Abstract
Autoimmune diseases (AIDs) are characterized by dysfunction and tissue destruction, and recent studies have shown that interleukin (IL)-37 expression is dysregulated in AIDs. Among cytokines of the IL-1 family, most are pro-inflammatory agents, and as an anti-inflammatory cytokine, IL-37 may have the potential to alleviate excessive inflammation and can be used as a ligand or transcription factor that is involved in regulating innate and adaptive immunity. IL-37 plays important roles in the development of AIDs. This review summarizes the biological characteristics and functions of IL-37 and discusses the potential of IL-37 as a therapeutic target for effective cytokine therapy and as a biomarker in AIDs.
Keywords: interleukin-37, autoimmune disease
1. Introduction
The interleukin (IL)-1 family of cytokines plays a major role in regulating the expression of genes related to inflammation in autoimmune diseases (AIDs) (1,2). IL-37 is also known as IL-1 family member 7 (IL-1F7), IL-1H4, and IL-1RP1. It is a novel anti-inflammatory cytokine with immunomodulatory effects. Specifically, it reduces the production of anti-inflammatory cytokines and thereby inhibits the inflammatory and immune responses by reducing the production of anti-inflammatory cytokines. IL-37 functions in three ways, i.e., by reducing the synthesis of pro-inflammatory cytokines, by lowering the expression of transcriptional cytokines, and by inhibiting the activation of kinase signaling (3,4). The aim of the present review was to summarize the immunomodulatory roles of IL-37, as well as relevant clinical studies based on the protective mechanisms of IL-37 in AIDs in order to develop therapeutic strategies for treatment of AIDs.
2. Biological characteristics of IL-37
Structure
IL-37, commonly known as IL-1F7, is a member of the IL-1 family of cytokines identified ten years ago (5). The IL-37 gene located at 2q12-q14.1 on chromosome 2, is typically composed of seven exons (6). There are five basic subtypes of IL-37, including IL-37a, IL-37b, IL-37c, IL-37d, and IL-37e. Exons 3, 4, 5, and 6 encode IL-37a. IL-37b is encoded by exons 1, 2, 4, 5, and 6, while IL-37c is encoded by exons 1, 2, 5, and 6. Exons 1, 4, and 6 encode IL-37d. IL-37e is encoded by exons 1, 5, and 6(7). It has been suggested that structural alterations induced in response to caspase-1-mediated cleavage are responsible for the production of a variety of IL-37 subtypes (8-10). The action of IL-37 is mediated by a β-barrel structural unit in its secondary structure. The 12-β-strand-containing proteins may be formed by amino acid sequences encoded by exons 4, 5, and 6 of IL-37a, IL-37b, and IL-37d (9,11). The 12-hypothetical β-strand structural units that constitute the primary secondary β-trefoil structure of IL-37 are responsible for the function of the protein. Other members of the IL-1 family have a similar barrel structure, which is intimately connected to the binding of the IL-1 receptor (12). This construct has been shown to be intimately involved in IL-1 receptor binding. Despite possessing the same β-trefoil secondary structure, differential regulation of signaling downstream of the receptor dictates differences in the activity of other cytokine members of the IL-1 family (10,13). However, further studies are required to reveal the detailed structural basis of this phenomenon.
An additional structural feature of IL-37 is its existence as a dimer (homodimer). It has been shown that this structure is found mostly in the IL-37b subtype. A symmetrical head-to-head IL-37b homodimer interface is created by the β3-β4 loops and β-trefoil sheet (β2-β3-β11) of each subunit. This dimer is a negative regulator of IL-37 activity, and the formation of such dimers weakens the anti-inflammatory effect of extracellular IL-37(14). It is possible that the binding of the homodimer to the IL-1 receptor is due to the formation of the 12-β-trefoil structure (15).
Distribution, expression, and release
IL-37 is widely expressed in multiple human tissues and organs, including the skin, heart, kidney, gut, lymph node, thymus, bone marrow, lung, testis, placenta, and uterus (16). However, the expression of distinct subtypes differs according to the specific tissues and organs involved. Under physiological conditions, IL-37a is mostly found in the lymph nodes, thymus, bone marrow, placenta, colon, lung, testicles, and brain, whereas IL-37b is mainly found in the peripheral blood, lymph nodes, placenta, colon, lung, testicles, and kidney. IL-37c is mostly expressed in the lymph nodes, placenta, colon, lung, testis, and heart, whereas IL-37d is predominantly expressed in the testis, bone marrow, blood system, umbilical cord tissue, and adipose tissue mesenchymal stem cells. The testicles and bone marrow are the primary sites of IL-37e expression. Cells from the aforementioned tissues may express IL-37 in a number of ways; for instance, monocytes, macrophages, B cells, plasma cells, endothelial cells, and skin keratinocytes are all capable of producing IL-37 (17,18).
IL-37 is expressed at low levels under physiological conditions, but can be upregulated in response to inflammatory stimuli and pro-cytokines. For example, IL-37 is mainly produced by macrophages in response to Toll-like receptor (TLR) activation (19), and lipopolysaccharide (LPS) can induce the expression of IL-37 in RAW264.7 mouse macrophage cells (20). Triptolide has been found to facilitate the expression of IL-37 in THP-1 cells through activation of the p38 and extracellular regulated protein kinase (ERK)1/2 pathways (21).
In different cells, such as peripheral blood mononuclear cells (PBMCs), RAW-IL-37 cells, dendritic cells (DCs), epithelial cells, endothelial cells, and T cells, IL-37 can be upregulated by various pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-1β, transforming growth factor-β1 (TGF-β1; low concentrations), IL-4, and IL-6(22). IL-12, IL-32, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are known to limit IL-37 production (5). In vivo evidence has shown that IL-37 can block the activity of Th1/Th2/Th17 cells via PBMCs, M1 macrophages, and DCs (23,24), while activating the function of Tregs (25,26). However, specific signaling pathways remain poorly understood.
3. Biological functions of IL-37
IL-37 primarily reduces innate and acquired immune responses through intracellular and extracellular inhibition by reducing the secretion of pro-inflammatory chemokines (11,27). IL-37 is a transcription factor that can be used to regulate gene expression in cells. Caspase-1 cooperates with the signal transduction protein Smad3 to regulate its transcription (17).
The IL-37a mRNA splicing site is positioned at the N-terminus of the amino acid sequence encoded by exon 3, which is located at the end of the exon. IL-37d also encodes the 12-β-strand-containing protein structure as it comprises exons 1, 4, 5, and 6, while IL-37b encodes a transcript variant containing exons 1 and 2, and includes an N-terminal pro-domain that comprises a potential caspase-1 cleavage site (28). Caspase-1 is primarily responsible for IL-37 splicing. Following translation, IL-37 exists in the form of an immature precursor peptide, which is subsequently cleaved by caspase-1 between amino acid residues D20 to E21 encoded by exon 1 of IL-37. This cleavage leads to the formation of active IL-37. Only mature IL-37 can perform biological tasks both extracellularly and intracellularly (8).
IL-37 binds to Smad3, dimers of which eventually enter the nucleus. Complexes formed in the cytoplasm by mature IL-37 and phosphorylated activated Smad3 translocate into the nucleus, where they are involved in regulating transcriptional activity (8,29). In response to the interaction between IL-37 and Smad3, the production of protein tyrosine phosphatases (PTPNs), which can prevent the activation of tyrosine phosphorylation-dependent signaling pathways, may be increased. PTPNs have been shown to inhibit a number of inflammation- and immune-related pathways, including ERK, mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), phosphatidylinositol-3-kinase (PI3K), nuclear factor-κB (NF-κB), and signal transduction and activator of transcription (STAT)3(17). IL-37/Smad3 complexes can compete with Smad2/3/4 complexes to reduce the phosphorylation of Smad2 and Smad4, allowing them to perform additional biological functions in the nucleus. However, the specific regulation remains to be elucidated (12).
The primary function of IL-37, an anti-inflammatory cytokine, is the secretion of proteins to the exterior of cells, which act as ligands for a functional receptor structure on the target cell membrane. The β-barrel structure of IL-37b binds to the α chain of the IL-18 receptor (IL-18R) and reduces the production of inflammatory mediators (30). Similar to IL-18, IL-37 is capable of non-competitively binding to the receptors IL-18Ra and IL-18BP to create trimeric complexes, which may then activate downstream transduction signals, such as the NF-κB, the mammalian target protein of rapamycin (mTOR), MAPK, ERK, AMP-dependent protein kinase (AMPK), and STAT3/6 signaling pathways (11,31,32). IL-37 also promotes the activation of M2 macrophages and tolerogenic DCs by downregulating MHC Class II, CD40, and CD86(33). These aforementioned studies indicated that IL-37 may regulate immunosuppressive responses by downregulating the expression of DC costimulatory molecules.
Formation of triplex complexes is critical for the anti-inflammatory effects of IL-37 and can coordinate innate immune responses by inducing the activation of myeloid differentiation factor 88 (MyD88) (34). IL-37 is not expressed in mice. Transgenic human IL-37 (hIL-37tg) and wild-type (WT) mice are widely used as animal models to investigate IL-37 pathology (10).
IL-37 forms a complex with IL-18Rα and IL-1R8 (previously TIR8 or SIGIRR), which can markedly reduce the anti-inflammatory activity of IL-37, indicating that the formation of the IL-37 receptor complex is required for IL-37 to fulfill its biological activities (35,36). Furthermore, IL-37 negatively regulates inflammatory responses in the innate immune system by downregulating IL-1 receptor-associated kinase 1 (IRAK), phosphatase and tensin homolog (PTEN), TNF receptor-associated factor 6 (TRAF6), NOD-like receptor family pyrin domain containing 3 (NLRP3), mTOR, and thymic stromal lymphopoietin (TSLP), and decreasing the levels of reactive oxygen species (ROS) (37,38).
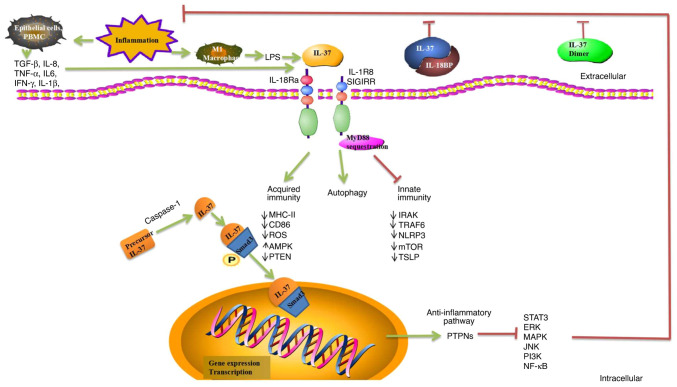
Currently, there is no evidence linking IL-37 to autophagy. However, the potential role of IL-37 in autophagy is currently unknown, although certain studies have revealed a potential link between them. IL-37 enhances autophagy and induces metabolic reprogramming by reducing mTOR expression and increasing the AMPK levels, resulting in changes in the cellular redox state, or by increasing oxidative phosphorylation (39,40). IL-37 may be involved in the progression of lung fibrosis by inhibiting TGF-β1 signaling and enhancing autophagy (41). IL-37/IL-1R8 exerts a pseudo-starvation effect on mTOR (36). The molecular mechanisms of IL-37-mediated autophagy also require investigation, and may provide new insights into the development of IL-37-mediated immunotherapy (Fig. 1).
Figure 1.
Role of IL-37 regulation of immunity. IL-37 has significant anti-inflammatory, anticancer, immuno-suppressive, and metabolic regulatory effects. IL-37 binds to IL-18Ra or IL-18BP, which can enhance inhibition of IL-18 and reduce inflammation. The homodimer of IL-37 is a negative regulator of extracellular anti-inflammatory activity. IL-37 is translocated to the nucleus after being processed by caspase-1, and precursor IL-37 is processed into mature IL-37. Complexes formed by mature IL-37 and phosphorylated activated Smad3 in the cytoplasm undergo nuclear translocation into the nucleus, where they play a role in regulating transcriptional activity. PTPNs are activated and numerous related inflammatory and immune pathways are inhibited, including ERK, MAPK, JNK, PI3K, NF-κB, and STAT3. IL-37 binds to its receptor IL-18Rα, recruiting the co-receptor IL-1R8 to form the IL-37/IL-18Rα/IL-1R8 complex at the plasma membrane induced by inhibiting MyD88-dependent signaling. IL-37 negatively regulates inflammatory responses in the innate and acquired immune system by downregulating IRAK, PTEN, ROS, TRAF6, NLRP3, mTOR, TSLP, MHC-II, and CD86. IL-37 acts as an anti-inflammatory cytokine by decreasing the production of pro-inflammatory cytokines and chemokines. IL-37 enhances autophagy and induces metabolic reprogramming by reducing the expression of mTOR and increasing the AMPK levels. However, molecular mechanisms of IL-37-mediated autophagy remain unknown. IL-18Ra, α-subunit of IL-18 receptor; IL-18BP, IL-18 binding protein; PTPNs, protein tyrosine phosphatases; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; PI3K, phosphatidylinositol-3-kinase; NF-κB, nuclear factor-κB; STAT, signal transduction and activator of transcription; MyD88, myeloid differentiation factor 88; IRAK, interleukin-1 receptor-associated kinase 1; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; TRAF6, TNF receptor-associated factor 6; NLRP3, NOD-like receptor family pyrin domain containing 3; mTOR, mammalian target of rapamycin; TSLP, thymic stromal lymphopoietin; AMPK, AMP-dependent protein kinase.
4. IL-37 and AIDs
Accumulating evidence shows that the expression of IL-37 is closely related to various AIDs such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren's syndrome (pSS), ankylosing spondylitis/spondyloarthritis (AS/SpA), vasculitis, gout, and osteoarthritis (OA) (Table I).
Table I.
IL-37 in autoimmune disease.
| Disease | Role of IL-37 | (Refs.) |
|---|---|---|
| RA | Associated with proinflammatory factors, TLR4 | (44,45,47,49-51) |
| Associated with disease activity | (44-46,49,51-53) | |
| Associated with activated T cell function | (46) | |
| Associated with bone loss | (49) | |
| Inhibits RAFLS proliferation and migration; induces RAFLS apoptosis by inhibiting the STAT3 pathway | (54) | |
| AS/SPA | Associated with bone density | (63) |
| Associated with disease activity | (63,64) | |
| GOUT | Associated with proinflammatory factors | (67,70) |
| Associated with disease activity | (67,69) | |
| Associated with tophi forming, kidney deterioration | (67) | |
| rhIL-37 suppressed MSU-induced innate immune responses by enhancing expression of Smad3 and IL-1R8 to trigger multiple intracellular switches to inhibit NLRP3 and the activation of SOCS3 | (68) | |
| Reduces the transcription of pyrophosphate-related proteins and release of inflammatory cytokines by enhancing phagocytosis of MSU, protects mitochondrial function, and mediates metabolic reprogramming in THP-1 cells treated by MSU, which depended on the mediation of GSK-3 β | (70) | |
| OA | Associated with VAS | (75,76) |
| Affects M1/M2-like macrophage polarization | (75) | |
| Associated with proinflammatory factors | (76,78) | |
| rhIL-37 may regulate the key downstream target MMP-3 | (78) | |
| SLE | Associated with disease activity (SLEDAI) | (80,82,83) |
| Associated with kidney damage and skin lesion. | (80,83) | |
| Associated with Asian race | (81) | |
| Associated with proinflammatory factors | (82) | |
| Negatively correlated with C3/C4, and antibodies | (83,84) | |
| Associated with C3 | (84) | |
| PSS | Associated with RF, antibodies, proinflammatory factors | (87) |
| BD | Negatively correlated with inflammatory response | (89-91) |
| rhIL-37 may reduce the levels of TSLP in vitro | (92) | |
| ITP | Positive correlate with the platelet count | (95) |
| Positive correlate with proinflammatory factors | (96,97) | |
| MS | Act as a part of a feed-back loop to control underlying inflammation | (99) |
| Positive correlate with disease activity | (99,100) | |
| Regulate autophagy, apoptosis | (101) |
RA, rheumatoid Arthritis; TLR, Toll-like receptor; RAFLS, fibroblast like synoviocytes; AS, ankylosing spondylitis; SPA, spondyloarthropathy; MSU, monosodium urate; NLRP3, NOD-like receptor family pyrin domain containing 3; SOCS3, suppressor of cytokine signaling 3; OA, osteoarthritis; VAS, visual analogue scale; MMP3, matrix metalloproteinase-3; SLEDAI, systemic Lupus Erythematosus Disease Activity Index; C, complement; PSS, primary Sjögren's syndrome; BD, Behcet's disease; RF, rheumatoid factor; TSLP, thymic stromal lymphopoietin; ITP, immune thrombocytopenic purpura; MS, multiple sclerosis.
IL-37 and inflammatory joint disease
Inflammatory arthritis (IA) is a prevalent joint inflammatory illness, in addition to RA, OA and spondyloarthritis (42).
RA
RA is a chronic inflammatory disease that can cause irreversible joint damage and physical disability. It can involve the skin, eyes, lungs, heart, and blood vessels (43).
As previously reported, the amount of IL-37 in normal human plasma and bodily fluids is exceedingly low, but is markedly increased in the synovial fluid, serum, and PBMCs of patients with RA (24,44-55). Increased IL-37 levels in the serum are positively associated with inflammatory markers [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)], rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (anti-CCP), disease activity score-28 (DAS28), bone loss, and pro-inflammatory cytokine expression (IL-6, IL-18, IL-4, IFN-γ, IL-17A).
The proportion of CD3+ CD26+ T cells is associated with disease activity, implying that IL-37 levels are positively correlated with activated T lymphocytes (46). It has been shown that IL-37 affects the activity and phenotype of DCs and suppresses the inflammatory responses mediated by Th17 and IL-17 in RA, while failing to inhibit Th-17 cell differentiation (24). In a study involving 70 patients with juvenile idiopathic arthritis (JIA), serum/synovial IL-37 levels and IL-37 mRNA expression in PBMCs were positively associated with disease activity and angiogenesis indicators [including vascular endothelial growth factor (VEGF) and VEGF receptors] (56).
A previous study indicated that angiogenesis is a crucial mechanism for the proliferation of synovial tissue and the formation of invasive pannus in the early onset of RA (57), and is associated with the regulation of VEGF and angiogenesis inhibitors. The inflammatory response of the RA synovial tissue is triggered by stimuli. Local macrophages and fibroblasts also respond to produce pro-inflammatory cytokines, which can modulate the expression of adhesion molecules, matrix metalloproteinases (MMPs), chemokines, TLRs, and growth factors that are important at different stages of angiogenesis (58). VEGF is a cytokine that acts on the vascular endothelium of the synovium, promotes angiogenesis, and binds to cognate receptors on endothelial cells (ECs), thereby activating these cells to produce greater levels of proteolytic enzymes. VEGF expression in the synovial tissue is regulated by angiogenesis (59). Redox signaling is closely associated with angiogenesis and can alter the angiogenic response of synovial cells. Downregulation of hypoxia-inducible factor-1α (HIF-1α) significantly reduces angiogenesis in VEGF-induced rheumatoid arthritis fibroblast-like synoviocytes (RAFLS) in macrophages of the synovial lining (60). Multiple studies have shown that blocking angiogenic pathways may reduce inflammatory cell infiltration and damage to joints (61,62). In animal models of RA, prophylactic treatment with anti-VEGF antibodies delays the onset, joint swelling, and vascularization in collagen-induced arthritis (CIA) (63). Inhibition of angiogenesis has emerged as a new option for the treatment of RA in recent years, and numerous drugs targeting RA angiogenesis have been developed (64).
In an in vitro study, recombinant IL-37 (rhIL-37) was used to stimulate PBMCs in RA patients. It was discovered that rhIL-37 considerably decreased the levels of TNF-α, IL-17, and IL-6 in RA patients (48).
The role of IL-37 single nucleotide polymorphisms (SNPs) in RA is debatable. A Chinese RA population study revealed that IL-37 rs3811047 is positively associated with disease activity, indicating that the prognosis of RA patients with various IL-37 genotypes varies (65). Two other studies involving a Han RA population revealed that no genotypes were associated with RA susceptibility (66,67). In future, it is important to expand sample sizes and ethnic diversity to identify distinct IL-37 phenotypes in patients with RA.
Collectively, IL-37 may be presented as a novel biomarker for predicting and monitoring disease severity/therapeutic targets in RA by reducing inflammation.
AS/SPA
AS/SPA is a chronic inflammatory illness that affects the sacroiliac joints, spine bony processes, paraspinal soft tissues, and peripheral joints, with extra-articular symptoms occurring in certain cases (68).
The level of IL-37 in the serum and PBMCs of patients with AS was revealed to be higher than that in healthy controls (HCs), and was was associated with ESR, CRP, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score (ASDAS), and bone density. It was found that IL-37 can inhibit the expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-17, and IL-23) in PBMCs of patients with AS, indicating a potential anti-inflammatory role of IL-37 in AS (69,70). RhIL-37 can significantly reduce LPS-stimulated PBMC proliferation and IL-6, IL-17, IL-23, and TNF-α production (70).
The A/G frequency of IL-37 rs3811047 in AS patients is significantly different from that observed in the general population, and there is a link between this and alcohol consumption (71). As it is related to the susceptibility to AS in the Han population, IL-37 A/G rs3811047 could be regarded as an independent risk factor.
In conclusion, the aforementioned studies suggested that IL-37 plays significant roles in the development of AS/SPA. IL-37 may be used as a predictive biomarker for AS, as well as to assess the degree of inflammation and bone loss.
Gout
Gout is caused by the precipitation of monosodium urate (MSU) crystals within joints and soft tissues that can progress to acute or chronic arthritis (72). Consequently, a negative feedback mechanism for MSU-induced inflammation may be present. IL-37 has been identified as a potential anti-inflammatory agent in response to MSU. However, the association between IL-37 and clinical markers and pro-inflammatory mediators in individuals with gout is not fully understood.
Several studies have shown that IL-37 expression is increased in PBMCs of patients with gout (73-75), and is positively correlated with ESR, CRP, tophi formation, and platelet counts.
Different doses of MSU have been shown to elicit dose-dependent overexpression of IL-37 protein and mRNA in PMBCs in vitro (75).
The mRNA level of pro-IL-37 in PBMCs from patients with acute gout (AG) was significantly higher than that in non-AG (NAG) PBMCs, indicating that IL-37 may act as a suppressor of MSU-induced inflammation. Additionally, this study demonstrated that rhIL-37 inhibited MSU-induced innate immune responses by increasing the expression of Smad3 and IL-1R8, both in vitro and in vivo. IL-37 controls MSU-induced inflammation, in part through a MERTK-dependent signaling pathway (74). IL-37 inhibits gout inflammation and exerts its effects in vitro by altering macrophage function (76).
Therefore, rhIL-37 has both preventive and therapeutic effects on gout, and the suppressive effect of IL-37-mediated inflammation may partially depend on the activation of MERTK (74,77). Recombinant human PDZ domain 1 protein (PDZK1) is a cytoskeletal protein expressed in renal tubular epithelial cells that interacts with a variety of uric acid transporters to control uric acid. A previous study revealed that the transcription of PDZK1 expression stimulated with various concentrations of IL-37 may regulate uric acid metabolism through the NF-κB signaling pathway in HK-2 cells (78).
Using a molecular inversion probe sequencing technique, four unusual IL-37 variations were detected in 675 individuals with gout. It is possible that carriers of p.(N182S) (rs752113534) are at a higher risk of developing gout and undergo early onset of disease (79).
These studies revealed that IL-37 may be a potentially valuable treatment option for patients with chronic gout, especially those with tophi and kidney damage.
OA
OA is one of the most frequent degenerative joint disorders that affects people globally (80). A link between the expression and function of IL-37 and OA remains unclear. IL-37 levels are elevated in the blood, synovial fluid, synovial cells from lesions, and chondrocytes of patients with OA. In addition, in patients with OA, IL-37 levels are favorably linked with ESR, CRP, visual analog scale (VAS) pain score, as well as other variables (81,82). Luo et al determined that IL-37a and IL-37b receptors are overexpressed in chondrocytes from patients with temporomandibular joint (TMJ) OA. IL-1R8 is required for IL-37b to exert anti-inflammatory effects on the TMJ. The therapeutic potential of IL-37b in the treatment of TMJ inflammation was also elucidated, suggesting that targeting the IL-37 pathway may provide a novel therapeutic strategy for treating inflammation in OA patients. RhIL-37b has also been shown to decrease the expression of inflammatory cytokines in the TMJ, which is associated with reduced inflammation and subchondral bone loss (81).
Ding et al revealed that IL-37 was significantly upregulated in erosive osteoarthritis (EIOA) [compared to primary generalized osteoarthritis (PGOA) and HCs], and that the release of pro-inflammatory cytokines in synovial cells treated with IL-37 was significantly inhibited in vitro (82).
Another study, using immunohistochemical assays revealed that the IL-37 protein was dysregulated in human OA chondrocytes, which could inhibit osteoclast differentiation directly (83). Overexpression of IL-37 in a model of adenovirus construct (ad-IL-37)-induced OA resulted in reduced levels of IL-1, IL-6, IL-8, and MMP3(84). MMP3 may be a critical downstream target of rhIL-37 when interfering with cartilage breakdown.
These studies indicated that IL-37 may prevent cartilage deterioration in individuals with OA. The presence of IL-37 may be a useful marker to distinguish EIOA from PGOA. It is anticipated that rhIL-37 will serve as a new therapeutic option for individuals with specific types of OA.
IL-37 and SLE
SLE is an autoimmune disease involving the activation of autoreactive B cells and the dysregulation of numerous other types of immune cells, including CD4+ T cells, DCs, macrophages, and neutrophils. SLE is highly heterogeneous in its various presentations and is characterized by multiple organ damage (85). The level of IL-37 in the serum, plasma, and PBMCs of SLE patients is elevated, and has been shown to be positively correlated with SLE disease activity index (SLEDAI) scores (especially renal disease activity), the degree of kidney and skin damage, and the levels of pro-inflammatory cytokines (IL-6, IL-18, and IFN-γ) (86-90). The opposite was observed in other studies, which found that the amount of IL-37 was negatively correlated with the level of complement proteins (89) and the levels of anti-Sm and anti-RNP antibodies (90). This may be attributed to the difference in sample sizes and cohorts.
Treatment with prednisone (1 mg/kg/day for 14 days) substantially decreased plasma IL-37 expression in SLE patients (88). RhIL-37 may play an essential role in SLE pathogenesis by modulating pro-inflammatory pathways in PBSCs from patients with SLE in vitro (89). Therefore, the level of IL-37 in Asian patients with SLE may serve as a marker of disease activity. Three IL-37 SNP variants (rs2723186, rs2723176, and rs4364030) may also be associated with SLE susceptibility (91).
IL-37 may play an important role in the inhibition of SLE pathogenesis. Thus, it is expected to serve as a diagnostic and prognostic tool. Further studies are required to identify the mechanisms underlying IL-37 regulation during the mediation of immune reactions in SLE.
IL-37 and pSS and Behçet's disease (BD)
pSS is an autoimmune disease characterized by focal lymphocytic infiltration of exocrine glands such as the salivary and lacrimal glands. Inflammatory response immune aberrations underlying pSS are mediated by B and mast cells (MCs) (92). Liuqing et al revealed that patients with pSS have increased IL-37 expression compared to HCs; IL-37 expression was positively correlated with disease activity and RF, IL-4, and IL-12 levels (93). Treatment with IL-37 may reduce glandular inflammation and decrease systemic inflammatory responses.
BD is a multisystem disorder characterized by primary vasculitis of unknown etiology. Vasculitis and thrombotic events are the most common causes of death (94). Currently, only a few studies have examined the association between IL-37 levels and BD. According to a previous study, the level of IL-37 in the cerebral fluid of patients with neuro-BD (NBD) was increased and positively linked with the level of TGF-β, indicating that IL-37 may be a significant NBD biomarker (95).
Ye et al revealed that the amount of IL-37 in the PBMCs of patients with active BD was considerably lower than that in patients from the HC group. When DCs were stimulated with rIL-37, the production of IL-6, IL-1, TNF-α, and ROS was reduced. These stimulated DCs prevented the activation of ERK1/2, JNK, and P38 MAPK (96). Treatment of patients with corticosteroids is associated with increased IL-37 expression (97).
TSLP is upregulated during the acute phase of BD, and is associated with skin lesions. It has been shown that rhIL-37 may reduce the levels of TSLP in vitro (98). Patients with BD tend to develop ophthalmia. An IL-37 SNP (rs3811047) and IL-18RAP SNP (rs2058660) have been shown to be associated with susceptibility to BD onset (1,063 cases) instead of Vogt-Koyanagi-Hrada (VKH) uveitis (419 cases) in a case-control study involving the Chinese Han population (99).
IL-37 alters immune dysregulation in pSS and BD. It can be considered as a new biomarker with potential therapeutic application.
IL-37 and immune thrombocytopenia (ITP)
ITP is an autoimmune disorder characterized by isolated thrombocytopenia (platelet count <100x109/l), in the absence of other causes and disorders that may be associated with thrombocytopenia (100).
Thus, IL-37 may be involved in ITP pathogenesis. Current studies suggest that IL-37 levels are elevated in the PBMCs or serum of patients with ITP (101-103). Serum IL-37 levels and IL-18Rα+/CD4+ T-cell ratios are negatively correlated with PLT counts (102). IL-37 expression is significantly higher in patients with active ITP, and can regulate the expression of several cytokines to exert anti-inflammatory effects (103). In terms of the possible mechanisms involving IL-37 in the pathogenesis of ITP, it is speculated that IL-37 may promote Th2 cell function by influencing cytokine expression and inhibiting the immune response effects of Th1 and Th17, thereby alleviating the inflammatory responses. This may account for elevated IL-37 expression in patients with active ITP. Thus, IL-37 may represent an important factor for ITP diagnosis and treatment. Furthermore, rhIL-37 may exert therapeutic effects with respect to refractory ITP.
IL-37 and multiple sclerosis (MS)
MS is one of the most prevalent neurological dysfunctions and is an autoimmune disease that affects the central nervous system (CNS), often leading to severe physical or cognitive loss and neurological problems in patients (104).
Studies have shown that IL-37 is aberrantly expressed in MS patients (105,106). There is a significant correlation between serum IL-37 levels and MS disease severity, and IL-37 may be involved in a feedback loop that controls the underlying inflammation in MS pathogenesis (105).
The serum levels of IL-37 and sVEGFR2 and the circulatory number of VEGFR2-expressing cells were higher in patients with MS than in HCs (106). Serum levels of IL-37 and sVEGFR2 may represent important prognostic biomarkers for MS. IL-37 plays a key role in the regulation of oxidative stress, autophagy, and apoptosis markers in periodontal ligament cells from patients after hypoxic preconditioning (107). Thus, IL-37 may serve as a nucleator in a panel of new biomarkers associated with MS.
5. Outlook
AIDs refer to the set of ailments that arise when the immune system of the body launches an assault on self-tissues. This involves the production of aberrant antibodies. IL-37, a member of the IL-1 family, can decrease both congenital inflammation and acquired immunological responses. Although the mechanism underlying IL-37 function is not entirely understood, it is known to be associated with the development of AIDs. IL-37 plays a crucial role in protecting tissues from damage in AIDs by suppressing excessive inflammatory responses. A transgenic IL-37tg mouse model showed that IL-37 exerts considerable anti-inflammatory effects (108). Therefore, further elucidation of the mechanism by which IL-37 is involved in other AIDs is critical for the therapeutic use of this cytokine. Currently, clinical studies involving IL-18 inhibitors [glycogen synthase kinase 1070806(109), ABT-325(110), and rIL-18 binding protein (111)] are underway, and a clinical study involving an IL-33 inhibitor (CNTO-7160) has commenced (112). IL-37 is considered to be an AID biomarker, a predictive factor, and a possible AID treatment.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was financially supported in part by research grants from the Sanming Project of Medicine in Shenzhen (grant no. SZSM201602087), the Shenzhen Science and Technology Project (grant no. JCYJ20180302145033769), and the Research on Public Welfare Project in Futian District, Shenzhen (grant no. FTWS 2021062).
Availability of data and materials
Not applicable.
Authors' contributions
HZ, KZ and ZY designed the study and wrote the manuscript. HZ and KZ performed literature review. KZ and ZY conceived the review and edited the manuscript. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti P, Lessiani G, Kritas SK, Ronconi G, Caraffa A, Theoharides TC. Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell. 2017;49:393–400. doi: 10.1016/j.tice.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Xu K, Chen S, Li Y, Li M. Role of interleukin-37 in inflammatory and autoimmune diseases. Iran J Immunol. 2018;15:165–174. doi: 10.22034/IJI.2018.39386. [DOI] [PubMed] [Google Scholar]
- 5.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sims JE, Smith DE. The IL-1 family: Regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 7.Pan G, Risser P, Mao W, Baldwin DT, Zhong AW, Filvaroff E, Yansura D, Lewis L, Eigenbrot C, Henzel WJ, Vandlen R. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13:1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Kulk N, Nold MF, Gräf R, Kim SH, Reinhardt D, Dinarello CA, Bufler P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 9.Quirk S, Agrawal DK. Immunobiology of IL-37: Mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 2014;10:1703–1709. doi: 10.1586/1744666X.2014.971014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allaire JM, Poon A, Crowley SM, Han X, Sharafian Z, Moore N, Stahl M, Bressler B, Lavoie PM, Jacobson K, et al. Interleukin-37 regulates innate immune signaling in human and mouse colonic organoids. Sci Rep. 2021;11(8206) doi: 10.1038/s41598-021-87592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev. 2018;281:179–190. doi: 10.1111/imr.12605. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Li Y, Guo C, Wang L, Chu H, Zhu F, Li Y, Wang X, Wang Q, Zhao W, et al. IL-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis. 2018;9(582) doi: 10.1038/s41419-018-0664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA. Introduction to the interleukin-1 family of cytokines and receptors: Drivers of innate inflammation and acquired immunity. Immunol Rev. 2018;281:5–7. doi: 10.1111/imr.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei Y, Liu H. IL-37: An anti-inflammatory cytokine with antitumor functions. Cancer Rep (Hoboken) 2019;2(e1151) doi: 10.1002/cnr2.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellisdon AM, Nold-Petry CA, D'Andrea L, Cho SX, Lao JC, Rudloff I, Ngo D, Lo CY, Soares da Costa TP, Perugini MA, et al. Homodimerization attenuates the anti-inflammatory activity of interleukin-37. Sci Immunol. 2017;2(eaaj1548) doi: 10.1126/sciimmunol.aaj1548. [DOI] [PubMed] [Google Scholar]
- 16.Smithrithee R, Niyonsaba F, Kiatsurayanon C, Ushio H, Ikeda S, Okumura K, Ogawa H. Human β-defensin-3 increases the expression of interleukin-37 through CCR6 in human keratinocytes. J Dermatol Sci. 2015;77:46–53. doi: 10.1016/j.jdermsci.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Bai J, Li Y, Li M, Tan S, Wu D. IL-37 as a potential biotherapeutics of inflammatory diseases. Curr Drug Targets. 2020;21:855–863. doi: 10.2174/1389450121666200429114926. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Wen X, Hao D, Wang Y, Wang L, He G, Jiang X. The role of IL-37 in skin and connective tissue diseases. Biomed Pharmacother. 2020;122(109705) doi: 10.1016/j.biopha.2019.109705. [DOI] [PubMed] [Google Scholar]
- 19.Theoharides TC, Tsilioni I, Conti P. Mast cells may regulate the anti-inflammatory activity of IL-37. Int J Mol Sci. 2019;20(3701) doi: 10.3390/ijms20153701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti P, Caraffa A, Mastrangelo F, Tettamanti L, Ronconi G, Frydas I, Kritas SK, Theoharides TC. Critical role of inflammatory mast cell in fibrosis: Potential therapeutic effect of IL-37. Cell Prolif. 2018;51(e12475) doi: 10.1111/cpr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, Liang Z, Zhao F, Peng L, Chen Z. Modulation of IL-37 expression by triptolide and triptonide in THP-1 cells. Cell Mol Immunol. 2015;12:515–518. doi: 10.1038/cmi.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tete S, Tripodi D, Rosati M, Conti F, Maccauro G, Saggini A, Cianchetti E, Caraffa A, Antinolfi P, Toniato E, et al. IL-37 (IL-1F7) the newest anti-inflammatory cytokine which suppresses immune responses and inflammation. Int J Immunopathol Pharmacol. 2012;25:31–38. doi: 10.1177/039463201202500105. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Shen Y, Li C, Liu C, Wang ZH, Li YS, Ke X, Hu GH. IL-37 attenuates allergic process via STAT6/STAT3 pathways in murine allergic rhinitis. Int Immunopharmacol. 2019;69:27–33. doi: 10.1016/j.intimp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Ye L, Jiang B, Deng J, Du J, Xiong W, Guan Y, Wen Z, Huang K, Huang Z. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-triggering cytokine production and limiting Th17 cell proliferation. J Immunol. 2015;194:5110–5119. doi: 10.4049/jimmunol.1401810. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Neff CP, Barber K, Hong J, Luo Y, Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA. 2015;112:2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An B, Liu X, Li G, Yuan H. Interleukin-37 ameliorates coxsackievirus B3-induced viral myocarditis by modulating the Th17/regulatory T cell immune response. J Cardiovasc Pharmacol. 2017;69:305–313. doi: 10.1097/FJC.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 27.Xu WD, Zhao Y, Liu Y. Insights into IL-37, the role in autoimmune diseases. Autoimmun Rev. 2015;14:1170–1175. doi: 10.1016/j.autrev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Gao X, Pan X, Peng X, Li Y, Li M. High-level expression and one-step purification of a soluble recombinant human interleukin-37b in Escherichia coli. Protein Expr Purif. 2015;108:18–22. doi: 10.1016/j.pep.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Ding F, Zhai Y, Tao W, Bi J, Fan H, Yin N, Wang Z. IL-37 is protective in allergic contact dermatitis through mast cell inhibition. Int Immunopharmacol. 2020;83(106476) doi: 10.1016/j.intimp.2020.106476. [DOI] [PubMed] [Google Scholar]
- 30.Robuffo I, Toniato E, Tettamanti L, Mastrangelo F, Ronconi G, Frydas I, Caraffa Al, Kritas SK, Conti P. Mast cell in innate immunity mediated by proinflammatory and antiinflammatory IL-1 family members. J Biol Regul Homeost Agents. 2017;31:837–842. [PubMed] [Google Scholar]
- 31.Wu W, Wang W, Wang Y, Li W, Yu G, Li Z, Fang C, Shen Y, Sun Z, Han L, et al. IL-37b suppresses T cell priming by modulating dendritic cell maturation and cytokine production via dampening ERK/NF-κB/S6K signalings. Acta Biochim Biophys Sin (Shanghai) 2015;47:597–603. doi: 10.1093/abbs/gmv058. [DOI] [PubMed] [Google Scholar]
- 32.Conti P, Caraffa A, Ronconi G, Kritas SK, Mastrangelo F, Tettamanti L, Frydas I, Theoharides TC. Mast cells participate in allograft rejection: Can IL-37 play an inhibitory role? Inflamm Res. 2018;67:747–755. doi: 10.1007/s00011-018-1166-3. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Cai X, Liu S, Wang S, Nold-Petry CA, Nold MF, Bufler P, Norris D, Dinarello CA, Fujita M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111:15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan Q, Zeng Q, Song R, Zhai Y, Xu D, Fullerton DA, Dinarello CA, Meng X. IL-37 suppresses MyD88-mediated inflammatory responses in human aortic valve interstitial cells. Mol Med. 2017;23:83–91. doi: 10.2119/molmed.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo C, Shu Y, Luo J, Liu D, Huang DS, Han Y, Chen C, Li YC, Zou JM, Qin J, et al. Intracellular IL-37b interacts with Smad3 to suppress multiple signaling pathways and the metastatic phenotype of tumor cells. Oncogene. 2017;36:2889–2899. doi: 10.1038/onc.2016.444. [DOI] [PubMed] [Google Scholar]
- 36.Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC, et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 37.Luo P, Peng S, Yan Y, Ji P, Xu J. IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatology (Oxford) 2020;59:3070–3080. doi: 10.1093/rheumatology/keaa192. [DOI] [PubMed] [Google Scholar]
- 38.Kim SK, Choe JY, Park KY. Activation of CpG-ODN-induced TLR9 signaling inhibited by interleukin-37 in U937 human macrophages. Yonsei Med J. 2021;62:1023–1031. doi: 10.3349/ymj.2021.62.11.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Zhu D, Mou T, Guo Z, Pu J, Wu Z. Interleukin-37 induces apoptosis and autophagy of SMMC-7721 cells by inhibiting phosphorylation of mTOR. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:440–445. (In Chinese) [PubMed] [Google Scholar]
- 40.Hou T, Sun X, Zhu J, Hon KL, Jiang P, Chu IM, Tsang MS, Lam CW, Zeng H, Wong CK. IL-37 ameliorating allergic inflammation in atopic dermatitis through regulating microbiota and AMPK-mTOR signaling pathway-modulated autophagy mechanism. Front Immunol. 2020;11(752) doi: 10.3389/fimmu.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MS, Baek AR, Lee JH, Jang AS, Kim DJ, Chin SS, Park SW. IL-37 attenuates lung fibrosis by inducing autophagy and regulating TGF-β1 production in mice. J Immunol. 2019;203:2265–2275. doi: 10.4049/jimmunol.1801515. [DOI] [PubMed] [Google Scholar]
- 42.Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP, Hurkmans E, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:1251–1260. doi: 10.1136/annrheumdis-2018-213585. [DOI] [PubMed] [Google Scholar]
- 43.Ngian GS. Rheumatoid arthritis. Aust Fam Physician. 2010;39:626–628. [PubMed] [Google Scholar]
- 44.Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9(e95346) doi: 10.1371/journal.pone.0095346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia T, Zheng XF, Qian BH, Fang H, Wang JJ, Zhang LL, Pang YF, Zhang J, Wei XQ, Xia ZF, Zhao DB. Plasma interleukin-37 is elevated in patients with rheumatoid arthritis: Its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis Markers. 2015;2015(795043) doi: 10.1155/2015/795043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragab D, Mobasher S, Shabaan E. Elevated levels of IL-37 correlate with T cell activation status in rheumatoid arthritis patients. Cytokine. 2019;113:305–310. doi: 10.1016/j.cyto.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Song L, Wang Y, Sui Y, Sun J, Li D, Li G, Liu J, Li T, Shu Q. High interleukin-37 (IL-37) expression and increased mucin-domain containing-3 (TIM-3) on peripheral T cells in patients with rheumatoid arthritis. Med Sci Monit. 2018;24:5660–5667. doi: 10.12659/MSM.909254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia L, Shen H, Lu J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: Attenuated the production of inflammatory cytokines. Cytokine. 2015;76:553–557. doi: 10.1016/j.cyto.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Zhang J, Tao J, Tao J, Lu T. Elevated serum levels of Interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS. 2015;123:1025–1031. doi: 10.1111/apm.12467. [DOI] [PubMed] [Google Scholar]
- 50.Wan LL, Wang XD, Ci ZC. Expression of TLR4 in peripheral blood of patients with 274 rheumatoid arthritis and its correlation with IL-37 level. World J Complex Med. 2016;2:23–25. [Google Scholar]
- 51.Chen X, Tian J, Zhang J, Su J. Expression and clinical significance of serum IL-37 and soluble PD-1 in patients with rheumatoid arthritis. Chin J Immunol. 2017;33:422–425. [Google Scholar]
- 52.Akram N, Jamal A, Ullah S, Waqar AB, Iqbal K. Expression level of serum interleukin-37 in rheumatoid arthritis patients and its correlation with disease activity score. Adv Life Sci. 2018;5:159–165. [Google Scholar]
- 53.Ke Q, Huang Z, Yu H, et al. Expression and significance of interleukin-37 in PBMCs from rheumatoid arthritis patients. Int J Lab Med. 2020;41:754–757. (In Chinese) [Google Scholar]
- 54.Liu Y, Gao W. Interleukin-37 inhibits proliferation, migration and induces apoptosis of rheumatoid arthritis fibroblast-like synoviocytes (RAFLS) by inhibiting STAT3. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36:236–241. (In Chinese) [PubMed] [Google Scholar]
- 55.Zhu J, Xie C, Qiu H, Shi L. Correlation between level of interleukin-37 and rheumatoid arthritis progression. Int J Gen Med. 2021;14:1905–1910. doi: 10.2147/IJGM.S309436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Barbary AM, Hussein MS, Almedany SH, Rageh EM, Alsalawy AM, Aboelhawa MA, Elkholy RM, Shafik NM, Elharoun AS. Role of interleukin 37 as a novel proangiogenic factor in juvenile idiopathic arthritis. J Clin Rheumatol. 2019;25:85–90. doi: 10.1097/RHU.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 57.Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18:433–448. doi: 10.1007/s10456-015-9477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacDonald IJ, Liu SC, Su CM, Wang YH, Tsai CH, Tang CH. Implications of angiogenesis involvement in arthritis. Int J Mol Sci. 2018;19(2012) doi: 10.3390/ijms19072012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabi EM, Singh A, Althafar ZM, Behl T, Sehgal A, Singh S, Sharma N, Bhatia S, Al-Harrasi A, Alqahtani HM, Bungau S. Elucidating the role of hypoxia-inducible factor in rheumatoid arthritis. Inflammopharmacology. 2022;30:737–748. doi: 10.1007/s10787-022-00974-4. [DOI] [PubMed] [Google Scholar]
- 60.Hu F, Mu R, Zhu J, Shi L, Li Y, Liu X, Shao W, Li G, Li M, Su Y, et al. Hypoxia and hypoxia-inducible factor-1α provoke toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann Rheum Dis. 2014;73:928–936. doi: 10.1136/annrheumdis-2012-202444. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Wu H, Deng R. Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur J Pharmacol. 2021;910(174500) doi: 10.1016/j.ejphar.2021.174500. [DOI] [PubMed] [Google Scholar]
- 62.Ba X, Huang Y, Shen P, Huang Y, Wang H, Han L, Lin WJ, Yan HJ, Xu LJ, Qin K, et al. WTD attenuating rheumatoid arthritis via suppressing angiogenesis and modulating the PI3K/AKT/mTOR/HIF-1α pathway. Front Pharmacol. 2021;12(696802) doi: 10.3389/fphar.2021.696802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J, Su C, Chen Y, Hao X, Jiang J. Electroacupuncture on ST36 and GB39 acupoints inhibits synovial angiogenesis via downregulating HIF-1α/VEGF expression in a rat model of adjuvant arthritis. Evid Based Complement Alternat Med. 2019;2019(5741931) doi: 10.1155/2019/5741931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng X, Chen Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J Drug Target. 2018;26:845–857. doi: 10.1080/1061186X.2018.1433680. [DOI] [PubMed] [Google Scholar]
- 65.Pei B, Xu S, Liu T, Pan F, Xu J, Ding C. Associations of the IL-1F7 gene polymorphisms with rheumatoid arthritis in Chinese Han population. Int J Immunogenet. 2013;40:199–203. doi: 10.1111/iji.12007. [DOI] [PubMed] [Google Scholar]
- 66.Zhang XY, Zuo Y, Li C, Tu X, Xu HJ, Guo JP, Li ZG, Mu R. IL1F7 gene polymorphism is not associated with rheumatoid arthritis susceptibility in the Northern Chinese Han population: A case-control study. Chin Med J (Engl) 2018;131:171–179. doi: 10.4103/0366-6999.222340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi LP, He Y, Liu ZD. Correlation between single nucleotide polymorphism of rs3811047 in IL-1 F7 gene and rheumatoid arthritis susceptibility among Han population in central plains of China. Asian Pac J Trop Med. 2013;6:73–75. doi: 10.1016/S1995-7645(12)60204-1. [DOI] [PubMed] [Google Scholar]
- 68.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, Haroon N, Borenstein D, Wang R, Biehl A, et al. 2019 Update of the American college of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (Hoboken) 2019;71:1285–1299. doi: 10.1002/acr.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fawzy RM, Ganeb SS, Said EA, Fouad NA. Serum level of interleukin-37 and expression of its mRNA in ankylosing spondylitis patients: Possible role in osteoporosis. Egypt J Immunol. 2016;23:19–29. [PubMed] [Google Scholar]
- 70.Chen B, Huang K, Ye L, Li Y, Zhang J, Zhang J, Fan X, Liu X, Li L, Sun J, et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J Transl Med. 2015;13(36) doi: 10.1186/s12967-015-0394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge R, Pan F, Liao F, Xia G, Mei Y, Shen B, Zhang T, Gao J, Zhang L, Duan Z, et al. Analysis on the interaction between IL-1F7 gene and environmental factors on patients with ankylosing spondylitis: A case-only study. Mol Biol Rep. 2011;38:2281–2284. doi: 10.1007/s11033-010-0359-9. [DOI] [PubMed] [Google Scholar]
- 72.Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016;388:2039–2052. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 73.Ding L, Li H, Sun B, Wang T, Meng S, Huang Q, Hong X, Liu D. Elevated interleukin-37 associated with tophus and pro-inflammatory mediators in Chinese gout patients. Cytokine. 2021;141(155468) doi: 10.1016/j.cyto.2021.155468. [DOI] [PubMed] [Google Scholar]
- 74.Liu L, Xue Y, Zhu Y, Xuan D, Yang X, Liang M, Wang J, Zhu X, Zhang J, Zou H. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Res Ther. 2016;18(268) doi: 10.1186/s13075-016-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng M, Dang W, Chen B, Qing Y, Xie W, Zhao M, Zhou J. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin Rheumatol. 2016;35:2251–2258. doi: 10.1007/s10067-015-3109-5. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Zhao T, Yang X, Cao L, Xu R, Liu J, Lin C, Yu Y, Xuan D, Zhu X, et al. IL-37 blocks gouty inflammation by shaping macrophages into a non-inflammatory phagocytic phenotype. Rheumatology (Oxford) 2022;(keac009) doi: 10.1093/rheumatology/keac009. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 77.Onuora S. IL-37 linked to gout pathogenesis and treatment. Nat Rev Rheumatol. 2020;16(250) doi: 10.1038/s41584-020-0410-8. [DOI] [PubMed] [Google Scholar]
- 78.Wan W, Shi Y, Ji L, Li X, Xu X, Zhao D. Interleukin-37 contributes to the pathogenesis of gout by affecting PDZ domain-containing 1 protein through the nuclear factor-kappa B pathway. J Int Med Res. 2020;48(300060520948717) doi: 10.1177/0300060520948717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klück V, van Deuren RC, Cavalli G, Shaukat A, Arts P, Cleophas MC, Crișan TO, Tausche AK, Riches P, Dalbeth N, et al. Rare genetic variants in interleukin-37 link this anti-inflammatory cytokine to the pathogenesis and treatment of gout. Ann Rheum Dis. 2020;79:536–544. doi: 10.1136/annrheumdis-2019-216233. [DOI] [PubMed] [Google Scholar]
- 80.Mandl LA. Osteoarthritis year in review 2018: Clinical. Osteoarthritis Cartilage. 2019;27:359–364. doi: 10.1016/j.joca.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Luo P, Feng C, Jiang C, Ren X, Gou L, Ji P, Xu J. IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif. 2019;52(e12692) doi: 10.1111/cpr.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding L, Hong X, Sun B, Huang Q, Wang X, Liu X, Li L, Huang Z, Liu D. IL-37 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Sci Rep. 2017;7(11601) doi: 10.1038/s41598-017-11397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Geffen EW, van Caam APM, Schreurs W, van de Loo FA, van Lent PLEM, Koenders MI, Thudium CS, Bay-Jensen AC, Blaney Davidson EN, van der Kraan PM. IL-37 diminishes proteoglycan loss in human OA cartilage: Donor-specific link between IL-37 and MMP-3. Osteoarthritis Cartilage. 2019;27:148–157. doi: 10.1016/j.joca.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 84.van Geffen EW, van Caam AP, van Beuningen HM, Vitters EL, Schreurs W, van de Loo FA, van Lent PL, Koenders MI, Blaney Davidson EN, van der Kraan PM. IL37 dampens the IL1β-induced catabolic status of human OA chondrocytes. Rheumatology (Oxford) 2017;56:351–361. doi: 10.1093/rheumatology/kew411. [DOI] [PubMed] [Google Scholar]
- 85.Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med. 2020;172:ITC81–ITC96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 86.Tawfik MG, Nasef SI, Omar HH, Ghaly MS. Serum interleukin-37: A new player in lupus nephritis? Int J Rheum Dis. 2017;20:996–1001. doi: 10.1111/1756-185X.13122. [DOI] [PubMed] [Google Scholar]
- 87.Godsell J, Rudloff I, Kandane-Rathnayake R, Hoi A, Nold MF, Morand EF, Harris J. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep. 2016;6(34604) doi: 10.1038/srep34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song L, Qiu F, Fan Y, Ding F, Liu H, Shu Q, Liu W, Li X. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33:111–117. doi: 10.1007/s10875-012-9791-z. [DOI] [PubMed] [Google Scholar]
- 89.Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu T, Chen B, Zhang J, Ding L, et al. IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: Its correlate with disease activity. J Transl Med. 2014;12(69) doi: 10.1186/1479-5876-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu GC, Li HM, Wang JB, Leng RX, Wang DG, Ye DQ. Elevated plasma interleukin-37 levels in systemic lupus erythematosus patients. Lupus. 2016;25:1377–1380. doi: 10.1177/0961203316646462. [DOI] [PubMed] [Google Scholar]
- 91.Wu Q, Zhou J, Yuan ZC, Lan YY, Xu WD, Huang AF. doi: 10.1080/08820139.2020.1869254. Association between IL-37 and systemic lupus erythematosus risk. Immunol Invest: Jan 17, 2021 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 92.Bowman SJ. Primary Sjögren's syndrome. Lupus. 2018;27 (1 Suppl):S32–S35. doi: 10.1177/0961203318801673. [DOI] [PubMed] [Google Scholar]
- 93.Liuqing W, Liping X, Hui S, Jing L. Elevated IL-37, IL-18 and IL-18BP serum concentrations in patients with primary Sjögren's syndrome. J Investig Med. 2017;65:717–721. doi: 10.1136/jim-2016-000301. [DOI] [PubMed] [Google Scholar]
- 94.Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, Gaudric J, Gul A, Kötter I, Leccese P, et al. 2018 Update of the EULAR recommendations for the management of Behçet's syndrome. Ann Rheum Dis. 2018;77:808–818. doi: 10.1136/annrheumdis-2018-213225. [DOI] [PubMed] [Google Scholar]
- 95.Ben Dhifallah I, Borhani-Haghighi A, Hamzaoui A, Hamzaoui K. Decreased level of IL-37 correlates negatively with inflammatory cytokines in cerebrospinal fluid of patients with neuro-Behcet's disease. Iran J Immunol. 2019;16:299–310. doi: 10.22034/IJI.2019.80281. [DOI] [PubMed] [Google Scholar]
- 96.Ye Z, Wang C, Kijlstra A, Zhou X, Yang P. A possible role for interleukin 37 in the pathogenesis of Behcet's disease. Curr Mol Med. 2014;14:535–542. doi: 10.2174/1566524014666140414210831. [DOI] [PubMed] [Google Scholar]
- 97.Bouali E, Kaabachi W, Hamzaoui A, Hamzaoui K. Interleukin-37 expression is decreased in Behçet's disease and is associated with inflammation. Immunol Lett. 2015;167:87–94. doi: 10.1016/j.imlet.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Kacem O, Kaabachi W, Dhifallah IB, Hamzaoui A, Hamzaoui K. Elevated expression of TSLP and IL-33 in Behçet's disease skin lesions: IL-37 alleviate inflammatory effect of TSLP. Clin Immunol. 2018;192:14–19. doi: 10.1016/j.clim.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 99.Tan H, Deng B, Yu H, Yang Y, Ding L, Zhang Q, Qin J, Kijlstra A, Chen R, Yang P. Genetic analysis of innate immunity in Behcet's disease identifies an association with IL-37 and IL-18RAP. Sci Rep. 2016;6(35802) doi: 10.1038/srep35802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ, Grainger J, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhan Y, Cheng L, Wu B, Ji L, Chen P, Li F, Cao J, Ke Y, Yuan L, Min Z, et al. Interleukin (IL)-1 family cytokines could differentiate primary immune thrombocytopenia from systemic lupus erythematosus-associated thrombocytopenia. Ann Transl Med. 2021;9(222) doi: 10.21037/atm-20-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Z, Qu W, Wang HQ, Xing LM, Wu YH, Liu ZY, Zhang Y, Liu H, Dong XF, Tao JL, Shao ZH. Relationship of peripheral blood IL-37 expression with T lymphocytes subsets and NK cells in patients with primary immune thrombocytopenia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27:1201–1207. doi: 10.19746/j.cnki.issn.1009-2137.2019.04.034. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 103.Liu L, Feng K, Wang ML, Shu XH, Zhou KS, Zhou H, Liu XJ, Song YP. Expression of IL-37 in peripheral blood of adults with primary immune thrombocytopenia. Zhonghua Xue Ye Xue Za Zhi. 2017;38:628–631. doi: 10.3760/cma.j.issn.0253-2727.2017.07.016. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olek MJ. Multiple sclerosis. Ann Intern Med. 2021;174:ITC81–ITC96. doi: 10.7326/AITC202106150. [DOI] [PubMed] [Google Scholar]
- 105.Farrokhi M, Rezaei A, Amani-Beni A, Etemadifar M, Kouchaki E, Zahedi A. Increased serum level of IL-37 in patients with multiple sclerosis and neuromyelitis optica. Acta Neurol Belg. 2015;115:609–614. doi: 10.1007/s13760-015-0491-3. [DOI] [PubMed] [Google Scholar]
- 106.Kouchaki E, Tamtaji OR, Dadgostar E, Karami M, Nikoueinejad H, Akbari H. Correlation of serum levels of IL-33, IL-37, soluble form of vascular endothelial growth factor receptor 2 (VEGFR2), and circulatory frequency of VEGFR2-expressing cells with multiple sclerosis severity. Iran J Allergy Asthma Immunol. 2017;16:329–337. [PubMed] [Google Scholar]
- 107.Giacoppo S, Thangavelu SR, Diomede F, Bramanti P, Conti P, Trubiani O, Mazzon E. Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB J. 2017;31:5592–5608. doi: 10.1096/fj.201700524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schauer AE, Klassert TE, von Lachner C, Riebold D, Schneeweiß A, Stock M, Müller MM, Hammerschmidt S, Bufler P, Seifert U, et al. IL-37 causes excessive inflammation and tissue damage in murine pneumococcal pneumonia. J Innate Immun. 2017;9:403–418. doi: 10.1159/000469661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McKie EA, Reid JL, Mistry PC, DeWall SL, Abberley L, Ambery PD, Gil-Extremera B. A study to investigate the efficacy and safety of an anti-interleukin-18 monoclonal antibody in the treatment of type 2 diabetes mellitus. PLoS One. 2016;11(e0150018) doi: 10.1371/journal.pone.0150018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Argiriadi MA, Xiang T, Wu C, Ghayur T, Borhani DW. Unusual water-mediated antigenic recognition of the proinflammatory cytokine interleukin-18. J Biol Chem. 2009;284:24478–24489. doi: 10.1074/jbc.M109.023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabay C, Fautrel B, Rech J, Spertini F, Feist E, Kötter I, Hachulla E, Morel J, Schaeverbeke T, Hamidou MA, et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Ann Rheum Dis. 2018;77:840–847. doi: 10.1136/annrheumdis-2017-212608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nnane I, Frederick B, Yao Z, Raible D, Shu C, Badorrek P, van den Boer M, Branigan P, Duffy K, Baribaud F, et al. The first-in-human study of CNTO 7160, an anti-interleukin-33 receptor monoclonal antibody, in healthy subjects and patients with asthma or atopic dermatitis. Br J Clin Pharmacol. 2020;86:2507–2518. doi: 10.1111/bcp.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.