profiles in nephrotic syndrome [published online ahead of print December 3, 2021]. Am J Kidney Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al. : Global glomerulosclerosis with nephrotic syndrome; The clinical importance of age adjustment. Kidney Int 93: 1175–1182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani LH, Martini S, Barisoni L, Canetta PA, Troost JP, Hodgin JB, et al. : Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transplant 33: 310–318, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royal V, Zee J, Liu Q, Avila-Casado C, Smith AR, Liu G, et al. : Ultrastructural characterization of proteinuric patients predicts clinical outcomes. J Am Soc Nephrol 31: 841–854, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nast CC, Lemley KV, Hodgin JB, Bagnasco S, Avila-Casado C, Hewitt SM, et al. : Morphology in the digital age: Integrating high-resolution description of structural alterations with phenotypes and genotypes. Semin Nephrol 35: 266–278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barisoni L, Gimpel C, Kain R, Laurinavicius A, Bueno G, Zeng C, et al. : Digital pathology imaging as a novel platform for standardization and globalization of quantitative nephropathology. Clin Kidney J 10: 176–187, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA: Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int 94: 170–177, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zee J, Mansfield S, Mariani LH, Gillespie BW: Using all longitudinal data to define time to specified percentages of estimated GFR decline: A simulation study. Am J Kidney Dis 73: 82–89, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnamian A, Millard K, Banks SN, White L, Richardson M, Pasher J: A systematic approach for variable selection with random forests: Achieving stable variable importance values. IEEE Geosci Remote Sens Lett 14: 1988–1992, 2017 [Google Scholar]
- 29.Altmann A, Toloşi L, Sander O, Lengauer T: Permutation importance: A corrected feature importance measure. Bioinformatics 26: 1340–1347, 2010 [DOI] [PubMed] [Google Scholar]
- 30.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, et al. : Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes MB, D’Agati VD: Morphologic variants of focal segmental glomerulosclerosis and their significance. Adv Chronic Kidney Dis 21: 400–407, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, et al. : Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Eom M, Hudkins KL, Alpers CE: Foam cells and the pathogenesis of kidney disease. Curr Opin Nephrol Hypertens 24: 245–251, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins J, Brodsky SV, Satoskar AA, Nadasdy G, Nadasdy T: The relevance of periglomerular fibrosis in the evaluation of routine needle core renal biopsies. Arch Pathol Lab Med 135: 117–122, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts ISD, Troyanov S, et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Barbour SJ, Coppo R, Zhang H, Liu Z-H, Suzuki Y, Matsuzaki K, et al. ; International IgA Nephropathy Network : Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med 179: 942–952, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas M: Evolving criteria for the diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Nephrol Hypertens 27: 137–143, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. : The Banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant 20: 2318–2331, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mengel M, Loupy A, Haas M, Roufosse C, Naesens M, Akalin E, et al. : Banff 2019 meeting report: Molecular diagnostics in solid organ transplantation—Consensus for the Banff human organ transplant (B-HOT) gene panel and open source multicenter validation. Am J Transplant 20: 2305–2317, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. : A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation 102: 1795–1814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. : International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int 44: 411–422, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Loupy A, Aubert O, Orandi BJ, Naesens M, Bouatou Y, Raynaud M, et al. : Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366: l4923, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. : Podocytopathies. Nat Rev Dis Primers 6: 68, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.