Abstract
Endometriosis is a common female gynecological disease that is characterized by the presence of functional endometrial tissue outside the uterine cavity. At present, many animal models have been established. However, previous studies consistently use human endometrial tissue implanted in the subcutaneous or abdominal cavity for modeling and rarely use endometrial cells. In the present study, we ascertained whether immortalized stromal and/or epithelial endometrial cells are able to induce subcutaneous endometriosis in nude mice. Mixed human immortalized endometriosis stromal and epithelial cells, but not the cells of Group 1 or Group 2, were successfully constructed and led to endometriotic-like lesions. The endometriosis-like lesions observed in nude mice consisted of endometriosis-like glands lined with columnar epithelial cells and surrounded by stromal cells in the fibrous fatty connective tissue. Immunofluorescence analysis showed that glandular epithelial cells were intensely stained for E-cadherin and cytokeratin 7, and surrounding stromal cells were mildly stained for neprilysin (CD10) and vimentin. Moreover, the cells present in the endometriosis-like lesions were of human origin. Our data indicate that the mixture of human immortalized endometriosis stromal cells and epithelial cells is able to establish subcutaneous endometriosis lesions in nude mice. This model could be used to understand the molecular mechanisms involved in the occurrence and development of endometriosis.
Keywords: endometriosis, T HESCs, epithelial cells, subcutaneous endometriotic-like lesions, nude mice
Introduction
Endometriosis is a common female gynecological disease that is estrogen-dependent and is characterized by the presence of functional endometrial tissue outside the uterine cavity (1,2). Dysmenorrhea, dyspareunia and infertility caused by endometriosis seriously affect the physical and mental health and quality of life of women worldwide (3). Although there are many existing theories, the understanding of the pathogenesis of endometriosis is relatively poor (4,5). In addition, the recurrence rate following drug therapy and surgical treatment is high. The ethical basis for studying the developmental process of the disease in human trials is not sufficient, and invasive observational studies cannot be carried out. Hence, animal models play an important role in the study of the occurrence, development, pathophysiology and treatment of this disease.
At present, many animal models, such as rabbits, mice, rats and primates, have been established domestically and overseas. Primates have pelvic anatomical structure and reproductive physiological characteristics similar to those of humans, and their regular menstrual cycle can spontaneously form endometriosis, which are ideal animal models with which to study the pathogenesis, pathophysiology and treatment of endometriosis (6-8). However, due to the low molding rate, long cycle, limited quantity and high price of such models, they are difficult to popularize in experiments. In addition, the rodent model is the most commonly used model to study endometriosis. Since rodents cannot spontaneously form ectopic lesions, most of these models are induced by surgical transplantation or intraperitoneal injection of endometrium, uterine fragments, decidua, or menstrual blood (9,10). Among them, the nude mouse model with congenital thymus deficiency is the most widely used due to the lower immune rejection response to transplanted human tissues (11-13). However, previous studies consistently use human endometrial tissue implanted in the subcutaneous or abdominal cavity for modeling and rarely use endometrial cells (14-16).
Thus, in the present study, we aimed to identify a superior animal model for endometriosis by using mixed cultures of stromal and epithelial endometrial cells. Our aim was to determine the ability of a mixed population of human endometrial stromal cells (T HESCs; ATCC CRL-4003) and epithelial cells (EECs) to induce subcutaneous endometriosis-like lesions in nude mice. Furthermore, we used hematoxylin and eosin (H&E) staining and histo-immunofluorescence to identify and compare the histomorphology of these induced lesions in nude mice with spontaneous endometriosis lesions in women.
Materials and methods
Experimental animals
A total of 20 female nude mice, approximately six weeks of age and weighing 14-16 g, were purchased from Hunan SJA Laboratory Animal Co. Ltd (China). Animals were housed in specific pathogen-free conditions at a monitored ambient temperature of 22-24˚C and a humidity of 40-70%. Animals were maintained under a light/dark cycle of 12/12 h and fed sterile maintenance fodder. Animal care and procedures were performed following the approval of the Laboratory Animal Ethics Committee of Nanchang Royo Biotech Co., Ltd. (Nanchang, China) (approval no. RYE2019051001).
Injection of human immortalized endometrial cells into nude mice
In the present study, we used the human immortalized endometrial stromal cells (T HESCs; ATCC CRL-4003) and immortalized human endometriosis epithelial cells (EECs; GuangZhou Jennio Biotech Co., Ltd.) to induce subcutaneous endometriosis in nude mice (17). The T HESCs was maintained in DMEM/F12 medium (Beijing Solarbio Science & Technology Co., Ltd.) and EECs were maintained in MEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Beijing Solarbio Science & Technology Co., Ltd.). Both cell lines were cultured at 37˚C in a humidified incubator containing 5% CO2.
One week before injection, a sterile 60-day-release pellet of E2 (estradiol; Innovative Research of America) was subcutaneously implanted on the back of the nude mouse. On the day of transplantation, the endometriosis epithelial cells (EECs) and stromal cells (T HESCs) were trypsinized and counted, and then distributed into three groups: Group 1, 2x106 T HESCs only; Group 2, 2x106 EECs only; Group 3, 2x106 T HESCs+2x106 EECs. The cells in each group were resuspended in medium and mixed with Matrigel (BD Biosciences) at a 1:1 ratio and made into a final volume of 100 µl. The cells were then maintained on ice and quickly transplanted into the nude mice. All the mixed cells were subcutaneously injected on the left flank of each nude mouse. Each mouse in each group received only one type of implantation in a single injection.
Histological analysis of endometriosis lesions in nude mice
Nude mice were placed in a separate ventilation system for observation and reared for 30 days, and then they were euthanasia by cervical dislocation, according to the AVMA Guidelines for Euthanasia (18). The ectopic tissues formed subcutaneously (only from group 3, n=8) were carefully stripped and then immersed in 10% formalin fluid. The tissues were sent to the pathology department for embedding, sectioning, and hematoxylin and eosin (H&E) staining. Briefly, the entire tissue was sectioned serially at a thickness of 5 µm, and then tissue sections were deparaffinized, rehydrated, stained with hematoxylin for 10 min and eosin for 2 min. Images were captured with the use of a Digital Slide Scanner Pannoramic Scan (3DHISTECH, Inc.).
Immunofluorescence
Paraffin-embedded sections were heated for antigen retrieval in citrate buffer (0.01 M, pH 6.0), and they were incubated with 5% (v/v) goat serum (Zhongshan Jinqiao Biotec) for 30 min to block non-specific binding sites. The primary antibodies including mouse anti-cytokeratin 7 (1:500 dilution, 66483-1, ProteinTech Group, Inc.), mouse anti-E-cadherin (1:500 dilution, ab40772, Abcam), rabbit anti-human CD10 antibodies (1:250 dilution, 18008-1-AP, ProteinTech Group, Inc.) and rabbit anti-human vimentin (1:500 dilution, ab45939, Abcam) were used to identify stromal and epithelial cells and incubated overnight at 4˚C. The slides were incubated with 10 µg/ml FITC-conjugated goat anti-rabbit secondary antibody (1:1,000 dilution, P0186, Beyotime Institute of Biotech) or 10 µg/ml Cy3-conjugated goat anti-mouse secondary antibody (1:1,000 dilution, P0186, Beyotime Institute of Biotech) for 1 h at 37˚C. Nuclei were counterstained with DAPI. Negative control included sections stained with a nonimmune serum in the absence of the primary antibody. Fluorescent images were captured with an inversed fluorescent microscope (IX-71, Olympus Corp.) at room temperature. All images were evaluated with the same setting for brightness and contrast at original magnifications of x100 and x200.
DNA extraction and PCR
DNA was extracted from the paraffin-embedded tissue sections using the DNA NucleoSpin Tissue Kit (Omega) as described previously (19). DNA concentration and quality were quantified by absorbance readings taken at 260 and 280 nm using a Nanodrop One Spectrophotometer (Thermo Fisher Scientific, Inc.).
In this study, we respectively designed two species-specific primers of the mouse and human to determine the origin of cells detected in the endometriotic-like lesions in mice (Table I). For amplification condition, we used regular and touchdown PCR techniques to amplify the purified DNA. For touchdown PCR, the cycle condition was as follows: an initial denaturation step of 95˚C for 10 min, 20 cycles of 95˚C for 30 sec, touchdown 65-55˚C for 30 sec (decrease 0.5˚C by per cycle), 72˚C for 30 sec, followed by 15 cycles of denaturation at 95˚C for 30 sec, annealing at 55˚C for 30 sec, elongation at 72˚C for 30 sec, and a final extension step of 72˚C for 10 min. For regular PCR, the cycle condition was as follows: an initial denaturation step of 95˚C for 10 mins, 30 cycles of 95˚C for 30 sec, annealing 60˚C for 30 sec, elongation 72˚C for 30 sec, and a final extension step of 72˚C for 10 min. All the reagents used for regular and touchdown PCR were from TaKaRa LA Taq (RR02MA, TaKaRa). The PCR products were examined by electrophoresis on 2% agarose gels w/v, stained with ethidium bromide and visualized under a UV illumination system (Chemi DOC XRS, Bio-Rad Laboratories, Inc.).
Table I.
Primer sequences for PCR.
| Primer | Sequence | PCR product (bp) | Note |
|---|---|---|---|
| Mouse GAPDH-F1: | 5'-CAGGTTGTCTCCTGCGACTT-3' | 571 | Touchdown PCR |
| Mouse GAPDH-R1: | 5'-CAGCTGGATGTCAGAGCCAA-3' | ||
| Mouse GAPDH-F2: | 5'-AAGGGCATCTTGGGCTACAC-3' | 549 | Touchdown PCR |
| Mouse GAPDH-R2: | 5'-CCTGCTTCACCTCCCCATAC-3' | ||
| Human GAPDH-F1: | 5'-GGCTCTTAAAAAGTGCAGGGTC-3' | 327 | Touchdown PCR |
| Human GAPDH-R1: | 5'-ATGGTACATGACAAGGTGCGG-3 | ||
| Human GAPDH-F2: | 5'-TAACTGTCTGCTTCTCTGCTGTAGGC-3' | 772 | Regular PCR |
| Human GAPDH-R2: | 5'-GCTTCACCACCTTCTTGATGTCATCA-3 |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; F, forward; R, reverse.
Statistical analysis
The percentage of induced endometriosis-like lesions was calculated. Student's t-test has been applied to the results. P<0.05 was considered as indicative of a statistically significant result.
Results
Formation of endometriosis-like lesions requires both glandular epithelial and stromal cells
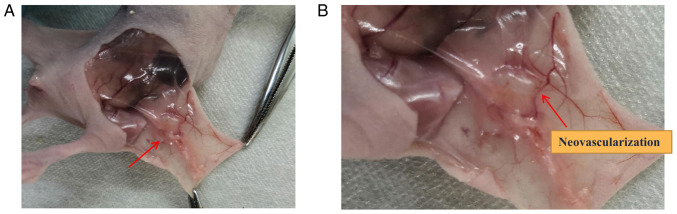
To evaluate the capacity of cell line T HESCs, EEC or mixed epithelial and stromal cells to form endometriosis-like lesions in vivo, 2 million cells were injected subcutaneously to E2-supplemented mice. The results shown in Table II suggest that both T HESCs and epithelial cells needed to be injected subcutaneously in the nude mice for the successful construction of subcutaneous endometriosis-like lesions. If only a single cell line, either the mesenchymal cells or epithelial cells, inoculated subcutaneously into nude mice, none of the model were successfully constructed. The survival rate of the nude mice and induction of subdermal endometriosis in group 3 was 100% (Table II), and the subcutaneous anatomy of nude mice is shown in Fig. 1. As showed in Fig. 1B, the endometriosis-like lesion was accompanied by the growth of blood vessels that supplied the lesion.
Table II.
Injection of immortalized human endometriosis epithelial and stromal cells into nude mice.
| Group | Injected cells | Total number of nude mice | Number of endometriosis lesions |
|---|---|---|---|
| 1 | T HESCs | 6 | 0 |
| 2 | EECs | 6 | 0 |
| 3 | T HESCs + EECs | 8 | 8 (100%)a,b |
aP<0.05 vs. group 1;
bP<0.05 vs. group 2. T HESCs, human endometrial stromal cells; EECs, epithelial cells. Group 1, 2x106 T HESCs only; Group 2, 2x106 EECs only; Group 3, 2x106 T HESCs+2x106 EECs.
Figure 1.
Representative photo images of endometriosis-like lesions in Group 3. (А) Original image; arrow indicates the endometrioid lesion. (В) Partial enlarged image; arrow indicates the blood vessel formation. Group 3: 2x106 T HESCs+2x106 EECs.
Histomorphology of endometriosis-like lesions
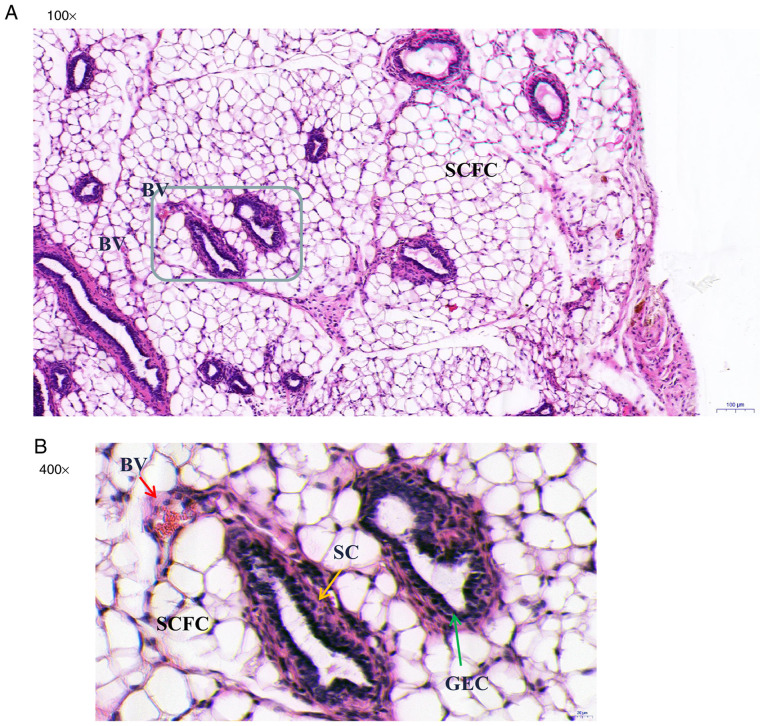
In group 3, the endometriotic-like lesions observed in the nude mice consisted of endometriosis-like glands (grey-lined area) lined with columnar epithelial cell and surrounded by stromal cells in the fibrous fatty connective tissue (Fig. 2A). Blood vessels were observed around the glands (Fig. 2B; red arrow). Histomorphologic analyses demonstrated that most of the endometriosis glands were developed and fully organized glands consisting of typical glandular structure (acini) lined with glandular epithelial cells (green arrow) (Fig. 2B) surrounded by stromal cells (yellow arrow).
Figure 2.
Histologic characteristics of endometriosis-like lesions in nude mice, detected by hematoxylin and eosin (H&E) staining. Cross section of embedded endometriosis-like lesions is shown at original magnifications of x100 and x400. (A) Note the presence of endometriosis glands (grey-lined area) in the fibrous fatty connective tissues. (B) Developed and organized endometriosis glands with acini lined with GEC (green arrow). These endometriosis-like glands are lined with columnar epithelial cells and surrounded by SC (yellow arrow). The BV (red arrow) are filled with red blood cells and lined with flattened endothelial cells. BV, blood vessels; SCFC, subcutaneous fat cells; GEC, glandular epithelial cells; SC, stromal cells.
Immunofluorescence analysis of endometriosis-like lesions
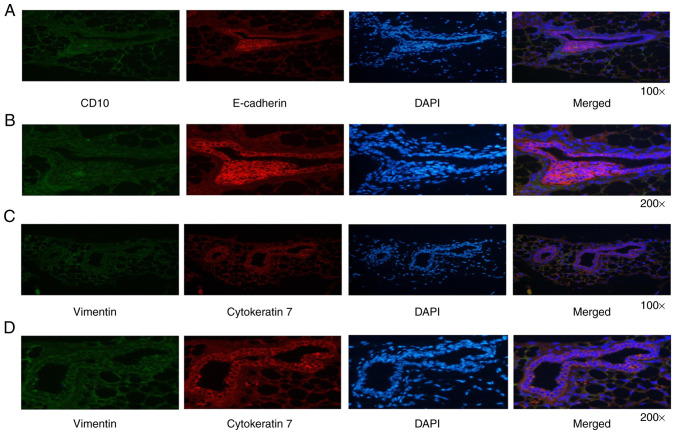
The human origin of cells detected in the endometriotic-like structure in nude mice was demonstrated by specific staining with anti-E-cadherin, anti-cytokeratin 7, anti-vimentin and anti-CD10 antibodies (Fig. 3). Either cytokeratin or E-cadherin represents a specific epithelial marker protein (20,21), whereas vimentin and CD10 are both considered as specific stromal cell marker proteins (22,23). As expected, glandular epithelial cells were intensely stained for E-cadherin and cytokeratin 7 (Fig. 3A and B), and surrounding stromal cells were mildly stained for CD10 and vimentin (Fig. 3C and D). These results confirm that the subcutaneous nodules were endometriotic-like lesions with the presence of endometriosis epithelial and stromal cells.
Figure 3.
Immunofluorescence characterization of the endometriosis-like lesions. (A and B) Glandular epithelial cells were intensely stained for E-cadherin and surrounding stromal cells were mildly stained for CD10. (C and D) Glandular epithelial cells were intensely stained for cytokeratin 7 and surrounding stromal cells were mildly stained for vimentin.
Identification of the cell origin of endometriosis-like lesions
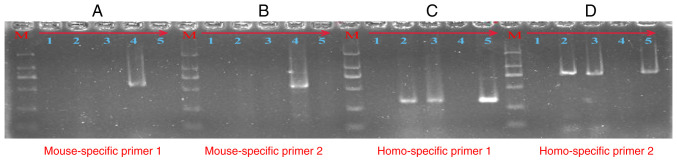
To confirm that the cells present in the endometriosis-like lesions were of human origin, we respectively designed species-specific primers of the mouse and human. The source of the cells present in the endometriosis-like lesions was determined by PCR technology. As showed in Fig. 4, the 571-bp (A) and 549-bp (B) PCR products were amplified by the mouse-specific GAPDH primers, and the 327-bp (C) and 772-bp (D) PCR products were amplified by the human-specific GAPDH primers. Lanes 1-5 respectively represent: no-template control, DNA from endometriosis-like lesion tissue (no. 2), DNA from endometriosis-like lesion tissue (no. 3), positive control of mouse DNA, and positive control of human peripheral blood DNA. As detailed in Fig. 4C and D, the PCR product in lane 2 and lane 3 yielded a clear band at the expected size for human GAPDH in the agarose gel electrophoresis following the regular and touchdown PCR protocol. However, there were no bands in lane 2 and lane 3 in Fig. 4A and B. These results confirm that the cells present in the endometriosis-like lesions were of human origin.
Figure 4.
Agarose gel electrophoresis analysis for the PCR products. The 571-bp (A) and 549-bp (B) PCR products were amplified by mouse-specific GAPDH primers. The 327-bp (C) and 772-bp (D) PCR products were amplified by human-specific GAPDH primers. Lanes 1-5, respectively, represent: the no-template control, DNA from endometriosis-like lesion tissue (no. 2), DNA from endometriosis-like lesion tissue (no. 3), positive control of mouse DNA, positive control of human peripheral blood DNA. Lane M is the DNA DL2000 marker: 2000 bp\1000 bp\750 bp\500 bp\250 bp\100 bp. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Endometriosis is formed by the growth and spread of endometrial tissues (glands and stroma) outside the uterine body, most commonly in the ovaries, with an incidence as high as 6-10%, which seriously affects the quality of life of these patients (3,24). At present, many animal models such as rabbits, rats and primates have been established at home and abroad (25-27). Animal models can be divided into two categories, according to the etiology of endometriosis. One is spontaneous animal models, which only occur in primates, but its application has been limited due to the high cost, high feeding requirements and low mold production rate (28,29). Another is induced animal models, in which mice are the most commonly used animal model for the study of endometriosis. Rodents have short and regular estrus cycle, early sexual maturity, strong fecundity, spontaneous ovulation, but no endometrial shedding, thus this can only be used to establish an induced endometriosis model (30-32).
In the induced endometriosis animal model, endometrial tissues or cells are transplanted to parts outside the uterine cavity of animals through various surgical procedures or endometrial fragmentation injection methods to form endometriosis lesions and induce the occurrence of disease (33). Autologous transplantation can be used in the research of immunology and drug therapy (34,35). However, there are differences between animal and human endometrium in term of biochemical characteristics and other aspects, thus the histological characteristics and biological response of autologous transplantation animals are not completely applicable to humans (26,36). Xenotransplantation involves transplanting human endometrial tissue or endometrial cells into immunocompromised mice. This method can preserve the biological characteristics of human endometrium, and it can be used to study the invasive ability of human endometrium, drug efficacy, side effects and so on (37,38).
The results of the present study demonstrated for the first time that single human immortalized endometriosis epithelial cells or stromal cells cannot form endometriosis-like lesions when transplanted subcutaneous into recipient nude mice. Subcutaneous ectopic endometriosis was established when the two types of cells were mixed and transplanted subcutaneously into nude mice. It can be seen that the interaction between glandular epithelial cells and stromal cells is essential in the formation of endometriosis lesions (39). Our results showed a 100% success rate in inducing endometriosis using mixed human immortalized endometriosis cells, which is better than other induced endometriosis models (40-42). These lesions are characterized by the presence of endometriosis glands lined with cylindrical and flattened epithelial cells and surrounded by dense stromal cells in the subcutaneous adipose tissue. Moreover, the cells present in the endometriosis-like lesions were of human origin identified by PCR technology. All of these results indicate the stability and reliability of this experimental model.
This experiment has its advantages and disadvantages compared with the animal models of ectopic abdominal cavity constructed by predecessors (43). Although intraperitoneal implantation creates an abdominal environment similar to human disease conditions for the growth of lesions, multiple exploratory laparotomy interfers with the formation of lesions to a certain extent, leading to the decline of animal life vitality, and it is difficult to evaluate the final results with scattered and variable-sized lesions. The subcutaneous implant used in this study can facilitate the continuous and intuitive observation of the growth and change of the lesions, which is convenient for operation and measurement. The success of the model can be determined by touching the subcutaneous injection site of the nude mouse. For the in vivo experiment of drug treatment for endometriosis, it is convenient to observe changes in the lesions on a daily basis. Human endometrial cells isolated and cultured in vitro are more similar to the characteristics of human pathology than animal models of homotransplantation, which can not only be used to observe the growth and angiogenesis of endometrial cells, but also study the cytochemistry and molecular biology. The subcutaneous xenotransplantation model constructed in this study can be used as a potential experimental model to understand the molecular mechanism of human endometriosis. For example, this model can be used to understand the heredity genes or abnormal expression of key functional proteins, including the MAPK signaling pathway and WNT signaling pathway, which improve our understanding of endometriosis (3). In this model, we can overexpression, knockdown or knockout the key genes in endometrial stromal cells, and then observe the influence on the formation of endometriosis lesions. However, the disadvantage of this animal model of endometriosis is that it cannot be used in the immunological study of endometriosis.
In summary, a mixture of human immortalized endometriosis stromal cells and epithelial cells was able to establish subcutaneous endometriosis lesions in nude mice. The model built in this study can be used as a valuable tool today to understand the molecular and cellular behavior of the pathogenesis of endometriosis. Furthermore, this model has good application value for the study of gene modification or abnormal protein expression in the pathogenesis of endometriosis and could be used to develop potential targeted therapy to treat endometriosis in women.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China (no. 82060274) and the Natural Science Foundation of Jiangxi Province (nos. 20202BABL216009 and 20181BAB215009).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL and YZ conceived and designed the study. LPL and ZML wrote the manuscript. ZZW, DMH, LPL, GC and BNC collected and analyzed the data. YL, ZML, YFC and YZ analyzed and interpreted the results. YL, YZ, YFC and ZML revised the manuscript in light of the findings. YL and LPL confirm the authenticity of all the raw data. All authors have been involved in revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments and animal care were performed under the approval of the Laboratory Animal Ethics Committee of Nanchang Royo Biotech Co. Ltd (Nanchang, China; approval no. RYE2019051001; May 9, 2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil Steril. 2019;111:327–340. doi: 10.1016/j.fertnstert.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Rolla E. doi: 10.12688/f1000research.14817.1. Endometriosis: Advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res 8: F1000 Faculty Rev-529, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–1256. doi: 10.1056/NEJMra1810764. [DOI] [PubMed] [Google Scholar]
- 4.Czyzyk A, Podfigurna A, Szeliga A, Meczekalski B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017;69:447–461. doi: 10.23736/S0026-4784.17.04048-5. [DOI] [PubMed] [Google Scholar]
- 5.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 6.D'Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckx PR. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) Biol Reprod. 1991;45:411–416. doi: 10.1095/biolreprod45.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Hastings JM, Fazleabas AT. A baboon model for endometriosis: Implications for fertility. Reprod Biol Endocrinol. 2006;4 (Suppl 1)(S7) doi: 10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor HS, Alderman Iii M, D'Hooghe TM, Fazleabas AT, Duleba AJ. Effect of simvastatin on baboon endometriosis. Biol Reprod. 2017;97:32–38. doi: 10.1093/biolre/iox058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns KA, Pearson AM, Slack JL, Por ED, Scribner AN, Eti NA, Burney RO. Endometriosis in the Mouse: Challenges and progress toward a ‘Best Fit’ murine model. Front Physiol. 2022;12(806574) doi: 10.3389/fphys.2021.806574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan H. In-vitro models of human endometriosis. Exp Ther Med. 2020;19:1617–1625. doi: 10.3892/etm.2019.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: The nude mouse as a xenographic host. Ann NY Acad Sci. 2002;955:328–342. doi: 10.1111/j.1749-6632.2002.tb02793.x. 396-406. [DOI] [PubMed] [Google Scholar]
- 12.Perello M, Gonzalez-Foruria I, Castillo P, Martínez-Florensa M, Lozano F, Balasch J, Carmona F. Oral administration of pentoxifylline reduces endometriosis-like lesions in a nude mouse model. Reprod Sci. 2017;24:911–918. doi: 10.1177/1933719116673198. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Hong S, Tan J, Ke P, Liang L, Fei H, Liu B, Liu L, Liu Y, Yu B. A red fluorescent nude mouse model of human endometriosis: Advantages of a non-invasive imaging method. Eur J Obstet Gynecol Reprod Biol. 2014;176:25–30. doi: 10.1016/j.ejogrb.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Ni HJ, Zhang Z, Dai YD, Zhang SY. Establishment of endometriosis subcutaneous model in immunodeficient nude mice. Zhonghua Yi Xue Za Zhi. 2016;96:2675–2677. doi: 10.3760/cma.j.issn.0376-2491.2016.33.016. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Ma X. Effects of estrogen and progestin on expression of MMP-2 and TIMP-2 in a nude mouse model of endometriosis. Clin Exp Obstet Gynecol. 2012;39:229–233. [PubMed] [Google Scholar]
- 16.Wu D, Lu P, Mi X, Miao J. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. Mol Hum Reprod. 2018;24:357–365. doi: 10.1093/molehr/gay019. [DOI] [PubMed] [Google Scholar]
- 17.Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Veterinary Medical Association 2020. AVMA Guidelines for the Euthanasia of Animals. 9th edition. American Veterinary Medical Association, Schaumburg, IL, 2020. [Google Scholar]
- 19.Luo Y, Zou Y, Wu J, Zhang ZY, Liu FY, Li LP, Huang OP. The mitochondrial DNA 4977-bp deletion and copy number alteration in Han Chinese samples with uterine fibroids. Ann Hum Genet. 2019;83:220–230. doi: 10.1111/ahg.12303. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri C, Franlund B, Boeryd B. Expression of cytokeratin 7 in simultaneous mucinous tumors of the ovary and appendix. Mod Pathol. 1995;8:573–576. [PubMed] [Google Scholar]
- 21.Mishra A, Galvankar M, Vaidya S, Chaudhari U, Modi D. Mouse model for endometriosis is characterized by proliferation and inflammation but not epithelial-to-mesenchymal transition and fibrosis. J Biosci. 2020;45(105) [PubMed] [Google Scholar]
- 22.Atkins HM, Lombardini ED, Caudell DL, Appt SE, Dubois A, Cline JM. Decidualization of endometriosis in macaques. Vet Pathol. 2016;53:1252–1258. doi: 10.1177/0300985816646433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PT. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. 2014;184:1930–1939. doi: 10.1016/j.ajpath.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimoto-Kakiuchi A, Netsu S, Matsuo S, Hayashi S, Ito T, Okabayashi S, Yasmin L, Yuzawa K, Kondoh O, Kato A, et al. Characteristics of histologically confirmed endometriosis in cynomolgus monkeys. Hum Reprod. 2016;31:2352–2359. doi: 10.1093/humrep/dew209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltan G, Suntar I, Ozbilgin S, Ilhan M, Demirel MA, Oz BE, Keleş H, Akkol EK. Viburnum opulus L: A remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J Ethnopharmacol. 2016;193:450–455. doi: 10.1016/j.jep.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Simitsidellis I, Gibson DA, Saunders PTK. Animal models of endometriosis: Replicating the aetiology and symptoms of the human disorder. Best Pract Res Clin Endocrinol Metab. 2018;32:257–269. doi: 10.1016/j.beem.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 28.D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 29.Harirchian P, Gashaw I, Lipskind ST, Braundmeier AG, Hastings JM, Olson MR, Fazleabas AT. Lesion kinetics in a non-human primate model of endometriosis. Hum Reprod. 2012;27:2341–2351. doi: 10.1093/humrep/des196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU, Kommagani R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod. 2019;34:1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Zhou Y, Taylor HS. MiR-451a inhibition reduces established endometriosis lesions in mice. Reprod Sci. 2019;26:1506–1511. doi: 10.1177/1933719119862050. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Li D, Yuan M, Li Q, Zhen Q, Li N, Wang G. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol Hum Reprod. 2019;25:5–16. doi: 10.1093/molehr/gay049. [DOI] [PubMed] [Google Scholar]
- 33.Bruner-Tran KL, Mokshagundam S, Herington JL, Ding T, Osteen KG. Rodent models of experimental endometriosis: Identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14:173–188. doi: 10.2174/1573404813666170921162041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korbel C, Menger MD, Laschke MW. Size and spatial orientation of uterine tissue transplants on the peritoneum crucially determine the growth and cyst formation of endometriosis-like lesions in mice. Hum Reprod. 2010;25:2551–2558. doi: 10.1093/humrep/deq201. [DOI] [PubMed] [Google Scholar]
- 35.Pelch KE, Sharpe-Timms KL, Nagel SC. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp. 2012;59(e3396) doi: 10.3791/3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallwiener D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: An explanation for the controversy on the use of autologous and alloplastic barriers? Fertil Steril. 1998;69:132–137. doi: 10.1016/s0015-0282(97)00429-9. [DOI] [PubMed] [Google Scholar]
- 37.Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, Smith SK, Tavaré S, Print CG, Charnock-Jones DS. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol. 2008;173:700–715. doi: 10.2353/ajpath.2008.071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hull ML, Prentice A, Wang DY, Butt RP, Phillips SC, Smith SK, Charnock-Jones DS. Nimesulide, a COX-2 inhibitor, does not reduce lesion size or number in a nude mouse model of endometriosis. Hum Reprod. 2005;20:350–358. doi: 10.1093/humrep/deh611. [DOI] [PubMed] [Google Scholar]
- 39.Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis: Adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74:306–312. doi: 10.1016/s0015-0282(00)00601-4. [DOI] [PubMed] [Google Scholar]
- 40.Eggermont J, Donnez J, Casanas-Roux F, Scholtes H, Van Langendonckt A. Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril. 2005;84:492–499. doi: 10.1016/j.fertnstert.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Mettlen M, Guillet A, Donnez J. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecol Obstet Invest. 2008;65:174–186. doi: 10.1159/000111148. [DOI] [PubMed] [Google Scholar]
- 42.Pereira FE, Almeida PR, Dias BH, Vasconcelos PR, Guimaraes SB, Medeiros Fd. Development of a subcutaneous endometriosis rat model. Acta Cir Bras. 2015;30:6–12. doi: 10.1590/S0102-86502015001000002. [DOI] [PubMed] [Google Scholar]
- 43.Banu SK, Starzinski-Powitz A, Speights VO, Burghardt RC, Arosh JA. Induction of peritoneal endometriosis in nude mice with use of human immortalized endometriosis epithelial and stromal cells: A potential experimental tool to study molecular pathogenesis of endometriosis in humans. Fertil Steril. 2009;91 (Suppl 5):S2199–S2209. doi: 10.1016/j.fertnstert.2008.06.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.