Abstract
Background
Postreperfusion liver biopsy (PRB) can assess the degree of ischemia/reperfusion injury (IRI) after orthotopic liver transplantation (OLT). The influence of IRI on graft outcomes and overall survival is controversial.
Aim
To determine the correlation between the severity of IRI in PRB and overall graft and patient survival and, secondarily, to identify factors on PRB that predict poor graft outcomes.
Methods
This is a retrospective analysis of all patients who underwent OLT using donation after brain death (DBD) with PRB. The severity of IRI in PRB was graded. Predictors of IRI were assessed using univariate and multivariate analysis and the Kaplan–Meier with log rank test for the graft and overall survival, respectively.
Results
We included 280 OLTs (64.7%). The histopathological assessment of IRI severity was as follows: no IRI (N = 96, 34.3%), mild IRI (N = 65; 23.2%), moderate IRI (N = 101; 36.1%), and severe IRI (N = 18; 6.4%). The incidence rates of initial good graft function (IGGF), primary nonfunction and early allograft dysfunction (EAD) were 32.5%, 3.9%, and 18.6%, respectively. Severe IRI was associated with a lower incidence of IGGF (OR: 0.34, 95% CI 0.12–0.92; P = 0.03). Patients with severe IRI tended to have a higher incidence of EAD (33.2% vs. 18.6, P = 0.23). The cold ischemia time was an independent predictor of severe IRI on the multivariate analysis. Severe IRI was associated with poor 1- and 5-year overall survival rates (67% and 44%, respectively, compared with 84 and 68% in nonsevere IRI). Patients with severe IRI exhibited worse graft and overall survival.
Conclusions
Cold ischemia time predicts the development of severe IRI. Patients with severe IRI show worse graft and overall survival and a lower incidence of IGGF, suggesting that histopathological findings could be useful for identifying patients at high risk of worse outcomes after OLT.
Keywords: postreperfusion biopsy, liver transplantation, ischemia reperfusion injury, cold ischemia time, early allograft dysfunction
Abbreviations: ALD, alcohol-related liver disease; ALF, acute liver failure; ALT, alanine aminotransferase; CIHD, chronic ischaemic heart disease; CNI, calcineurin inhibitors; COPD, chronic obstructive pulmonary disease; DBD, donation after brain death; EAD, early allograft dysfunction; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; H&E, hematoxylin and eosin; IGGF, initial good graft function; IRI, ischaemia/reperfusion injury; IQR, interquartile range; MELD, Model for End-stage Liver Disease; OLT, orthotopic liver transplantation; ONT, Organización Nacional de Transplantes; PBC, primary biliary cholangitis; PNF, primary nonfunction; PRB, postreperfusion liver biopsy; SD, standard deviation; STROBE, Strengthening the Reporting of Observational studies in Epidemiology
Orthotopic liver transplantation (OLT) represents the only curative treatment for patients with end-stage liver failure due to acute and chronic liver diseases and unresectable hepatocellular carcinoma. The 1- and 5-year survival rates exceed 90% and 70%, respectively.1 However, early post-OLT complications persist. Some of these complications are primary nonfunction (PNF) or early allograft dysfunction (EAD). EAD occurs in 8.7–36.3%2 of OLTs, and the PNF incidence ranges from 0.9 to 8.5%.2,3 These factors might decrease survival among transplant recipients or increase the need for retransplantation.4,5
Postreperfusion biopsy (PRB) is an intraoperative biopsy performed after complete revascularization. Histopathological assessment of PRB allows to determine the presence of steatosis and ischemia/reperfusion injury (IRI). IRI is an inherent early complication of OLT. It is a process characterized by activation of the innate immune system and release of inflammatory cytokines, leading to oxidative stress in the graft after reperfusion occurs. IRI might be favored by an increase in the selection of high risk organs.6
Warm ischemia affects liver tissue, leading to hepatocyte apoptosis, and cold ischemia affects nonparenchymal cells in the allograft, mainly sinusoidal endothelial cell and cells in the biliary duct.7,8
The broader acceptance of donors could have a significant impact on patient and graft survival. Therefore, identifying predictive factors of graft dysfunction is essential for achieving a better understanding of poor short- and medium-term graft survival.7 Studies analyzing the relationship between the PRB and graft outcomes are limited. Some studies have suggested the relationships with PNF, acute rejection, EAD, and biliary complications.9, 10, 11, 12, 13
The primary aim of our study was to determine the correlation between the severity of IRI in PRB and overall graft and patient survival. Secondary aim was to identify graft outcomes and predictive factors of IRI.
Patients and methods
Study Design and Study Population
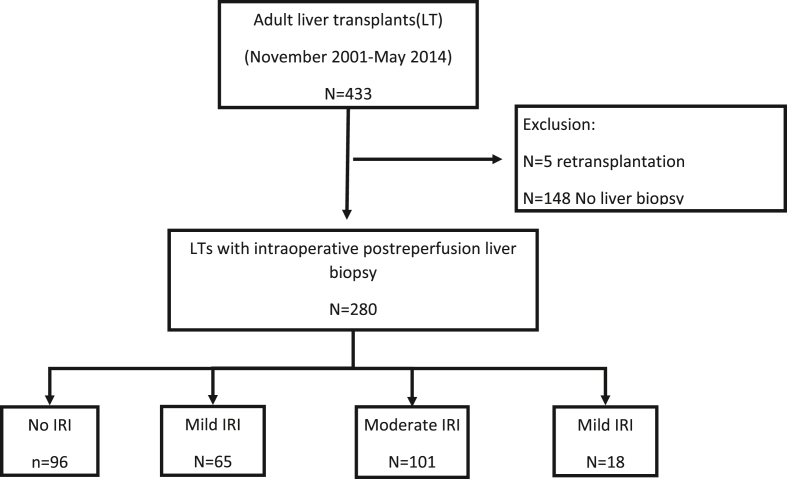
We conducted a retrospective study at a single tertiary center. All adult patients (≥18 years old) in whom PRB were performed during OLT between November 1, 2001 and May 31, 2014, and the follow-up was conducted at the same hospital were eligible. During the period from 2001 to 2005 and 2010 to 2014, the PRB was part of the LT protocol at our center. From 2006 to 2009, PRB was performed selectively at the discretion of the surgeon.
Exclusion criteria included transplantation without PRB, retransplantation and combined liver-kidney transplantation. The study was performed according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement14 and received approval from the local Institutional Review Board (PI167-19).
Data Collection
Data were extracted from patients' medical records. Donor and recipient information was obtained from a prospectively collected database. The following variables were recorded from comparison groups: age, sex, height and weight (and BMI) at the time of the transplant and five years post-OLT, date, Model for End-stage Liver Disease (MELD) and indication of transplant, total and cold ischemia time, comorbidities, histopathological assessment of PRB and graft function. Donor and recipient information was obtained from a retrospective database held in the centrally maintained database of the Spanish registry of ONT (Organización Nacional de Transplantes).
Definitions
-
•
Cold ischemia time: period between the interruption of blood supply of the graft in the donor and infusion of cold preservation solution until the time the graft is inserted into the abdominal cavity of the recipient.5
-
•
Total ischemia time: period from the cutting off of the blood supply until reperfusion after vascular anastomosis with hepatic artery perfusion in the recipient.5
Graft Function
-
•
Initial good graft function (IGGF): peak AST < 1.500 U/l or ALT < 1.000 U/l and PT (<25 sec) within the first week after OLT.15
-
•
EAD was defined as the presence of one or more of the following: total bilirubin >10 mg/dL, INR > 1.6 or ALT > 2000 U/l within the first week after OLT.5 This definition excluded the presence of vascular thrombosis, anastomotic strictures and rejection.
-
•
PNF: irreversible graft dysfunction requiring urgent retransplantation or unable to sustain life within the first week after OLT.16 This definition excluded the presence of vascular thrombosis, anastomotic strictures and rejection.
Donor Liver Preservation
Donor organs were procured from donation after brain death with standardized techniques. Organs were perfused and stored in cold University of Wisconsin solution (ViaSpan; Bristol-Myers Squibb Pharma, Garden City, NY).
Assessment of Postreperfusion Biopsy
PRBs were obtained intraoperatively via a wedge-shaped incision of the graft implant after reperfusion occurred with complete vascular anastomosis. Formalin-fixed, paraffin-embedded biopsy specimens were sectioned and stained with hematoxylin and eosin.
Assessment of biopsies was made by the pathologist and the study investigators. Pathologist and investigators were blinded to the clinical details of the patients.
The reperfusion injury observed in PRB was graded as severe and nonsevere. Then a subcategorization was made of nonsevere into no IRI, mild IRI, and moderate IRI. This classification was performed according to the following histological features in the periportal spaces: hepatocellular necrosis, neutrophilic exudate, congestion, and apoptosis.11 Mild IRI was characterized by occasional neutrophilic infiltrate. Moderate IRI includes the presence of neutrophilic infiltrate associated with some hepatocyte necrosis. Severe IRI included the presence of confluent hepatocyte necrosis, including perivenular location or periportal confluent necrosis.
Additionally, the presence of macro- or microvesicular steatosis and the degrees of steatosis, fibrosis, mononuclear infiltrate, and iron deposits were assessed (Figure 1). The severity of steatosis was recorded as none (0–5%), mild (6–33%), moderate (33–66%), and severe (more than 66%). Neutrophilic infiltration was categorized based on the presence or absence of neutrophilic aggregates in three stages: mild, moderate, and severe. The presence of necrosis was graded as absent, focal necrosis, or confluent necrosis.
Figure 1.
The histologic range of ischaemia reperfusion injury (IRI). (A) H&E staining (40×) of mild IRI demonstrating no large clusters of intrasinusoidal neutrophils among centrilobular hepatocytes. (B) H&E staining (40×) of moderate IRI demonstrating centrilobular necrosis of several clusters of neutrophils (asterisk) with hepatocyte damage (arrow). (C) H&E staining (10×) of severe IRI showing centrilobular coagulative necrosis and confluent cell loss (arrows).
Early Postoperative Period
Postoperative care for these patients was provided according to the following protocol: all patients received methylprednisolone 40 mg intravenously four times a day on a tapering schedule until 20 mg/day orally of prednisone and calcineurin inhibitors (CNIs) (tacrolimus or cyclosporine) based on dosage adjusted to blood concentration; Basiliximab 20 mg was administered intravenously on postoperative days 0 and 4 if patients presented serum creatinine ≥1.5 mg/dL or refractory ascites at the time of transplant for induction therapy. Mycophenolate mofetil was administered orally at 2000 mg per day combined with CNI.
Statistical Analysis
Descriptive statistics were used to report the characteristics of the patients. Continuous variables are shown as the mean ± standard deviation (SD); otherwise, they are shown as the median and interquartile range (IQR) whenever appropriate. Categorical variables are presented as numbers and percentages. Student's t or nonparametric (Mann–Whitney) tests were used to compare qualitative and quantitative continuous variables, and the X2 test or Fisher's exact test was used for categorical variables, wherever appropriate. All possible independent predictor factors of IRI were assessed using univariate and multivariate analysis. Survival analysis was determined using the Kaplan–Meier method and the log-rank test to compare subgroups. Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 24.0. IBM Corp, NY, USA). P-values <0.05 were considered statistically significant.
Results
Recipient and Donor Demographics
During this period, 433 patients underwent OLT at the Hospital Universitario Rio Hortega Transplant Unit. Intraoperative PRB were performed in 280 (64.7%) patients. The median age of the recipients was 56 years (IQR 49–61.2), and 217 (77.5%) were men. The median MELD score was 13.0 (IQR 10–17). The most common indication for OLT was decompensated cirrhosis (Child B–C) in 136 patients (48.6%), and alcohol-related liver disease was the main etiology of liver disease in 119 patients (42.5%). A total of 47 patients (16.8%) were admitted to the intensive care unit due to severe clinical conditions before OLT. The baseline characteristics are summarized in Table 1.
Table 1.
Baseline Recipient Characteristics.
| Total | No IRI | Mild IRI | Moderate IRI | Severe IRI | P | |
|---|---|---|---|---|---|---|
| Male gender (%) | 217 (77.5) | 70 (72.9) | 54 (83.1) | 77 (77) | 16 (84.2) | 0.42 |
| Tobacco (%) | 0.82 | |||||
| Former smoker | 93 (33.2) | 34 (37.8) | 20 (32.3) | 29 (32.2) | 7 (36.9) | |
| Smoker | 58 (20.7) | 23 (24) | 12 (19.4) | 19 (18.8) | 4 (22.2) | |
| Non-smoker | 129(46.1) | 39 (40.6) | 30 (48.4) | 53 (52.5) | 7 (38.9) | |
| Hypertension (%) | 85 (30.4) | 27 (28.4) | 23 (35.4) | 28 (27.7) | 7 (38.9) | 0.59 |
| Diabetes (%) | 88 (31.4) | 25 (26) | 28 (43.1) | 31 (30.7) | 4 (22.2) | 0.1 |
| CIHD (%) | 12 (4.3) | 4 (4.2) | 4 (6.2) | 3 (3) | 1 (5.6) | 0.79 |
| COPD (%) | 29 (10.4) | 13 (13.7) | 7 (10.8) | 9 (8.9) | 0 | 0.3 |
| MELD score (IQR) | 13.0 (10–17) | 13.8 (9–17) | 13.2 (10.2–17.5) | 13.9 (10–17.2) | 13.5 (9–17.8) | 0.45 |
| OLT indication (%) | 0.18 | |||||
| HCC | 103 (36.8) | 37 (38.9) | 22 (34.9) | 33 (33) | 11 (61.1) | |
| CHILD B–C cirrhosis | 136 (48.6) | 45 (47.4) | 31 (49.2) | 55 (55.5) | 3 (16.7) | |
| ALF | 22 (7.9) | 9 (9.5) | 4 (6.3) | 6 (6) | 3 (16.7) | |
| Others | 19 (6.7) | 4 (4.2) | 6 (9.5) | 6 (6) | 1 (5.6) |
ALD, alcohol-related liver disease; ALF, acute liver failure; CIHD, chronic ischaemic heart disease; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IQR, interquartile range; IRI, Ischaemia/reperfusion injury; MELD, Model for end-stage liver disease; PBC, primary biliary cholangitis, OLT, orthotopic liver transplantation.
The median donor age was 57.9 years (IQR 46–72), the median weight was 71 kg (IQR 65–80), and the mean BMI was 26.1 kg/m2 (SD ± 3.3).
The median cold ischemia time and warm ischemia time were 400.0 and 48 min (IQR 345–453 and 40–56), respectively.
Histopathological Findings
The severity of IRI in PRB was graded as follows: no IRI (N = 96; 34.3%), mild (N = 65; 23.2%), moderate (N = 101; 36.1%), and severe (N = 18; 6.4%) (Figure 2). The severity of steatosis was assessed as follows: none (N = 223; 79.6%); mild (N = 46; 16.4%%); moderate (N = 8; 2.9%) and severe (N = 3; 1.1%). Neutrophilic infiltration was categorized into three stages: mild (67.1%), moderate (18.9%) and severe (2.5%). Focal necrosis was found in 186 patients (66.4%) with grafts, and confluent necrosis was found in 27 patients (9.6%). More detailed data are shown in Table 2.
Figure 2.
Severity of ischaemia reperfusion injury according to the histopathological assessment of postreperfusion biopsy. IRI, ischaemia reperfusion injury.
Table 2.
Time-zero Biopsy IRI and Histological Findings in Postreperfusion Biopsy.
| No IRI 96 (34.3%) |
Mild IRI 65 (23.2%) |
Moderate IRI 101 (36.1%) |
Severe IRI 18 (6.4%) |
Total OLT (N = 280 | P Value | |
|---|---|---|---|---|---|---|
| Steatosis degree | ||||||
| None (0–5%) | 80 (76.3%) | 50 (80%) | 77 (76.2%) | 16 (88.9%) | 223 (79.6%) | 0.70 |
| Mild (6–33%) | 14 (14.6%) | 11 (16.9%) | 19 (18.8%) | 2 (11.1%) | 46 (16.4%) | |
| Moderate (33–66%) | 2 (2.1%) | 2 (3.1%) | 4 (3.7%) | 0 | 8 (2.8%) | |
| Severe (>66%) | 0 | 2 (3.1%) | 1 (1%) | 0 | 3 (1.1%) | |
| Portal fibrosis | 36 (37.5%) | 20 (30.8%) | 32 (31.7%) | 6 (33.3%) | 94 (33.6%) | 0.79 |
| Apoptosis | 26 (27.1%) | 12 (18.5%) | 31 (29.8%) | 6 (40%) | 75 (26.8%) | 0.24 |
| Congestion | 20 (20.8%) | 8 (12.3%) | 23 (22.1%) | 5 (33.3%) | 56 (20%) | 0.22 |
| Haemorrhage | 17 (17.7%) | 14 (21.5%) | 21 (20.8%) | 3 (16.7%) | 55 (19.6%) | 0.90 |
| Neutrophilia grade | 0.01 | |||||
| Mild | 77 (80.2%) | 45(69.2%) | 58 (57.4%) | 8 (44.4%) | 188 (67.1%) | |
| Moderate | 4 (4.2%) | 8 (12.3%) | 36 (35.6%) | 5 (27.8%) | 53 (18.9%) | |
| Severe | 0 | 0 | 2 (2%) | 5 (27.8%) | 7 (2.5%) | |
| Necrosis Grade | 0.01 | |||||
| Absence | 30 (31.3%) | 20 (30.8%) | 17 (16.8%) | 0 | 67 (23.9%) | |
| Focal | 66 (68.8%) | 45 (69.2%) | 70 (69.3%) | 6 (33.3%) | 186 (66.4%) | |
| Confluent | 0 | 0 | 14 (13.9%) | 12 (66.7%) | 27 (9.6%) |
IRI, Ischaemia/reperfusion injury; OLT, orthotopic liver transplantation.
Factors Associated with Severity of Ischemia/Reperfusion Injury
Univariate and multivariate analyses were used to assess the relationship between the severity of IRI and donor or recipient features. Age, weight, and donor factors nor histological factors were associated with the severity of IRI (Table 3).
Table 3.
Univariate Analysis of Predictors of Severe IRI.
| Variables | No IRI N = 96 |
Mild N = 65 |
Moderate N = 101 |
Severe N = 18 |
P |
|---|---|---|---|---|---|
| pre-transplant recipient ICU status (%) | 15(15.6) | 12 (18.4) | 17(16.8) | 3 (16.7) | 0.94 |
| Recipient age (±SD) | 52.8 (11.1) | 55.5 (10.1) | 55.6 (13.3) | 60.3 (25.6) | 0.12 |
| Recipient BMI (±SD) | 26.7 (4.7) | 26.4 (4.4) | 26.7 (4.1) | 27.5 (5.4) | 0.84 |
| Donor BMI (±SD) | 26.1 (3.5) | 25.5 (4.5) | 25.9 (3.3) | 25.3 (3.7) | 0.88 |
| MELD score | 13.8 (5.1) | 13.2 (4.9) | 13.9 (5.9) | 13.5 (6.5) | 0.90 |
| Cold ischemia time | 397.9 (99.1) | 416.5 (81.1) | 388.9 (84.9) | 447.6 (112.5) | 0.04 |
| Warm ischemia time | 56.3 (43.2) | 52 (45.9) | 54.2 (37.0) | 52.4 (14.9) | 0.92 |
| Donor age | 54.9 (18.7) | 58.7 (16.6) | 60.5 (16.5) | 56.7 (18.4) | 0.15 |
| Steatosis >6% | 16 (16.7) | 15 (23.5) | 24 (23.7) | 2 (11.1) | 0.42 |
IRI, Ischaemia/reperfusion injury; MELD, Model for End-stage Liver Disease; SD, Standard Deviation; BMI, body mass index; ICU, intensive care unit.
In the multivariate analysis, only the cold ischemia time was an independent predictor of severe IRI (Table 4).
Table 4.
Multivariate Analysis of Predictors of Severe IRI.
| CI 95% | P Value | |
|---|---|---|
| pre-transplant recipient ICU status | 0.83–1.07 | 0.40 |
| Recipient age | 0.990–1.047 | 0.21 |
| Recipient BMI | −0.006–0.009 | 0.626 |
| Donor BMI | 0.830–1.077 | 0.40 |
| MELD score | −0.047-0.057 | 0.404 |
| Cold ischemia time | 1.001–1.011 | 0.03 |
| Warm ischemia time | 0.98–1.03 | 0.91 |
| Donor age | −0.002–0.016 | 0.89 |
| Steatosis >6% | 0.385–8.88 | 0.44 |
IRI, Ischaemia/reperfusion injury; MELD, Model for End-stage Liver Disease; ICU, intensive care unit; BMI, body mass index; CI, confidence interval. Cold ischemia time was the only predictor of IRI in the multivariate analysis of predictors of severe IRI.
Histological Factors as Predictors of Short-term Graft Outcomes
The severity of steatosis in PRB was associated with the presence of graft dysfunction. Patients who presented >6% steatosis in PRB exhibited a significant association with EAD (OR 2.1, P = 0.024 95% CI 1.10–4.16) and were less likely to present IGGF (OR 0.48, P = 0.01 95% CI 0.26–0.86). No association was found with PNF.
Second, the association between neutrophilic infiltrate and graft dysfunction was not statistically significant. However, the presence of vascular congestion on the PRB was significantly associated with the development of EAD (OR 4.3, P = 0.001 95% CI 2.26–8.34) and less likely to have IGGF (OR: 0.42, P = 0.004 95%; CI 0.23–0.76). Neither the presence of periportal fibrosis nor the degree of necrosis were associated with worse graft function after liver transplantation.
Ischemia/Reperfusion Injury as a Predictor of Graft Function After Liver Transplantation
Fifty-four patients developed EAD (19.3%), and 11 patients suffered PNF (3.9%); 172 patients (62.8%) met the criteria for IGGF. The detailed distribution according to the degree of IRI is shown in Table 5.
Table 5.
Time-zero Biopsy Ischaemia/Reperfusion Injury and Graft Outcomes.
| Total N = 280 |
No IRI N = 96 |
Mild N = 65 |
Moderate N = 101 |
Severe N = 18 |
P Value | |
|---|---|---|---|---|---|---|
| Early allograft dysfunction | 54 (19.3%) | 14 (14.6%) | 14 (21.5%) | 20 (19.8%) | 6 (33.3%) | 0.27 |
| Primary nonfunction | 11 (3.9%) | 2 (2.1%) | 2 (3.1%) | 6 (5.8%) | 1 (6.7%) | 0.52 |
| Initial good graft function | 176 (62.8%) | 63 (65.6%) | 39 (60%) | 68 (67.3%) | 6 (33.3%) | 0.04 |
| Peak ALT during first 7 days | 831 (±800) | 604.6 (±368) | 850 (±712.9) | 1043 (±107.5) | 1308 (724.8) | 0.002 |
| Retransplantation within 90 days | 8 (2.9%) | 3 (3.1%) | 1 (1.5%) | 2 (2.0%) | 2 (11.1%) | 0.16 |
ALT, alanine aminotransferase; IRI, Ischaemia/reperfusion injury.
The correlation between the severity of IRI and graft function outcomes was evaluated. Patients with severe IRI exhibited a lower incidence of IGGF. Therefore, 33.3% patients with severe IRI in PRB and 59.9% of patients with nonsevere IRI met the criteria for IGGF (OR: 0.34, 95% CI 0.12–0.92; P = 0.03). Additionally, patients with severe IRI, compared with those with nonsevere IRI, tended to present a higher incidence of EAD (33.3% vs 18.3%, P = 0.23), although the difference was not statistically significant.
Moreover, patients with severe IRI had a higher peak ALT during the first week after the LT, so the mean peak ALT for patients with severe IRI was 1308 U/l, 1043 U/l for moderate IRI, 850 U/l for mild IRI and 604.6 U/l for no IRI (P = 0.002).
Eleven patients with PNF were identified in our study: one and ten patients with severe and nonsevere IRI (3.8% vs. 5.6%), respectively. However, nonsignificant differences were found in the incidence of PNF (P = 0.52) among patients with different degrees of IRI.
Ischemia/Reperfusion Injury as a Predictor of Patient and Graft Survival
We assessed overall survival according to the degree of IRI in PRB. The median overall survival times in patients with severe and nonsevere IRI were 38.2 months (IQR: 6.4–98.2) and 80 months (IQR: 28.4–105.6), respectively. A significantly poorer patient overall survival was demonstrated in patients with severe IRI in PRB than in those with nonsevere IRI on the Kaplan–Meier analysis (Log Rank P = 0.04) (Figure 3A). This was also demonstrated on graft survival, so patient with severe IRI had a worse graft survival on the Kaplan–Meier analysis (Log Rank P = 0.025) (Figure 3B).
Figure 3.
(A) Kaplan–Meier analysis of overall survival according to the grade of ischaemia/reperfusion injury. A significantly worse overall survival was observed in patients with severe ischaemia/reperfusion injury than in those with nonsevere injury (Log Rank P = 0.04). (B) Kaplan–Meier analysis of graft survival according to the grade of ischaemia/reperfusion injury. Notice that there is a significant worse outcome in patients with severe IRI (Log Rank P = 0.025).
The overall 1- and 5-year patient overall survival were assessed according to the degree of IRI. Patients with no IRI and mild, moderate, and severe IRI exhibited 88% and 85%, 81%, and 67% overall 1-year survival, respectively. Moreover, patients with severe IRI had poorer rates than the rest of the group at the fifth year after liver transplantation. The survival in patients with severe IRI was 44%, whereas patients with no IRI, mild, and moderate IRI exhibited 68%, 72%, and 66% 5-year overall survival, respectively.
The cause of death and graft loss are detailed in Table 6.
Table 6.
Causes of Death Among Patients With Non-severe IRI and Severe IRI.
| Non severe IRI | Severe IRI | P | |
|---|---|---|---|
| Time until graft loss | 37.5 (44.6) | 12 (15.6) | 0.008 |
| Time until death (mean ± SD) | 44.3 (49.1) | 21.7 (29.7) | 0.04 |
| Causes of death | N.S | ||
| Cardiovascular | 6 (2.3%) | 1 (5.6%) | |
| Hepatic | 46 (17.6%) | 4 (22.2%) | |
| Infections | 25 (9.5%) | 5 (27.8%) | |
| De novo neoplasia | 17 (2.5%) | 1 (5.6%) | |
| Others | 0 | 0 |
IRI, Ischaemia/reperfusion injury; SD, Standard deviation.
Discussion
The present study is a retrospective study including 280 patients undergoing intraoperative PRB to assess the relationships between histopathological findings and graft outcomes and overall survival. Our analysis suggests that histological assessment of PRB identifies recipients with early poor liver function and worse outcomes after OLT. Moreover, our study identified cold ischemia time as an independent factor of severe IRI. Biopsies with severe IRI in PRB were associated with a lower incidence of IGGF, showing a poor short-term outcome after OLT. Among histological features, a higher steatosis degree predicted a higher incidence of early graft dysfunction (EAD and IGGF).
Two larger studies have assessed the relationship between preservation injury and graft outcomes. Jason M. et al included 476 OLTs with PRBs and demonstrated that severe IRI was significantly associated with donor age, donation after circulatory death, prolonged cold ischemia time, and steatosis. When long-term outcomes were assessed, the 1-year graft and patient survival rates were only 55 and 68% with severe IRI, respectively, compared with 90 and 93% in the nonsevere IRI group.11 Comparable with Jason M et al, the results of our study demonstrated that patients with severe IRI presented worse 1-year overall survival, whereas milder forms of IRI did not influence overall survival.
More recently, Takahiro et al evaluated 506 patients with PRB and reported the relationship between IRI and EAD.17 Therefore, IRI severity, specifically moderate to severe IRI, was associated with a higher incidence of EAD and graft survival at 6 months.17 In our study, we found that patients with severe IRI tended to have a higher incidence of EAD, although the difference was not statistically significant. Additionally, patients with severe IRI showed a lower incidence of IGGF.
Cold ischemia time was found to be a predictor of worse preservation injury, although no relationship between donor age or the degree of steatosis was identified. Jadhav et al recently reported, in a small study including 52 patients, that severity of IRI was associated with worse 3-month mortality and that prolonged ischemia time was related to the development of IRI.18 Most interestingly, in our study, when overall survival was assessed, patients with severe IRI exhibited worse overall survival than those with no IRI and mild and moderate IRI. This difference was maintained in the 1- and 5-year overall survival rates.
Other studies have shown that cold ischemia can result in sinusoidal endothelial and bile duct epithelial cell damage. Therefore, there is a higher incidence of biliary complications in patients with preservation injury.19, 20, 21, 22
The role of liver graft steatosis before OLT may be important and can exacerbate the innate immune response after OLT and, therefore, IRI.6,23 To grade the severity of IRI in PRB, Ali et al11 also used neutrophilic infiltrate, apoptosis and hepatocyte dropout. Zanchet et al9 reported that steatosis, mononuclear and polymorphonuclear infiltrate and necrosis were associated with mortality but failed to identify an association between the degree of IRI and short-term outcomes and mortality after OLT. When the histological findings were assessed, we found that the severities of steatosis and vascular congestion were associated with worse graft outcomes after OLT, supporting the idea that early histological findings are important predictors of outcomes after OLT.
The indications for performing PRB are controversial. Extended donor criteria seem to be accepted in many centers and might have some impact on graft outcomes. Early graft dysfunction is more frequent in patients with moderate and severe steatosis.18,24,25 Therefore, having some early diagnostic and prognostic tool to identify grafts and patients at high risk of poor outcome may be important. Having a PRB may provide one of these important prognostic tools identifying patients with higher risk of poor graft outcomes. A strict follow up should be recommended in these patients to identify early complication that might be managed properly.
There are some limitations that should be mentioned. This study was a retrospective review of medical records and was therefore limited by the absence of prospective or randomized data. Secondly, the limited number of patients developing severe IRI and PNF made it difficult to establish concrete causal relationship. Thirdly, only OLTs from donors showing brain death were included in our series. Fourthly, the influence of preservation injury on the biliary duct is well known, but neither the histological changes in the bile ducts nor the assessment of non-anastomotic biliary strictures were assessed in this study.
In summary, based upon our results, patients with severe IRI in PRB exhibit a higher incidence of early graft dysfunction and poorer overall survival, suggesting that histopathological findings correlate with graft outcomes after OLT. PRB may provide a prognostic tool for identifying patients at high risk of graft dysfunction and pre-empt a strict follow-up for these patients.
Credit authorship contribution statement
Esteban Fuentes-Valenzuela designed and performed the research and wrote the paper; Javier Tejedor-Tejada designed the research and contributed to the analysis; Beatriz Madrigal Rubiales provided advice on pathology issues; Félix García-Pajares designed the research and supervised the report; Rodrigo Nájera-Muñoz, Carlos Maroto-Martín, and Laura Sánchez-Delgado contributed to the data collection; and Carmen Alonso-Martín and Carolina Almohalla-Álvarez provided clinical advice. Gloria Sánchez Antolín supervised the research.
Conflicts of interest
The authors have none to declare.
Acknowledgments
We acknowledge the Department of Pathology for their dedicated work and contribution to this study.
Funding
None.
References
- 1.Ahmed A., Keeffe E.B. Current indications and contraindications for liver transplantation. Clin Liver Dis. 2007 May;11:227–247. doi: 10.1016/j.cld.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Chen X.B., Xu M.Q. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014 Apr;13:125–137. doi: 10.1016/s1499-3872(14)60023-0. [DOI] [PubMed] [Google Scholar]
- 3.Uemura T., Randall H.B., Sanchez E.Q., et al. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver Transpl. 2007 Feb;13:227–233. doi: 10.1002/lt.20992. [DOI] [PubMed] [Google Scholar]
- 4.Neves D.B., Rusi M.B., Diaz L.G.G., Salvalaggio P. Primary graft dysfunction of the liver: definitions, diagnostic criteria and risk factors. Einstein (Sao Paulo) 2016;14:567–572. doi: 10.1590/S1679-45082016RW3585. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff K.M., Kulik L., Samstein B., et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010 Aug;16:943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 6.Gehrau R.C., Mas V.R., Dumur C.I., et al. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. Transplantation. 2015;99:2523–2533. doi: 10.1097/TP.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busquets J., Figueras J., Serrano T., et al. Postreperfusion biopsy changes predict biliary complications after liver transplantation. Transplant Proc. 2002;34:256–258. doi: 10.1016/s0041-1345(01)02750-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang G., Hu B., Li Z. Cold ischemia/reperfusion injury in a mouse model of partial liver transplantation. J Surg Res. 2013 May 15;181:337–341. doi: 10.1016/j.jss.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 9.Zanchet M.V., Silva LLG da, Matias J.E.F., Coelho J.C.U. Post-reperfusion liver biopsy and its value in predicting mortality and graft dysfunction after liver transplantation. Arq Bras Cir Dig. 2016 Jul 1;29:189–193. doi: 10.1590/0102-6720201600030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolondi G., Mocchegiani F., Montalti R., Nicolini D., Vivarelli M., De Pietri L. Predictive factors of short term outcome after liver transplantation: a review. World J Gastroenterol. 2016 Jul 14;22:5936–5949. doi: 10.3748/wjg.v22.i26.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali J.M., Davies S.E., Brais R.J., et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transplant. 2015 Apr 1;21:487–499. doi: 10.1002/lt.24072. [DOI] [PubMed] [Google Scholar]
- 12.Gaffey M.J., Boyd J.C., Traweek S.T., et al. Predictive value of intraoperative biopsies and liver function tests for preservation injury in orthotopic liver transplantation. Hepatology. 1997 Jan;25:184–189. doi: 10.1002/hep.510250134. [DOI] [PubMed] [Google Scholar]
- 13.Koçbiyik A., Demirhan B., Sevmis S., Budakoglu I., Karakayali H., Haberal M. Role of postreperfusion subcapsular wedge biopsies in predicting initially poor graft function after liver transplantation. Transplant Proc. 2009 Sep;41:2747–2748. doi: 10.1016/j.transproceed.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Vandenbroucke J.P., von Elm E., Altman D.G., et al. Strengthening the reporting of observational studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007 Oct 16;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makowka L., Gordon R.D., Todo S., et al. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19(1 Pt 3):2378. [PMC free article] [PubMed] [Google Scholar]
- 16.Ploeg R.J., D'Alessandro A.M., Knechtle S.J., et al. Risk factors for primary dysfunction after liver transplantation - a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Ito T., Naini B.V., Markovic D., et al. Ischemia reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am J Transplant. 2021 Feb;21:614–625. doi: 10.1111/ajt.16219. [DOI] [PubMed] [Google Scholar]
- 18.Jadhav P.V., Kothakota S.R., Sasidharan M., Kareem H., Nair A.K. Effect of donor hepatic steatosis on ischemia reperfusion injury in liver transplant recipient. J Clin Exp Hepatol. 2020 May 1;10:236–244. doi: 10.1016/j.jceh.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busquets J., Figueras J., Serrano T., et al. Postreperfusion biopsies are useful in predicting complications after liver transplantation. Liver Transpl. 2001 May;7:432–435. doi: 10.1053/jlts.2001.23868. [DOI] [PubMed] [Google Scholar]
- 20.Kakizoe S., Yanaga K., Starzl T.E., Demetris A.J. Evaluation of protocol before transplantation and after reperfusion biopsies from human orthotopic liver allografts: considerations of preservation and early immunological injury. Hepatology. 1990 Jun;11:932–941. doi: 10.1002/hep.1840110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kukan M., Haddad P.S. Role of hepatocytes and bile duct cells in preservation-reperfusion injury of liver grafts. Liver Transplant. 2001 May 1;7:381–400. doi: 10.1053/jlts.2001.23913. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y., Ji W.B., Duan W.D., Ye S., Dong J.H. Graft cholangiopathy: etiology, diagnosis, and therapeutic strategies. Hepatobiliary Pancreat Dis Int. 2014 Feb;13:10–17. doi: 10.1016/s1499-3872(14)60001-1. [DOI] [PubMed] [Google Scholar]
- 23.Linares I., Hamar M., Selzner N., Selzner M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation. 2019 Jan;103:78–90. doi: 10.1097/TP.0000000000002466. [DOI] [PubMed] [Google Scholar]
- 24.Nemes B., Gámán G., Polak W.G., et al. Extended criteria donors in liver transplantation Part I: reviewing the impact of determining factors. Expert Rev Gastroenterol Hepatol. 2016 Jul;10:827–830. doi: 10.1586/17474124.2016.1149061. [DOI] [PubMed] [Google Scholar]
- 25.Nemes B., Gámán G., Polak W.G., et al. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016 Jul;10:841–859. doi: 10.1586/17474124.2016.1149062. [DOI] [PubMed] [Google Scholar]