Abstract
The present study investigated the characteristics, diagnosis, treatment and prognosis of hepatic portal venous gas (HPVG) using the data of 20 patients from the Tongji University School of Medicine Affiliated with Yangpu Hospital (Shanghai, China). The aim of the present study was to optimize the management method and improve the prognosis of patients with HPVG. A total of 20 patients were selected using a CT scan to confirm HPVG. All patients were enrolled and identified via a search engine, which examined all CT radiology reports containing the words pneumatosis and/or portal venous gas/air. Data were collected and analyzed, including sex, age, laboratory evidence, etiologies at admission, therapeutic method and in-hospital mortality. The patients consisted of 14 women (mean age, 79.1 years) and six men (mean age, 67.8 years). The results demonstrated that HPVG indicated a higher inflammatory index. The etiologies of HPVG included abdominal infection, pulmonary infection and hemorrhage, whereas the comorbidities included hypertension, diabetes, coronary disease, cerebrovascular disease and renal insufficiency. The present study determined that intestinal obstruction, acute enteritis and pulmonary infection were the main causes of HPVG. Of the 20 patients enrolled in the present study, four patients received surgery and 16 patients received conservative treatment. The overall in-hospital mortality was 25%. The present study indicated that the causes of HPVG may be closely related to inflammation and blood vessel injury. It was also determined that hemodynamic disorders of the intestinal tract and the combination of different types of infection were important contributors towards patient mortality.
Keywords: hepatic portal venous gas, hepatic portal venous gas, portal vein, intestinal obstruction, enteritis
Introduction
Hepatic portal venous gas (HPVG) was first described in 1955 as abnormal gas found on abdominal plain radiographs in infants. In 1978 HPVG was reported to have a mortality rate of 75% (1,2). HPVG is associated with necrotizing colitis (2) and was therefore previously regarded as a lethal condition. However, HPVG is now known to not be a specific disease entity but merely a rare condition in patients suffering from acute abdomen (3). HPVG is mostly caused by intestinal ischemia, intra-abdominal abscesses, necrotizing enterocolitis, abdominal trauma, infectious enteritis and inflammatory bowel disease (4).
In recent decades, with an increase in the popularity of CT scans, the number of patients diagnosed with HPVG has increased and some patients have been treated without surgery (5). The clinical features and management of HPVG have greatly changed with the rapid development of diagnostic tools and treatments. However, numerous previous studies of HPVG are reported in the form of single case report and therefore lack representation. Therefore, the aim of the present study was to investigate the characteristics, diagnosis, treatment and prognosis of HPVG in 20 patients.
Materials and methods
Patients
In the present study, 20 patients with HPVG, which was diagnosed using a CT scan, were selected at the Tongji University School of Medicine affiliated with Yangpu Hospital (Shanghai, China) between December 2015 and December 2020. Of the patients selected 70% were female (mean age of all patients, 75.4 years). The main complaint from the patients was abdominal pain and among them patients with cancer had a history of surgery or chemotherapy. Moreover, none of the patients had a history of HPVG. The medical records and radiology films were reviewed retrospectively. All patients were enrolled and were identified using a search engine, whereby all CT radiology reports were examined that contained the words ‘pneumatosis’ and/or ‘portal venous’ ‘gas/air’. The data that were collected and analyzed included sex, age, laboratory evidence, etiologies at admission, therapeutic method and in-hospital mortality. All patients provided written informed consent. The research received approval from the ethics committee of Yangpu hospital (approval no. YP20201102023). The reporting of this study conformed to Strengthening the Reporting of Observational Studies in Epidemiology guidelines (6).
Diagnosis and treatment
Routine blood tests, biochemical examinations and CT scans were performed at admission. Blood tests and biochemical examination indicators identified red blood cells, white blood cells, platelets, hemoglobin, neutrophils, C-reactive protein (CRP), procalcitonin (PCT), D-dimer (DD-I), alanine aminotransferase and aspartate aminotransferase levels. The diagnosis of HPVG was confirmed by a radiologist and surgeon. Conservative treatment and/or surgery were selected according to the specific condition of each patient. Conservative treatment mainly included fluid infusion and antibiotic therapy, atomizing inhalation and gastrointestinal decompression. Surgery included enterodialysis and enterectomy, gastrointestinal cancer resection and hernioplasties of oblique hernia.
Statistical analysis
Continuous parameters are presented as the mean and ranges. Discrete variables are presented as numbers and percentages.
Results
A total of 20 patients were diagnosed with HPVG during the study period. Patient information is presented in Table I. The results demonstrated that the average CRP, PCT and DD-I levels were higher compared with normal levels. The etiologies of the 20 patients with HPVG included abdominal infection, pulmonary infection and hemorrhage. The comorbidities included hypertension, diabetes, coronary disease, cerebrovascular disease and renal insufficiency. The proportions of each etiology and comorbidity in the enrolled patients are summarized in Table II.
Table I.
Characteristics of 20 patients with HPVG.
| Characteristic | Value |
|---|---|
| Total | 20 |
| Sex (%) | |
| Female/Male | 14 (70%)/6 (30%) |
| Age (years) | |
| Average (range) | 75.4 (20-94) |
| Female/Male | 79 (20-94)/67.8 (48-87) |
| Combined with MVG (%) | 12 (60%) |
| RBC (1012/l) | |
| Normal value (Female/Male, range) | 3.5-5.0/4.0-5.5 |
| Average (range) | 3.9 (2.7-5.7) |
| WBC (109/l) | |
| Normal value (range) | 4-10 |
| Average (range) | 9.4 (2.5-20.2) |
| PLT (109/l) | |
| Normal value (range) | 100-400 |
| Average (range) | 198.8 (96-385) |
| HB (g/l) | |
| Normal value (Female/Male, range) | 110-150/120-160 |
| Average (range) | 119.6 (68-166) |
| N (Neutrophil, %) | |
| Normal value (range) | 50-70 |
| Average (range) | 75.4 (46.8-96) |
| CRP (mg/l) | |
| Normal value (range) | 0-5 |
| Average (range) | 46.1 (0.9-200) |
| PCT (ng/l) | |
| Normal value (range) | 0-0.3 |
| Average (range) | 8.5 (0.1-44.7) |
| DD-I (D-Dimer, mg/l) | |
| Normal value (range) | 0-0.5 |
| Average (range) | 4.2 (0.6-8.1) |
| ALT (U/l) | |
| Normal value (range) | 0-40 |
| Average (range) | 38.6 (6-140) |
| AST (U/l) | |
| Normal value (range) | 0-40 |
| Average (range) | 50.5 (16-229) |
HPVG, hepatic portal venous gas; MVG, mesenteric venous gas; RBC, red blood cell; WBC, white blood cell; PLT, platelet; HB, hemoglobin; N, neutrophil; CRP, C-reactive protein; PCT, procalcitonin; DD-I, D-Dimer; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table II.
Etiologies and comorbidities of 20 patients with HPVG.
| Type | Value (%) |
|---|---|
| Etiologies | |
| Abdominal infection | 15(75) |
| Strangulated intestinal obstruction | 2(10) |
| Simple intestinal obstruction | 3(10) |
| Simple intestinal obstruction with cerebral infarction | 1(5) |
| Simple intestinal obstruction with pulmonary infection | 1(5) |
| Acute enteritis | 7(35) |
| Pulmonary infection | 5(25) |
| Simple pulmonary infection | 2(10) |
| Pulmonary infection with simple intestinal obstruction | 1(5) |
| Pulmonary infection with pyloric obstruction | 1(5) |
| Hemorrhage | 2(10) |
| Hemorrhage of digestive tract | 1(5) |
| Cerebellar hemorrhage | 1(5) |
| Comorbidities | |
| Hypertension | 10(50) |
| Diabetes | 6(30) |
| Coronary disease | 6(30) |
| Cerebrovascular disease | 5(25) |
HPVG, hepatic portal venous gas.
In the present study, six patients required surgery; however, two of the patients rejected the operation. The remaining 16 patients received conservative treatment. For one patient with a strangulated intestinal obstruction, enterodialysis and an enterectomy were performed. For two patients with gastrointestinal cancer, resections were performed to relieve the symptoms of the obstruction. An inguinal hernia was treated with a hernioplasty in one patient. The overall in-hospital mortality rate was 25%. Among the 20 cases of HPVG, two patients with strangulated intestinal obstructions and three patients with a multiplicity of infection succumbed, which included simple intestinal obstruction with pulmonary infection, simple intestinal obstruction with cerebral infarction and severe pulmonary infection with simple intestinal obstruction. The rate of HPVG absorption ranged from 2-8 days, with a mean time of 4.2 days. This was determined to be unrelated to the outcome of the disease. The details that were collected and analyzed are presented in Table III.
Table III.
Treatment and prognosis of patients.
| Variable | Value |
|---|---|
| Meeting surgical indications | 6 (30%) |
| Operation | 4 (20%) |
| Enterodialysis and enterectomy | 1 (5%) |
| Gastrointestinal cancer resection | 2 (5%) |
| Hernioplasty of oblique hernia | 1 (5%) |
| Conservative treatment | 16 (80%) |
| In-hospital mortality (%) | 5 (25%) |
| Strangulated intestinal obstruction | 2 (10%) |
| Simple intestinal obstruction with pulmonary infection | 1 (5%) |
| Simple intestinal obstruction with cerebral infarction | 1 (5%) |
| Severe pulmonary infection with simple intestinal obstruction | 1 (5%) |
| Time of gas absorption, day | |
| Average (range) | 4.2 (2-8) |
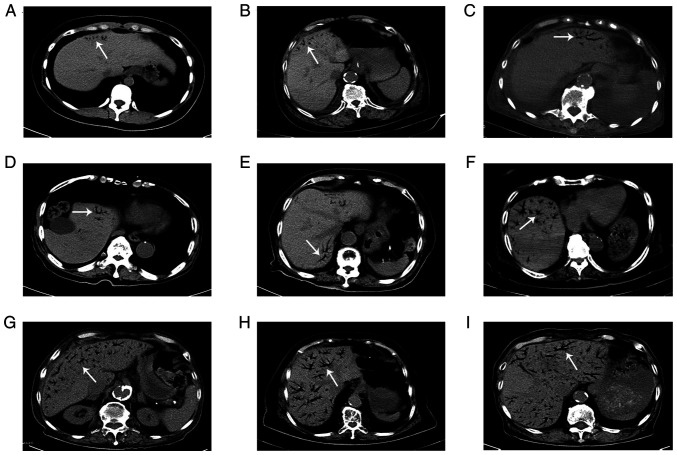
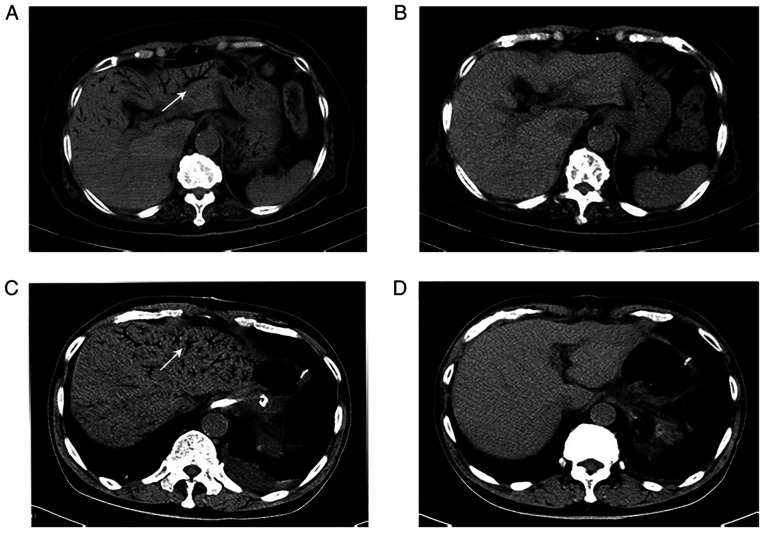
The imaging results for HPVG demonstrated branching radiolucency that extended to within 2 cm of the liver capsule. Furthermore, 60% of patients with HPVG also had mesenteric venous gas. Overall, nine typical imaging results of HPVG were identified in the present study and are presented in Fig. 1. The CT scans of two recovered patients, with different degrees of HPVG, were selected to demonstrate the changes before and after gas absorption; these images are presented in Fig. 2.
Figure 1.
CT scans of nine patients with various degrees of hepatic portal venous gas. (A-C) An abdominal CT scan (coronal view) demonstrated tiny amounts of gas existing within 2 cm of the liver capsule. (D-F) Partial gas was present within more than two liver segments. (G-I) Extensive gas was present throughout the entire portal venous system. White arrows indicate the presence of air in the venous system. CT, computed tomography.
Figure 2.
Absorption of venous gas in two patients with hepatic portal venous gas. (A) An abdominal CT scan (coronal view) demonstrated extensive gas within the portal venous system and mesenteric vein. (B) After two days the gas had disappeared. (C) Another abdominal CT scan (coronal view) demonstrated the extensive gas within the portal venous system and mesenteric vein. (D) After six days the gas had disappeared. White arrows indicate the presence of air in the venous system. CT, computed tomography.
Discussion
HPVG is rare radiological finding and can be indicative of several diseases, including inflammatory bowel disease and obstructive gastrointestinal disease, as well as potentially life-threatening conditions, such as mesenteric ischemia (4). With an increase in the use of CT scans during the early diagnosis of HPVG, the clinical outcome of patients has improved due to accurate diagnosis and early treatment. However, the pathogenesis of HPVG remains to be fully elucidated. At present two hypotheses of how HPVG originates have been proposed. One is that high-pressure gas from the bowel lumen circulates into the venous system via vascular endothelial injuries, such as abdominal trauma, bowel ischemia, or as a consequence of diagnostic or therapeutic invasive procedures, such as enteral nutrition. However, other studies have hypothesized that abdominal infections can release gas-producing bacteria into the portal venous system (7-11).
In the present study, patients with HPVG were relatively older with an average age of 75.4 years. However, the youngest patient included was 20 years old. These results indicated that old age may be an important factor in HPVG. Moreover, even though the majority of enrolled patients were women, there was no evidence that suggested that sex significantly determined which individuals would be more likely to get HPVG. A previous study concerning HPVG that was performed in Japan similarly reported that a statistical difference was found in the age of patients but not sex (12). In terms of inflammatory markers, PCT and CRP were demonstrated to be important. As a degradation product of cross-linked fibrin, DD-I induces venous thrombosis when inflammation occurs. In the present study, the average levels of CRP, PCT and DD-I were higher than the normal levels, which indicated a severe inflammatory state. From these results, it can be hypothesized that most etiologies have a close association with inflammation and vascular injury, which are potentially triggering factors for HPVG. In the present study abdominal infection, pulmonary infection and hemorrhage were major etiologies, whereas hypertension, diabetes, cardio-cerebrovascular disease and renal insufficiency were major comorbidities. These results demonstrated that both inflammation and injury of blood vessels may potentially induce HPVG, which was consistent with the aforementioned conclusion. However, a single case of hemorrhage in the present study was a result of cerebellar hemorrhage rather than abdominal trauma. Furthermore, five cases of infection were caused by pulmonary infection rather than abdominal infection. These results also indicated that not only hemorrhage of the digestive tract and abdominal infection can potentially induce HPVG, but also injury of the blood vessels and infection in other organs.
In a previous study it was reported that HPVG is a lethal condition and is related to mesenteric ischemia, which is associated with extended bowel necrosis (13). However, different conclusions were drawn from the results of the present study. Most of the enrolled patients relied on CT examination to confirm the diagnosis of HPVG. This is because CT has a high sensitivity and is considered to be the gold standard method for medical imaging (14). Wiesner et al (15) report that contrast-enhanced CT is a powerful investigatory tool to differentiate HPVG with acute mesenteric ischemia from HPVG with a non-ischemic pathology, which exhibits bowel wall thickening, either marked or absent enhancement of the bowel wall, intramural pneumatosis, mesenteric edema, ascites, mesenteric or portal venous gas and mesenteric arterial or venous thromboembolism. CT images in the present study demonstrated intrahepatic branching radiolucencies with various amounts of gas. Furthermore, the results determined that a hemodynamic disorder of the intestinal tract and a combination of different types of severe infection, which indicated a severe inflammatory state, were more likely to induce patient mortality. Moser et al (16) report that if HPVG is present in combination with a necrotic bowel, the mortality risk is >50%. Therefore, when ischemia is suspected, HPVG is an ominous sign that indicates progression to necrosis, especially when associated with a high level of lactate (17). However, it should be considered that HPVG alone does not lead to patient mortality. The present study demonstrated that the time of portal venous gas absorption ranged from 2-8 days, with a mean time of 4.2 days, which was unrelated to the outcome of the disease. Moreover, the results of the present study demonstrated that the prognosis of a patient was not directly proportional to the volume of HPVG or the number of liver segments containing the venous gas. However, Kinoshita et al (7) report that the involvement of three or more segments is an important sign that indicates a potentially lethal outcome and is correlated with a poor prognosis in 75% of cases. The main differential diagnosis for HPVG is pneumobilia, which is detected centrally within the liver rather than extending to the peripheral parenchyma (18). In certain cases, pneumobilia and HPVG may coexist depending on the etiology (7). Moreover, in the present study, 60% of patients were confirmed by abdominal CT to have gas present in the portal vein, which was accompanied by the presence of gas in the mesenteric vein. It can therefore be hypothesized that this phenomenon is related to their interlinked anatomic structure and that gas potentially moves via connected blood vessels.
Treatment for HPVG is dependent on the clinical presentation of the patient, imaging and laboratory evidence. Koami et al (19) report that HPVG is not a predictor of urgent surgery and high mortality if there is no obvious clinical evidence of ischemia or necrosis. Moreover, Wayne et al (17) demonstrate that treatment for HPVG should be directed at the underlying disease and the need for an emergency operation should be determined based on the primary disease, with consideration of the high mortality rate of HPVG associated with bowel necrosis. In the present study, six patients met the criteria for surgery; however, two patients rejected surgery because of the associated risk. Conservative treatment was therefore performed due to the absence of clinical and radiographic evidence of a hemodynamic disorder of the intestinal tract. An urgent exploratory laparotomy was only mandatory in one patient, in which intestinal ischemia or infarction was suspected based on clinical and radiologic evidence. Furthermore, a 92-year-old woman, who underwent enterodialysis and an enterectomy, succumbed with a strangulated intestinal obstruction, which had been suspected based on clinical and radiologic evidence. It was hypothesized that old age and complicated underlying diseases may have been the cause of the mortality. However, the HPVG disappeared within two days but the patient still succumbed. In addition, a previous study reports that for a patient with extensive comorbidities, in old age, surgery may also not be survivable (17). In the present study, the overall in-hospital mortality rate was determined to be 25%, which was consistent with other previous studies where mortality rates decreased in the range of 27-47% (13,20). From the aforementioned results it can be determined that surgical intervention is not a requisite therapeutic method but should still be investigated. Furthermore, surgery should not generally be performed if the patient is clinically stable and there is only radiological evidence of HPVG (21,22). At present, gastrointestinal decompression and antibiotic treatment are routine conservative therapies. Previous studies have reported that certain cases of HPVG occur without necrosis and these cases can be treated successfully with medication, which is consistent with the conclusion of the present study (23,24). Moreover, emergency surgery should be performed immediately in the presence of an obvious peritonitis symptom, which may indicate the presence of a hemodynamic disorder of the intestinal tract, even if there are no signs of ischemia on the CT scan (25).
In conclusion, in the present study, the limited number of HPVG cases restricted any deeper analysis and the conclusions may be partly controversial, such as the relationship between HPVG levels or the time of HPVG absorption and the prognosis of the disease. Furthermore, patients with HPVG caused by abdominal trauma and/or a consequence of diagnostic or therapeutic invasive procedures, such as colonoscopy or enteral nutrition, were not identified and enrolled in the present study. Large-scale data on HPVG is needed for future investigations.
Acknowledgements
The authors thank Dr Cui Tang (Department of Radiology, Yangpu Hospital, Tongji University, Shanghai, China) who provided technical assistance.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YZ analyzed the patient data and wrote the manuscript. HLL designed the present study. HLL and MBL confirm the authenticity of all the raw data. MT and HW participated in data analysis and interpretation. HHJ was involved in the design of the study and revised the manuscript. MBL made substantial contributions to conception and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study received ethics approval from the ethics committee of Yangpu Hospital (approval no. YP20201102023) and informed consent was obtained from all participants.
Patient consent for publication
All patients provided written informed consent. They consented to the images being taken for the purpose of research and also consented to their publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med. 1955;74:486–489. [PubMed] [Google Scholar]
- 2.Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic-portal venous gas in adults: Etiology, pathophysiology and clinical significance. Ann Surg. 1978;53:231–234. doi: 10.1097/00000658-197803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capolupo GT, Masciana G, Carannante F, Caricato M. Hepatic portal venous gas after colonoscopy: A case report and review. Int J Surg Case Rep. 2018;51:54–57. doi: 10.1016/j.ijscr.2018.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alqahtani S, Coffin CS, Burak K, Chen F, Gregor JM, Beck P. Hepatic portal venous gas: A report of two cases and a review of the epidemiology, pathogenesis, diagnosis and approach to management. Can J Gastroenterol. 2007;21:309–313. doi: 10.1155/2007/934908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou SK, Chern CH, How CK, Chen JD, Wang LM, Lee CH. Hepatic portal venous gas: Clinical significance of computed tomography findings. Am J Emerg Med. 2004;22:214–218. doi: 10.1016/j.ajem.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. STROBE Initiative. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita H, Shinozaki M, Tanimura H, Umemoto Y, Sakaguchi S, Takifuji K, Kawasaki S, Hayashi H, Yamaue H. Clinical features and management of hepatic portal venous gas: Four case reports and cumulative review of the literature. Arch Surg. 2001;136:1410–1141. doi: 10.1001/archsurg.136.12.1410. [DOI] [PubMed] [Google Scholar]
- 8.Shah PA, Cunningham SC, Morgan TA, Daly BD. Hepatic Gas: Widening Spectrum of Causes Detected at CT and US in the Interventional Era. Radiographics. 2011;31:1411–1413. doi: 10.1148/rg.315095108. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Takeuchi Y, Ishihara R, Kawakami H. Hepatic portal venous gas following colonic endoscopic submucosal dissection. Internal Med. 2019;58:755–756. doi: 10.2169/internalmedicine.1771-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solakoglu T, Sari SO, Koseoglu H, Basaran M, Akar M, Buyukasik S, Ersoy O. A case of hepatic portal venous gas after colonoscopy. Arab J Gastroenterol. 2016;17:140–142. doi: 10.1016/j.ajg.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka T, Kobayashi K, Lefor AK, Sasaki J, Shinozaki H. Mesenteric ischemia with pneumatosis intestinalis and portal vein gas associated with enteral nutrition: A series of three patients. Clin J Gastroenterol. 2020;13:1160–1164. doi: 10.1007/s12328-020-01206-4. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi C, Michihata N, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality for hepatic portal venous gas: Analysis of 1590 patients using a japanese national inpatient database. World J Surg. 2018;42:816–822. doi: 10.1007/s00268-017-4189-y. [DOI] [PubMed] [Google Scholar]
- 13.McElvanna K, Campbell A, Diamond T. Hepatic portal venous gas-three non-fatal cases and review of the literature Ulster Med. J. 2012;81:74–78. [PMC free article] [PubMed] [Google Scholar]
- 14.Moussa M, Marzouk I, Abdelmoula K, Manamani A, Dali N, Farhat LC, Hendaoui L. Role of Computed tomography in predicting prognosis of Hepatic portal venous gas. Int J Surg Case Rep. 2017;30:177–182. doi: 10.1016/j.ijscr.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226:635–650. doi: 10.1148/radiol.2263011540. [DOI] [PubMed] [Google Scholar]
- 16.Moser A, Stauffer A, Wyss A, Schneider C, Essig M, Radke A. Conservative treatment of hepatic portal venous gas consecutive to a complicated diverticulitis: A case report and literature review. Int J Surg Case Rep. 2016;23:186–189. doi: 10.1016/j.ijscr.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayne E, Ough M, Wu A, Liao J, Andresen KJ, Kuehn D, Wilkinson N. Management algorithm for pneumatosis intestinalis and portal venous gas: Treatment and outcome of 88 consecutive cases. J Gastrointest Surg. 2010;14:437–448. doi: 10.1007/s11605-009-1143-9. [DOI] [PubMed] [Google Scholar]
- 18.Soon WC, Liu KY, Blunt D. Hepatic portal venous gas. Clin Case Rep. 2015;3:518–519. doi: 10.1002/ccr3.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koami H, Isa T, Ishimine T, Kameyama S, Matsumura T, Yamada KC, Sakamoto Y. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today. 2015;45:156–161. doi: 10.1007/s00595-014-0941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AL, Millington TM, Sahani D, Chung RT, Bauer C, Hertl M, Warshaw AL, Conrad C. Hepatic portal venous gas: The ABCs of management. Arch Surg. 2009;144:575–581. doi: 10.1001/archsurg.2009.88. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Al Furajii H, Cahill RA. Symptomatic pneumatosis intestinalis (including portal venous gas) after laparoscopic total colectomy. World J Gastrointest Endosc. 2014;6:564–567. doi: 10.4253/wjge.v6.i11.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai N, Handa O, Naito Y, Dohi O, Okayama T, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, et al. Stenotic ischemic enteritis with concomitant hepatic portal venous gas and pneumatosis cystoides intestinalis. Internal Med. 2018;57:1995–1999. doi: 10.2169/internalmedicine.0367-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong I, Hong SW, Chang YG, Lee B, Lee WY, Ohe HJ, Kim YK. Successful conservative management of hepatic portal venous gas due to anastomosis leakage after a sigmoidectomy. Ann Coloproctol. 2019;35:282–284. doi: 10.3393/ac.2018.03.23.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuda T, Yagi N, Nakahata Y, Kurobe T, Yasuda Y, Omatsu T, Obora A, Kojima T. A case of phlegmonous gastritis with hepatic portal venous gas caused by Aeromonas hydrophila successfully treated with medication. Clin J Gastroenterol. 2020;13:281–286. doi: 10.1007/s12328-019-01020-7. [DOI] [PubMed] [Google Scholar]
- 25.Dibra R, Picciariello A, Trigiante G, Labellarte G, Tota G, Papagni V, Martines G, Altomare DF. Pneumatosis intestinalis and hepatic portal venous gas: Watch and wait or emergency surgery? a case report and literature review. Am J Case Rep. 2020;21(e923831) doi: 10.12659/AJCR.923831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.