Abstract
Background
Due to lack of targeted treatment options and inconsistent utilization of histologic endpoints among clinical trials, identifying efficacious pharmacotherapies for nonalcoholic steatohepatitis [NASH] has proven challenging.
Methods
A thorough systematic review and frequentist random-effects network meta-analysis was performed across all randomized clinical trials reporting a pharmacotherapeutic intervention on biopsy-proven NASH. Primary outcomes were based on the most current, up-to-date recommended histologic endpoints.
Results
A total of 40 RCTs were identified including 6593 total patients. The most effective and statistically significant treatment interventions for minimum two-point improvement in NAFLD Activity Score were aldafermin 1 mg [RR 7.69, 95% CI 2.00; 29.57], vitamin E 800 IU in combination with pioglitazone 45 mg [RR 3.38, 95% CI 1.88; 6.07], pioglitazone 45 mg [RR 3.29, 95% CI 1.74; 6.22], vitamin E 800 IU [RR 2.06, 95% CI 1.33; 3.18], resmetirom 80 mg [RR 1.74, 95% CI 1.03; 2.94], obeticholic acid 25 mg [RR 1.63, 95% CI 1.32; 2.01], and obeticholic acid 10 mg [RR 1.31, 95% CI 1.02; 1.67]). The most robust pharmacotherapies for NASH resolution without worsening fibrosis were found to be aldafermin 1 mg [RR 5.77, 95% CI 1.48; 22.51], pioglitazone 45 mg [RR 2.65, 95% CI 1.43; 4.91], vitamin E 800 IU in combination with pioglitazone 45 mg [RR 2.64, 95% CI 1.36; 5.12], pioglitazone 30 mg [RR 2.46, 95% CI 1.56; 3.88], vitamin E 800 IU [RR 1.90, 95% CI 1.20; 3.00], and obeticholic acid 25 mg [RR 1.52, 95% CI 1.03; 2.23]). Obeticholic acid had a significant improvement on fibrosis. Multiple interventions were found to improve individual histologic scores across secondary outcome analyses and are detailed below.
Conclusion
This novel systematic review and network meta-analysis represents the most comprehensive investigation to date regarding the pharmacotherapeutic options for biopsy-proven NASH using current recommended histologic endpoints.
Keywords: nonalcoholic steatohepatitis, NASH, histology, biopsy, network meta-analysis
Abbreviations: CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; IQR, interquartile range; MD, mean difference; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD Activity Score; NASH, nonalcoholic steatohepatitis; NASH CRN, Nonalcoholic Steatohepatitis Clinical Research Network; PRISMA, Preferred Reporting Item for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; Rob 2, revised Cochrane risk of bias tool; RR, relative risk
The incidence of nonalcoholic fatty liver disease [NAFLD] continues to rise on a global scale.1 Accordingly, nonalcoholic steatohepatitis [NASH] has become an increasingly ubiquitous cause of cirrhosis, hepatocellular carcinoma, and liver transplantation.2 Lifestyle modifications, weight loss, and treatment of concomitant manifestations of metabolic syndrome have become cornerstones of management for NASH. Much investigation regarding pharmacologic intervention for the treatment of this disease process has been undertaken, and a myriad of these pharmacologic agents, aimed at a multitude of cellular targets and signaling pathways, are detailed in Table 1.
Table 1.
Current Pharmacotherapeutics for NASH. Current Pharmacotherapeutics for Nonalcoholic Steatohepatitis, Cellular Targets, and their Proposed Mechanism of Action.
| Agent [alternative or experimental name] | Proposed mechanism of action |
|---|---|
| Aldafermin [NGM282] | FGF19 analog |
| Belapectin | Galectin 3 inhibitor |
| Cenicriviroc | C–C chemokine receptor type 2 and 5 dual antagonist |
| Colesevelam | Bile acid sequestrant, thus increasing turnover of hepatic cholesterol to the formation of bile acids; colesevelam also has been shown to increase GLP-1 levels |
| Elafibranor | Dual PPAR-α/δ agonist |
| Emricasan | Pan-caspase inhibitor |
| Ezetimibe | Inhibits small intestine absorption of cholesterol |
| Liraglutide | Long-acting GLP-1 analogue |
| Losartan | Angiotensin II receptor blocker |
| Metadoxine | Reduces oxidative stress (by restoring NADH, GSH, and ATP levels) and anti-inflammatory effects by decreasing activity of proline hydroxylase, TNF-⍺, and procollagen |
| Metformin | Improves insulin sensitivity and decreases hepatic gluconeogenesis |
| MSDC0602K | 2nd generation TZD; Insulin sensitizer that preferentially targets the mitochondrial pyruvate carrier and has only minimal direct interaction with PPAR-γ |
| Obeticholic acid | Selective FXR agonist |
| omega-3 PUFA | Multiple identified cellular targets including sterol response element-binding protein, interleukin 6, angiotensin 2, and nitric oxide-mediated signaling, thus improving hepatic steatosis, insulin sensitivity, and inflammation reduction |
| Pentoxifylline | Inhibits TNF-⍺ and phosphodiesterase, which exhibits anti-inflammatory and vasodilator properties, respectively |
| Pioglitazone | 1st generation TZD; Insulin sensitizer in addition to potent and selective PPAR-γ agonist |
| Resmetirom [MGL-3196] | Selective thyroid hormone receptor β agonist |
| Rosiglitazone | 1st generation TZD; Insulin sensitizer in addition to potent and selective PPAR-γ agonist |
| Saroglitazar | Dual PPAR-⍺/γ agonist |
| Selonsertib | ASK1 inhibitor |
| Semaglutide | GLP-1 receptor agonist |
| Silymarin | Antioxidant |
| Simtuzumab | Monoclonal antibody that binds and inhibits LOXL2 |
| Sitagliptin | DPP-4 inhibitor |
| Telmisartan | Angiotensin II receptor blocker |
| UDCA | Lowers bile acid levels, reduces oxidative stress, and also has demonstrated anti-apoptotic properties |
| Vitamin E | Antioxidant |
| Volixibat [SHP626; LUM002] | ASBT inhibitor |
Abbreviations: ASBT, apical sodium-dependent bile acid transporter; ASK1, apoptosis signal-regulating kinase 1; ATP, adenosine triphosphate; DPP-4, dipeptidyl peptidase-4; FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; GSH, glutathione; LOXL2, lysyl oxidase-like 2; NADH, nicotinamide adenine dinucleotide; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator activated receptor; PUFA, polyunsaturated fatty acid; TNF-⍺, tumor necrosis factor alpha; TZD, thiazolidinedione; UDCA, ursodeoxycholic acid.
Despite the recent flurry of pharmacotherapeutics development for NASH, an overall lack of targeted treatment options remains. One reason for the paucity of therapeutic strategies may be explained by a complex pathophysiologic disease process, interwoven by a variety of aberrant signaling pathways that are not reversed by a single pharmacologic agent. Another etiology for this phenomenon is explained best by the inconsistency among clinical endpoints over the years among RCTs. There has been a recent call for updated histologic endpoints among RCTs for NASH to more uniformly delineate the clinical efficacy of these pharmacotherapeutic options.3, 4, 5, 6 The most up-to-date recommendations for histologic endpoints include the following: a minimum two-point improvement in NAFLD activity score [NAS], NASH resolution without worsening fibrosis, and fibrosis improvement without NASH worsening.
The primary aim of this manuscript is to conduct a thorough systematic review and network meta-analysis to determine the clinical impact of pharmacotherapeutic options for NASH. No such network meta-analysis based on the current recommendations for histologic endpoints has been performed to date.
Methods
Literature Search
Three major databases, including MEDLINE/PubMed, EMBASE, and CENTRAL [Cochrane Central Register of Controlled Trials], were searched for clinical studies dated from inception to November 12, 2020. To broadly identify randomized, controlled trials detailing pharmacologic interventions among patients with biopsy-proven NASH, the following search criteria were utilized: “(nonalcoholic fatty liver OR nonalcoholic steatohepatitis OR nafld OR nash) AND randomi∗ AND control∗.” All data extraction performed was conducted according to the Preferred Reporting Item for Systematic Reviews and Meta-Analyses [PRISMA] extension statement for incorporating network meta-analyses.7
Inclusion Criteria
Articles and clinical trials that met the following inclusion criteria were eligible for this meta-analysis: (1) studies performed in adult, human subjects; (2) randomized, controlled clinical studies or trials, irrespective of the phase of the clinical trial; (3) biopsy-proven NASH; (4) histologic criteria assessed using the NASH CRN scoring system.8
Exclusion Criteria
Studies with the following characteristics were excluded from this meta-analysis: (1) studies in non-human subjects; (2) studies that were not a clinical trial, such as a review paper, letter, case report, proposal, or protocol design; (3) studies that were out of scope of the study question detailed earlier; (4) studies that lacked proper controls; (5) studies that did not provide raw data to perform quantitative meta-analysis; (6) studies conducted in pediatric subjects; (7) studies written only in a language other than English; (8) studies that did not have an available manuscript; (9) studies that were duplicates; (10) studies that were ongoing or not completed.
Outcomes and Endpoints
The primary outcomes for this network meta-analysis were as follows:
-
1.
Minimum two-point improvement in NAFLD Activity Score [NAS]
-
2.
Resolution of NASH without worsening fibrosis
-
3.
Improvement of fibrosis without worsening of NASH
-
4.
Improvement of fibrosis
As detailed in the NASH CRN scoring system,8 the NAFLD activity score [NAS] ranges from a score of 0–8 and is the unweighted summation of individual scores for steatosis, lobular inflammation, and hepatocellular ballooning. The primary outcomes were developed in accordance with current recommendations regarding endpoints for NASH in clinical trials.3, 4, 5, 6 NASH resolution was defined as a disappearance of hepatocellular ballooning [score of 0] in addition to either a disappearance or mild persistence of lobular inflammation [score of 0 or 1]. Improvement in fibrosis was defined as a minimum one-stage decrease in fibrosis scoring. Worsening NASH and fibrosis were defined as any increase in lobular inflammation/hepatocellular ballooning score or fibrosis stage, respectively. For this network meta-analysis, the endpoint of improvement in fibrosis alone was also conducted since fibrosis has been demonstrated to be the strongest predictor of liver-related mortality among patients with NAFLD/NASH.9,10
Secondary outcomes for this network meta-analysis include the following:
-
1.
All-cause mortality
-
2.Mean change from baseline of individual histologic components based on NASH CRN scoring system:
-
•NAS
-
•Steatosis
-
•Lobular inflammation
-
•Hepatocellular ballooning
-
•Fibrosis
-
•
Data Extraction
Ambiguity in the reported data was attempted to be resolved by emailing the corresponding author of the study where appropriate. Author names, dates, study type, setting of study, number and characteristics of patients, and treatment outcomes were gathered for all included studies (Supplementary Materials, Appendices 1 and2). Of note, the following comparisons were merged into a single intervention entitled “placebo”: placebo, control, or RCT treatment arm with no additional interventions aside from pharmacologic agent implemented in other treatment arm(s).
Risk of Bias
Since all included studies were randomized, the revised Cochrane risk of bias tool [RoB 2]11 was used to assess the risk of bias (Supplementary Materials, Appendix 6). RoB 2 tool assessed the risk of bias through the presence of bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Each form of bias was awarded either a “low risk of bias,” “some concerns,” or “high risk of bias.”
Data Synthesis and Statistical Analysis
A conventional pairwise meta-analysis utilizing DerSimonian and Laird random-effects model was implemented for all direct comparisons.12 Pooled relative risk [RR] was used as a summary measure of efficacy for dichotomous data, and pooled mean differences [MD] were used for continuous variables. 95% confidence intervals [CI] were reported for both measures. Data were considered statistically insignificant if the 95% CI of RR includes 1.00 or MD includes zero. If medians, interquartile ranges [IQR], 95% CI, and/or P values were provided in the included RCTs instead of mean and standard deviations, the mean and standard deviations were imputed as described previously,13, 14, 15 or as additionally described in the Cochrane Handbook for Systematic Reviews.16 Heterogeneity was assessed through visual inspection of the forest plots in addition to calculating the I2 statistic. I2 values of ≥50% were deemed as significant heterogeneity.17
A frequentist random-effects network meta-analysis was performed to analyze direct and indirect comparisons (Supplementary Materials, Appendix 4).18 Multi-arm RCTs were not excluded in this network meta-analysis as long as they satisfied the inclusion criteria detailed earlier. If a particular study was not connected to the network, it was subsequently removed from the quantitative analysis to conduct the network meta-analysis for each outcome. A treatment hierarchy was achieved through calculation of P-scores across all outcomes included in the network meta-analysis (Supplementary Materials, Appendix 5).19 Relative ranking of interventions was performed in the frequentist meta-analysis and based on a continuous P-score scale, which ranged from 0 [worst intervention] to 1 [best intervention], and a validated equivalent to the surface under the cumulative ranking curve [SUCRA] score.19 Depending on the presence of dichotomous or continuous variables reported, a RR or MD was calculated for each treatment, in addition to the corresponding 95% confidence interval. To assess small study effects, the assessment was performed using a comparison-adjusted funnel plot and corresponding Egger test for each outcome (Supplementary Materials, Appendix 11).18,20
Inconsistency, also referred to as incoherence among network meta-analyses, was meticulously assessed using several measures. First, global inconsistency across each network was utilized via the design-by-treatment model for each outcome (Supplementary Materials, Appendix 7).21 Second, the node-splitting method was performed to compare direct and indirect evidence.17,22 Inconsistency was visualized by forest plots of the node-splitting (Supplementary Materials, Appendix 9), in addition to network heat plots for each network (Supplementary Materials, Appendix 8). A loop-specific approach was implemented to calculate the ratio or absolute difference between direct and indirect estimates for endpoints reporting dichotomous or continuous variables, respectively (Supplementary Materials, Appendix 10). For each of these closed loops, the 95% CI and P values were also calculated to determine if any statistical inconsistency was present.
Summary of evidence was performed with each network estimate using the Grading of Recommendations, Assessment, Development, and Evaluation [GRADE] approach, which could lower the certainty of included RCTs based upon limitations in risk of bias, imprecision, inconsistency, indirectness, and publication bias (Supplementary Materials, Appendix 12).23 High-quality studies could potentially be downgraded to moderate, low, or very low quality, depending on their specific limitations. Using this approach, all direct, followed by indirect, comparisons were rated and cataloged. The quality and summary of evidence for the network estimate were based on the superior rating from either the direct and indirect evidence, or in some instances, only one of the two if either direct or indirect data was not present.24
This network meta-analysis was planned to be performed by evaluating primary and secondary outcomes detailed earlier. Additionally, subgroup analyses were outlined to surmise any further changes in treatment effect (Supplementary Materials, Appendices 13–15). This was performed by duplicating the network meta-analysis in the presence of excluding RCTs with one of the three following treatment variables:
-
1.
All patients with cirrhosis at baseline
-
2.
All patients with diabetes mellitus at baseline
-
3.
Length of treatment intervention reported as six months or less
This network meta-analysis was performed using R Studio [Version 1.4.1106] for all statistical analyses. CINeMA [Version 1.9.1] was utilized for thoroughly categorizing summary of evidence measures across all outcomes for network meta-analysis.25,26
Results
Study Selection
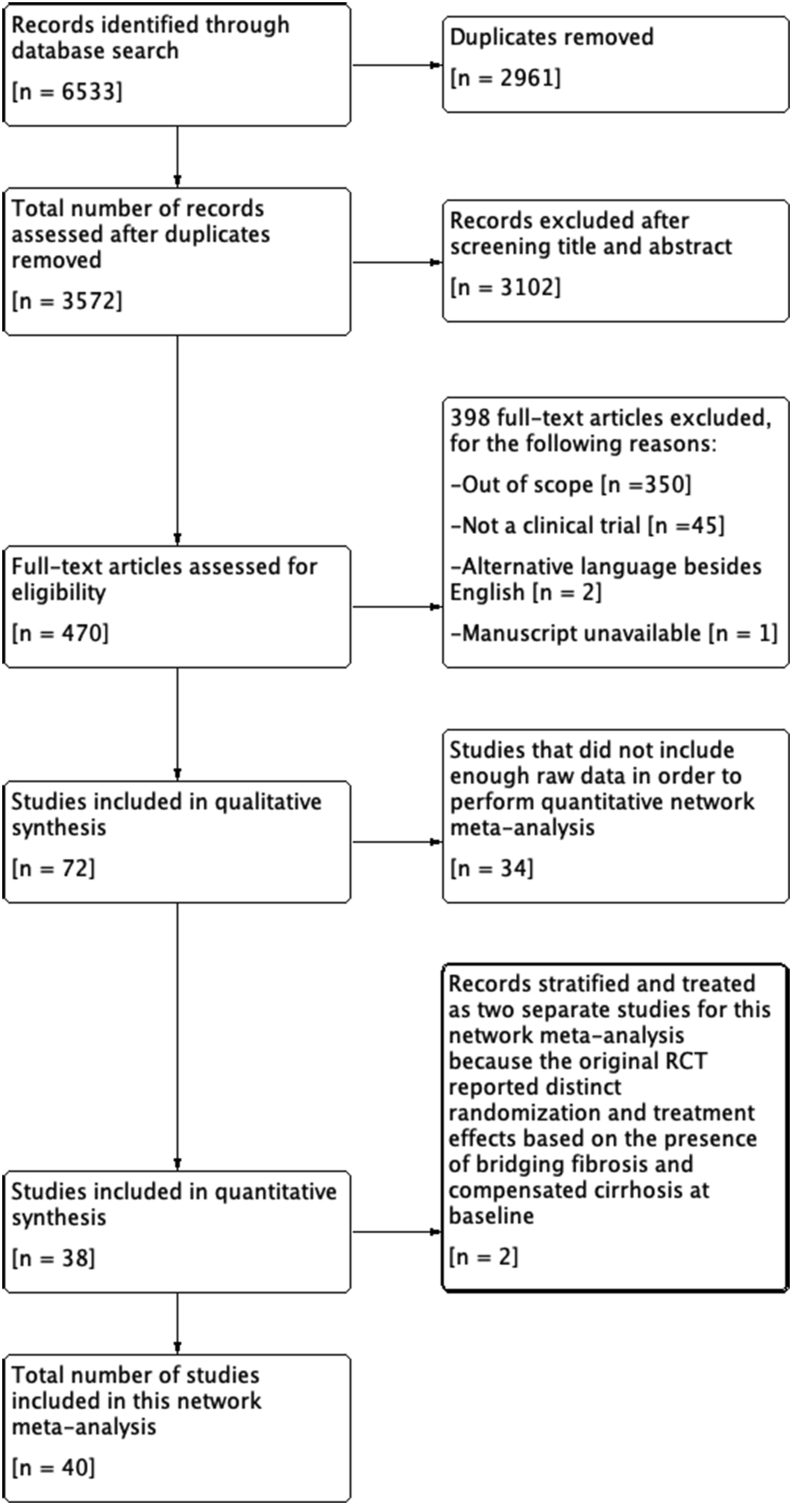
The data search, literature review, and study selection are outlined in Figure 1. 6533 studies were identified via the thorough literature search strategy detailed above. After the removal of duplicates and studies not satisfying the predefined inclusion criteria, only 72 RCTs remained. 34 studies did not include enough raw data to conduct a quantitative network meta-analysis. Two studies stratified outcomes based on patients with bridging fibrosis and cirrhosis at baseline,27,28 and thus were split into two separate studies for this network meta-analysis. In summation, a total of 40 RCTs were included in this network meta-analysis comprised of 6593 total patients (Supplementary Materials, Appendices 1 and 2).
Figure 1.
Study flow diagram. Study flow diagram detailing the thorough literature search and rationale for inclusion/exclusion of clinical studies.
Network Meta-analysis Results of Primary and Secondary Outcomes
Network plots for each outcome are listed in Supplementary Materials, Appendix 3. Overall results for individual primary and secondary outcomes are characterized in Table 2, Table 3, respectively. The network estimate and relative treatment rank are reported for each outcome. The treatment effect with respect to the GRADE summary of evidence is designated in the corresponding legend for each table. Further results, tables, and figures are detailed and specifically categorized in Supplementary Materials, Appendices 3–16.
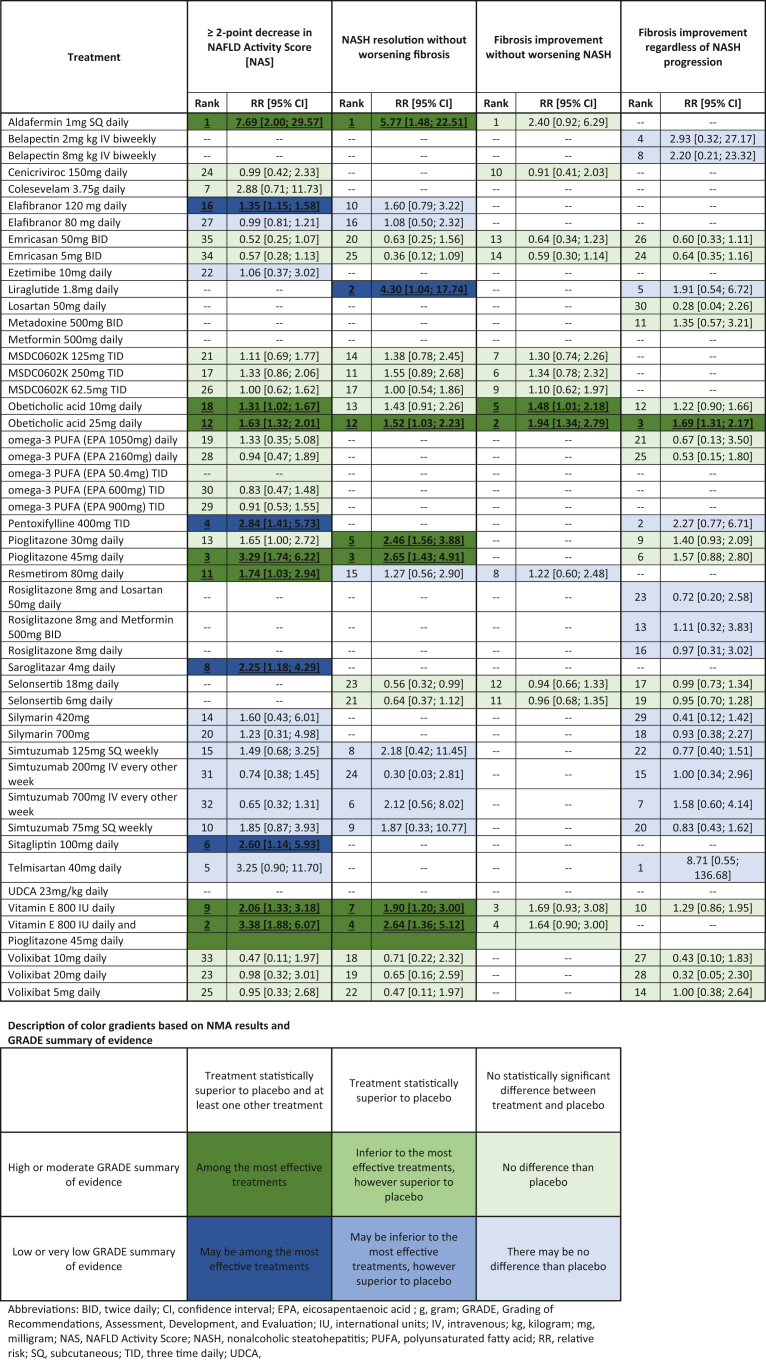
Table 2.
Network Meta-analysis Results for Primary Outcomes Listed as Treatment Intervention Compared to Placebo for All Endpoints. If Present for the Network, a Treatment Rank, Treatment Effect, and 95% Confidence Interval are Provided. The Quality and GRADE Summary of Evidence for Each Intervention is also Provided Using the Color Gradient Described.
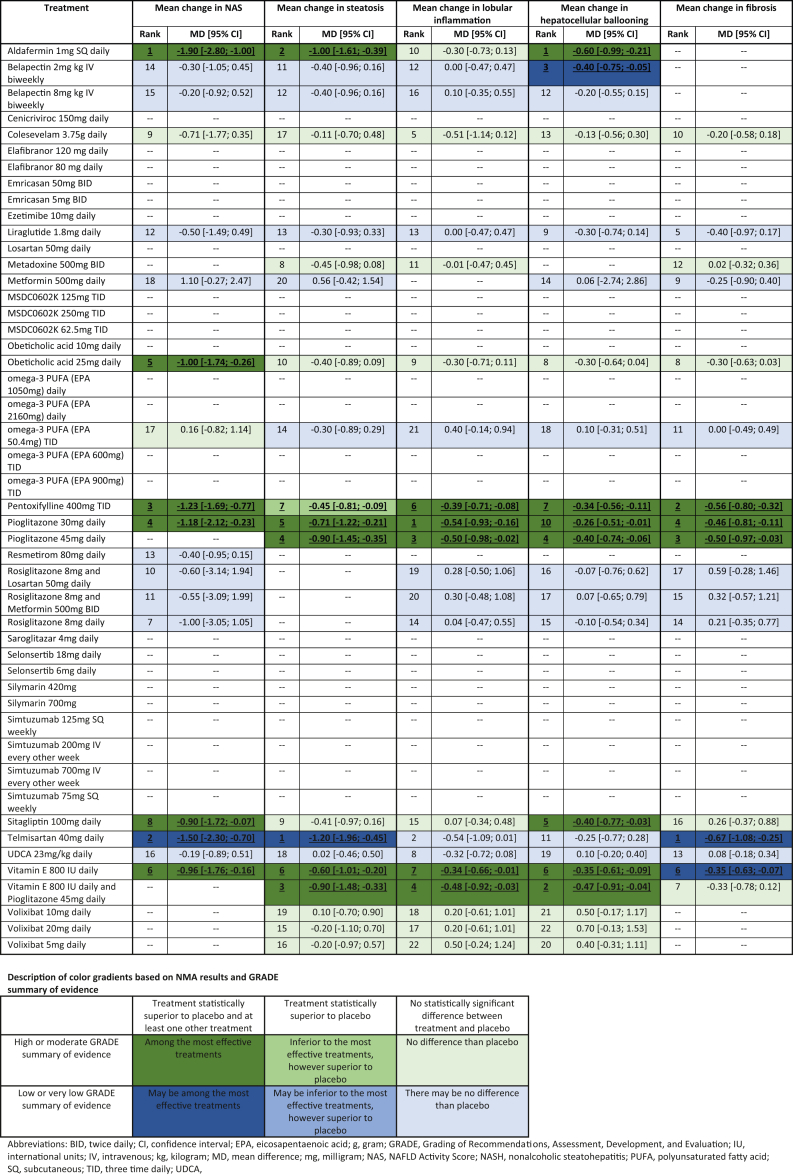
Table 3.
Network Meta-analysis Results for Secondary Outcomes Listed as Treatment Intervention Compared to Placebo for All Endpoints. If Present for the Network, a Treatment Rank, Treatment Effect, and 95% Confidence Interval are Provided. The Quality and GRADE Summary of Evidence for Each Intervention is also Provided Using the Color Gradient Described.
Minimum Two-point Improvement in NAS Score
A total of 25 RCTs report the histologic endpoint of ≥2-point decrease in NAS across 36 treatment comparisons. Overall, several treatments were found to be superior to placebo (Table 2). In order of treatment rank, the pooled RR of aldafermin 1 mg [RR 7.69, 95% CI 2.00; 29.57], vitamin E 800 IU and pioglitazone 45 mg [RR 3.38, 95% CI 1.88; 6.07], pioglitazone 45 mg [RR 3.29, 95% CI 1.74; 6.22], vitamin E 800 IU [RR 2.06, 95% CI 1.33; 3.18], resmetirom 80 mg [RR 1.74, 95% CI 1.03; 2.94], obeticholic acid 25 mg [RR 1.63, 95% CI 1.32; 2.01], and obeticholic acid 10 mg [RR 1.31, 95% CI 1.02; 1.67] were found to be statistically superior and of high or moderate GRADE summary of evidence, making them the most effective treatments in this network meta-analysis. Pentoxifylline 400 mg TID [RR 2.84, 95% CI 1.41; 5.73], sitagliptin 100 mg [RR 2.60, 95% CI 1.14; 5.93], saroglitazar 4 mg [RR 2.25, 95% CI 1.18; 4.29], and elafibranor 120 mg [RR 1.35, 95% CI 1.15; 1.58] may be among the most effective treatments given their statistical superiority, however, comprised low or very low GRADE summary of evidence. No heterogeneity was found with reported I2 = 0%.
NASH Resolution without Worsening Fibrosis
A total of 16 RCTs analyzed the endpoint of resolution of NASH without worsening fibrosis, which was performed across 26 treatments (Table 2). Aldafermin 1 mg [RR 5.77, 95% CI 1.48; 22.51], pioglitazone 45 mg [RR 2.65, 95% CI 1.43; 4.91], vitamin E 800 IU and pioglitazone 45 mg [RR 2.64, 95% CI 1.36; 5.12], pioglitazone 30 mg [RR 2.46, 95% CI 1.56; 3.88], vitamin E 800 IU [RR 1.90, 95% CI 1.20; 3.00], and obeticholic acid 25 mg [RR 1.52, 95% CI 1.03; 2.23] were among the most effective therapies due to its high or moderate GRADE evidence and statistical superiority. Only one intervention was statistically superior and among low or very low GRADE evidence, which includes liraglutide 1.8 mg with pooled RR 4.30 [95% CI 1.04; 17.74]. Overall, no heterogeneity was identified across this network with I2 = 0%.
Improvement of Fibrosis without Worsening of NASH
Nine RCTs reported improvement of fibrosis without worsening NASH. Fifteen total treatment interventions were implemented across this network (Table 2). Only two interventions, obeticholic acid 25 mg [RR 1.94, 95% CI 1.34; 2.79] and obeticholic acid 10 mg [RR 1.48, 95% CI 1.01; 2.18], were found to be statistically superior. These treatments comprised high or moderate quality GRADE evidence, making them the most effective treatments. No heterogeneity was noted across this network with I2 = 0.4%.
Improvement of Fibrosis
Twenty-one RCTs including 31 different treatment interventions investigated the histologic improvement of fibrosis among biopsy-proven NASH (Table 2). Obeticholic acid 25 mg [RR 1.69, 95% CI 1.31; 2.17] was the only intervention statistically superior and was found to be of high or moderate GRADE evidence. No significant heterogeneity was reported across the network with I2 = 0%.
Mean Change in NAFLD Activity Score [NAS]
Nineteen different interventions reported across nineteen RCTs exist that studied the mean change in NAS as compared from end of treatment to baseline. Overall, there are several interventions found to be significantly superior detailed in Table 3, most notably aldafermin 1 mg [MD −1.90, 95% CI −2.80; −1.00], pentoxifylline 400 mg TID [MD −1.23, 95% CI −1.69; −0.77], pioglitazone 30 mg [MD −1.18, 95% CI −2.12; −0.23], obeticholic acid 25 mg [MD −1.00, 95% CI −1.74; −0.26], vitamin E 800 IU [MD −0.96, 95% CI −1.76; −0.16], and sitagliptin 100 mg [MD −0.90, 95% CI −1.72; −0.07]. Telmisartan 40 mg was found to be superior as well with pooled MD −1.50 [95% CI −2.30; −0.70]; however, this was based on low or very low GRADE quality evidence. No significant heterogeneity was present with I2 = 36.8%.
Mean Change in Steatosis
Nineteen RCTs compared the mean change in steatosis score across 25 various treatments [Table 3]. Aldafermin 1 mg [MD −1.00, 95% CI −1.61; −0.39], vitamin E 800 IU and pioglitazone 45 mg [MD −0.90, 95% CI -1.48; −0.33], pioglitazone 45 mg [MD −0.90, 95% CI −1.45; −0.35], pioglitazone 30 mg [MD −0.71, 95% CI −1.22; −0.21], vitamin E 800 IU [MD −0.60, 95% CI −1.01; −0.20], and pentoxifylline 400 mg TID [MD −0.45, 95% CI −0.81; −0.09] were all found to be interventions statistically superior with respect to reduction of the steatosis score. Telmisartan 40 mg was also superior to placebo with pooled MD −1.20 [95% CI −1.96; −0.45]; however, this was based on low-quality GRADE evidence. No significant heterogeneity was noted with I2 = 46.6%.
Mean Change in Lobular Inflammation
Twenty RCTs among 23 interventions analyzed the histologic endpoint of mean change in lobular inflammation scores from baseline to end of treatment (Table 3). Several treatments demonstrated statistical superiority, including pioglitazone 30 mg [MD −0.54, 95% CI −0.93; −0.16], pioglitazone 45 mg [MD −0.50, 95% CI −0.98; −0.02], vitamin E 800 IU and pioglitazone 45 mg [MD −0.48, 95% CI −0.92; −0.03], pentoxifylline 400 mg TID [MD −0.39, 95% CI −0.71; −0.08], and vitamin E 800 IU [MD −0.34, 95% CI −0.66; −0.01]. Overall I2 = 41.9% indicating that no significant heterogeneity was present.
Mean Change in Hepatocellular Ballooning
Twenty-three different treatments were described across 20 RCTs investigating the mean change in hepatocellular balloon scoring (Table 3). Multiple treatment measures were found to be statistically superior including aldafermin 1 mg [MD −0.60, 95% CI −0.99; −0.21], vitamin E 800 IU and pioglitazone 45 mg [MD −0.47, 95% CI −0.91; −0.04], pioglitazone 45 mg [MD −0.40, 95% CI −0.74; −0.06], sitagliptin 100 mg [MD −0.40, 95% CI −0.77; −0.03], vitamin E 800 IU [MD −0.35, 95% CI −0.61; −0.09], pentoxifylline 400 mg TID [MD −0.34, 95% CI −0.56; −0.11], and pioglitazone 30 mg [MD −0.26, 95% CI −0.51; −0.01]. Belapectin 2 mg also demonstrated a statistically superior response with pooled MD −0.40 [95% CI −0.75; −0.05]; however, this was based on low-quality GRADE evidence. No statistical heterogeneity was present across the network with I2 = 30%.
Mean Change in Fibrosis
Nineteen RCTs including eighteen different treatments compared mean change fibrosis scores from baseline to end of treatment (Table 3). Of data with high or moderate GRADE evidence, pentoxifylline 400 mg TID [MD −0.56, 95% CI −0.80; −0.32], pioglitazone 45 mg [MD −0.50, 95% CI −0.97; −0.03], and pioglitazone 30 mg [MD −0.46, 95% CI −0.81; −0.11] established a statistically superior improvement of fibrosis scoring. Telmisartan 40 mg [MD −0.67, 95% CI −1.08; −0.25] and vitamin E 800 IU [MD −0.35, 95% CI −0.63; −0.07] also were found to be statistically superior; however, this was based on low-quality evidence after careful GRADE assessment. I2 = 14.6% thus substantiating that no statistical heterogeneity was present.
Mortality
Unfortunately, there was insufficient evidence to perform a network meta-analysis for the endpoint of all-cause mortality. The overwhelming majority of RCTs did not report any deaths within the timeframe of their study. Only seven RCTs remained that had reported cases of mortality, with most treatment arms still having no observed events. Thus, it was concluded that there was insufficient evidence to perform a comprehensive network meta-analysis for all-cause mortality.
Network Consistency
Results of global inconsistency as calculated by the design-by-treatment interaction model are reported in Supplementary Materials, Appendix 7. Overall, no significant global inconsistency was found among the networks for each individual outcome. Local inconsistency among networks for specific outcomes was visualized by network heat plots (Supplementary Materials, Appendix 8) and forest plots of node splitting (Supplementary Materials, Appendix 9), which did not reveal any overt inconsistency. Closed-loop data with both direct and indirect evidence were also analyzed (Supplementary Materials, Appendix 10). Overall, there was no evidence of inconsistency among these network meta-analyses, with one exception occurring within the secondary outcome of mean change in fibrosis where the comparison of vitamin E 800 IU daily versus placebo was found to have a statistically significant absolute difference of 0.84 [95% CI 0.04, 1.63; P = 0.04].
Small Study Effects
No asymmetry of comparison-adjusted funnel plots was noted to suggest evidence of small study effects, which was validated by the corresponding Egger test for each outcome (Supplementary Materials, Appendix 11).
Subgroup Analysis
Overall, there were no major trends or shifts in treatment rank order upon performing subgroup analyses for primary outcomes, and furthermore, there were no treatments that demonstrated statistically improved outcomes upon removal of these individual patient populations (Supplementary Materials, Appendices 13–15). This trend was also demonstrated across secondary outcomes with the one exception of the mean change in lobular inflammation endpoint. Upon removal of RCTs with 100% patients reported with diabetes mellitus, the following interventions went from insignificant to statistically significant compared to placebo with respect to improvement of lobular inflammation scores: telmisartan 40 mg daily, pioglitazone 45 mg daily, UDCA 23 mg/kg daily, obeticholic acid 25 mg daily, and aldafermin 1 mg SQ daily. Similarly, for the length of treatment subgroup analysis performed upon removal of RCTs with a length of treatment reported as 6 months or less, the following treatments become statistically superior to placebo for improvement of lobular inflammation: telmisartan 40 mg daily, UDCA 23 mg/kg daily, and obeticholic acid 25 mg daily.
Discussion
The paradigm for the management of patients with NASH remains dynamic. The flurry of recent RCTs will continue to fuel clinicians for greater pharmacotherapeutic options towards an increasingly prevalent disease. However, to date, there has been an overall inconsistent utilization of histologic endpoints among clinical trials. There have been two previous network meta-analyses that attempt to identify a hierarchy of pharmacologic treatments among patients with NASH; however, the recommend histologic endpoints of a minimum two-point improvement in NAS, NASH resolution without worsening fibrosis, and fibrosis improvement without NASH worsening. The first network meta-analysis was performed in 2015 where results of nine RCTs were identified including interventions of vitamin E, thiazolidinediones, pentoxifylline, and obeticholic acid.29 Primary outcome analyzed was an improvement in fibrosis, regardless of concomitant NASH resolution or progression. Secondary outcomes included improvement in individual histologic scores in steatosis, lobular inflammation, and balloon degeneration. All four interventions were lumped together in this network meta-analysis, demonstrating improvement in steatosis across all interventions, ballooning for all interventions except pentoxifylline, and only obeticholic acid as significantly improving fibrosis score. A more recent network meta-analysis was conducted across 26 RCTs and 23 different interventions.30 The primary outcome was an improvement in the fibrosis stage, and secondary outcomes included NASH resolution, without mention or consideration of a concomitant change in fibrosis. It was demonstrated that obeticholic acid, and possibly lanifibranor, were the most effective treatments for improvement in fibrosis, and semaglutide, liraglutide, and vitamin E plus pioglitazone had the highest treatment ranks for NASH resolution. Some of these conclusions identified are validated in this network meta-analysis as well. Not only are the findings in this current systematic review and network meta-analysis more recent and up-to-date, but there is a more exhaustive assessment of recommended histologic endpoints that recovers deficiencies made in prior network meta-analyses. Additionally, there are 48 interventions assessed over a large number of primary and secondary endpoints, making identification of treatment efficacy and rank much more robust. While there was no inclusion or exclusion criteria based on the clinical trial phase, only randomized controlled clinical trials were included in this meta-analysis, representing the most robust, impactful studies in the field. In the end, this systematic review and network meta-analysis provides the most novel, comprehensive, and accurate assessment of pharmacotherapeutic interventions on biopsy-proven NASH to date.
The overall results illuminate the current treatment options for NASH based upon current recommended histologic endpoints. Several themes are noted in the network meta-analysis across primary outcomes (Table 2). Aldafermin 1 mg was the highest-ranked treatment for the minimum two-point decrease in NAS and overall NASH resolution without worsening fibrosis. It was also the highest-ranked intervention for fibrosis improvement without worsening NASH; however, this was not found to be statistically significant. Vitamin E 800 IU alone or in combination with pioglitazone 45 mg were found to be statistically significant and of the most effective treatments. Pioglitazone alone, in both 45 mg and 30 mg doses, was also found to be effective intervention. Obeticholic acid was also found to be one of the most effective treatment strategies. Obeticholic acid 10 mg was statistically superior for two-point minimum improvement of NAS as well as fibrosis improvement without worsening NASH. Meanwhile, obeticholic acid 25 mg dose was a statistically effective treatment intervention across all primary outcomes. Resmetirom 80 mg also demonstrated promise with statistical superiority with respect to a two-point minimum decrease in NAS. There were treatments, including elafibranor 120 mg, liraglutide, saroglitazar 4 mg, and sitagliptin 100 mg, which demonstrated statistically significant findings among isolated outcomes for primary measures. However, these four interventions were comprised of low to very low GRADE evidence. Across secondary endpoints, several interventions found statistical benefit across the network. Significant interventions include aldafermin 1 mg, pioglitazone [either 30 mg or 45 mg], vitamin E 800 IU, vitamin E 800 IU in combination with pioglitazone 45 mg, obeticholic acid 25 mg, and pentoxifylline 400 mg TID. Telmisartan 40 mg daily also had a significant effect on many secondary endpoints; however, this was found to be low to very low GRADE evidence. Yet, many of these interventions were observed among low-quality RCTs. Overall across all networks, no significant heterogeneity was present. No statistical inconsistency was found, apart from the vitamin E 800 IU daily versus placebo comparison observed in the secondary outcome of mean change in fibrosis score [P = 0.04]. Sensitivity analysis through the subgroups of cirrhosis, diabetes mellitus, and length of treatment was broadly unrevealing. However, there did appear to be an isolated effect for several pharmacotherapeutic agents with respect to the secondary outcome of mean change in lobular inflammation scores upon subgroup analysis excluding RCTs with 100% patients with diabetes mellitus.
The area of pharmacotherapeutics and drug development for NASH remains a rapidly evolving topic of research. Since the completion of this literature search, several high-impact clinical trials have shown promise among NASH histologic endpoints. One recent RCT reports improvement in NASH resolution among patients receiving semaglutide for 72 weeks.31 Another phase 2 RCT assessing the effect of aldafermin 1 mg daily among patients with NASH was recently published.32 The primary endpoint was a change in absolute liver fat content after 24 weeks of treatment, which aldafermin demonstrated a greater reduction of −7.7% over placebo −2.7% with [P = 0.002]. Secondary endpoints included histologic assessment. While there was an absolute reduction in both endpoints of NASH resolution without worsening fibrosis [24% aldafermin group vs 9% placebo] and fibrosis improvement without worsening NASH [38% aldafermin group vs 18% placebo], neither of these endpoints achieved statistical significance. These findings would not appear to be congruent with the robust association of aldafermin found in this network meta-analysis with respect to these two endpoints. However, aldafermin was demonstrated to be statistically significant over placebo in a variety of other endpoints. 22% patients receiving aldafermin had NASH resolution and fibrosis improvement as compared to 0% of patients in the placebo group [P = 0.015]. 62% of patients receiving aldafermin had a minimum two-point improvement in NAS without worsening fibrosis as compared to 9% among placebo group [P < 0.001]. For individual histologic components, aldafermin demonstrated superiority for patients achieving a minimum one-point improvement in steatosis score [70% vs 18%; P < 0.001], ballooning score [58% vs 18%; P = 0.002], and inflammation [52% vs 23%; P = 0.021]. Overall, aldafermin demonstrated significant improvement in the primary endpoint of this RCT in addition to a number of alternative histologic endpoints. However, future studies are required to further investigate its validated effect specifically towards endpoints of NASH resolution without worsening fibrosis and fibrosis improvement without worsening NASH. Similarly, phase 2 data for resmetirom has shown promise among secondary endpoints for improvement in NAS and fibrosis scoring,33 and further testing in a phase 3 RCT is currently underway.
While this systematic review and network meta-analysis are comprehensive, it is not without limitations. First, the number of overall RCTs included following the literature search is dependent on the type of histologic endpoints reported. Because the primary objective of this network meta-analysis was to identify RCTs implementing current, up-to-date histologic endpoints, this did severely limit the overall number of studies included. Second, despite the recent call for these current histologic endpoints based on previous inconsistency among RCTs, there still remains some obscurity over their global utility. Even from the most recent AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD,6 there still is not an absolute, true consensus on the definition for the “worsening of NASH.” Also, there is a slightly adjusted set of endpoints recommended for RCTs exclusively reporting NASH cirrhosis, which was addressed in this study with the subgroup analysis. Third, there are relatively few head-to-head treatment interventions among RCTs, with most evidence available comparing one pharmacotherapeutic intervention to a placebo. In the end, this generates a large portion of indirect evidence among the networks. Fourth, limitations with respect to risk of bias and GRADE summary of evidence were present and cataloged. Likely a function of a sound literature search and inclusion/exclusion criteria, but no overt, glaring trends were observed to account for this. Fifth, inconsistency was largely absent across all networks in this analysis. However, there was a statistically significant inconsistency observed in the secondary outcome of mean change in fibrosis score for the specific comparison of vitamin E 800 IU daily versus placebo [P = 0.04]. Sixth, the final limitation is the mere fact that some RCTs only reported findings that applied to secondary rather than primary outcomes identified in this network meta-analysis. This makes the direct comparison more difficult when attempting to compare treatment effects across all included endpoints.
This systematic review and network meta-analysis represents unique and novel findings in a booming area of NASH research. Complex pathophysiology mechanisms, lack of targeted treatment interventions, and inconsistent utilization of histologic endpoints in clinical trials have posed challenges in the management of this disease. This current network meta-analysis exhausts all present pharmacotherapies and demonstrates several robust treatment options for currently recommended histologic endpoints. Based on this thorough systematic review and network meta-analysis, the most promising treatment interventions include aldafermin 1 mg, pioglitazone 45 mg, pioglitazone 30 mg, vitamin E 800 IU, vitamin E 800 IU in combination with pioglitazone 45 mg, obeticholic acid 25 mg, obeticholic acid 10 mg, and resmetirom 80 mg. RCTs moving forward should shift their focus towards the currently recommended histologic endpoints of minimum two-point improvement in NAS, resolution of NASH without worsening fibrosis, and improvement of fibrosis without worsening of NASH. Future studies ideally will continue to bolster the current evidence to identify targeted pharmacotherapeutic options for NASH.
Credit authorship contribution statement
AK provided substantial contributions to the conception and design of the study, acquisition of data/analysis, interpretation of data, drafting of the article, critical revisions of the article, and final approval of the article.
Conflicts of interest
The author has none to declare.
Acknowledgements
The author does not have any further acknowledgments at this time.
Funding
This systematic review and network meta-analysis did not receive funding, grant support, or writing assistance of any kind.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.01.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cotter T.G., Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Sheka A.C., Adeyi O., Thompson J., et al. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal A.J., Brunt E.M., Kleiner D.E., et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleiner D.E., Bedossa P. Liver histology and clinical trials for nonalcoholic steatohepatitis-perspectives from 2 pathologists. Gastroenterology. 2015;149:1305–1308. doi: 10.1053/j.gastro.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V. A critical review of endpoints for non-cirrhotic NASH therapeutic trials. J Hepatol. 2018;68:353–361. doi: 10.1016/j.jhep.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Rinella M.E., Tacke F., Sanyal A.J., et al. Report on the AASLD/EASL Joint Workshop on clinical trial endpoints in NAFLD. Hepatology. 2019;70:1424–1436. doi: 10.1002/hep.30782. [DOI] [PubMed] [Google Scholar]
- 7.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner D.E., Brunt E.M., Van Natta M., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z.M., Stepanova M., Rafiq N., et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M., Hagström H., Nasr P., et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 11.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5 doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D., Wan X., Liu J., et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 15.Wan X., Wang W., Liu J., et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 17.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaimani A., Higgins J.P., Mavridis D., et al. Graphical tools for network meta-analysis in STATA. PLos One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rücker G., Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salanti G., Ades A.E., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Jackson D., Barrett J.K., et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias S., Welton N.J., Caldwell D.M., et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 23.Puhan M.A., Schünemann H.J., Murad M.H., et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 24.Brignardello-Petersen R., Bonner A., Alexander P.E., et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papakonstantinou T., Nikolakopoulou A., Higgins J.P., et al. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16:e1080. doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison S.A., Wong V.W., Okanoue T., et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Harrison S.A., Abdelmalek M.F., Caldwell S., et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Singh S., Khera R., Allen A.M., et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology. 2015;62:1417–1432. doi: 10.1002/hep.27999. [DOI] [PubMed] [Google Scholar]
- 30.Majzoub A.M., Nayfeh T., Barnard A., et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther. 2021;54:880–889. doi: 10.1111/apt.16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newsome P.N., Buchholtz K., Cusi K., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 32.Harrison S.A., Neff G., Guy C.D., et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–231.e1. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Harrison S.A., Bashir M., Moussa S.E., et al. Effects of resmetirom on noninvasive endpoints in a 36-week phase 2 active treatment extension study in patients with NASH. Hepatol Commun. 2021;5:573–588. doi: 10.1002/hep4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.