Abstract
The emergence of carbapenem-resistant organisms posed considerable threat to global health while only limited treatment options are available and led to efforts to discover a novel way to treat them. To evaluate in vitro synergistic activity of meropenem plus ertapenem, a total of 203 carbapenem-resistant strains, collected from 12 provinces and municipalities in China, were examined with a dual carbapenem combination therapy. The statistical software R was used for analysis. Two hundred and one (201) of carbapenem-resistant strains mainly produced four types of carbapenemase: KPC-2 (n = 142, 69.95%), OXA-232 (n = 7, 3.45%), NDM (n = 38, 18.72%; 36 NDM-1, 1 NDM-4, 1 NDM-5), and IMP (n = 15, 7.39%; 1 IMP-26, 10 IMP-30, 4 IMP-4). Fifty-one out of two hundred and three (51/203 or 25.12%) of the examined strains showed a synergistic effect for the meropenem plus ertapenem combination throughout the checkerboard method, while only three isolates showed potential clinically relevant synergy (3/203, 1.48%). An additive effect was observed in 55/203 (27.09%) of the examined strains. Ninety-seven of the examined isolates (47.78%) showed fractional inhibitory concentration (FIC) greater or equal to 2 (indicating antagonism). The synergistic activity of meropenem plus ertapenem combination suggests this combination can be a possible way to treat the infection caused by the carbapenem-resistant organisms, especially for IMP or NDM producer with a lesser minimum inhibitory concentration (MIC) and the infected individual who was not recommended to use colistin or tigecycline.
Keywords: Carbapenem-resistant, Double-carbapenem regimen, Combination, Synergistic activity, In vitro
1. Introduction
The prevalence of multidrug-resistant (MDR) bacteria which cause significantly high morbidity and mortality rates continued to increase on a global basis (Siegel et al., 2007). Carbapenems, such as ertapenem, meropenem, doripenem and imipenem, were considered as the last-line antibiotics used in clinical settings for the treatment of MDR pathogen infections. However, with the continuous consumption of carbapenems, carbapenem-resistant Gram-negative bacteria (CR-GNB) such as Pseudomonas aeruginosa (P. aeruginosa), Acinetobacter baumannii (A. baumannii) and Enterobacterales were constantly increasing (Chen et al., 2011, Kizny Gordon et al., 2017, Zhang et al., 2018). CR-GNB frequently cause severe infections leading to high mortality rate. For example, approximately 32% patients of bloodstream infections, which were caused by carbapenem-resistant Enterobacterales (CRE), died within 14 days (Tamma et al., 2017).
Carbapenem resistance in Gram-negative bacteria is mainly due to intrinsic and/or acquired resistant mechanisms including the changes of bacterial penicillin-binding proteins, enhanced efflux pump and expression, alteration in the outer membrane proteins, and production of various carbapenemases (Meletis, 2016, Codjoe and Donkor, 2017). Among them, the production of carbapenemases is the most important clinically since carbapenemases are able to hydrolyze essentially all beta-lactams antibiotics (Queenan and Bush, 2007). Also, the transferability of carbapenemase genes which were encoded on plasmids or transposons significantly expanded their host range and posed serious threat to public health (Meletis, 2016). Carbapenemases are commonly categorized using the Ambler classification scheme, into molecular class A, B, and D β-lactamases (Ambler, 1980). The KPC type (class A), NDM, VIM and IMP types (class B) and OXA-48 (class D) carbapenemases are among the most important carbapenemases in terms of carbapenem hydrolysis activity and geographical dissemination (Poirel et al., 2012, Meletis, 2016).
While carbapenem resistance was at relatively low prevalence among P. aeruginosa and A. baumannii, the prevalence among the Enterobacterales was growing, leading to the concern for a potential rise of untreatable community-acquired infections (Tangden and Giske, 2015). Imipenem/relebactam, meropenem/vaborbactam, ceftazidime/avibactam, approved to treat CR-GNB infections currently, are capable of hydrolysing Ambler class A and C enzymes but are not effective against class B/D β-lactamases (Wong and van Duin, 2017, Yu et al., 2021, Jean et al., 2022). Ceftolozane/tazobactam have been approved for the treatment of infections caused by MDR. However, recent studies reported that only 1.9–7.1% of CR-GNB were susceptible to ceftolozane-tazobactam (Yin et al., 2019). Cefiderocol is a newly approved, siderophore cephalosporin with potent in vitro activity against CR-GNB, while limited clinical data were available for the in vitro efficacy of cefiderocol against CR-GNB in China to date. Antibiotics, including colistin, tigecycline, gentamicin and fosfomycin, remain effective for the treatment of infections caused by CR-GNB, while the renal toxicity and poor diffusion in the urinary tract of antibiotics such as colistin and tigecycline constrain their clinical application (Karaiskos and Giamarellou, 2014, Trecarichi and Tumbarello, 2017).
Alternatively, pioneer studies have demonstrated that combinations of multiple carbapenems, such as ertapenem plus either doripenem, meropenem or imipenem, provided a synergistic efficacy over monotherapies, against CR-GNB infections in vitro and in animal studies (Bulik and Nicolau, 2011, Ceccarelli et al., 2013, Giamarellou et al., 2013). Combinations therapy of ertapenem with another carbapenem were prompted by the evidence that ertapenem, as a suicide antibiotic, could bind to the active site of carbapenemase in a high affinity which further prevented the hydrolysis of the other carbapenem molecule and preserved its bactericidal activity. To our knowledge, the effectiveness of dual carbapenem combinations have only been tested in vitro among carbapenem-resistant Klebsiella pneumoniae and A. baumannii (Poirel et al., 2015, Nordmann et al., 2020). Here, we conducted a comprehensive study to evaluate the in vitro activity of double carbapenem combination with meropenem and ertapenem against a collection of CR-GNB strains encoding diverse carbapenemases.
2. Materials and methods
2.1. Strain collection
CRE are a type of Enterobacterales that test resistant to at least one of the carbapenem antibiotics (ertapenem, meropenem, doripenem or imipenem) or produce a carbapenemase. According to this definition based on CDC, a collection of 203 clonally unrelated clinical CR-GNB isolates with a meropenem MIC ≥ 4 μg/mL, or an ertapenem MIC ≥ 2 μg/mL were included in this study (Table S1). These strains were isolated in 2018 from intestinal tract (n = 40, 19.70%) and respiratory tract (n = 163, 80.30%) specimens from ICU departments in hospitals located in 12 provinces and municipalities in China (Anhui, Chongqing, Fujian, Henan, Hunan, Jiangxi, Jilin, Liaoning, Shanghai, Tianjin, Yunnan and Zhejiang). All strains have been previously identified using the matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany) and fully characterized for their carbapenemase genes by PCR and sequencing (Poirel et al., 2011).
2.2. Checkerboard synergy testing and statistical analysis
Checkerboard synergy testing was performed with the broth microdilution assay as described previously (Elemam et al., 2010). Graded concentrations of meropenem and ertapenem were mixed, with each antibiotic ranging from 0.5–8 μg/mL and 0.5–32 μg/mL, respectively. Clinical and Laboratory Standards Institute-recommended interpretive breakpoints were used for all categorical interpretations (CLSI, 2020). The experiment was conducted in duplicate.
Fractional inhibitory concentration (FIC) indexes were calculated according to the formula ΣFIC = FIC of drug A + FIC of drug B, where FIC of drug A/B = MIC of drug A/B in combination divided by the MIC of drug A/B alone (Poirel et al., 2015). The lowest FIC index value (FICI-MIN) in each checkerboard array was used to determine whether the combination was synergistic. The results was interpreted based on the following: FIC values of ≤0.5 indicate complete synergy, FIC values of >0.5–1 indicate partial synergy, FIC values of 1 (inclusive) to 2 indicate additivity and FIC values of ≥2 indicate antagonism (Oliva et al., 2016). If the FICI-MINs of both antibiotics were within the susceptible or intermediate category, potential clinically relevant synergy was considered.
2.3. Data analysis
Statistical analysis was performed using R software v3.6.1 (R Foundation, Vienna, Austria). Categorical variables were compared by using the Fisher's exact test. Multiple comparison after Fisher's exact test was performed using the Bonferroni method.
2.4. Ethics statement
The study was approved by the Ethics Committee of Second Affiliated Hospital, Zhejiang University School of Medicine (2019–074). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
2.5. Biosafety statement
All concerns related to the safe and appropriate use of human-derived materials and infectious agents were approved by the Institutional Biosafety Committee of Second Affiliated Hospital of Zhejiang University, School of Medicine. All experiments were conducted under the guidelines from the Biological Agent Reference Sheet.
3. Results
3.1. Overview of the clinical isolates
The 203 CR-GNB isolates included 134 K. pnenmoniae, 7 K. aerogenes, 1 K. michiganensis, 30 E. coli, 2 C. freundii, 2 C. koseri, 4 E. hormaechei, 1 P. rettgeri, 10 P. aeruginosa, 10 S. marcescens and 2 R. planticola (Table S1). They produced four main types of carbapenemase, including KPC-2 (n = 142, 69.95%), OXA-232 (n = 7, 3.45%), NDM (n = 38, 18.72%; 36 NDM-1, 1 NDM-4, 1 NDM-5) and IMP (n = 15, 7.39%; 1 IMP-26, 10 IMP-30, 4 IMP-4), with 1 K. michiganensis co-producing KPC-2 and NDM-1 and 2 isolates (1 K. aerogenes and 1 K. pnenmoniae) carried no carbapenemase gene (Table 1). The remaining 6 K. aerogenes and all the S. marcescens and P. aeruginosa contained KPC-2 while the E. coli included in this study contained three types of carbapenemase (IMP-26, KPC-2, NDM). The K. pnenmoniae included in this study contained the most gene (IMP, KPC, NDM, OXA), and other examined strains contained KPC or NDM.
Table 1.
Species and carriage of carbapenemase genes of clinical carbapenem-resistant Gram-negative isolates in this study.
| Bacterial species | Total no. (percentage) | No. (percentage) of blaKPC-2-positive strains | No. (percentage) of blaNDM-positive strains |
No. (percentage) of blaOXA-232-positive strains | No. (percentage) of blaIMP-positive strains |
||||
|---|---|---|---|---|---|---|---|---|---|
| blaNDM-1 | blaNDM-4 | blaNDM-5 | blaIMP-4 | blaIMP-26 | blaIMP-30 | ||||
| E. coli | 30 (14.78%) | 17 (8.37%) | 11 (5.42%) | 0 | 1 (0.49%) | 0 | 0 | 1 (0.49%) | 0 |
| K. aerogenes | 7 (3.45%) | 6 (2.96%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K. pnenmoniae | 134 (66.01%) | 93 (45.81%) | 18 (8.87%) | 1 (0.49%) | 0 | 7 (3.45%) | 4 (1.97%) | 0 | 10 (4.93%) |
| P. aeruginosa | 10 (4.93%) | 10 (4.93%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. marcescens | 10 (4.93%) | 10 (4.93%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Othersa | 12 (5.91%) | 6 (2.96%) | 7 (3.45%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 203 | 142 (69.95%) | 36 (17.73%) | 1 (0.49%) | 1 (0.49%) | 7 (3.45%) | 4 (1.97%) | 1 (0.49%) | 10 (4.93%) |
Others included K. michiganensis, E. hormaechei, P. rettgeri, R. planticola, C. freundii and C. koseri.
3.2. Overview of the in vitro experiment outcomes
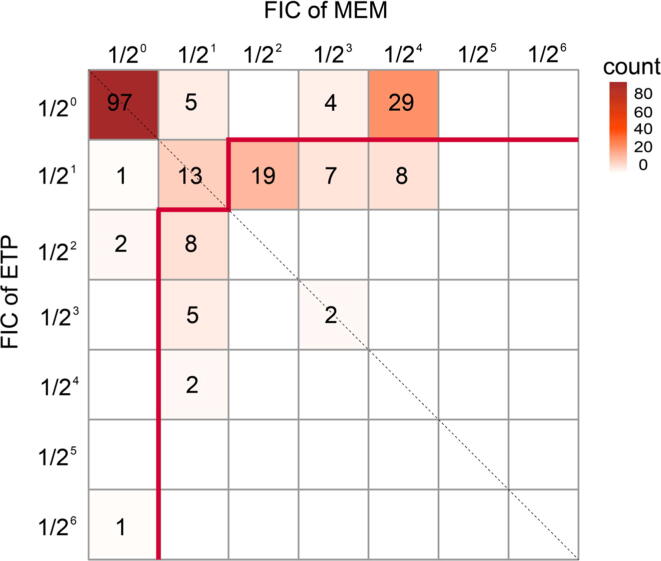
Antimicrobial susceptibility testing for the 203 CR-GNB showed that all isolates were resistant to meropenem or ertapenem. The isolate co-producing KPC-2 and NDM-1 showed high MICs of both carbapenems, which were 8 mg/L for meropenem and 32 mg/L for ertapenem, respectively (Table S1). FIC of drug meropenem, FIC of drug ertapenem and the ΣFIC for all the isolates were presented in Table S1. In vitro synergistic activity was systematically observed in 51 (25.12%) isolates, including 1 (0.49%) complete synergy and 50 (24.63%) partial synergy. Among these, only 8 isolates showed potential clinically relevant synergy (3.94%). Additivity was observed in 55 (27.09%) strains. Antagonism (defined by a FIC ≥ 2) was noticed with 97 (47.78%) of the examined isolates (Fig. 1). Intriguingly, all isolates exhibiting antagonism were highly resistant to both meropenem and ertapenem, with MICs ≥ 8 mg/L and 32 mg/L, respectively.
Fig. 1.
Total fractional inhibitory concentrations (ΣFIC) of meropenem and ertapenem for carbapenem-resistant Gram-negative bacteria (CR-GNB). ΣFIC in each box was calculated by adding the value of the horizontal axis (FIC of meropenem) and that of the longitudinal axis (FIC of ertapenem). The color of each box and the number in each box represent the total count of the isolates with the corresponding ΣFIC. The area below the red line represents isolates that performed complete or partial synergy (ΣFIC<1) in the checkerboard synergy testing. The area above the red line represents isolates that exhibited antagonism (ΣFIC = 2, top-left box) and additivity (1 ≤ ΣFIC < 2, the remaining boxes).
3.3. Evaluation of meropenem-ertapenem combination on different CR-GNB species
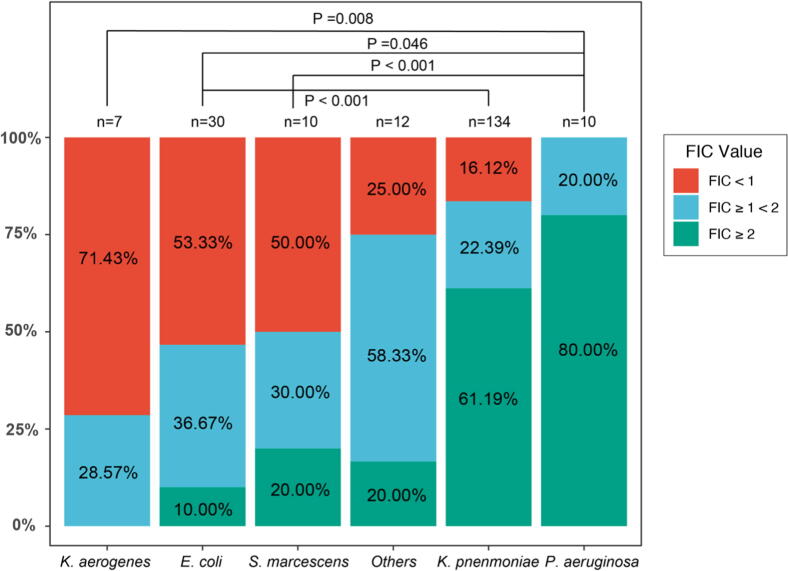
The 51 isolates that exhibited synergistic activity with meropenem-ertapenem combination included 16 (53.33%) E. coli, 22 (16.12%) K. pnenmoniae, 5 (71.43%) K. aerogenes, 1 (25.00%) E. hormaechei, 1 (50.00%) C. koseri, 5 (50.00%) S. marcescens and 1 (50.00%) R. planticola. Additivity was observed among 2 (28.57%) K. aerogenes, 11 (36.67%) E. coli, 30 (22.39%) K. pnenmoniae, 2 (100.00%) C. freundii, 3 (75.00%) E. hormaechei, 1 (50.00%) C. koseri, 2 (20.00%) P. aeruginosa, 3 (30.00%) S. marcescens and 1 (50.00%) R. planticola. Isolates among which antagonism was noticed included 3 (10.00%) E. coli, 82 (61.19%) K. pnenmoniae, 8 (80.00%) P. aeruginosa, 2 (20.00%) S. marcescens, 1 (100.00%) P. rettgeri and 1 K. michiganensi. Pairwise comparison of the checkerboard synergy testing results indicated the proportion of isolates exhibiting synergy, additivity and antagonism differed significantly among different bacterial species (P < 0.05). Such divergence was observed between isolates of K. aerogenes and P. aeruginosa (P = 0.008), E. coli and P. aeruginosa (P = 0.046), E. coli and K. pnenmoniae (P < 0.001), and S. marcescens and P. aeruginosa (P < 0.001) (Fig. 2).
Fig. 2.
Distribution of FIC values in different bacterial species. Horizontal axis represents the species of isolates. Others in the horizontal axis include species K. michiganensis, E. hormaechei, P. rettgeri, R. planticola, C. freundii and C. koseri, for each of which <5 strains were tested. The red, blue and green boxes represented the FIC values of <1, 1 (inclusive) ∼2 and ≥2, respectively. The longitudinal axis is a proportional scaler. P value calculated between species pairs that exhibited significant difference (P < 0.05) in the checkerboard synergy testing were labeled on the top.
3.4. Evaluation of meropenem-ertapenem combination on different carbapenemase genes
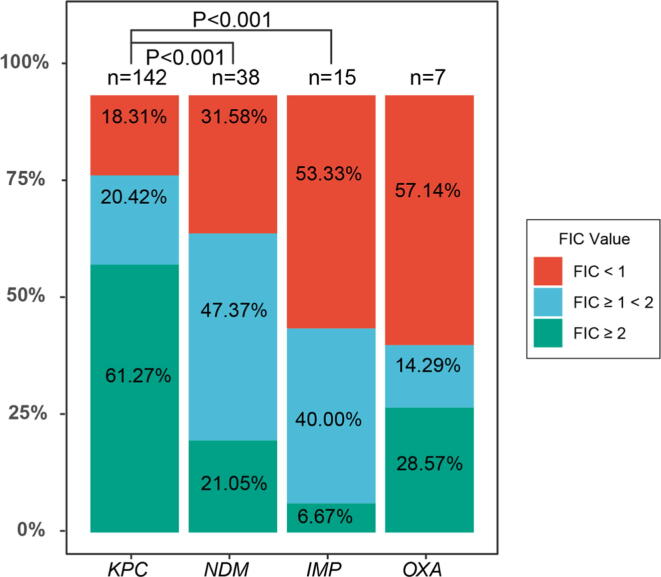
Synergistic activity of meropenem-ertapenem combination was observed in 8 (53.33%) IMP-producing, 26 (18.31%) KPC-producing, 12 (31.58%) NDM-producing and 4 (57.14%) OXA-producing isolates. On the other hand, 6 (40.00%) IMP-producing, 29 (20.42%) KPC-producing, 18 (47.37%) NDM-producing and 1 (14.29%) OXA-producing isolates displayed additive activity. Antagonism was noticed in 1 (6.67%) IMP-producing, 87 (61.72%) KPC-producing, 8 (21.05%) NDM-producing and 2 (28.57%) OXA-producing isolates. The proportion of isolates exhibiting synergy, additivity and antagonism differed significantly among isolates producing KPC and NDM, and KPC and IMP (P < 0.001) (Fig. 3). For KPC, E. coli, K. pnenmoniae, P. aeruginosa as well as S. marcescens exhibited synergistic activity; K. aerogenes, E. coli, K. pnenmoniae, C. freundii and P. aeruginosa exhibited additivity; antagonistic effect was noticed in K. aerogenes, E. coli, K. pnenmoniae, C. freundii, E. hormaechei, C. koseri, P. aeruginosa, S. marcescens and K. michiganensis. For NDM, synergistic activity was only noticed in E. coli and K. pnenmoniae; E. coli, K. pnenmoniae, E. hormaechei, P. rettgeri as well as R. planticola exhibited additivity; while K. michiganensis, E. coli, K. pnenmoniae, E. hormaechei and R. planticola exhibited antagonistic. For IMP, synergistic activity was only noticed in E. coli and K. pnenmoniae; additive and antagonistic effect was only noticed in K. pnenmoniae. As for OXA, due to the limited number of samples included in this study, synergistic activity, additive or antagonistic was only displayed in K. pnenmoniae.
Fig. 3.
Distribution of FIC values in isolates producing different carbapenemase. Horizontal and longitudinal axes represent the type of carbapenemase and the proportional scaler, respectively. The red, blue and green boxes represented the FIC values of <1, 1 (inclusive) ∼2 and ≥2, respectively. P < 0.05 means that there was statistical difference. P value calculated between carbapenemase pairs that exhibited significant difference (P < 0.05) in the checkerboard synergy testing were labeled on the top.
4. Discussion
The widespread of CR-GNB isolates have constituted a major public health problem (Zhang et al., 2018). Although there are a few novel agents such as ceftazidime/avibactam, imipenem/relebactam, and meropenem/vaborbactam, they are not universally active against all CR-GNB and are cost-prohibitive or are not readily available in certain settings (Wong and van Duin, 2017, Yu et al., 2021, Jean et al., 2022). More importantly, KPC-producing K. pneumoniae isolates resistant to ceftazidime/avibactam have been reported recently, including isolates with emergence of resistance following exposure to this agent (Shi et al., 2020). Currently, the majority of clinical research concluded that the combination treatment with two or more antimicrobials was a preferred approach for infections caused by CR-GNB isolates (Trecarichi and Tumbarello, 2017, Papst et al., 2018). The phenomenon of antimicrobial synergy is that two agents exert better than additive activity when use them together, and may offer the ability to treat pathogens which are resistant to all now available and acceptable therapies. Meropenem, colistin, tigecycline, aminoglycosides and fosfomycin are the most commonly used agents as part of a combination regimen, among them colistin/tigecycline-containing combinations displays a high level of synergism, especially with regard to colistin + tigecycline (Oliva et al., 2017, Paul et al., 2018, Papoutsaki et al., 2020). Unfortunately, toxicity, poor penetration in some tissues and emergence of resistance may raise the concerns when in polymyxin and tigecycline using (Pogue et al., 2011, Satlin et al., 2011). Furthermore, the emergence of MCR or tet(X)-harboring plasmid leads a great challenge in the treatment of CR-GNB infections after the extensive use of these two antibiotics (Chen et al., 2019, He et al., 2019). Recently, combinations of two synergistic carbapenems (ertapenem plus either meropenem, imipenem or doripenem) has been suggested as the rescue treatment for CR-GNB infections (Bulik and Nicolau, 2011, Ceccarelli et al., 2013, Giamarellou et al., 2013). The effectiveness of combinations was validated predominantly with retrospective clinical studies, with only a few studies focusing on in vitro evaluations (De Pascale et al., 2017, Souli et al., 2017, Piedra-Carrasco et al., 2018). Besides, the majority of previous in vitro studies had focused on CRE, leaving the effect of dual carbapenem combinations on non-Enterobacterales being scarcely understood. We thus conducted this work to comprehensively study the in vitro activity of dual carbapenem combinations (meropenem plus ertapenem) against diverse CR-GNB, since doripenem is not used as frequently as meropenem, aiming to provide supporting data for future clinical treatments.
The percentage of CR-GNB isolates exhibiting synergy, additivity and antagonism with the dual carbapenem combination was 25.12%, 27.09% and 47.78%, respectively. All isolates showing antagonism were highly resistant to meropenem and ertapenem. In other words, this may be one of the reasons that the dual carbapenem combination was ineffective for such isolates, among which KPC take a large proportion (87/97, 89.69%). This phenomenon also coordinates with the indication from a recent study that the significant favorable impact was observed during the combinations only if meropenem MIC was ≤8 mg/L (Tumbarello et al., 2012). However, our data disagree with the result observed by Brennan-Krohn et al. (2017), who noticed that meropenem/ertapenem combination may not provide reliable benefit.
Significant heterogeneity in synergistic testing was observed against different strains, which was consistent with the previous study (Brennan-Krohn et al., 2017). The difference was statistically significant between K. aerogenes and P. aeruginosa, E. coli and P. aeruginosa, S. marcescens and P. aeruginosa, as well as E. coli and K. pnenmoniae (P < 0.05). That is to say the synergy rate of the dual carbapenem combination in K. aerogenes, E. coli as well as S. marcescens was much higher than that in P. aeruginosa; the synergy rate in E. coli was much higher than that in K. pnenmoniae.
Analysis of the synergies among four main types of carbapenemase showed the statistical differences between KPC and IMP; KPC and NDM, indicating that the synergy rate in IMP was much higher than that in KPC, and that the synergy rate in NDM was much higher than that in KPC. This phenome might due to the mechanism of action of metallo-β-lactamases, which differs significantly from that of serine-based carbapenemases (Ambler 1980). However, these results disagree with the observations made by Poirel et al. (2015), who noticed that synergies were frequently observed with KPC producers, and to a lesser extent with OXA-48 producers, while not with NDM (a metallo-β-lactamase) producers. This might be because of the different prevalence of CR-GNB genotypes in China.
The mechanism with which the combination of ertapenem and meropenem works is that ertapenem, binding with high affinity to the active site of carbapenemase, would prevent the hydrolysis of the meropenem, which can preserve its bactericidal activity against the pathogen (Poirel et al., 2015). The larger ertapenem consumes the carbapenemase, the higher concentration of the associated drug will be active in the infection site, which also means a lower amount of meropenem will be available to degrade the administered antibiotic. This principle was also confirmed in our study (Fig. 1). The total counts on the right of dotted line were much larger than that on the left of dotted line, in other words meropenem was decreased more folds than ertapenem.
Overall, the comparatively low synergy rate in our results and the observations made by De Pascale et al. (2017) were not perfectly aligned, who hold that the association of ertapenem plus meropenem provides a survival benefit, and with Souli et al. (2017), who noticed a successful outcome of the meropenem/ertapenem combination applied for 27 patients infected with a KPC-2-producing K. pneumoniae. These two completely opposite results were likely because of the different carbapenemase enzyme subgroups and the selection of highly resistant strains in this research. In addition, human immune system may also play an important role in those CR-GNB infected patients and lead a successful outcome after the dual carbapenem regimen. Although only 25.12% of the isolates in this study were synergy (FIC < 1), over half of them showed at least one fold decrease in either meropenem or ertapenem. Hence, caution has to be used when double-carbapenem therapy was considered as an alternative therapy for CRE infection, especially when these pathogenic strains showed high-level resistance to meropenem or ertapenem.
However, there are still some limitations in the current study. First, checkerboard array testing does not provide bactericidal or time–kill data, which may more accurately reflect in vivo synergy. Second, only 203 clinical CR-GNB isolates were included in this study while the number of different kinds of strains was in a large disparity. Third, MLST analysis was not involved in this research, therefore meropenem-ertapenem combination on different epidemic strains is yet unknow.
In conclusion, we demonstrated the in vitro synergistic effect of the combination of meropenem with ertapenem, which can be an alternative regimen in patients for whom the use of colistin or tigecycline was not recommended because of potential nephrotoxicity or resistance and the infection caused by CRE with lesser MIC. When the MIC of meropenem or ertapenem for CRE was many folds higher than the breakpoint, this combination may not be effective. In vitro synergy tests also can be a referable way to perform in cases of infections due to CRE to select the best concentration in antimicrobial combinations to guide the clinical prescription.
Declaration of Competing Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This work was supported by National Natural Science Foundation of China (81772250, 81861138052 and 31761133004).
Author contributions
JL and YQ did strain characterization and participated in manuscript writing. ND and CL did statistical analysis. YZ and QS participated in collecting the checkerboard synergy test data. QS, LH, YW and HZ did checkerboard synergy testing. ZS participated in the research design, data interpretation, and manuscript writing. RZ designed and supervised the study, interpreted the data and wrote the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2022.03.007.
Contributor Information
Zhangqi Shen, Email: szq@cau.edu.cn.
Rong Zhang, Email: zhang-rong@zju.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ambler R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Brennan-Krohn T., Truelson K.A., Smith K.P., Kirby J.E. Screening for synergistic activity of antimicrobial combinations against carbapenem-resistant Enterobacteriaceae using inkjet printer-based technology. J. Antimicrob. Chemother. 2017;72(10):2775–2781. doi: 10.1093/jac/dkx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik C.C., Nicolau D.P. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011;55:3002–3004. doi: 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli G., Falcone M., Giordano A., Mezzatesta M.L., Caio C., Stefani S., Venditti M. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013;57(6):2900–2901. doi: 10.1128/AAC.00188-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-W., Tang H.-J., Chen C.-C., Lu Y.-C., Chen H.-J., Su B.-A., Weng T.-C., Chuang Y.-C., Lai C.-C. The Microbiological Characteristics of Carbapenem-Resistant Enterobacteriaceae Carrying the mcr-1 Gene. J. Clin. Med. 2019;8(2):261. doi: 10.3390/jcm8020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhou Z., Jiang Y., et al. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Glob. Antimicrob. Resist. 2011;66:1255–1259. doi: 10.1016/j.jgar.2018.07.008. [DOI] [PubMed] [Google Scholar]
- CLSI . CLSI; Wayne, PA: 2020. Performance Standards for Antimicrobial Susceptibility Testing. M100-S30. [Google Scholar]
- Codjoe F., Donkor E. Carbapenem Resistance: A Review. Med. Sci. (Basel) 2017;6(1):1. doi: 10.3390/medsci6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascale G., Martucci G., Montini L., Panarello G., Cutuli S.L., Di Carlo D., Di Gravio V., Di Stefano R., Capitanio G., Vallecoccia M.S., Polidori P., Spanu T., Arcadipane A., Antonelli M. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: a two-center, matched case–control study. Crit. Care. 2017;21(1) doi: 10.1186/s13054-017-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemam A., Rahimian J., Doymaz M. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 2010;48:3558–3562. doi: 10.1128/jcm.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H., Galani L., Baziaka F., Karaiskos I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013;57(5):2388–2390. doi: 10.1128/AAC.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T., Wang R., Liu D., Walsh T.R., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., Shen Y., Wang G., Sun L., Lei L., Lv Z., Li Y., Pang M., Wang L., Sun Q., Fu Y., Song H., Hao Y., Shen Z., Wang S., Chen G., Wu C., Shen J., Wang Y. Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4(9):1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- Jean S.-S., Lu M.-C., Ho M.-W., et al. Non-susceptibilities to antibiotics against important Gram-negative bacteria, and imipenem-relebactam, meropenem-vaborbactam against carbapenem non-susceptible Enterobacterales and Pseudomonas aeruginosa isolates implicated in complicated intra-abdominal and urinary tract infections in Taiwan, 2019. Int. J. Antimicrobial Agents. 2022:106521. doi: 10.1016/j.ijantimicag.2022.106521. [DOI] [PubMed] [Google Scholar]
- Karaiskos I., Giamarellou H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin. Pharmacother. 2014;15:1351–1370. doi: 10.1517/14656566.2014.914172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizny Gordon A.E., Mathers A.J., Cheong E.Y.L., Gottlieb T., Kotay S., Walker A.S., Peto T.E.A., Crook D.W., Stoesser N. The Hospital Water Environment as a Reservoir for Carbapenem-Resistant Organisms Causing Hospital-Acquired Infections-A Systematic Review of the Literature. Clin. Infect. Dis. 2017;64(10):1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016;3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Perler J., Kieffer N., Poirel L. In-vitro evaluation of a dual carbapenem combination against carbapenemase-producing Acinetobacter baumannii. J. Infect. 2020;80(1):121–142. doi: 10.1016/j.jinf.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Oliva A., Gizzi F., Mascellino M.T., Cipolla A., D'Abramo A., D'Agostino C., Trinchieri V., Russo G., Tierno F., Iannetta M., Mastroianni C.M., Vullo V. Bactericidal and synergistic activity of double-carbapenem regimen for infections caused by carbapenemase-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2016;22(2):147–153. doi: 10.1016/j.cmi.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Oliva A., Scorzolini L., Cipolla A., Mascellino M.T., Cancelli F., Castaldi D., D’Abramo A., D’Agostino C., Russo G., Ciardi M.R., Mastroianni C.M., Vullo V. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: the activity of the double-carbapenem regimen is related to meropenem MIC value. J. Antimicrob. Chemother. 2017;72(7):1981–1984. doi: 10.1093/jac/dkx084. [DOI] [PubMed] [Google Scholar]
- Papoutsaki V., Galani I., Papadimitriou E., Karantani I., Karaiskos I., Giamarellou H. Evaluation of in vitro methods for testing tigecycline combinations against carbapenemase-producing Klebsiella pneumoniae isolates. J. Glob. Antimicrob. Resist. 2020;20:98–104. doi: 10.1016/j.jgar.2019.07.028. [DOI] [PubMed] [Google Scholar]
- Papst L., Beović B., Pulcini C., Durante-Mangoni E., Rodríguez-Baño J., Kaye K.S., Daikos G.L., Raka L., Paul M., Abbo L., Abgueguen P., Almirante B., Azzini A.M., Bani-Sadr F., Bassetti M., Ben-Ami R., Beović B., Béraud G., Botelho-Nevers E., Bou G., Boutoille D., Cabié A., Cacopardo B., Cascio A., Cassir N., Castelli F., Cecala M., Charmillon A., Chirouze C., Cisneros J.M., Colmenero J.D., Coppola N., Corcione S., Daikos G.L., Dalla Gasperina D., De la Calle Cabrera C., Delobel P., Di Caprio D., Durante Mangoni E., Dupon M., Ettahar N., Falagas M.E., Falcone M., Fariñas M.C., Faure E., Forestier E., Foti G., Gallagher J., Gattuso G., Gendrin V., Gentile I., Giacobbe D.R., Gogos C.A., Grandiere Perez L., Hansmann Y., Horcajada J.P., Iacobello C., Jacob J.T., Justo J.A., Kernéis S., Komnos A., Kotnik Kevorkijan B., Lebeaux D., Le Berre R., Lechiche C., Le Moxing V., Lescure F.X., Libanore M., Martinot M., Merino de Lucas E., Mondain V., Mondello P., Montejo M., Mootien J., Muñoz P., Nir-Paz R., Pan A., Paño-Pardo J.R., Patel G., Paul M., Pérez Rodríguez M.T., Piroth L., Pogue J., Potoski B.A., Pourcher V., Pyrpasopoulou A., Rahav G., Rizzi M., Rodríguez-Baño J., Salavert M., Scheetz M., Sims M., Spahija G., Stefani S., Stefos A., Tamma P.D., Tattevin P., Tedesco A., Torre-Cisneros J., Tripolitsioti P., Tsiodras S., Uomo G., Verdon R., Viale P., Vitrat V., Weinberger M., Wiener-Well Y. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin. Microbiol. Infect. 2018;24(10):1070–1076. doi: 10.1016/j.cmi.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Paul M., Daikos G.L., Durante-Mangoni E., Yahav D., Carmeli Y., Benattar Y.D., Skiada A., Andini R., Eliakim-Raz N., Nutman A., Zusman O., Antoniadou A., Pafundi P.C., Adler A., Dickstein Y., Pavleas I., Zampino R., Daitch V., Bitterman R., Zayyad H., Koppel F., Levi I., Babich T., Friberg L.E., Mouton J.W., Theuretzbacher U., Leibovici L. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet. Infect. Dis. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- Piedra-Carrasco N., Miguel L., Fàbrega A., Viñado B., Campany D., Mir A., Fox M.L., Almirante B., Larrosa N., Ruiz-Camps I., González-López J.J. Effectiveness of a double-carbapenem regimen in a KPC-producing Klebsiella pneumoniae infection in an immunocompromised patient. Microb. Drug Resist. 2018;24(2):199–202. doi: 10.1089/mdr.2017.0129. [DOI] [PubMed] [Google Scholar]
- Pogue J.M., Lee J., Marchaim D., Yee V., Zhao J.J., Chopra T., Lephart P., Kaye K.S. Incidence of and Risk Factors for Colistin-Associated Nephrotoxicity in a Large Academic Health System. Clin. Infect. Dis. 2011;53(9):879–884. doi: 10.1093/cid/cir611. [DOI] [PubMed] [Google Scholar]
- Poirel L., Kieffer N., Nordmann P. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2015;71:156–161. doi: 10.1093/jac/dkv294. [DOI] [PubMed] [Google Scholar]
- Poirel L., Potron A., Nordmann P. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 2012;67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Queenan A.M., Bush K. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satlin M.J., Kubin C.J., Blumenthal J.S., Cohen A.B., Furuya E.Y., Wilson S.J., Jenkins S.G., Calfee D.P. Comparative Effectiveness of Aminoglycosides, Polymyxin B, and Tigecycline for Clearance of Carbapenem-Resistant Klebsiella pneumoniae from Urine. Antimicrob. Agents Chemother. 2011;55(12):5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Yin D., Han R., Guo Y., Zheng Y., Wu S., Yang Y., Li S., Zhang R., Hu F. Emergence and Recovery of Ceftazidime-avibactam Resistance in blaKPC-33-Harboring Klebsiella pneumoniae Sequence Type 11 Isolates in China. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020;71(Supplement_4):S436–S439. doi: 10.1093/cid/ciaa1521. [DOI] [PubMed] [Google Scholar]
- Siegel J.D., Rhinehart E., Jackson M., Chiarello L. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control. 2007;35(10):S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Souli M., Karaiskos I., Masgala A., Galani L., Barmpouti E., Giamarellou H. Double-carbapenem combination as salvage therapy for untreatable infections by KPC-2-producing Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36(7):1305–1315. doi: 10.1007/s10096-017-2936-5. [DOI] [PubMed] [Google Scholar]
- Tamma P.D., Goodman K.E., Harris A.D., Tekle T., Roberts A., Taiwo A., Simner P.J. Comparing the Outcomes of Patients With Carbapenemase-Producing and Non-Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin. Infect. Dis. 2017;64(3):257–264. doi: 10.1093/cid/ciw741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangden T., Giske C.G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J. Intern. Med. 2015;277:501–512. doi: 10.1111/joim.12342. [DOI] [PubMed] [Google Scholar]
- Trecarichi E.M., Tumbarello M. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence. 2017;8:1–15. doi: 10.1080/21505594.2017.1292196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello M., Viale P., Viscoli C., Trecarichi E.M., Tumietto F., Marchese A., Spanu T., Ambretti S., Ginocchio F., Cristini F., Losito A.R., Tedeschi S., Cauda R., Bassetti M. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. 2012;55(7):943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- Wong D., van Duin D. Novel Beta-Lactamase Inhibitors: Unlocking Their Potential in Therapy. Drugs. 2017;77:615–628. doi: 10.1007/s40265-017-0725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Wu S., Yang Y., et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the Activities of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Clinical Isolates of and. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02431-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Luo Q., Shen P., et al. New options for bloodstream infections caused by colistin- or ceftazidime/avibactam-resistant Klebsiella pneumoniae. Int. J. Antimicrobial Agents. 2021;58:106458. doi: 10.1016/j.ijantimicag.2021.106458. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Q.i., Yin Y., Chen H., Jin L., Gu B., Xie L., Yang C., Ma X., Li H., Li W., Zhang X., Liao K., Man S., Wang S., Wen H., Li B., Guo Z., Tian J., Pei F., Liu L.i., Zhang L., Zou C., Hu T., Cai J., Yang H., Huang J., Jia X., Huang W., Cao B., Wang H. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob. Agents Chemother. 2018;62(2) doi: 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.