Abstract
Liver injury effects of green tea-based products have been reported in sporadic case reports. However, no study has examined systematically such adverse effects in an unbiased manner. We examined the potential effects of a high, sustained oral dose of green tea extract (GTE) on liver injury measures in a randomized, placebo-controlled, double-blinded phase II clinical trial, which enrolled 1,075 women with the original aim to assess the effect of daily GTE consumption for 12 months on biomarkers of breast cancer risk. The present analysis examined the effect of GTE consumption on liver injury in 1021 participants (513 in GTE and 508 in placebo arm) with normal baseline levels of liver enzymes. Among women in the GTE arm, alanine aminotransferase (ALT) increased by 5.4 U/L [95% confidence interval (CI) =3.6–7.1] and aspartate aminotransferase increased by 3.8 U/L (95% CI=2.5–5.1), which were significantly higher than those among women in the placebo arm (both P <0.001). Overall, 26 (5.1%) women in GTE developed moderate or more severe abnormalities in any liver function measure during the intervention period, yielding an odds ratio of 7.0 (95% CI = 2.4–20.3) for developing liver function abnormalities as compared with those in the placebo arm. ALT returned to normal after dechallenge and increased again after one or more rechallenges with GTE. The rise-fall pattern of liver enzyme values following the challenge-dechallenge cycles of GTE consumption strongly implicates the effect of high-dose GTE on liver enzyme elevations.
Trial registration
clinicaltrials.gov Identifier: NCT00917735
Keywords: green tea extract, catechins, liver injury, liver enzyme elevation, randomized clinical trial
INTRODUCTION
Tea, obtained from the plant Camellia sinensis, is the second most popular beverage in the world. Several types of tea are produced by different post-harvest processing methods. Green tea is a product of fresh tea leaves that are heated or steamed, rolled and dried with minimal enzymatic oxidation after harvest. Water-extractable components from green tea contain about 30–40% tea catechins in dry weight, with (–)-epigallocatechin gallate (EGCG) comprising on average two-thirds of total tea catechins (1). Extensive in vitro and animal studies have consistently shown the inhibitory activities of green tea extract (GTE) and green tea catechins, especially EGCG, against tumorigenesis at different organ sites (2). Epidemiological studies suggest that drinking green tea offers some protection against the risk of several cancers including liver cancer (3–5). Tea consumption has also been associated with reduced mortality due to cardiovascular disease, body weight reduction and metabolic syndrome alleviation (6, 7).
The potential health benefits associated with the consumption of GTE and other green tea products have been attributed partially to the antioxidant and other properties of catechins, especially EGCG (8). However, EGCG at high doses has been shown to induce hepatotoxicity in animal models (9, 10). More than 50 case reports on hepatic adverse events associated with the consumption of high-dose green tea-herbal products have been described during the past 15 years, 19 of them with GTE as the major constituents (11, 12). Recently, we found that among participants with positive serology of hepatitis B surface-antigen (HBsAg), urinary excretion of epigallocatechin (EGC) at levels equivalent to five cups of green tea per day was associated with a significant 2-fold increased risk of hepatocellular carcinoma compared with undetectable EGC in the Shanghai Cohort Study (13).
Despite the inconsistent results between the potential protective effect of drinking green tea on liver cancer observed in epidemiological studies and hepatic adverse effect of high-dose green tea-containing herbal supplements in case reports, there have been no data from randomized clinical trials with large sample size and long-term treatment with a high dose of GTE (i.e., higher than 500 mg EGCG, equivalent to three 240 mL servings of green tea per day) on measures of liver injury in humans (6, 14). In the Minnesota Green Tea Trial (MGTT), a higher proportion of women in the treatment than the placebo arm developed gastrointestinal-related adverse events, including liver function measures (15). Utilizing this dataset (16), we conducted a detailed analysis to determine the time course and the possible effects of a high, sustained dose of encapsulated GTE on liver injury. In addition, we evaluated the potential modifying effects of lifestyle factors and concurrent use of medications on the association between GTE consumption and liver injury.
MATERIALS AND METHODS
Participants
The design of the MGTT has been described in detail previously (16). In brief, the MGTT was a randomized, double-blinded, placebo-controlled phase II clinical trial designed to investigate the effects of oral intake of a high dose of GTE as a dietary supplement on biomarkers of breast cancer risk. Postmenopausal women aged 50–70 years with heterogeneously dense (51–75% glandular) or extremely dense (>75% glandular) breasts otherwise in good health were eligible. Participants were excluded before randomization if she tested serological positive for either hepatitis B surface antigen (HBsAg) or antibodies to hepatitis C virus (HCV), which indicate chronic infection with hepatitis B or C virus, respectively; had elevated liver enzyme alanine aminotransferase (ALT) greater than 1.5 times the upper limit of normal (ULN); was diagnosed with diabetes mellitus, or any other lifestyle factors (alcohol drinking and cigarette smoking) that may influence metabolism of sex hormones and oxidative stress outcomes. The study enrolled 1075 women from 2009 through 2013 in the Minneapolis-St. Paul metropolitan area; 538 were randomly assigned to the GTE and 537 to the placebo group. At the baseline visit, each participant completed a self-administered questionnaire requesting information on demographics, smoking and alcohol use, medical history, medication use and dietary intake during the past 12 months. Body weight, height, and waist and hip circumferences were measured at the research clinic. Blood samples were collected at baseline and in clinical visits during the 12-month intervention period for monitoring liver function.
This present analysis on the liver injury effect of GTE was restricted to 1021 participants (513 in the GTE and 508 in the placebo arm) with normal baseline serum ALT (≤75 U/L). We excluded: 1) 3 participants (all in the GTE arm) with missing baseline values; and 2) 51 participants (22 in the GTE and 29 in the placebo group) who withdrew from the study before the first monthly liver panel test was conducted (Figure 1).
Figure 1.
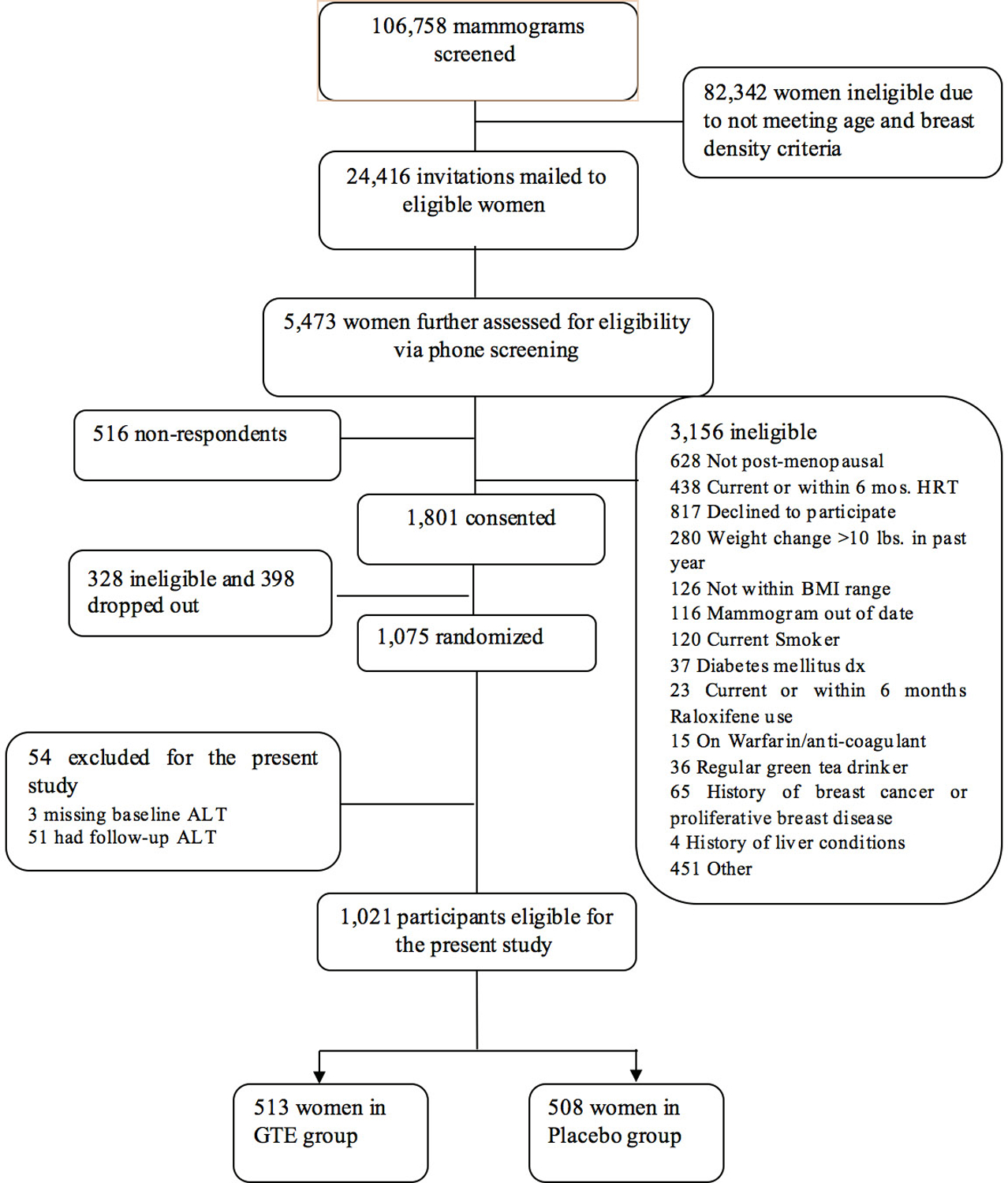
Flow diagram of participant screening, enrollment, randomization, and eligible for the present sub-study, the Minnesota Green Trial, 2009–2015(16). GTE, green tea extract; ALT Alanine aminotransferase.
Supplementation with Green Tea Extracts
The original source of green tea was from the Youshan Tea Farm (Fuzhou, Fujian Province) in China. The compounds were extracted from dried green tea leaves with water and ethyl acetate, decaffeinated and spray-dried in the Taiyo Green Power Co. Ltd. (Wuxi, Jiangsu Province, China). The extracted compounds were imported to the U.S. by Taiyo International Inc. (Minneapolis, MN) and were encapsulated by Corban Laboratories/Eniva Nutraceutics (Plymouth, MN).
GTE and placebo capsules were identical in appearance and were produced and provided by Corban Laboratories. The catechin contents of all eight batches of GTE were quantified using high-performance liquid chromatography technology at Covance Laboratories (Madison, WI), and confirmed in the laboratory of C.S. Yang. Mean (standard deviation, SD) of specific catechins per GTE capsule was 328.8 (28.9) mg total catechins that included 210.7 (11.0) mg EGCG, 50.6 (18.5) mg epicatechin gallate (ECG), 26.7 (29.7) mg EGC, and 26.8 (5.9) mg epicatechin (EC). Each GTE capsule also contained 3.9 mg caffeine. Each placebo capsule contained 204 mg maltodextrin (50%), 202 mg cellulose (49.5%), and 2 mg magnesium stearate (0.5%), but did not contain any tea catechins or caffeine. GTE and placebo capsules were stored at ambient temperature (20–25°C) in 40–45% humidity prior to dispensing to study participants by the investigational drug services pharmacy at the University of Minnesota.
Participants were blinded to treatment assignment and were instructed to take two capsules at breakfast and two capsules at dinner daily for 12 months. The mean daily dose (SD) of tea catechins was 1315 (115.4) mg total catechins including 843 (44.1) mg EGCG, 202 (74.0) mg ECG, 106 (118.8) mg EGC, 107 (23.4) mg EC, and 15.8 mg caffeine. The amount of EGCG was equivalent to approximately five cups (equivalent to 8 fluid ounces or 240 mL) of brewed green tea per day (17).
Liver Injury Measure
The hepatic panel, including ALT, aspartate aminotransferase (AST), alkaline phosphatase (AKP), albumin globulin, total proteins, albumin/globulin ratio, and indirect, direct and total bilirubin were tested in serum collected from study participants at baseline and in follow-up clinical visits (Quest Diagnostics, Wood Dale, IL). The original protocol requested a monthly blood draw, which was changed to months 1, 2, 3, 4, 5, 6, 9, and 12 for participants enrolled after first two years given that ALT elevation was rare after it had been normal in the first 6 months of monitoring.
Since our study participants were healthy postmenopausal women, the liver injury grading system developed by the Acquired Immune Deficiency Syndrome Clinical Trials Group was used for this analysis (18). Based on the highest value of 4 liver function measures during the entire 12-month intervention period, individuals were classified as grade 0 or no abnormal liver function if all 3 liver enzymes were <1.25x ULN (ULN: ALT = 60, AST = 35, AKP = 135 U/L) and total bilirubin ≤1x ULN (ULN: ≤1.2 mg/dl), grade 1 abnormal liver function if any liver enzyme was 1.25–2.5x ULN or total bilirubin >1–1.5x ULN, grade 2 (mild) abnormal liver function if any liver enzyme was >2.5–5.0x ULN or total bilirubin >1.5–2.5x ULN, grade 3 (moderate) abnormal liver function if any liver enzyme was >5–10x ULN or total bilirubin >2.5–5x ULN, or grade 4 (severe) abnormal liver function if any liver enzyme was >10x ULN or total bilirubin >5x ULN (Supplemental Table S1). Liver injury would be considered if patient had jaundice or signs of hepatic or organ failure or hospitalized.
According to the protocol of this primary intervention study, any ALT value above 1.5 times ULN (>90 U/L) during the treatment period, a pre-defined adverse event, triggered the change of treatment course and required participants to stop taking study capsules immediately. The repeated liver function tests were conducted once every two weeks for all those participants until their ALT returned to below 1.5x ULN (≤90 U/L). If the peak ALT value was ≤5x ULN (≤ 300 U/L), the participant was requested to resume intake of study capsules. If the peak ALT value was >5x ULN, the participant was permanently withdrawn from the study, but follow-up continued to the end of planned intervention period.
Statistical Analysis
The distributions of demographic characteristics at baseline (age, race, education, body mass index (BMI), smoking and alcohol status), dietary nutrients, use of medication, and serum concentrations of lipids were compared between participants in the GTE and in the placebo groups. Differences were examined by Student’s t (for continuous variables) or χ2 test (for categorical variables).
Changes in levels of serum ALT, AST, AKP and total bilirubin from baseline to the peak value observed during the entire intervention period were compared between the GTE and placebo groups using a generalized linear mixed effect model with adjustment for age, education, BMI, and smoking status (never versus former smokers) at baseline. The effects of obesity, alcohol use and other potential modifiers on ALT and AST during the course of intervention were examined using the same regression model.
The unconditional logistic regression method was employed to calculate the odds ratios (ORs) and their 95% confidence intervals (CIs) for overall and different grades of abnormal liver function measures associated with the intake of GTE. Potential confounding variables were adjusted.
Statistical computing was conducted using SAS version 9.4 (SAS Inc., Cary, NC). All P values were two-sided. P values < 0.05 were considered to be statistically significant.
RESULTS
All baseline characteristics of study participants in the two treatment groups were comparable except for serum total triglyceride concentration, which was lower in the GTE than in the placebo arm (P = 0.01) (Table 1). At baseline, women using statins had statistically significant higher levels of serum ALT. In the placebo arm, weekly drinkers of alcoholic beverages had lower levels of serum AST compared with non-drinkers, and women with BMI ≥30 kg/m2 had higher level of serum ALT compared with women with BMI <30 kg/m2. Other characteristics did not have significant effects on either ALT or AST concentrations (Supplemental Table S2).
Table 1.
Distributions of characteristics at baseline among study participants in the green tea extract and placebo group, The Minnesota Green Tea Trial, 2009–2015
| Characteristics | Placebo | GTE | P a |
|---|---|---|---|
| No. of total participants, n (%) | 508 (100) b | 513 (100) b | |
| Age at baseline (year), mean (SD) | 59.6 (5.0) | 59.9 (5.0) | 0.32 |
| Caucasian race, n (%) | 491 (97.2) | 501 (98.0) | 0.39 |
| Level of education, n (%) | 0.13 | ||
| High school or below | 37 (7.3) | 30 (5.9) | |
| Some college | 84 (16.7) | 110 (21.5) | |
| College graduate | 221 (43.9) | 229 (44.8) | |
| Postgraduate/professional degree | 162 (32.1) | 142 (27.8) | |
| Body Mass Index (kg/m2), mean (SD) | 25.1 (3.8) | 25.2 (3.7) | 0.79 |
| <30, n (%) | 459 (90.4) | 447 (87.1) | 0.10 |
| ≥30, n (%) | 49 (9.7) | 66 (12.9) | |
| Former smokers, n (%) | 146 (31.7) | 149 (32.6) | 0.78 |
| No. years of quitting smoking, mean (SD) | 26.2 (11.3) | 25.8 (10.2) | 0.52 |
| Current use of alcohol (<7 drinks/week), n (%) | 426 (84.2) | 410 (79.9) | 0.08 |
| No. of drinks per week, mean (SD) | 3.4 (3.0) | 3.3 (3.0) | 0.92 |
| Use of medication, n (%) | |||
| Acetaminophen | 137 (27.0) | 150 (29.3) | 0.42 |
| Aspirin | 140 (27.6) | 136 (26.6) | 0.71 |
| Non-aspirin NSAIDs | 338 (66.7) | 354 (69.1) | 0.40 |
| Statin | 106 (20.9) | 115 (22.5) | 0.55 |
| Total cholesterol (mg/dl), mean (SD) | 209.3 (31.5) | 206.5 (30.6) | 0.22 |
| <200, n (%) | 189 (40.0) | 197 (42.8) | 0.63 |
| 200–240, n (%) | 212 (44.8) | 200 (43.5) | |
| >240, n (%) | 72 (15.2) | 63 (13.7) | |
| Total triglyceride (mg/dl), mean (SD) | 101.8 (48.5) | 93.5 (43.2) | 0.01 |
| <150, n (%) | 409 (86.5) | 414 (90.0) | 0.09 |
| ≥50, n (%) | 64 (13.5) | 46 (10.0) |
GTE, green tea extract; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation.
2-sided P values were derived from t-test (for continuous variables) or chi-squared test (for categorical or nominal variables).
The sum of some variables may be less than the total due to missing values.
The mean levels of ALT, AKP and total bilirubin at baseline were comparable between the GTE and placebo arms, while the baseline AST mean in the placebo was slightly higher than in the GTE arm. GTE intake significantly increased both ALT and AST during the treatment period from baseline levels. These changes were significantly greater than the same enzyme changes in the placebo arm (both P < 0.001). On the contrary, such changes of AKP and total bilirubin were not statistically significant between GTE and placebo arms (Table 2).
Table 2.
Change of serum liver functional tests during the intervention period from baseline in all participants, the Minnesota Green Tea Trial, 2009–2015
| Placebo | GTE | P | |
|---|---|---|---|
| No. of participants | 508 | 513 | |
| ALT (U/L) | |||
| Mean (95% CI) at baseline | 18.0 (17.5–18.7) | 17.6 (17.0–18.7) | 0.25 |
| Mean change (95% CI) from baseline | −0.5 (−2.2–1.3) | 5.4 (3.6–7.1) | <0.001a |
| AST (U/L) | |||
| Mean (95% CI) at baseline | 20.7 (20.2–21.2) | 20.0 (19.6–20.4) | 0.04 |
| Mean change (95% CI) from baseline | −0.2 (−1.5–1.1) | 3.8 (2.5–5.1) | <0.001a |
| AKP (U/L) | |||
| Mean (95% CI) at baseline | 69.8 (68.2–71.3) | 69.1 (67.4–70.8) | 0.29 |
| Mean change (95% CI) from baseline | 0.8 (−0.6–2.1) | 0.2 (−1.1–1.5) | 0.19a |
| Total Bilirubin (mg/dl) | |||
| Mean (95% CI) at baseline | 0.51 (0.49–0.53) | 0.50 (0.48–0.52) | 0.32 |
| Mean change (95% CI) from baseline | 0.02 (−0.001–0.04) | 0.02 (−0.001–0.04) | 0.95a |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; CI, confidence interval; GTE, green tea extract.
2-sided P values were derived from generalized linear mixed models comparing the changes of liver functional tests during the intervention period from baseline among women in the GTE group to the corresponding changes in the placebo group after adjustments for age, level of education, body mass index, and smoking status.
Among 513 women with normal baseline ALT in the GTE arm, 44 (8.6%) had elevated ALT above 1.25x ULN compared with 9 (1.8%) of 508 women in the placebo arm; yielding an OR of 5.1 (95% CI=2.5–10.7). Furthermore, 17 (3.3%) women in the GTE but none in the placebo developed moderate or severe liver enzyme elevation (ALT >2.5x ULN) (Table 3). Among these 17 women, 5 had moderate (>5–10x ULN ALT) and 3 had severe liver enzyme elevation (>10x ULN ALT). We further explored if subjects with elevated ALT (n=44) had different characteristics at baseline than those with normal ALT with GTE supplement (n = 469). We did not detect any statistically significant difference in age, race, education, BMI, use of alcohol, history of smoking, use of aspirin, other NSAIDs or acetaminophen, and use of statin. The baseline mean levels of total cholesterol, total triglyceride, AKP and total bilirubin were comparable between these two groups (Supplemental Table S3). Though all baseline ALT were within normal range, subjects whose ALT increased above 1.25x ULN during the GTE treatment period had slightly higher baseline ALT mean 20.8, SD 9.7 U/L), compared to subjects with consistently normal ALT (mean 17.3, SD 6.3 U/L).
Table 3.
Odds ratio of developing abnormal liver functional tests for women assigned to the GTE versus placebo group during the 12-month treatment period, the Minnesota Green Tea Trial, 2009–2015
| Placebo | GTE | OR (95% CI)a | |
|---|---|---|---|
| Alanine aminotransferase (ALT) | |||
| Normal (< 75 U/L) | 499 | 469 | 1.0 |
| Abnormal (≥75 U/L)b | 9 | 44 | 5.1 (2.5–10.7) |
| Grade 1 (75–150 U/L) | 9 | 27 | 3.2 (1.5–6.8) |
| Grade 2 or above (>150 U/L) | 0 | 17 | 25.5 (5.5, N/A)c |
| Aspartate aminotransferase (AST) | |||
| Normal (< 43.75 U/L) | 486 | 447 | 1.0 |
| Abnormal (≥43.75 U/L)b | 22 | 66 | 3.4 (2.0–5.5) |
| Grade 1 (43.75–87.5 U/L) | 21 | 40 | 2.1 (1.2–3.7) |
| Grade 2 or above (>87.5 U/L) | 1 | 26 | 28.6 (3.9–212.5) |
| Alkaline Phosphatase (AKP) | |||
| Normal (< 162.5 U/L) | 507 | 508 | 1.0 |
| Abnormal (≥162.5 U/L)b,d | 1 | 5 | 4.2 (0.5–36.7) |
| Total Bilirubin | |||
| Normal (≤ 1.20 mg/dl) | 483 | 492 | 1.0 |
| Abnormal (>1.20 mg/dl) | 25 | 21 | 0.8 (0.5–1.5) |
| Grade 1 (1.20–1.80mg/dl) | 22 | 20 | 0.9 (0.5–1.7) |
| Grade 2 (>1.8 mg/dl) | 3 | 1 | 0.3 (0.03–1.4) |
| Summed Liver Injury Measure | |||
| All normale | 459 | 428 | 1.0 |
| Any abnormal | 49 | 85 | 1.8 (1.3–2.7) |
| Grade 1 | 45 | 59 | 1.4 (0.9–2.1) |
| Grade 2 or above | 4 | 26 | 7.0 (2.4–20.3) |
GTE, green tea extract; CI, confidence interval; OR, odds ratio; ULN upper limit normal; BMI body mass index.
Odds ratios were derived from unconditional logistic regression models that also included age, BMI, smoking status and level of education.
Abnormal hepatic aminotransferases was defined as having at least one elevation above ULN during the monthly liver function tests after randomization.
Odds ratio was derived from exact logistic regression model, and upper confidence interval was not estimated.
No participants in grade 2 or above.
All of liver function tests are in grade 0.
Similar to ALT, AST level increased in 66 (12.8%) women in the GTE and 22 (4.3%) women in the placebo during the intervention period (OR = 3.4, 95% CI=2.0–5.5) (Table 3). AST was highly correlated with ALT. After excluding participants with >1.25 × ULN ALT, 24 women in the GTE and 14 women in the placebo had abnormal AST elevation (OR = 2.0, 95% CI=1.0–3.9) (data not shown). GTE intake had no effect on AKP and total bilirubin (Table 3). When the 4 measures for drug-induced liver function abnormalities were considered together, 26 participants in the GTE arm and 4 in the placebo arm had one or more moderate liver enzyme elevations, yielding an OR of 7.0 (95% CI: 2.4–20.3). The participants (23 in the GTE arm and 21 in the placebo arm) with baseline liver function tests (LFT) results greater than ULN defined in Supplemental Table S1 did not show a differential effect of GTE on ALT elevation level during the intervention period (P for interaction = 0.41) (Supplemental Table S4).
The effect of GTE intake on ALT and AST elevation was stronger in obese women, but less in weekly alcohol drinkers (Table 4). The impact of GTE on ALT increase was less in current users of non-aspirin NSAID than non-users (P for interaction = 0.003) whereas serum concentrations of triglycerides or cholesterol, or use of aspirin, acetaminophen and statin were not associated with the differential effect of GTE on the changes of ALT or AST (Supplemental Table S5).
Table 4.
Change of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) during the intervention period from baseline in subgroups stratified by selected baseline characteristics, the Minnesota Green Tea Trial, 2009–2015
| Placebo | GTE | P b | Pint c | |||
|---|---|---|---|---|---|---|
| No. | Mean (95% CI)a | No. | Mean (95% CI)a | |||
| Mean changes in ALT (U/L) from baseline | ||||||
| BMI (kg/m2) | 0.08 | |||||
| <25 | 278 | −0.6 (−2.3, 1.2) | 282 | 5.1 (3.2, 6.9) | <0.0001 | |
| 25–<30 | 181 | −0.3 (−2.2, 1.6) | 165 | 5.0 (3.1, 6.9) | <0.0001 | |
| ≥30 | 49 | −0.8 (−3.2, 1.6) | 66 | 7.5 (5.3, 9.8) | <0.0001 | |
| P d | 0.54 | 0.005 | ||||
| Current Alcohol Drinker | 0.002 | |||||
| No | 80 | −1.2 (−3.3, 0.9) | 103 | 7.2 (4.2, 9.3) | <0.0001 | |
| Yes | 426 | −0.3 (−2.0, 1.5) | 410 | 5.0 (3.2, 6.8) | <0.0001 | |
| P d | 0.22 | 0.002 | ||||
| Mean changes in AST (U/L) from baseline | ||||||
| BMI (kg/m2) | 0.15 | |||||
| <25 | 278 | 0.001 (−1.4, 1.4) | 282 | 3.7 (2.4, 5.1) | <0.0001 | |
| 25–<30 | 181 | −0.3 (−1.7, 1.1) | 165 | 3.4 (2.4, 5.1) | <0.0001 | |
| ≥30 | 49 | −0.5 (−2.3, 1.3) | 66 | 5.1 (3.4, 6.8) | <0.0001 | |
| P d | 0.70 | 0.04 | ||||
| Current Alcohol Drinker | <0.001 | |||||
| No | 80 | −1.0 (−2.6, 0.6) | 103 | 5.5 (3.9, 7.1) | <0.0001 | |
| Yes | 426 | 0.1 (−1.2, 1.4) | 410 | 3.5 (2.1, 4.8) | <0.0001 | |
| P d | 0.07 | 0.0001 | ||||
GTE, green tea extract; BMI, body mass index; CI, confidence interval; NASID, Nonsteroidal anti-inflammatory drug; COMT, catecheol-O-methyltransferase; All means are from Least Squares Means.
2-sided P values were derived from generalized linear mixed models comparing the changes of liver enzyme (ALT or AST) during the intervention period from baseline of women in the GTE group to the corresponding changes in the placebo group after adjustments for age, BMI, smoking status and level of education.
2-sided P values were derived from the same model assessing the interaction between the intervention (GTE or placebo) and the stratifying variables on the liver enzyme (ALT or AST) with the same adjustments.
2-sided P values were derived from the same model comparing the differences in changes of liver enzyme (ALT or AST) from baseline among different levels of stratifying variables within the GTE or placebo group.
During the intervention period, ALT concentrations increased above 90 U/L for 36 women in the GTE and 4 women in the placebo arm, the predetermined level that required the change of treatment course (Supplemental Table S6). Among these women, the median time interval from initial GTE intake to the first detected ALT ≥90 U/L was 106 days (range: 56–365 days). The mean peak value of ALT was 238 U/L (range: 93–2,055) U/L, approximately 12 times the mean value of ALT at baseline. ALT returned to < 90 U/L after a mean of 32 days (range: 13–92 days) of discontinuing intake of GTE capsules for all 36 women. Twenty women resumed GTE intake after their ALT returned to < 90 U/L. Among them, 9 (45%) showed ALT below 90 U/L for the rest of the treatment period while 11 (55%) had ALT increased above 90 U/L again. There were 8 individuals whose ALT increased about 5x ULN (>300 U/L), a severe ALT elevation, however none of them reported liver injury symptoms and signs such as jaundice or liver failure (Supplemental Table S7).
ALT concentrations increased and decreased following the GTE challenge/dechallenge pattern. Overall, 17 women - all in the GTE arm - had ALT above 2.5x ULN (moderate or more severe liver enzyme elevations) during the course of intervention (Figure 2 and Supplemental Table S6). Six of the 17 resumed GTE intake after their ALT fell below 90 U/L; all six ALT increased above 90 U/L again after resuming GTE intake for 58 (23–114) days and returned to below 90 U/L after discontinuing GTE intake for 30 (16–43) days. Three women (IDs: G26, G27, G32) resumed GTE intake for a third time; ALT increased above 90 U/L after resuming GTE intake for 38 (32–46) days for all three participants and returned to below 90 U/L after discontinuing intake of GTE capsules for 21 (16–32) days for a third time (Figure 2b).
Figure 2.
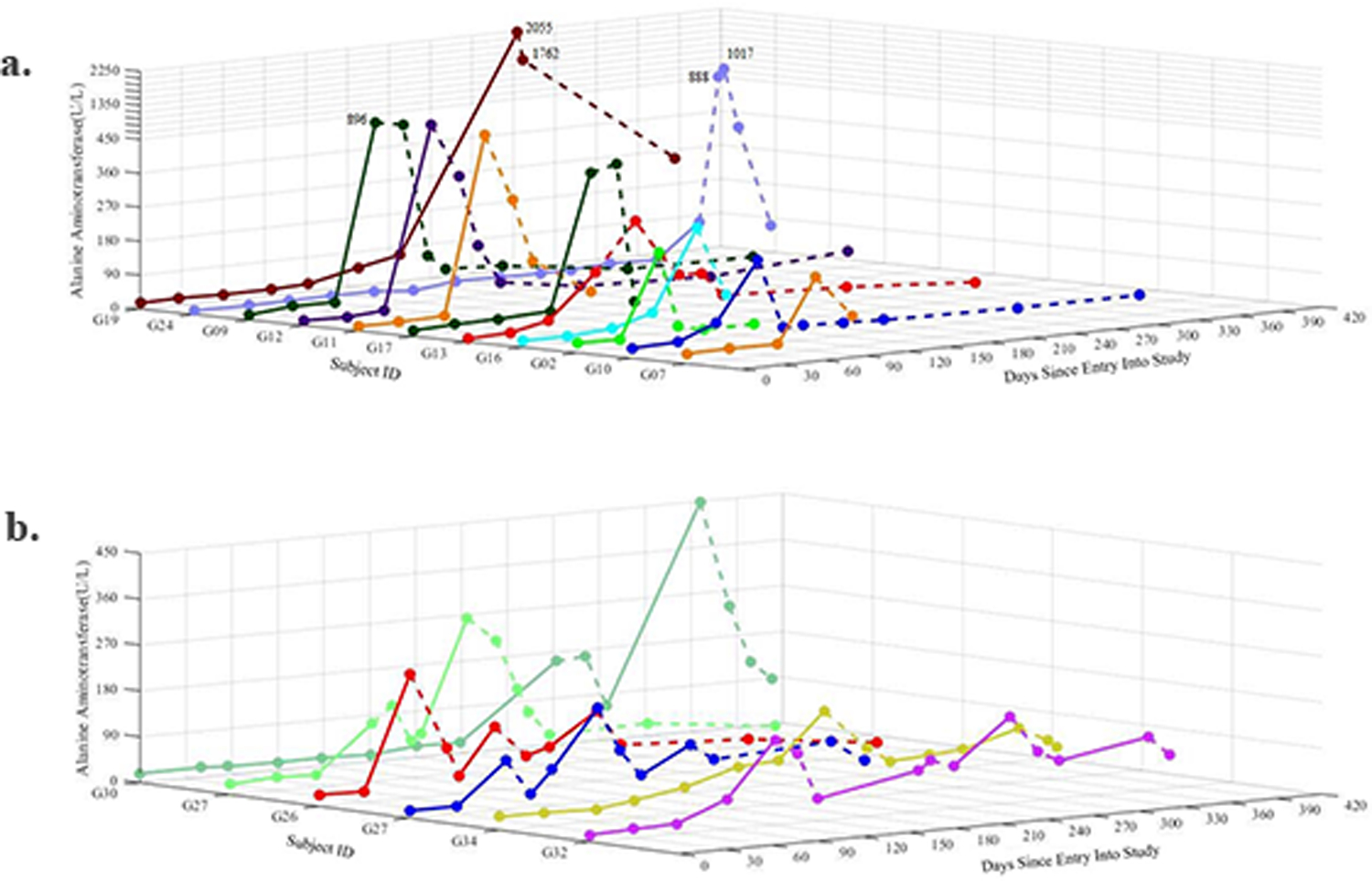
Levels of serum alanine aminotransferase (ALT) in participants whose ALT increased above 150 U/L (2.5 × ULN) over the course of treatment period. (a) Participants in the green tea extract arm with a single ALT peak > 150 U/L; (b) participants in the green tea extract arm with at least one ALT peak > 150 U/L and underwent one or more dechallenge-rechallenge cycles of GTE treatment; solid line denotes the period for GTE consumption and dashed line the period for withdrawal from consumption.
DISCUSSION
The present analysis demonstrates that sustained consumption of a high daily dose of GTE as a dietary supplement (approximately 1300 mg total tea catechins, 800 mg EGCG) may relate with mild to severe liver enzyme elevation in a small percentage (5.1%) of healthy postmenopausal women who were negative for both HBsAg and anti-HCV in serum at baseline and had reported no history of chronic liver disease, current tobacco use, or consumption of >7 alcoholic beverages weekly. Compared with women in the placebo group, participants randomized to receive GTE capsules were approximately 7 times more likely to develop mild or more severe (i.e., grade 2 or higher) liver enzyme elevation. The induction period from the initial intake to the manifestation of mild or more severe liver enzyme elevation varied widely among different participants, from a minimum of 2 months to a maximum of one year. Abnormal liver function tests induced by GTE were transient; ALT levels returned to below 1.5 times ULN on average within about one month after cessation of GTE intake. Among women who experienced initial liver enzyme elevation (i.e., ALT ≥90 U/L), more than half of women who resumed GTE intake a second time according to the study protocol and all the women who resumed GTE intake a third time experienced elevated ALT above 1.5 times ULN again. The rise-fall pattern of serum liver enzyme levels following the dechallenge-rechallenge cycles of GTE treatment strongly implicates a possible association of this high-dose GTE capsule regimen on liver laboratory abnormalities in a subset of postmenopausal women who are sensitive to GTE. Green tea-based products are the fifth most commonly consumed dietary supplement in the US (19). Some brands of green tea supplements contain as much as 1350 mg tea catechins in a single tablet. The public should be aware of the potential liver injury from consumption of high-dose green tea-based products.
Our findings are based on a well-designed and carefully implemented randomized, double-blinded, placebo-controlled phase II clinical trial of GTE in a large population of healthy U.S. women. These unequivocal results ruled out possibilities of confounding effects inherent in previous case reports on the liver injury of green tea-based products (11, 12). All study participants had normal baseline liver function panels, had no infection with hepatitis B or C virus or history of liver disease. Both GTE and placebo capsules were produced by the same GMP-certified facility with high standard of quality control. The contents of both GTE and placebo capsules were well characterized and quantified. In addition, compliance was monitored rigorously (16). Thus, the liver enzyme elevations are easily interpretable and attributable to GTE.
The biological mechanism whereby GTE provokes liver injury is not well-understood. In vitro and in vivo animal experiments support the pro-oxidant property of tea catechins; EGCG facilitates generation of reactive oxygen species (ROS), depletion of glutathione, and collapse of the mitochondrial membrane potential of hepatocytes (20). Excess production of ROS may alter intracellular proteins, increase cell membrane permeability, and induce apoptosis (21). It has been demonstrated that glutathione-depleted cells are susceptible to oxidation and further cell damage (22). EGCG-related ROS formation occurs biphasically, i.e., at low doses EGCG decreases ROS production while at high doses EGCG increases ROS generation and damages the mitochondrial membrane of hepatocytes (23). Mice given an oral dose of 1500 mg/kg EGCG showed 8- to 108-fold increases in serum ALT levels after 24 and 48 hours as compared to the control group, along with increased oxidative stress damage, hepatic necrosis and increased plasma interleukin 6 (9).
Obesity and its complications, including dyslipidemia, inflammation and oxidative stress, are strong metabolic risk factors for fatty liver disease (24, 25). In one longitudinal study in a Mexican population, weight gain increased ALT levels by more than 3 times (26). The high prevalence of obesity in the U.S. has led to high incidence of nonalcoholic fatty liver disease and chronic liver disease (27). Green tea-based products are widely used as a dietary supplement for weight loss (28–30). However, high doses of tea catechins may enhance liver injury, especially for obese individuals along with oxidative stress (9, 31). As shown in Table 4, the interaction term between BMI and GTE intake was borderline statistically significant; the change in ALT by GTE was greater in women with BMI ≥30 kg/m2 than women with BMI <30 kg/m2 (P for interaction = 0.08), suggesting that obesity may exacerbate the adverse effect of high-dose GTE on the liver enzyme elation. However, it should be noted that the effects of GTE on ALT elevation in women with BMI <25 kg/m2 and 25–<30 kg/m2 were statistically significant.
The present study, similar to previous case reports, showed that the use of statins was significantly associated with elevated ALT at baseline. Among 53 case reports of liver injury associated with intake of GTE or tea-based products, 28 were consuming other medications concurrently (11, 12). Thus, the use of medications or preexisting conditions that require medication may exacerbate GTE-induced liver abnormal tests. In a small clinical trial involving 11 patients with multiple sclerosis (32), five of the six patients assigned to receive Polyphenon E (a mixed GTE formulation similar to ours) at a daily dose of 800 mg EGCG developed abnormal ALT compared to one in five control subjects who had a similarly abnormal ALT. The high incidence of liver enzyme elevation in these patients could be due to pre-existing disease and/or concurrent use of medications.
The major limitation of the present study is its generalizability. All study participants were healthy postmenopausal women and virtually all were Caucasian. BMI range was limited to 19–40 kg/m2 and women consuming 7 or more drinks of alcoholic beverages per week were excluded to avoid potential adverse effect of obesity and/or alcohol intake on liver enzyme in subjects in the GTE group, thus the generalizability may be limited. We do not know if the impact of GTE on liver enzyme elevation would be stronger in women who drink alcohol more frequently (e.g., >7 drinks per week). The present study demonstrates that effect of GTE intake on liver enzyme elevation was present in both women with normal BMI (<25 kg/m2) and never users of alcohol, suggesting there could be even higher impacts of high-dose GTE intake on liver enzyme elevation in the U.S. general population with high prevalence of obesity and high level of alcohol intake because obesity or heavy alcohol use may exacerbate the adverse effect of GTE on liver health. Moreover, the effects of specific ingredients in commercial green tea supplements on liver enzyme elevation cannot be ascertained from this study. The MGTT study protocol did not call for further pathological evaluation of women who experienced ALT elevation to the level of severe liver injury. Future studies are warranted to elucidate the biological mechanisms for GTE on liver injury.
This randomized, double-blinded, placebo-controlled phase II clinical trial clearly demonstrates that high-dose of GTE intake for approximately 12 months associated with liver enzyme elevation in a small proportion of healthy postmenopausal women. The public should be informed about the potential risk of liver injury with a high, sustained dose of GTE as a dietary supplement, especially for those who are obese or have preexisting liver disease.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the study participants, the data collection team and study administrative staff at the Human Nutrition Research Clinic, Department of Food Science and Nutrition, University of Minnesota; the Oncology Research Department of the Park Nicollet Institute (St. Louis Park, MN), and the Investigational Drug Services pharmacy of the University of Minnesota Medical Center, Fairview. We also thank Corban Laboratories/Eniva Nutraceutics (Plymouth, MN) for providing the study supplement.
Grant Support
The Minnesota Green Tea Trial was supported by the U.S. National Cancer Institute (R01 CA127236 to M.S. Kurzer). The present work was partially supported by the U.S. National Cancer Institute (UM1 CA182876 to J.M.Y.; R35 CA197222 to T.W.K.; T32CA132670 to A.M.D) and Department of Defense/US Army Medical Research (W81XWH-11-1-0013 to HS). Both the National Cancer Institute’s Cancer Center Support Grants of the University of Minnesota Masonic Cancer Center (P30 CA077598) and the University of Pittsburgh Cancer Institute (P30 CA047904) partially supported this work.
REFERENCE
- 1.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Preventive medicine. 1992;21:334–50. [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–22. [DOI] [PubMed] [Google Scholar]
- 3.Huang YQ, Lu X, Min H, Wu QQ, Shi XT, Bian KQ, et al. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition. 2016;32:3–8. [DOI] [PubMed] [Google Scholar]
- 4.Ni CX, Gong H, Liu Y, Qi Y, Jiang CL, Zhang JP. Green Tea Consumption and the Risk of Liver Cancer: A Meta-Analysis. Nutrition and cancer. 2017;69:211–20. [DOI] [PubMed] [Google Scholar]
- 5.Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. The American journal of clinical nutrition. 2013;98:1676s–81s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. Jama. 2006;296:1255–65. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS, Zhang J, Zhang L, Huang J, Wang Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol Nutr Food Res. 2016;60:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu Rev Nutr. 2013;33:161–81. [DOI] [PubMed] [Google Scholar]
- 9.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010;48:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Wang Y, Wan X, Yang CS, Zhang J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicology and applied pharmacology. 2015;283:65–74. [DOI] [PubMed] [Google Scholar]
- 11.Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Archives of toxicology. 2015;89:1175–91. [DOI] [PubMed] [Google Scholar]
- 12.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. European journal of clinical pharmacology. 2009;65:331–41. [DOI] [PubMed] [Google Scholar]
- 13.Butler LM, Huang JY, Wang R, Lee MJ, Yang CS, Gao YT, et al. Urinary biomarkers of catechins and risk of hepatocellular carcinoma in the Shanghai Cohort Study. American journal of epidemiology. 2015;181:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. International journal of cancer. 2007;120:1344–50. [DOI] [PubMed] [Google Scholar]
- 15.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol. 2015;83:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer causes & control. 2015;26:1405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagwat S, Haytowitz D, Holden J. USDA database for the flavonoid content of selected foods. 2015.
- 18.National Institute of Disease and Digestive and Kidney Disease. Severity grading in drug induced liver injury. 2016. https://livertox.nih.gov/Severity.html [PubMed]
- 19.Smitha T, Kawab K, Ecklb V, Johnsonc J. Sales of Herbal Dietary Supplements in US Increased 7.5% in 2015 Consumers spent $6.92 billion on herbal supplements in 2015, marking the 12th consecutive year of growth. J Am Botanical Council. 2016;111:67–73. [Google Scholar]
- 20.Galati G, Lin A, Sultan AM, O’Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free radical biology & medicine. 2006;40:570–80. [DOI] [PubMed] [Google Scholar]
- 21.Wan C, Han R, Liu L, Zhang F, Li F, Xiang M, et al. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicology and applied pharmacology. 2016. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochemical pharmacology. 2003;66:1769–78. [DOI] [PubMed] [Google Scholar]
- 23.Kucera O, Mezera V, Moravcova A, Endlicher R, Lotkova H, Drahota Z, et al. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxidative medicine and cellular longevity. 2015;2015:476180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koppe SW. Obesity and the liver: nonalcoholic fatty liver disease. Translational research : the journal of laboratory and clinical medicine. 2014;164:312–22. [DOI] [PubMed] [Google Scholar]
- 25.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clinics in liver disease. 2016;20:205–14. [DOI] [PubMed] [Google Scholar]
- 26.Flores YN, Auslander A, Crespi MC, Rodriguez M, Zhang ZF, Durazo F, et al. Longitudinal association of obesity, metabolic syndrome and diabetes with risk of elevated aminotransferase levels in a cohort of Mexican health workers. Journal of digestive diseases. 2016; 17:304–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:524–30. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso GA, Salgado JM, Cesar Mde C, Donado-Pestana CM. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. Journal of medicinal food. 2013;16:120–7. [DOI] [PubMed] [Google Scholar]
- 29.Narotzki B, Reznick AZ, Navot-Mintzer D, Dagan B, Levy Y. Green tea and vitamin E enhance exercise-induced benefits in body composition, glucose homeostasis, and antioxidant status in elderly men and women. Journal of the American College of Nutrition. 2013;32:31–40. [DOI] [PubMed] [Google Scholar]
- 30.Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. The Cochrane database of systematic reviews. 2012;12:Cd008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World journal of gastroenterology. 2014;20:14205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovera J, Ramos A, Devier D, Garrison V, Kovner B, Reza T, et al. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. Journal of the neurological sciences. 2015;358:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


