Abstract
Cognitive decline is one of the serious complications associated with diabetes mellitus (T2DM) of type-2. In this reported work, the effect of aqueous sukkari dates seed extract (ASSE) was evaluated in T2DM-induced rats. T2DM was induced using intraperitoneal injection of nicotinamide and streptozocin (STZ) administration. The diabetic rats were then treated orally with 200 mg/kg and 400 mg/kg of dates seed extract for 30 days and results were compared with metformin-treated groups. The memory functions were assessed using three maze models. Glucose and insulin levels in the blood and acetylcholine, acetylcholinesterase brain homogenates were estimated. The results showed a significant reduction in transfer latency (TL) (p < 0.001) during the elevated plus maze (EPM) test. The novel object recognition (NOR) test revealed a longer exploration time (p > 0.05) with novel objects and a higher discrimination index (p > 0.05). The Y-maze test also showed a significant increase in the number of entries to the novel arm (p > 0.05) and the total number of entries in the trial (p > 0.01) as well as in test (p > 0.05) sessions. Reduction in blood glucose (p > 0.05) and improvement in blood insulin (p > 0.05) levels were also noted. Improvement in ACh levels (p > 0.001) with 400 mg/kg of ASSE and reduction in AChE (p > 0.001) with both doses of ASSE were also observed in the brain homogenates. The results of ASSE were found comparable with the metformin-treated rats. The estimation of phytochemical constituents displayed a significant presence of phenolic content. Further, molecular modeling studies showed ellagic acid, catechin, and epicatechin as the potential molecule interacting with GSK-3β, α-amylase, and AChE and may be responsible for observed bioactivity. In conclusion, ASSE has the ability to alleviate T2DM-related cognitive impairments.
Keywords: Sukkari seeds, Type 2 diabetes, Memory deficits, Acetylcholine, Acetylcholinesterase, Insulin, Molecular modeling
1. Introduction
Diabetes Mellitus (DM) is a global pandemic and is characterized by hyperglycemia among the people suffering from it. The prevalence of DM is on the rise across the globe. In the year 2013, nearly 382 million people were affected and the number is anticipated to increase up to 592 million by the year 2035 (International Diabetes Federation, 2017). Particularly, type II DM (T2DM) is characterized by resistance of insulin receptors to the insulin molecule. As per the report of the international diabetes federation revealed in the year 2015, approximately 415 million adults were affected and the number is expected to rise by 640 million in 2040 (Ogurtsova et al., 2017). DM is associated but is not limited to complications with cardiovascular systems, retinopathy, nephropathy, foot damage, skin conditions, hearing impairment, and neuropathy. DM is also documented as a risk factor for memory impairment (Gold et al., 2007, Takeda et al., 2010, De Felice and Ferreira, 2014). The effect of long-term T2DM condition on cognitive decline was demonstrated in animal models as well as on humans and is ready to impact the available health resources. It is also documented that the T2DM condition results in a 50% increased risk of cognitive decline (Zilliox et al., 2016).
Of all the cases of dementia, Alzheimer's disease (AD) accounts for 60% of them. Vascular dementia is the second most frequent cause of cognitive impairment, accounting for 20% of all cases. (Rizzi et al., 2014). DM has constituted 19% of the cases in the last 20 years when compared to the cases not associated with DM (Rawlings et al., 2014). DM is an important risk factor linked to vascular dementia and AD. The memory impairment under persistent DM conditions results from the changes in the CNS (Takeda et al., 2010). The etiology of DM-dependent cognitive decline is associated with brain insulin resistance, cerebral microvascular damage, and neuroinflammation (Baglietto-Vargas et al., 2016). There are pieces of evidence suggesting a strong relationship between DM and brain dysfunction. Kodl and Seaquist suggested memory impairment under T2DM conditions depends on the factors like age, comorbidity, the effectiveness of the therapy being utilized, and type of diabetes (Kodl and Seaquist, 2008). Studies have demonstrated the effect of DM on dopaminergic, glutamatergic, purinergic, and cholinergic neurotransmission systems (Ezzeldin et al., 2014, Pérez‐Taboada et al., 2020). The cholinergic system plays a vital role in (i) the functions of the CNS, (ii) sleep control, and (iii) learning and memory (Haam and Yakel, 2017). A previous report showed increased acetylcholinesterase (AChE) mRNA and reduced cholinergic M1 and M3 receptors levels among diabetic rats. Besides the increase in AChE activity has also been demonstrated in diabetic rats (Antony et al., 2010). Recent reports have suggested, “type 3 diabetes mellitus” refers to AD resulting from insulin resistance (Mittal and Katare, 2016). The hyperglycemic condition causes worsening of brain cells and their functions, causing mild memory dysfunction and thus an early stage of AD. Hyperglycemic condition besides increasing production of amyloid-beta also increases tau phosphorylation and later two parameters are the hallmark for the characterization of AD (de la Monte and Wands, 2008).
Date palm (Phoenix dactylifera L.) is a fruit crop that consists of good nutritional value with a wide range of potential health benefits and also it is considered a staple food in the Arab Gulf regions (Ali et al., 2018, Tang et al., 2013). Various parts of the date palm which include leaves, fruits, pollen, trunk, and seeds are the source of cure to many ailments for human beings. Because of its high nutritional value and being a source of many remedies date palm has earned high recognition among the plant kingdom (Tang et al., 2013). In terms of nutritional value, date palms carry a very little amount of fat and cholesterol (Al-Abdoulhadi et al., 2011). Date palm is known to be a rich source of dietary fibers and is considered to be good for patients with digestive problems and heart conditions. As reported, seeds are the major waste product of the data industry and have the potential to offer a solution for developing agents for medicinal value. Besides being a rich source of minerals like sodium, potassium, and calcium, the seeds of the date palm are also rich in secondary metabolites like polyphenols, flavonoids, carotenoids, antioxidants, and phenolic acids (Zihad et al., 2021). The date palm also has many medicinal uses including the treatment of colds, fever, cystitis, edema, sore throat, bronchial catarrh, liver cancer, low sperm count, etc (Ali et al., 2018). The hepatoprotective, nephroprotective, hypolipidemic, and gastroprotective effect of date palm seeds extract has been documented (Baliga et al., 2011, Khalid et al., 2017). Aqueous extract of the date palm is reported to regulate glucose, insulin, and glycosylated hemoglobin levels in the blood of diabetic rats. It was also reported to restore liver and kidney functions in addition to relieving oxidative stress with an aqueous extract (Hasan and Mohieldein, 2016). Kalantaripour et al. reported the protection of cortical neurons due to the antioxidant properties of dates seed extract (Kalantaripour et al., 2012). Subash et al. reported reversal in the memory deficit, anxiety-related behavior, and motor coordination in a transgenic mouse model for AD supplemented with food of dietary fibers from dates fruits (Subash et al., 2015). Besides treatment also resulted in the lowering of Aβ protein deposition. Sukkari is one of the premium and famous varieties among the dates known to Arabs. It is mainly known for its good monetary returns to the consumers and farmers and It is mainly grown in the regions of Iraq and central regions of the Kingdom of Saudi Arabia (Siddeeg et al., 2019). Because there is a lack of research report for the effect of Sukkari dates seeds on CNS, we have demonstrated the effects of aqueous Sukkari seed extract (ASSE) on the reversal of the cognitive decline in the T2DM rat model. The effect of ASSE on cognitive deficits was demonstrated using maze models such as EPM, Y-maze, NOR. The level of acetylcholine and acetylcholinesterase was also estimated from the brain sample in order to demonstrate the effect of ASSE on the cholinergic system of diabetic rats. The study also demonstrated the outcome of ASSE on blood glucose and plasma insulin levels.
2. Material and methods
2.1. Plant material and extraction
The dates were acquired from farms in the Saudi Arabian province of Al-Qassim. The authenticity of Sukkari dates was confirmed with Professor Mohammed Motawei, Professor in Genetics Molecular from College of Agriculture and Veterinary, Qassim University. The seeds were collected from the pulp, washed in water, dried under shade at room temperature for two to three days. The coarse powder of seeds was prepared with the help of a grinder and soaked into the water as per the measurement of 1 l/100 g and kept for three days. The aqueous sukkari dates seed extract (ASSE) was then filtered and dried under vacuum using a rotary evaporator (BUCHI UK Ltd, Newmarket Suffolk, UK) for further use (Hasan and Mohieldein, 2016).
2.2. Quantitative estimation of the total polyphenolics, total flavonoids, and total tannins
The total polyphenols, total flavonoids, and total tannins contents of the ASSE were quantitatively measured as equivalents to gallic acid (GAE), quercetin (QE), and catechin (CE), respectively. For the polyphenolics quantification, 0.2 ml of the Folin–Ciocalteu reagent (diluted 1:5 in distilled water) was added to 0.2 ml sodium carbonate (10% w/v) and 1.6 ml of the ASSE (0.1 mg/ml). The mixture was thoroughly mixed, incubated for 30 min at room temperature, and spectrophotometrically measured at 760 nm. The method was conducted in triplicates, and total polyphenolic contents were expressed as gallic acid equivalent (GAE) per gram of the dried ASSE using the slope equation of the gallic acid calibration curve (Mohammed et al., 2021).
The contents of total flavonoids were measured according to the method of Do et al, (2014) as follows: exactly, 0.1 ml of the potassium acetate (0.1 mM) and 0.1 ml of the aluminum chloride (10 % in distilled water) were thoroughly mixed with 2 ml of the ASSE (0.1 mg/ml) in test tubes. The mixtures were incubated for 30 min at room temperature before measuring the absorbance of resulted color at 415 nm. The total flavonoids contents were calculated from three independent measurements and expressed as quercetin equivalent per gram of the dried ASSE.
The tannins contents in the ASSE were measured by the method described in the literature (Priyanthi et al., 2021). Briefly, 1.5 ml of the vanillin solution (4 % in methanol) and 750 µL of the hydrochloric acid (HCl) were thoroughly mixed with the ASSE (200 µL of the 0.1 mg/mL solution of ASSE). The mixtures were incubated at room temperature for 15 min and the absorbance was measured at 500 nm. The total tannins contents were calculated from three independent measurements and expressed as catechin equivalent per gram of the dried ASSE.
2.3. Drugs and chemicals
Streptozocin (STZ), nicotinamide, and metformin hydrochloride were purchased from Cayman Chemical, Ann Arbor, Michigan, USA. The enzyme-linked immunosorbent assay (ELISA) kits to evaluate the levels of insulin, acetylcholine (ACh), and acetylcholinesterase (AChE) were purchased from Cloud-Clone Corp., Katy, Texas, USA. Solvents and other chemicals were used according to analytical grades.
2.4. Vehicle
ASSE as well as metformin hydrochloride were dissolved in normal saline (NS, 0.9% w/v) and gavaged orally to targeted groups. The 0.1 M, cold citrate buffer (pH = 4.5) was freshly prepared and used to dissolve STZ. Nicotinamide was prepared as a solution with NS (0.9% w/v). The STZ and nicotinamide solutions were administered intraperitoneally (i.p.).
2.5. Experimental animals
Thirty Sprague Dawley (SD) adult male rats with the age of three months (body weight, 200–250 g) were used. The animals were used obtained from the College of Pharmacy’s animal house Qassim University. The experimental design was permitted by the animal ethical committee of the College of Pharmacy (Approval ID 2020-CP-5) and the Deanship of Scientific Research of Qassim University referred the grant number Pharmacy-2019-2-2-I-5643. The rats were separated into five groups (n = 6) and the first group was labeled as control, normal animals. The second group was labeled as a negative control or diabetic-induced group. The third group of diabetic rats was treated with standard drug metformin, and the fourth, as well as fifth groups of diabetic rats, were treated with 200 mg/kg and 400 mg/kg of ASSE, respectively. The animals were kept under standard laboratory conditions for seven days before starting of experiments. Three animals per propylene case were housed and allowed food (rodent pellet food from First Milling Company, Saudi Arabia) and water as ad libitum. After the induction of T2DM, the treatment with metformin and above mention doses of ASSE was continued for thirty days. After thirty days of treatment, the animals were sacrificed and samples were collected for ELISA analysis.
2.6. Acute toxicity study
The acute toxicities were carried out following the Organization for Economic Cooperation and Development's recommendations (OECD 423) (Mani et al., 2021). Three female rats of similar weight and age were allocated for each step. Generally, female rats have been observed slightly more sensitive in conventional LD50 studies as compared to the male gender (Lipnick et al., 1995). Primarily, the rats were treated with the dose of 5 mg/kg p.o. and were monitored for toxicity signs for the first four hours. For any mortality, the rats were observed next three days. As per the guidelines, if two of the three animals are dead, the dose is considered toxic. If one of the three animals is dead, the dose is repeated to confirm the toxic effects and if none of the animals is dead the toxicity experiments are repeated at the higher doses. For ASSE., the toxicity experiments were performed at the dose level of 50, 300, and 2000 mg/kg.
2.7. Induction of diabetes
Except for control animals, other groups were induced diabetes. The animals were kept on overnight fasting before the induction of diabetes. T2DM was induced by i.p. injections of nicotinamide 120 mg/kg followed by STZ 60 mg/kg within 15 min time intervals. Blood glucose levels were measured after 72 h and on day 7 to confirm the diabetes state (Fig. 1). Diabetic rats were designated for further studies if their fasting blood sugar levels were higher than 126 mg/dl. (Aboonabi et al., 2014). Glucose levels were determined using an Accu-Chek glucometer (Roche, Mannheim, Germany).
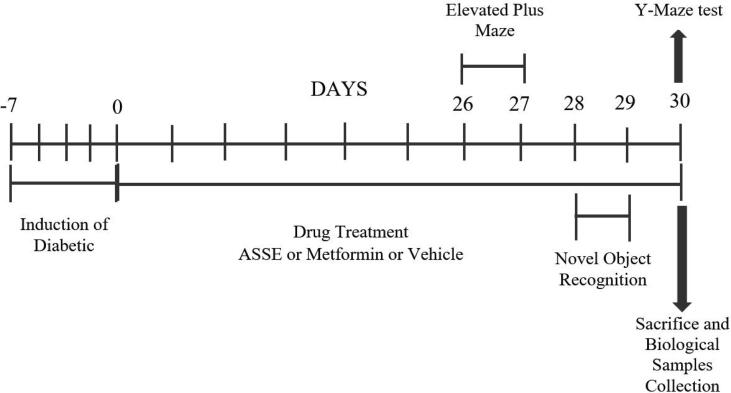
Fig. 1.
Timeline of diabetic induction, administration of drugs (ASSE or Metformin or Vehicle), behavioral assessments, and isolation of brain samples. Except for control rats, other groups were induced diabetic with STZ and nicotinamide on day −7. From day 0 to day 30 were considered treatment days, groups of rats were administrated with vehicle or metformin or ASSE. Regarding memory assessments, the EPM (days 26–27), NOR (days 28–29), and Y-maze (day 30) tests were conducted on respective days of the treatment schedule. At the end of the Y-maze test (day 30), all the rats were sacrificed and biological samples were collected for further ELISA assays.
2.8. Experimental design and drug treatments
The total number of 30 rats was divided into five groups (n = 6) for treatment, behavioral assessments, and biochemical estimations. The control and diabetic-induced groups were provided with normal saline for 30 days. The group of animals was devoid of any treatment. The standard group of animals was orally treated with metformin (200 mg/kg). The fourth and fifth groups of animals were treated with 200 and 400 mg/kg of ASSE (ASSE 200 and ASSE 400). At the end of 30 days of treatments, blood and brain tissues were preserved for biochemical assays. During the treatment, the tests for spatial memory assessments were conducted. The elevated plus maze (EPM) was conducted on the 26th and 27th days while the novel object recognition (NOR) test was conducted on the 28th and 29th days, and the Y-maze test was conducted on the 30th day. During the treatment, the animals' body weight and any mortality were measured every fifth day and the blood glucose level was measured on the 1st, 15th, and 30th days using a glucometer, as mentioned early (Fig. 1).
2.9. Elevated plus maze (EPM) test
The EPM was a wooden structure that stood 50 cm off the ground. It is made up of four equal-sized arms, each measuring 50 cm and 10 cm in length and width, respectively. Two of the arms were surrounded by walls of 40 cm in height and were ninety degrees to the two open arms in the opposite direction (Ahmad et al., 2014, Mani, 2021). On day 26, a test session was conducted by placing them one of the ends of the open arm and away from the intersection of the four arms. The animal is expected to move to the closed arm within 90 s and in the case, the animal is not moving on itself, a gentle push towards the closed arm was given. The animal was given a time of two minutes to explore the closed arm of the maze. On day 27, the transfer latency (TL) was used to assess the retention of learned task memory (Mani, 2021).
2.10. Novel object recognition (NOR) test
A box made up of wood with the dimension of 80 X 60 X 40 was used. The experiment was conducted in three sessions i.e. habituation on the 28th day, and training as well as a test on the 29th day of treatment (Malik et al., 2013, Mani, 2021). Each of the animals was permitted to explore in the empty box (without any object) for five minutes during habituation. The training session was performed after 24 h. In this session, the animals were allowed to explore with two familiar objects (FO1 and FO2) placed in the wooden box for five minutes. The exploration time by the animals for FO1 and FO2 was recorded (Mani, 2021). The test session was conducted after a gap of four hours concerning the training sessions. One of the FO was substituted by a novel object (NO) in the test session. Each rat was given another five minutes to investigate the two objects, NO and FO1. The exploration time during the test session was also recorded. The FO1 and FO2 were rectangular while NO was cylindrical. The objects used in the NOR test were tall and firm, difficult to be displaced by the animals. The discrimination index of both objects in the test session was also calculated (Mani, 2021).
2.11. Y-Maze test
The Y-maze has three arms at an angle of 120°, with the dimensions of 50 X 10 X 18 cm. The apparatus was placed on the floor and at the end of each arm, a picture was pasted. The pictures in each arm contained different patterns. Y-maze has also been conducted in two sessions i.e. trial session and test session (day 30). During the trial session, one of the three arms was closed and the remaining two arms were allowed to be explored by the animals for five minutes. The test session was conducted after four hours of the training session. The rats were allowed five minutes to investigate all three arms during this session. During the trial and test sessions, the number of entries was recorded, as well as the length of time spent in each arm during the test session was also noted (Tripathi et al., 2017, Mani, 2021).
2.12. Blood and brain sample collection
After the completion of scheduled treatment days, all of the animals were sacrificed using the cervical decapitation procedure under light ether anesthesia. The blood and brain tissues were collected from each animal. The blood was collected in the heparin-coated tube by retro-orbital puncture and the plasma was separated from blood tissues by centrifuging at 4000 rpm for 10 min. The collected brain samples were homogenized in phosphate buffer (pH 7.6) followed by centrifugation at 4000 rpm for ten minutes. Biuret colorimetry was used to determine the total protein content of the homogenates (Crescent Diagnostics, Saudi Arabia). The plasma sample was used for measuring the insulin levels and brain samples were used for the estimation of ACh and AChE levels.
2.13. Estimation of plasma insulin level
ELISA kit was procured for the estimation of plasma insulin levels. The method was followed from the manufacturer's manual without any modifications (Cloud-Clone Corp., Katy, Texas, USA). The assays were performed in duplicate and absorbance data were collected at 450 nm using ELx800 Absorbance Microplate Reader (BioTek Instruments, Santa Clara, California, USA).
2.14. Determination of brain acetylcholine (ACh) and acetylcholinesterase (AChE) levels
The ACh and AChE levels were estimated using ELISA kits based on sandwich enzyme immunoassay. The assays were performed in duplicate and the procedure was followed as per the manufacturer's protocol (Cloud-Clone Corp., Katy, Texas, USA). Finally, the absorbance was documented at 450 nm using ELx800 Absorbance Microplate Reader (BioTek Instruments, Santa Clara, California, USA).
2.15. Statistical analysis
The standard error of the mean (SEM) is used to express the results of in vivo experiments and ELISA assays. One-way ANOVA was used to examine the data, and the Tukey–Kramer post hoc test was performed to establish the significant levels (Graph Pad version 9, USA). In the NOR test, the student's unpaired 't'-test was employed to relate matching each group of exploration times. Statistical significance was defined as a p-value of<0.05.
2.16. Molecular docking
Molecular docking was performed using AutoDock vina integrated with PyRx (Trott and Olson, 2010). The crystallized proteins with PDB code 2OQV, 1Q41, 3BAJ, 1DX6, and 6OD6 were downloaded from the protein data bank. Protein preparation was performed by removing crystalized water, removing hetero atoms, adding polar hydrogen, adding Kollman charges. The three-dimensional structures of ligands were downloaded from the PubChem database in the SDF format. Ligands preparation was performed by minimizing them using a universal force field, adding gasteiger charges and polar hydrogens, defining trosions. The grids were defined by keeping the co-crystalized ligand in the center with 25 Angstrom distance in X, Y, and Z directions. Protein and ligand preparation was performed using AutoDock Tool (ADT), bundled with the MGLTools package (version 1.5.6) (Morris et al., 2009). The docking methodology was validated by reproducing the binding mode of cocrystallized ligands. Potential binding affinity is defined as kcal/mol. Protein-ligand interactions were analyzed using pymol.
3. Results
3.1. Total polyphenolics, flavonoids, and tannins contents in the ASSE
The quantitative measurements of the total phenolic contents, i.e., polyphenols, flavonoids, and tannins in the ASSE revealed the presence of a considerable amount of the polyphenols which were been measured at 38.38 ± 0.86 mg GAE/gm of the plant extract. The total flavonoids and tannins were measured as 4.12 ± 0.01 mg QE and 8.12 ± 0.51 mg CE per gm of the dried plant extract (Table 1).
Table 1.
Quantitative measurements of the total polyphenols, total flavonoids, and total tannins contents in the ASSE.
| TPC | TFC | TTC |
|---|---|---|
| mg/gm of the dried plant extract | ||
| 38.38 ± 0.86 | 4.12 ± 0.01 | 8.12 ± 0.51 |
All the measurements were conducted in triplicate; the mean ± standard deviations were calculated. TPC, total phenolic contents calculated in mg/g gallic acid equivalent; TFC, total flavonoid contents calculated in mg/g quercetin equivalent; TTC, total tannins contents calculated in mg/gm catechin equivalent.
3.2. Acute toxicity study
Following OECD 423 guidelines, no indication of toxicity or animal death was observed until 2000 mg/kg of ASSE. For subsequent studies, 200 and 400 mg/kg were chosen for oral administration.
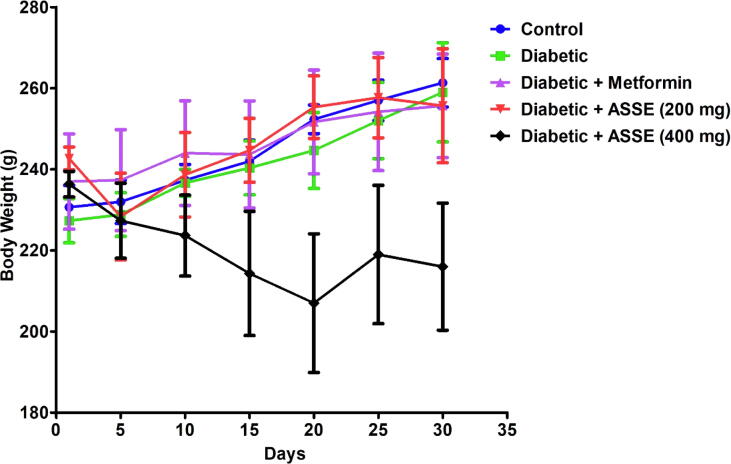
3.3. Administration of ASSE did not alter the bodyweight of diabetic-induced rats
The body weights of the animals were taken every fifth day i.e. 5th, 10th, 15th, 20th, 25th 30th days as referred to in Fig. 2. As a parallel to the control group, the other treated groups did not alter the animals’ bodyweight during the entire experiment. Additionally, Fig. 3 shows that there was no mortality of rats during diabetic induction and continuous treatment with metformin or ASSE (200 or 400 mg/kg).
Fig. 2.
Effect of ASSE and metformin on body weight of STZ-nicotinamide-induced diabetic rats. The values are mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 0.8272, p > 0.05 for day 1; F(4,25) = 0.2001, p > 0.05 for day 5; F(4,25) = 0.7042, p > 0.05 for day 10; F(4,25) = 1.507, p > 0.05 for day 15; F(4,25) = 2.268, p > 0.05 for day 20; F(4,25) = 1.876, p > 0.05 for day 25; F(4,25) = 2.257, p > 0.05 for day 30] followed by Tukey-Kramer multiple comparisons test. There were no statistically significant differences found between the groups in body weight.
Fig. 3.
Effect of metformin or ASSE on STZ-nicotinamide-induced diabetic rats during the 30 days of the study. There was no mortality recorded during 30 days of observation.
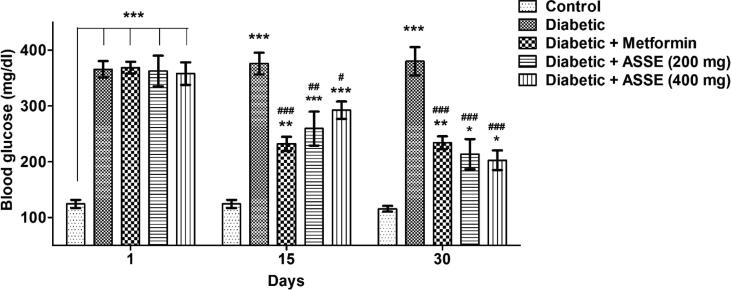
3.4. Administration of ASSE reduced blood glucose levels in diabetic-induced rats
The data collected for glucose levels on the 1st day, 15th day, and 30th day are represented in Fig. 4. One way ANOVA analysis of glucose level showed significant difference among the groups on day 1 (F(4,25) = 37.20, p < 0.001, 15th day (F(4,25) = 24.05, p < 0. 0.001) and on 30th day (F(4,25) = 25.04, p < 0.001). The diabetic-induced group exhibited a major difference (p < 0. 0.001) in the glucose levels on days 1, 15, and 30, confirming the appropriate induction of DM condition. The ASSE (200 mg/kg, p.o.) treatment group displayed a considerable reduction in the glucose levels of blood on day 15 (p < 0.01) and day 30 (p < 0.001) when associated with the diabetic rats. Also, the other dose of ASSE (400 mg/kg, p.o.) suggestively reduced the blood glucose levels on day 15 (p < 0.05) and day 30 (p < 0.001) as paralleled to the diabetic rats. As expected, the administration of metformin controlled the glucose levels (p < 0.001) on day 15 and day 30 in diabetic-induced rats. Unfortunately, no one group was not revised the blood glucose level as equivalent to control on day 15 as well as day 30.
Fig. 4.
Effect of ASSE and metformin on blood glucose levels of STZ-nicotinamide-induced diabetic rats. The values are mean ± SEM (n = 6). Administration of metformin and ASSE significantly reduced the blood glucose levels on day 15 and day 30 assessment. One-way ANOVA [F(4,25) = 37.20, p < 0.001 for day 1; F(4,25) = 24.05, p < 0.001 for day 15; F(4,25) = 25.04, p < 0.001 for day 30] followed by Tukey-Kramer multiple comparisons test. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 as compared to the diabetic-induced group.
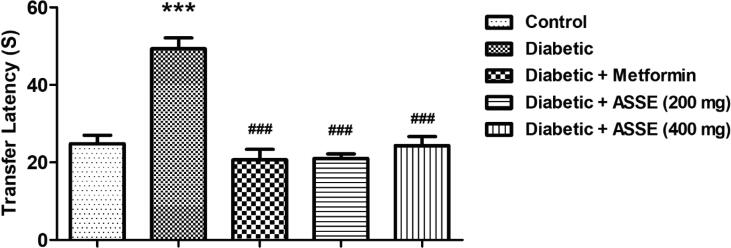
3.5. Administration of ASSE reduces the TL of diabetic-induced rats in the EPM test
The data collected for TL using the EPM test is shown in Fig. 5. The analysis among the group shows significant differences [F(4,25) = 27.02, p < 0.001]. When allied with the control group, the diabetes group had significantly longer TL values ((p < 0.001). These longer values of TL confirmed the cognitive deficit among the animals in the diabetic group. The treatment of animals with two doses of ASSE resulted in a significant (p < 0.001) reduction of TL values thus indicating the reversal of cognitive deficit in diabetic animals. The group of diabetic rats treated with metformin showed a similar reduction (p < 0.001) in TL value as compared to the diabetic group. There were no significant differences in TL values as compared between control, metformin, and both dose of ASSE. It highlighted the efficacious reversal of diabetic-induced memory impairment by treatment of ASSE.
Fig. 5.
Effect of ASSE and metformin on transfer latency of STZ-nicotinamide-induced diabetic rats using elevated plus-maze. Administration of ASSE and metformin shorten the TL in diabetic rats. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 27.02, p < 0.001] followed by Tukey-Kramer multiple comparisons test. ***p < 0.001 as compared to the control group; ###p < 0.001 as compared to the diabetic-induced group.
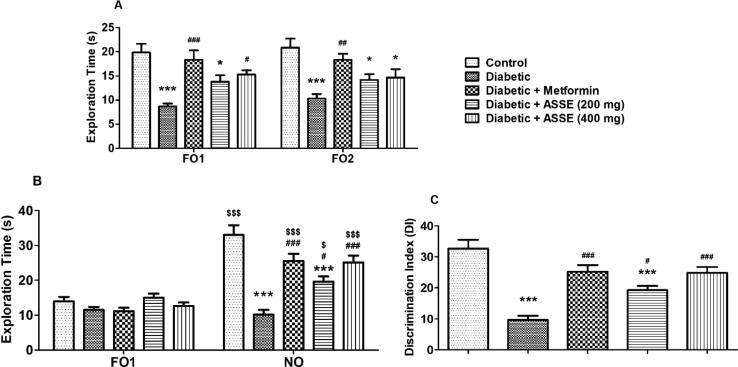
3.6. Administration of ASSE enhanced cognitive functions of diabetic-induced rats in NOR test
The effect of ASSE or metformin on various cognitive behaviors of diabetic rats during the NOR test is represented in Fig. 6. The mean exploration time for the familiar objects showed a significant difference (F(4,25) = 9.621, p < 0.001 for FO1 and F(4,25) = 7.932, p < 0.001 for FO2) among the groups during the training session (Fig. 6A). Further post-hoc analysis showed a remarkable reduction (p < 0.001) in exploration time for the FO1 and FO2 among the animals of the diabetic group as associated with the control rats. The exploration time for the ASSE treated groups were also exhibited substantial variances (p < 0.05) as related to the control group. As linked to the diabetic rats, only the higher dose of ASSE (400 mg/kg, p.o.) showed a considerable variation (p < 0.05) with exploration time of FO1. Besides, a comparison of exploration for the FO1 and FO2 showed similarities. Furthermore, treatment of metformin has resulted in the improvement of exploration time (p < 0.001 for FO1; p < 0.01 for FQ2) in diabetic-induced rats. There were no remarkable variations between the two doses of ASSE treatments in the exploration time of objects FO1 and FO2.
Fig. 6.
Effect of ASSE and metformin on (A) exploration time of two familiar objects (FO1 and FO2) during the training session, (B) exploration time of familiar (FO1) and novel (NO) objects during the test session, (C) discrimination index of STZ-nicotinamide-induced diabetic rats model using novel object recognition test. Treatment of metformin and ASSE significantly increased the exploration time of NO and DI performance of diabetic rats in the test session. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 9.621, p < 0.001 for FO1 and F(4,25) = 7.932, p < 0.001 for FO2 during training session; F(4,25) = 2.378, p > 0.05 for FO1 and F(4,25) = 17.76, p < 0.001 for NO during test session; F(4,25) = 17.89, p < 0.001 for DI] followed by Tukey-Kramer multiple comparisons test for comparisons of within the groups. The student's unpaired ‘t’ test was used to compare of correspond to each group of exploration time. $p < 0.05 and $$$p < 0.001 as compared to the corresponding group; *p < 0.05, and ***p < 0.001 as compared to the control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 as compared to diabetic -induced group.
In the test session, excluding the diabetic-induced group, other groups such as control (p < 0.001), metformin (p < 0.001), and ASSE (p < 0.05 for 200 mg/kg; p < 0.001 for 400 mg/kg) treatment resulted in significant improvement of exploration time for NO as parallel to corresponding groups of FO1, using student's unpaired ‘t’-test (Fig. 6B). These results explain the improvement in the exploration behavior of rats to a novel object. The significant differences among the group for exploration time of NO (F(4,25) = 17.76, p < 0.001) were noted. However, similar differences were not observed for FO1 (F(4,25) = 2.378, p > 0.05). The comparison for the exploration time of NO was found to be a considerable reduction (p < 0.001) with the diabetic group and low dose of ASSE (200 mg/kg) when compared to the control group. For ASSE treated groups observed with dose-dependent significant (p < 0.05 and p < 0.001 for 200 and 400 mg/kg, respectively) improvement in the exploration time for NO as related to the diabetic rats. However, there were no significant changes occurred as compared between the two doses. Metformin treatment also resulted in significant improvement in exploration time for NO (p < 0.001) as parallel to diabetic-induced animals.
Fig. 6C depicts the data collected for DI between familiar and novel objects. Significant [F(4,25) = 17.89, p < 0.001] differences for DI among the groups resulted. A considerable (p < 0.001) reduction in the DI was noted for the diabetic-induced group and ASSE (200 mg/kg) treated group as related to the control group. Treatment with 400 mg/kg of ASSE resulted in the improvement of DI values. The group of animals treated with 400 mg/kg of ASSE showed reduction (p < 0.001) in DI values when matched to the diabetic-induced group. Additionally, a considerable reduction in DI values (p < 0.05) with 200 mg/kg of ASSE was recorded. Still, the DI values of both doses were comparable and there were no significant changes between them. The treatment of metformin also displayed the reversal of DI (p < 0.001) values in diabetic-induced rats.
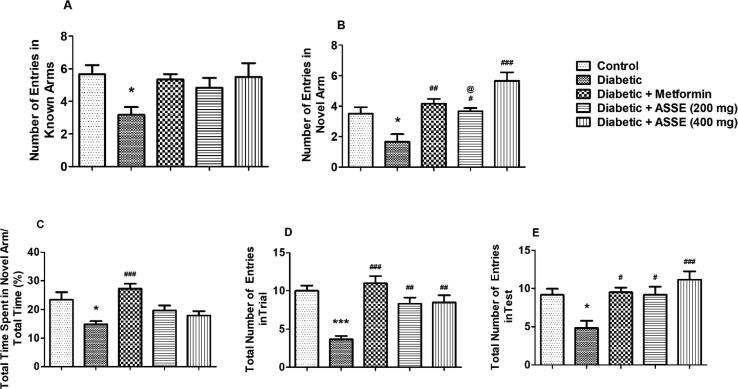
3.7. Administration of ASSE enhanced cognitive functions of diabetic-induce rats in Y-maze test
Fig. 7 shows the results of behavioral assessment studies using Y-maze. Among, Fig. 7A and 7B show the number of entries made by the animal into the known and novel arm respectively during the test session. One-way ANOVA showed notable [F(4,25) = 2.998, p < 0.05] variations in the number of entries for known arms among the groups. Similarly significant [F(4,25) = 11.76, p < 0.001] differences were observed for the novel arm entries among the group. In association with the control group of rats, the diabetic-induced group demonstrated a decrease in the number of entries for both known (p < 0.05) and novel (p < 0.01) Y-maze arms. The treatment with two doses of ASSE and metformin substantial improvement only in the number of entries to novel arm concerning with diabetic-induced group of animals. With metformin treatment, the significance level was found to be p < 0.001. Also, for two dosages of ASSE the significance values were found to be p < 0.05 and p < 0.001, respectively. Considering the number of novel arm entries, there was a significant difference (p < 0.05) between the effects of two doses of ASSE.
Fig. 7.
Effect of ASSE and metformin on (A) number of entries in known arms in test, (B) number of entries in novel arm in test, (C) percentage of time spent in the novel arm in test, (D) the total number of entries in the trial, and (E) the total number of entries in STZ-nicotinamide-induced diabetic rats model using Y-maze test. Treatment of metformin and ASSE increased the number of entries in the novel arm and the total number of entries in both sessions. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 2.998, p < 0.05 for the number of entries in known arm; F(4,25) = 11.76, p < 0.001 for the number of entries in novel arms; F(4,25) = 7.217, p < 0.001 for the percentage of time spend in novel arm; F(4,25) = 13.18, p < 0.001 for the total number of entries in the trial; F(4,25) = 6.531, p < 0.001 for the total number of entries in test] followed by Tukey-Kramer multiple comparisons test. *p < 0.05 and ***p < 0.001 as compared to the control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 as compared to the diabetic-induced group; @p < 0.05 as compared to the Diabetic + ASSE (400 mg).
Fig. 7C shows the ratio of time spent by the rats in the novel arm. There were notable changes [F(4,25) = 13.18, p < 0.001] among the treatment groups in time spent in the novel arm. As expected, the diabetic-induced animals that spent lesser time (p < 0.05) in the novel arm as associated with the control group of animals. The group treated with ASSE 200 mg/kg and 400 mg/kg of ASSE did not show any improvement in the time present in the novel arm.
Fig. 7D and 7E show the overall entries into the arms during the trial and test periods using the Y-maze. The significance levels for the trial and test sessions were found to be F(4,25) = 13.18, p < 0.001, and F(4,25) = 6.531, p < 0.001; respectively, when matched among the groups. The diabetic rats exhibited a decrease in the total number of entries associated with the respective control groups during the trial (p < 0.001) and test (p < 0.05) sessions. Two doses of ASSE and metformin showed substantial improvements as related to the diabetic group for the total entries during both trial and test sessions. For metformin, the deviations were p < 0.001 and p < 0.05 for trial and test sessions. Both doses of ASSE resulted in an improvement (p < 0.01) in the total entries of diabetic rats in the trial session. Similarly, in the test session, both doses (p < 0.05 for 200 mg/kg and p < 0.001 for 400 mg/kg) improves the entries in the diabetic group as related to the diabetic-induced group. There were no significant differences observed in the total number of entries in both trial and test when compared between both doses of ASSE.
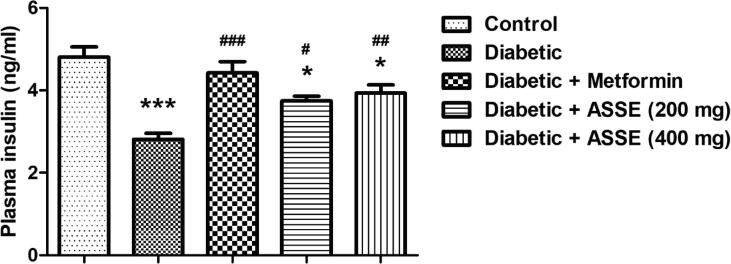
3.8. Administration of ASSE increased plasma insulin levels in diabetic-induced rats
Fig. 8 shows the data collected for the plasma insulin levels for the animals under study. There were significant differences in the plasma insulin levels among the group (F(4,25) = 13.19, p < 0.001). The diabetic group showed a major decrement (p < 0.001) in the plasma insulin levels as associated with the control group. The treatment of animals with 200 and 400 mg/kg of ASSE was reported in the development of plasma insulin levels. The significance levels concerning the diabetic-induced group were found to be p < 0.05, and p < 0.01, respectively. Remarkably, the effects of both doses were comparable, significantly there was no difference between the treated groups. Additionally, the elevation in the plasma insulin levels was found to be comparable with the group of animals treated with standard drug metformin. The significance level for the metformin-treated group was found to be p < 0.001 as associated with the diabetic group of animals.
Fig. 8.
Effect of ASSE and metformin on plasma insulin levels of STZ-nicotinamide-induced diabetic rats. The plasma insulin levels were improved in both metformin and ASSE treatment. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 13.19, p < 0.001] followed by Tukey-Kramer multiple comparisons test. *p < 0.05 and ***p < 0.001 as compared to the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 as compared to the diabetic-induced group.
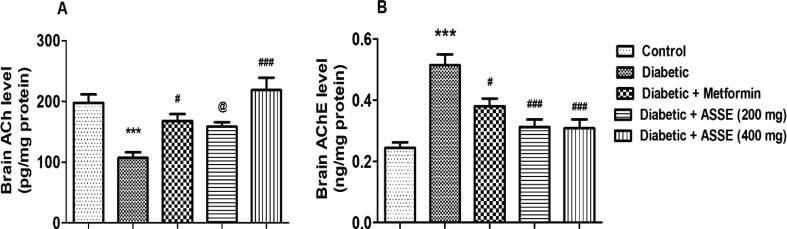
3.9. Administration of ASSE elevated ACh and reduced AChE activities in diabetic-induced rats brains
Fig. 9A and 9B represent the results of the level of ACh and AChE respectively in brain homogenate of animals after 30 days of treatments. The analysis of the comparison between all the groups revealed significant differences in ACh and AChE levels among the groups, as F(4,25) = 15.17, p < 0.001, and F(4,25) = 10.50, p < 0.001, respectively. The diabetic group of animals reveal significantly lower levels of ACh (p < 0.001) and higher levels of AChE (p < 0.001) as associated with the control. The metformin (p < 0.05) and a higher dose of ASSE (p < 0.001 for 400 mg/kg) treated groups revealed significant restoration of ACh in the brain. The group of rats treated with a lower dose of ASSE (200 mg/kg, p.o.) did not show any significant changes in ACh levels in brain homogenate as allied with the diabetic group. On the other hand, there were significant reductions in AChE levels with metformin (p < 0.05), and both doses of ASSE (p < 0.001) as related to the diabetic rats. In the reduction of AChE levels, both doses of ASSE resulted in comparable effects.
Fig. 9.
Effect of ASSE and metformin on (A) acetylcholine (ACh) and (B) acetylcholinesterase (AChE) levels of STZ-nicotinamide-induced diabetic rats. Treatment of ASSE improved the cholinergic transmission in diabetic-induced mouse brain. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F(4,25) = 15.17, p < 0.001 for ACh; F(4,25) = 10.50, p < 0.001 for AChE] followed by Tukey-Kramer multiple comparisons test. ***p < 0.001 as compared to the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 as compared to the diabetic-induced group; @p < 0.05 as compared to the Diabetic + ASSE (400 mg).
3.10. Molecular modeling studies
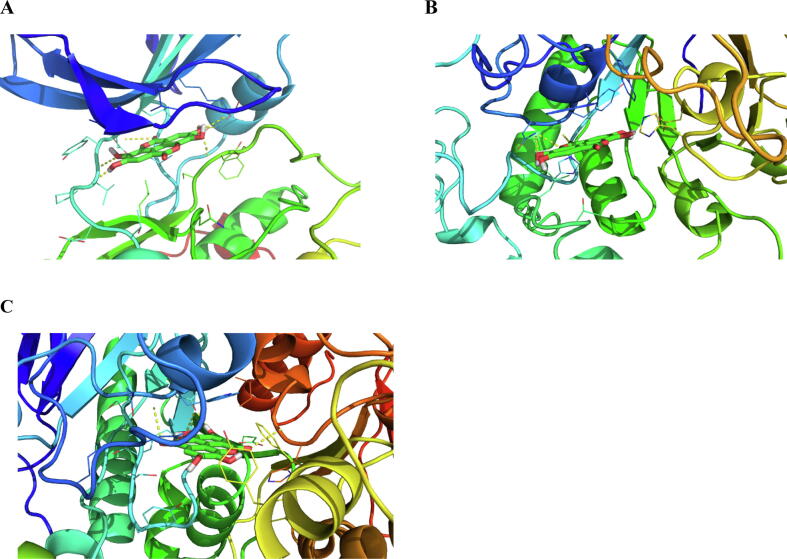
As mentioned above dates seeds are rich in polyphenols, flavonoids, fatty acids, amino acids etc. The estimation of phytochemical constituents displayed a significant amount of phenolic content whereas tannin content and flavonoid content were significantly less. Therefore, molecular modeling studies were conducted on the reported phenolic contents. The reported phenolic compounds are pyrogallol, cinnamic acid, benzoic acid, ellagic acid, catechol, gallic acid, protocatechuic acid, syringic acid, vanillic acid, epicatechin, and catechin (Mohammadi et al., 2018). Therefore, exploration of the mechanism started with the inverse docking/target fishing using pharmMapper (Wang et al., 2017). The pharmMapper is a web-based server that explores plausible targets for the particular molecule. The exploration of the above-mentioned molecules indicated dipeptidyl peptidase 4 (DPP-4), glycogen synthase kinase 3 (GSK-3) α-amylase as antidiabetic targets, whereas acetylcholinesterase (AChE) and beta-secretase 1 (BACE 1) was identified as target implicated in the cholinergic transmission for most of the molecule. Therefore the molecular docking studies were performed for the above-mentioned enzymes. The crystal structures utilized for DPP-4, GSK-3, α-amylase, AChE, and BACE 1 are 2OQV, 1Q41, 3BAJ, 1DX6, and 6OD6 respectively. The docking scores of the phenolics are listed in Table 2 and were compared with the co-crystallized ligand. The docking scores for the co-crystallized ligands were found to be −10.1, −10.2, −9.1, −10.5, and −9.3 kcal/mol for DPP-4, GSK-3, α-amylase, AChE, and BACE 1 respectively. The docking score for the phytochemical constituents varied from ∼ 4 to ∼ 11 kcal mol and hence, the ∼ -8 kcal/mol and above were considered for the binding mode analysis. In this reference ellagic acid, epicatechin, and catechin showed significant binding potential for GSK-3, α-amylase, and AChE. The binding score of ellagic acid for GSK-3, α-amylase, and AChE was noted to be −9.8, −8.6, and −10.2 kcal/mol respectively. Similarly, the binding score for epicatechin was noted to be −8.8, −8.6, and −9.7 kcal/mol respectively. The catechin showed the binding score of −8.6, −8.7, and −9.8 kcal/mol respectively. The binding mode analysis of docked ligands with GSK-3 showed hydrogen bond interaction with Asp 133, Val 135, and Arg141. The hydrophobic interactions were observed with the residues Ile62, Val70, Ala83, Lys85, Val110, Leu 132, Tyr134, Leu154 (Arfeen et al., 2015, Arfeen et al., 2016). The Binding mode analysis of docked ligand with α-amylase showed hydrogen bond interaction with Tyr62 and Gln 63, His305. The hydrophobic interaction was observed with residues Trp59, Thr163, Gly164. Trp59 was noted to be involved in π-πinteraction (Yousuf et al., 2020). Similarly, the binding mode analysis of docked ligand with AChE displayed the hydrogen bond interaction with Tyr70, Trp84, Asp 85, Tyr130, Ser122, His 440. The hydrophobic interactions displayed are Gly117, Gly118, and Trp84. The indole ring of tryptophan is also involved in π-π interaction with docked ligand (Tallini et al., 2018). Fig. 10 shows the binding mode of ellagic acid in the active site of GSK-3β, α-amylase and AChE.
Table 2.
Docking score of reported phenolic compounds for the five potential targets.
| Sr. No. | Ligands | 2OQV | 1Q41 | 3BAJ | 1DX6 | 6OD6 |
|---|---|---|---|---|---|---|
| 1 | Co-crystallized ligand | −10.1 | −10.2 | −9.1 | −10.5 | −9.3 |
| 2 | Ellagic acid | −7.3 | −9.8 | −8.6 | −10.2 | −7.2 |
| 3 | Epicatechin | −7.7 | −8.8 | −8.6 | −9.7 | −6.9 |
| 4 | Catechin | −7.5 | −8.6 | −8.7 | −9.8 | −7.1 |
| 5 | Pyrogallol | −5.7 | −4.9 | −5.3 | −5.4 | −4.4 |
| 6 | Syringic acid | −5.5 | −5.8 | −5.5 | −6.3 | −5 |
| 7 | Vanillic acid | −5.9 | −5.8 | −5.7 | −6.3 | −5.2 |
| 8 | Benzoic acid | −6 | −5.4 | −5.3 | −6.4 | −5 |
| 9 | Catechol | −5.1 | −4.7 | −5.1 | −5.4 | −4.3 |
| 10 | Cinnamic acid | −5.9 | −6.3 | −6.1 | −7.2 | −5.4 |
| 11 | Gallic acid | −6.3 | −5.7 | −6.1 | −6.3 | −5.2 |
Fig. 10.
Predicted binding mode of Ellagic acid in the active site of (A) GSK-3 (PDB code 1Q41), (B) α-amylase (PDB code 3BAJ), and (C) AChE (PDB code 1DX6). The yellow color dashed line represents hydrogen bond interaction.
4. Discussion
Cognitive impairment is one of the complications of DM. In the present work, we have demonstrated, (i) effects of ASSE on blood glucose levels, plasma insulin levels improvement in cognition resulting from T2DM (ii) effect on cholinergic transmission, and (iii) total phenolic contents, flavonoid contents, and total tannin contents. In this study, T2DM was induced by administering nicotinamide followed by STZ. The toxin STZ results in pancreatic β-cells destruction. STZ is entered preferentially to β-cells via the glucose transporter GLUT2 receptor, causing DNA damage. The DNA damage further results in the enhancing activity of poly(ADP-ribose) polymerase (PARP-1) enzyme for its repair and thus induces depletion of ATP and NAD+ present intracellularly resulting in the necrosis of insulin-secreting cells (Szkudelski, 2012). Prior administration of nicotinamide provides partial protection to the β-cells of the pancreas through inhibition of PARP-1 enzyme thus maintaining the required concentration of intracellular ATP and NAD+ (Aboonabi et al., 2014). Besides nicotinamide also serves as the precursor of NAD+ thus aiding the increased levels of intracellular NAD+. Because of the above-mentioned mechanism, the blood glucose levels in the STZ induced diabetic animals can be controlled from mild hyperglycemic condition to severely hyperglycemic condition depending upon the dose of STZ and time of examination after the injection of nicotinamide and STZ. Similarly, the level of insulin in the blood can also be controlled (Szkudelski, 2012). In this report single i.p. injection of 120 mg/kg of nicotinamide followed by 60 mg/kg of STZ injection resulted in a successful T2DM condition. Blood glucose levels were measured on the 1st, 15th, and 30th days of the experiment to validate the animals' hyperglycemic status. Besides, insulin levels were measured using ELISA, which further confirmed the diabetic condition. The oral treatment of animals with 200 and 400 mg/kg of ASSE for 30 days resulted in the improvement of T2DM condition by restoring the hyperglycemic condition and hypoinsulism.
Insulin and its receptors (IRs) present in the brain are associated with extensive biological activities including energy hemostasis by controlling metabolism in several organs. Besides, defective insulin signaling is linked with neuronal survival, cognitive impairment, and AD. Furthermore, IRs are expressed in neurons, and in the brain, it is particularly expressed in the hippocampus. Zhao et. al reported improvement of memory functions in diabetic animal models upon administration of peripheral insulin (Zhao et al., 2004). The report further emphasized the significance of IRs found in areas of the brain like the cortex and hippocampus, which are maintaining learning and memory functions. Our results showed significantly reduced levels of insulin in the diabetic group. However, treatment of diabetic animals with 200 and 400 mg/kg of ASSE resulted in a notable improvement in the plasma insulin levels. It is pertinent to mention that the results of animals treated with 400 mg/kg of ASSE are comparable to the metformin-treated groups. This effect can be correlated with the ASSE protective effect on pancreatic β-cells against the lethal effect of STZ. Besides, the protective effect of dates seed extract on liver cells against the cytotoxic effect of DM condition can also be held responsible. It is stated that the protective effect of dates seed extract on liver cells may result in resolving the metabolism of glucose and insulin and thus restoring their levels. The results further establish the ASSE extract has the potential to improve T2DM conditions.
In the present study, nicotinamide and STZ induced hyperglycemic condition is linked to cognitive impairment has been demonstrated through different behavioral tests like EPM, NOR, and Y-maze tests. Our results from these tests have also shown impairment of normal cognitive functions among the diabetic group of animals and are in line with some of the previous studies (Wang et al., 2019, Semuyaba et al., 2017). In this work, we have treated two doses such as 200 and 400 mg/kg of ASSE on diabetic-induced rats, respectively for the cognitive deficit. Our results showed reversal of memory impairment at both dose levels. EPM was used as one of the tools to evaluate the behavior and cognitive functions of animals. The results from the EPM tests were indicated in terms of transfer latency (TL) time. Our results showed longer TL time for diabetic animals in contrast to the control group of animals. However, the TL time was found to be reduced for the animals treated with two doses of ASSE and metformin. The improvement in TL time was found to be significant at both the dose level of ASSE thus indicating the protective effect of extracts on hippocampus neurons. The treated group may have increased availability of acetylcholine and insulin, which improved the cognitive performance among the animals during the behavioral tasks as evident from current results and are parallel with the previous reports (Subash et al., 2015).
NOR test has been used to differentiate a novel object (NO) from a familiar object (FO). The animals were allowed to explore two identical items (FO1 and FO2) during the training session to help them remember familiar things as their working memory (Silvers et al., 2007). The results of the training session (using FO1 and FO2) showed a considerable reduction in the exploration time for the diabetic group of animals when referred to the control group. The animals treated with ASSE showed some improvement in exploration time in the training session but were not comparable with the control group. From the above-mentioned statements, it is evident that the T2DM condition affects the capacity of retaining the previously exposed task in working memory. However, there were no changes in exploration time for FO1 and FO2 among all the groups indicated neither diabetic condition nor treatment of diabetic animals with ASSE alters the capacity to remember the similarity of objects. In the test session, used FO1 and NO to evaluate the discrimination ability of the animals. Our results showed a considerable increment in the exploration time for the NO when compared to the corresponding group concerning the exploration time of the FO. The results showed animals' preference to spend a long time exploring the NO than the FO suggesting the capacity to retain and discriminate the objects. As expected the diabetic group of animals showed lower exploration times with the NO confirming the lower retention and discriminating ability of animals under T2DM conditions. The metformin and ASSE treated groups of animals showed improved exploration time for the NO as related to the diabetic group of animals indicating the reversal of cognitive deficit among the T2DM animals. As per Silvers et. al., superior cognitive skills are a prerequisite to distinguish novel objects from familiar objects or to execute a task in a different environment (Silvers et al., 2007). Our results are in line with the published reports (Seibenhener and Wooten, 2015, Ennaceur, 2010). Our results of DI provided additional proof for the discriminating capability of animals during the test session. Groups treated with two doses of ASSE and metformin showed better DI values than the diabetic group. The values for DI were found to be comparable with the control group of animals.
The animals' spatial working memory was assessed using the Y-maze test. During the Y-maze test, the animal uses most of its brain areas, which include the basal forebrain, hippocampus, and prefrontal cortex (Liet et al., 2015). The Y-maze test is also conducted in two phases which are the trial and test phases. During the trial phase, one arm of the Y-maze was closed and the rat was free to explore the remaining two arms, while in the test phase rats were permitted to freely explore all three arms of the Y-maze. In principle, the tendency of animals to enter into the third (previously blocked), also called a novel arm frequently in comparison to the arms visited during the training session highlights the prefrontal cortical functions. Our results T2DM condition in animals causes the reduced entries to both known as well as novel arms indicating the deficits in spatial memory (Tripathi et al., 2017). The treatment with two doses of ASSE and metformin showed improved the novel arm entries as referred to the diabetic group confirming the improvement in impaired spatial memory induced by the diabetic. Consider the test session proportion of the time present in the novel arm to the entire time was also calculated to identify the coping behavior of animals in different environments (Poimenova et al., 2010). Besides, a rise in anxiety behavior is also inversely related to this coping behavior. The diabetic group of animals displayed lower coping behavior as related to the control which indicates the anxiety behavior of the diabetic animals in the new environment. Unfortunately, the oral treatment of two doses of ASSE failed to increase the ratio of time present in the novel arm in diabetic-induced rats. The comparison of the total entries during the trial and test sessions shows curiosity behavior (Kraeuter and Guest, 2019). The treatment group of animals showed improved curiosity behavior in comparison to the diabetic animals which further indicates improvement in the memory functions because of the ASSE.
At present, there is a very inadequate quantity of reports for the effect of dates on memory impairment. Subhash et al. in the year 2015 suggested that the dietary supplement of dates fruit can cause a reversal of memory impairment, improvement in anxiety-related behavior, and better motor coordination in the transgenic mouse model for AD (Subash et al., 2015). The other report suggested that a renewal of learning and memory impairment were among the rats treated with dates extract (Bamy Mozafaty rutab) for twelve days. In this report, the Alzheimer experiment model was induced in rats by a single ICV injection of Aβ25-35 and the memory assessment was done using Morris water maze (Dehghanian et al., 2017). Our results are in conjugation with these cited reports.
ACh is an important neurotransmitter for activating the nicotinic receptors in the hippocampus. It is also known for memory formation and preserving memory for the long term (Yakel, 2012). Levels of ACh in the hippocampus are also connected with various memory functions. It is very well stated in the literature that a decrease in hippocampus ACh levels is responsible for the decline of age-related cognitive functions. It is also stated that ACh levels in the hippocampus increase during the tasks related to spatial memory (Stancampiano et al., 1999), and damage to the medial septum results in the decline of ACh levels in the hippocampus thus impairing the spatial memory (Herzog et al., 2000). The enzyme AChE present in the synaptic cleft is very well known for degrading the ACh to choline and acetate and thus inhibits cholinergic transmission (Ahmad et al., 2014). Earlier studies have reported elevated levels of AChE in the critical areas of the brain, which includes the hippocampus, cortex, and striatum (Mushtaq et al., 2014). Our results showed decreased ACh and elevated AChE activities in the brain homogenate of diabetic animals. However, the group of animals treated with metformin and ASSE showed improved levels of ACh and restored the level of AChE enzyme. Our results are in agreement with the previously published studies (Herzog et al., 2000, Ahmad et al., 2014, Mushtaq et al., 2014).
The concentration of neurotransmitter in different brain regions are altered because of the T2DM condition and these alterations can lead to various diseases of CNS. In terms of the relationship between insulin level and ACh level, it can be safely stated that they are directly related i.e. lower levels of insulin result in lower levels of ACh in the brain. The low levels of ACh in the brain because of low levels of insulin are because of lower supplies of choline and acetyl-Co-A which increase insulin stimulation (Rivera et al., 2005). The earlier studies have also demonstrated a direct relationship between insulin stimulation on ACh levels in AD. Thus alteration in insulin signaling results causes cognitive dysfunction further leading to AD. A study reported by Dubey et al. demonstrated that an increase in glucose uptake and insulin signaling along with the use of ACh agonist increase the level of ACh in the brain thus further supporting the relationship of insulin and ACh levels (Dubey et al., 2020). Our results also demonstrated significantly improved levels of insulin and ACh in plasma and brain respectively thus further confirming the direct relationship between insulin and ACh.
The measurement of phytochemical constituents showed the presence of a considerable amount of the polyphenols (38.38 ± 0.86 mg GAE), which were nearly similar to the reported polyphenols in the Sukkari dates (Zihad et al., 2021). However, the flavonoid contents and tannin content was found to be at a lower level (4.12 ± 0.01 mg QE) and (8.12 ± 0.51 mg CE) as compared to the previous reports which can be attributed to the nature of the extract. The inverse docking of the reported phenolic content revealed DPP4, GSK-3β, and α-amylase as the potential antidiabetic targets, whereas AChE was BACE-1 was revealed as the target which could lead to the improvement of cholinergic transmission. Further molecular docking clarified ellagic acid, epicatechin, and catechin as the potential phytochemical constituents responsible for the anti-diabetic and improved cholinergic activity. The molecular docking also elucidated that the ellagic acid, Epicatechin, and catechin have the significant binding potential for GSK-3β, α-amylase, and AChE but not for DPP-4 and BACE-1. It is pertinent to mention that GSK-3β is a well-established target for drug discovery against diabetes as well as Alzheimer’s (Arfeen and Bharatam, 2013). Therefore it can be envisaged that the observed antidiabetic effect and improvement in the cognitive impairment could be because of the action of ellagic acid, epicatechin, and catechin on the GSK-3β, α-amylase, and AChE.
5. Conclusions
Our results demonstrate, the promising effect of ASSE on cognitive impairment induced due to the T2DM in addition to the restoration of the hyperglycemic condition among a rat model. The cognitive deficit was assessed using different maze models. EPM demonstrated shorter TL time, NOR showed improved exploration time and DI for a novel object. Y-maze displayed reversal in the number of entries for the novel arm, improved couping, and curiosity behavior for the animals. Besides lower blood glucose and elevated plasma insulin levels were observed. Improved levels of ACh and reduced levels of AChE were also detected in the brain homogenates. Estimation of phytochemical constituents showed the presence of phenolic content in a significant amount. Inverse docking and molecular docking analysis revealed Ellagic acid, epicatechin and catechin have the potential to bind with GSK-3β, α-amylase, and AChE and thus may be responsible for observed antidiabetic effect and improvement in cognitive impairment. In conclusion, ASSE has the potential to treat T2DM dependent cognitive impairment. However, further illustrative mechanistic investigation is required to validate the primary finding.
Funding
This research was funded by the Deanship of Scientific Research, Qassim University, Saudi Arabia under the project number (Pharmacy-2019-2-2-I-5643) during the academic year 1440AH/2019AD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research on the financial support for this research under the number (Pharmacy-2019-2-2-I-5643) during the academic year 1440AH/2019AD.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aboonabi A., Rahmat A., Othman F. Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Toxicol. Reports. 2014;1:915–922. doi: 10.1016/j.toxrep.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Ramasamy K., Jaafar S.M., Majeed A.B.A., Mani V. Total isoflavones from soybean and tempeh reversed scopolamine-induced amnesia, improved cholinergic activities and reduced neuroinflammation in brain. Food Chem. Toxicol. 2014;65:120–128. doi: 10.1016/j.fct.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Al-Abdoulhadi I.A., Al-Ali S., Khurshid K., Al-Shryda F., Al-Jabr A.M., Ben Abdallah A. Assessing fruit characteristics to standardize quality norms in date cultivars of Saudi Arabia. Indian J. Sci. Technol. 2011;4:1262–1266. doi: 10.17485/ijst/2011/v4i10/30169. [DOI] [Google Scholar]
- Ali A., Waly M.I., Musthafa M.E., Devarajan S. Nutritional and medicinal value of date fruit. Dates. 2018;380–395 doi: 10.1201/b11874-30. [DOI] [Google Scholar]
- Antony S., Peeyush Kumar T., Mathew J., Anju T.R., Paulose C.S. Hypoglycemia induced changes in cholinergic receptor expression in the cerebellum of diabetic rats. J. Biomed. Sci. 2010;1–9 doi: 10.1186/1423-0127-17-7. https://jbiomedsci.biomedcentral.com/articles/10.1186/1423-0127-17-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfeen M., Bhagat S., Patel R., Prasad S., Roy I., Chakraborti A.K., Bharatam P.V. Design, synthesis and biological evaluation of 5-benzylidene-2-iminothiazolidin-4-ones as selective GSK-3β inhibitors. Eur. J. Med. Chem. 2016;121:727–736. doi: 10.1016/j.ejmech.2016.04.075. [DOI] [PubMed] [Google Scholar]
- Arfeen M., Patel R., Khan T., Bharatam P.V. Molecular dynamics simulation studies of GSK-3β ATP competitive inhibitors: Understanding the factors contributing to selectivity. J. Biomol. Str. Dyn. 2015;33(12):2578–2593. doi: 10.1080/07391102.2015.1063457. [DOI] [PubMed] [Google Scholar]
- Arfeen M., Bharatam P.V. Design of glycogen synthase Kinase-3 Inhibitors : An overview on recent advancements. Curr. Pharm. Des. 2013;19:73–77. doi: 10.2174/1381612811319260007. [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D., Shi J., Yaeger D.M., Ager R., LaFerla F.M. Diabetes and Alzheimer's disease crosstalk. Neurosci. Biobehav. Rev. 2016;64:272–287. doi: 10.1016/j.neubiorev.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Baliga M.S., Baliga B.R.V., Kandathil S.M., Bhat H.P., Vayalil P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.) Food Res. Int. 2011;44(7):1812–1822. doi: 10.1016/j.foodres.2010.07.004. [DOI] [Google Scholar]
- De Felice F.G., Ferreira S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- Dehghanian F., Kalantaripour T.P., Esmaeilpour K., Elyasi L., Oloumi H., Pour F.M., Asadi-Shekaari M. Date seed extract ameliorates β-amyloid-induced impairments in hippocampus of male rats. Biomed. Pharmacother. 2017;89:221–226. doi: 10.1016/j.biopha.2017.02.037. [DOI] [PubMed] [Google Scholar]
- Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S.K., Lakshmi K.K., Krishna K.V., Agrawal M., Singhvi G., Saha R.N., Saraf S., Saraf S., Shukla R., Alexander A. Insulin mediated novel therapies for the treatment of Alzheimer’s disease. Life Sci. 2020;249:117540. doi: 10.1016/j.lfs.2020.117540. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010;215(2):244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ezzeldin E., Souror W.A.H., El-Nahhas T., Soudi A.N.M.M., Shahat A.A. Biochemical and neurotransmitters changes associated with tramadol in streptozotocin-induced diabetes in rats. Biomed. Res. Int. 2014;2014:1–9. doi: 10.1155/2014/238780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S.M., Dziobek I., Sweat V., Tirsi A., Rogers K., Bruehl H., Tsui W., Richardson S., Javier E., Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50(4):711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Haam J., Yakel J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017;142:111–121. doi: 10.1111/jnc.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Mohieldein A. In vivo evaluation of anti-diabetic, hypolipidemic, antioxidative activities of Saudi date seed extract on streptozotocin induced diabetic rats. J. Clin. Diagnostic Res. 2016;10:FF06-FF12. doi: 10.7860/JCDR/2016/16879.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C.D., Gandhi C., Bhattacharya P., Walsh T.J. Effects of intraseptal zolpidem and chlordiazepoxide on spatial working memory and high-affinity choline uptake in the hippocampus. Neurobiol. Learn. Mem. 2000;73(2):168–179. doi: 10.1006/nlme.1999.3928. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation, 2017. eighth ed. 2017, IDF Diabetes Atlas.
- Kalantaripour T.P., Asadi-Shekaari M., Basiri M., G.n.a., Cerebroprotective effect of date seed extract (Phoenix dactylifera) on focal cerebral ischemia in male rats. J. Biol. Sci. 2012;148:180–185. https://scialert.net/abstract/?doi=jbs.2012.180.185 [Google Scholar]
- Khalid S., Khalid N., Khan R.S., Ahmed H., Ahmad A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017;63:60–69. doi: 10.1016/j.tifs.2017.02.009. [DOI] [Google Scholar]
- Kodl C.T., Seaquist E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A., Guest P.C. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. 10.1007%2F978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- Liet C., Amenouche F., Freret T., Boulouard M., Mauvieux B., Lelong-Boulouard V., Bocca M.-L. Effects of acute administration of melatonin on attentional, executive, and working memory processes in rats. Fundam. Clin. Pharmacol. 2015;29(5):472–477. doi: 10.1111/fcp.12134. [DOI] [PubMed] [Google Scholar]
- Lipnick R.L., Cotruvo J.A., Hill R.N., Bruce R.D., Stitzel K.A., Walker A.P., Chu I., Goddard M., Segal L., Springer J.A., Myers R.C. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem. Toxicol. 1995;33(3):223–231. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- Malik J., Kumar M., Deshmukh R., Kumar P. Ameliorating effect of lyophilized extract of Butea frondosa leaves on scopolamine-induced amnesia in rats. Pharm. Biol. 2013;51(2):233–239. doi: 10.3109/13880209.2012.717229. [DOI] [PubMed] [Google Scholar]
- Mani V. Betahistine protects doxorubicin-induced cognitive impairment by improving cholinergic activity and attenuating proinflammatory cytokines in mouse brain. Int. J. Pharmacol. 2021;17:584–595. https://scialert.net/abstract/?doi=ijp.2021.584.595 [Google Scholar]
- Mani V., Sajid S., Rabbani S.I., Alqasir A.S., Alharbi H.A., Alshumaym A. Anxiolytic-like and antidepressant-like effects of ethanol extract of Terminalia chebula in mice. J. Tradit. Complement. Med. 2021;11(6):493–502. doi: 10.1016/j.jtcme.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K., Katare D.P. Shared links between type 2 diabetes mellitus and Alzheimer's disease: A review. Diabetes Metab. Syndr. 2016;10(2):S144–S149. doi: 10.1016/j.dsx.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Mohammed H.A., Ali H.M., Qureshi K.A., Alsharidah M., Kandil Y.I., Said R., Mohammed S.A.A., Al-Omar M.S., Rugaie O.A., Abdellatif A.A.H., Abd-Elmoniem E., Abbas M.M., Mohany K.M., Khan R.A. Comparative phytochemical profile and biological activity of four major medicinal halophytes from Qassim flora. Plants. 2021;10(10):2208. doi: 10.3390/plants10102208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., Soltani M., Siahpoosh A., Hosseini Shekarabi S.P., Shamsaie Mehrgan M., Lymbery A. Effect of date palm (Phoenix dactylifera) seed extract as a dietary supplementation on growth performance immunological haematological biochemical parameters of common carp. Aquacult. Res. 2018;49(8):2903–2912. doi: 10.1111/are.13760. [DOI] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comp. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq N., Schmatz R., Pereira L.B., Ahmad M., Stefanello N., Vieira J.M., Abdalla F., Rodrigues M.V., Baldissarelli J., Pelinson L.P., Dalenogare D.P., Reichert K.P., Dutra E.M., Mulinacci N., Innocenti M., Bellumori M., Morsch V.M., Schetinger M.R. Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2014;32(3):287–293. doi: 10.1002/cbf.3014. [DOI] [PubMed] [Google Scholar]
- Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Pérez‐Taboada I., Alberquilla S., Martín E.D., Anand R., Vietti‐Michelina S., Tebeka N.N., Cantley J., Cragg S.J., Moratalla R., Vallejo M. Diabetes causes dysfunctional dopamine neurotransmission favoring nigrostriatal degeneration in mice. Mov. Disord. 2020;35(9):1636–1648. doi: 10.1002/mds.28124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A., Markaki E., Rahiotis C., Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Priyanthi C., Sivakanesan R., Castillo A. The total antioxidant capacity and the total phenolic content of rice using water as a solvent. Int. J. Food Sci. 2021;2021:1–6. doi: 10.1155/2021/5268584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings A.M., Sharrett A.R., Schneider A.L.C., Coresh J., Albert M., Couper D., Griswold M., Gottesman R.F., Wagenknecht L.E., Windham B.G., Selvin E. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann. Intern. Med. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera E.J., Goldin A., Fulmer N., Tavares R., Wands J.R., de la Monte S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J. Alzheimer’s Dis. 2005;8(3):247–268. doi: 10.3233/JAD-2005-8304. [DOI] [PubMed] [Google Scholar]
- Rizzi L., Rosset I., Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res. Int. 2014;2014:1–8. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener M.L., Wooten M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015;1–6 doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semuyaba I., Safiriyu A.A., Tiyo E.A., Niurka R.F. Memory Improvement Effect of Ethanol Garlic (A. sativum) Extract in Streptozotocin-Nicotinamide Induced Diabetic Wistar Rats Is Mediated through Increasing of Hippocampal Sodium-Potassium ATPase, Glutamine Synthetase, and Calcium ATPase Activities. Evid.-Based Complement. Alternat. Med. 2017;2017:1–7. doi: 10.1155/2017/3720380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddeeg A., Zeng X.-A., Ammar A.-F., Han Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019;56(2):754–762. doi: 10.1007/s13197-018-3534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.M., Harrod S.B., Mactutus C.F., Booze R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods. 2007;166(1):99–103. doi: 10.1016/j.jneumeth.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancampiano R., Cocco S., Cugusi C., Sarais L., Fadda F. Serotonin and acetylcholine release response in the rat hippocampus during a spatial memory task. Neuroscience. 1999;89(4):1135–1143. doi: 10.1016/S0306-4522(98)00397-2. [DOI] [PubMed] [Google Scholar]
- Subash S., Essa M.M., Braidy N., Awlad-Thani K., Vaishnav R., Al-Adawi S., Al-Asmi A., Guillemin G.J. Diet rich in date palm fruits improves memory, learning and reduces beta amyloid in transgenic mouse model of Alzheimer’s disease. J. Ayurveda Integr. Med. 2015;6:111–120. doi: 10.4103/0975-9476.159073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012;237(5):481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- Takeda S., Sato N., Uchio-Yamada K., Sawada K., Kunieda T., Takeuchi D., Kurinami H., Shinohara M., Rakugi H., Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. U. S. A. 2010;107(15):7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z.-X., Shi L.-E., Aleid S.M. Date fruit: chemical composition, nutritional and medicinal values, products. J. Sci. Food Agric. 2013;93(10):2351–2361. doi: 10.1002/jsfa.6154. [DOI] [PubMed] [Google Scholar]
- Tallini L., Bastida J., Cortes N., Osorio E., Theoduloz C., Schmeda-Hirschmann G. Cholinesterase inhibition activity, alkaloid profiling and molecular docking of Chilean rhodophiala (Amaryllidaceae) Molecules. 2018;23(7):1532. doi: 10.3390/molecules23071532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A., Paliwal P., Krishnamurthy S. Piracetam attenuates LPS-induced neuroinflammation and cognitive impairment in rats. Cell. Mol. Neurobiol. 2017;37(8):1373–1386. doi: 10.1007/s10571-017-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S.M., Wands J.R. Alzheimer’s disease is type 3 diabetes- evidence reviewed. J. Diabetes Sci. Technol. 2008;2(6):1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Song F., Xu K., Liu Z., Han S., Li F., Sun Y.u. Irisin attenuates neuroinflammation and prevents the memory and cognitive deterioration in streptozotocin-induced diabetic mice. Mediators Inflamm. 2019;2019:1–8. doi: 10.1155/2019/1567179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Shen Y., Wang S., Li S., Zhang W., Liu X., Lai L., Pei J., Li H. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45:356–360. doi: 10.1093/nar/gkx374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J.L. Nicotinic ACh receptors in the hippocampus: Role in excitability and plasticity. Nicotine Tob. Res. 2012;14(11):1249–1257. doi: 10.1093/ntr/nts091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf H., Shamim S., Khan K.M., Chigurupati S., Kanwal, Hameed S., Khan M.N., Taha M., Arfeen M. Dihydropyridines as potential α-amylase and α-glucosidase inhibitors: Synthesis, in vitro and in silico studies. Bioorg. Chem. 2020;96:103581. doi: 10.1016/j.bioorg.2020.103581. [DOI] [PubMed] [Google Scholar]
- Zhao W.-Q., Chen H., Quon M.J., Alkon D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490(1-3):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Zihad S.M.N.K., Uddin S.J., Sifat N., Lovely F., Rouf R., Shilpi J.A., Sheikh B.Y., Göransson U. Antioxidant properties and phenolic profiling by UPLC-QTOF-MS of Ajwah, Safawy and Sukkari cultivars of date palm. Biochem. Biophys. Reports. 2021;25:100909. doi: 10.1016/j.bbrep.2021.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilliox L.A., Chadrasekaran K., Kwan J.Y., Russell J.W. Diabetes and cognitive impairment. Curr. Diab. Rep. 2016;16:1–11. doi: 10.1007/s11892-016-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]