Abstract
Laparoscopic surgery for malignant solid tumors is still in the stage of clinical exploration. Neuroblastoma is a common solid tumor in children. The present study discussed significance and feasibility of complete resection of stage III neuroblastoma by laparoscopic surgery and its safety and effectiveness was compared with traditional surgery. For children suffering from neuroblastoma with large tumor volume and vascular invasion, preoperative chemotherapy can be given and minimally invasive laparoscopic surgery can be one option to be considered when the tumor volume is <6 cm. During the operation, the tumor tissue can be removed by segmental resection and the removal of as much tumor tissue as possible is an important factor in improving the prognosis. Laparoscopic minimally invasive surgery is associated with minimal surgical trauma and quick recovery of patients, and children can receive postoperative chemotherapy as early as possible, which is conducive to good recovery. Basically, the prerequisite and requirements for performing this operation are professional laparoscopic skills and an experienced team.
Keywords: laparoscopy, neuroblastoma, children
Introduction
Neuroblastoma is the most common solid tumor in children (1). With the development of pediatric anesthesia and endoscopic minimally invasive technology, children's abdominal tumors can be completely removed by laparoscopy. Laparoscopic minimally invasive surgery has little trauma and quick recovery and children can receive postoperative chemotherapy as early as possible, which is conducive to good recovery. However, laparoscopic surgery for malignant solid tumors is still in the stage of clinical exploration. The laparoscopic surgical approach can be intraperitoneal and retroperitoneal. The two approaches are safe and effective in the resection of adrenal neurogenic tumors. The transperitoneal approach is the standard approach for the treatment of adrenal tumors. When the tumor diameter is >6 cm, the tumor is malignant, or the tumor surrounds important blood vessels or invades surrounding tissues, it is more suitable to choose the transperitoneal approach.
At present, there are few literature reports on the successful treatment of stage III-IV neuroblastoma by laparoscopy. The present study discussed the significance and feasibility of complete resection of stage III neuroblastoma by laparoscopic surgery while comparing its safety and effectiveness with traditional surgery.
Case report
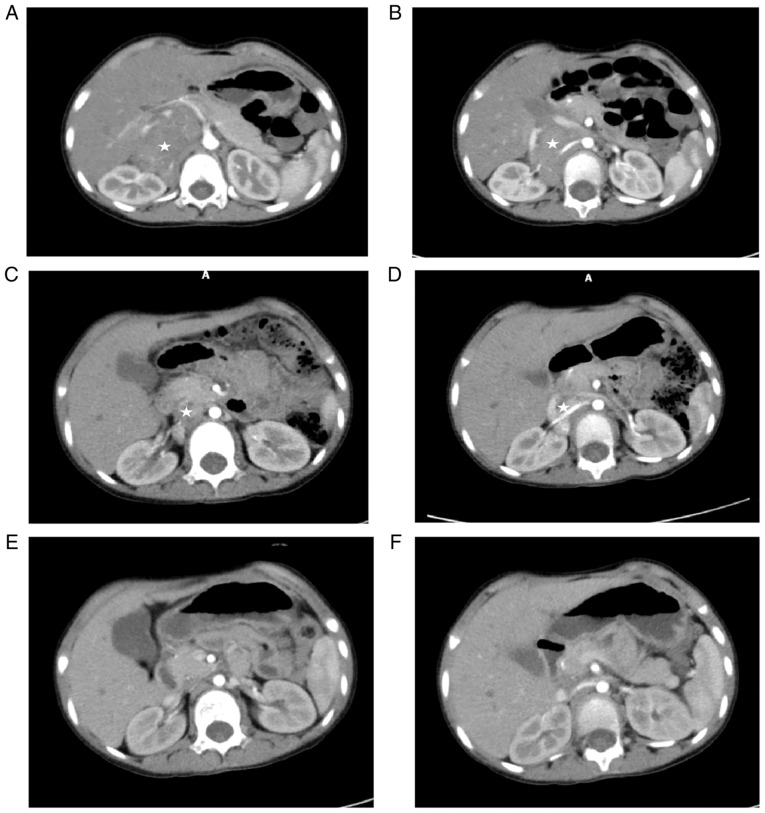
A 4-year-old girl was admitted to the First Affiliated Hospital of Xiamen University on 26 February 2021 with a 6-month history of right intercostal pain. Physical examination revealed that right abdominal distention and a hard mass could be palpable under the right lower costa. Abdominal enhancement computed tomography scan (CT) on 1 March 2021 had revealed a large malignant mass in the right retroperitoneal and adrenal area, with a size of 8.9x6.5x4.6 cm. The possibility of neuroblastoma was taken into consideration; it had caused an serious effect on the inferior vena cava, bilateral renal veins and right renal artery and multiple enlarged lymph nodes retroperitoneally (Fig. 1A and B). Positron emission tomography (PET)-CT (4 March 2021) revealed a large right adrenal neuroblastoma surrounding right renal artery, right renal vein and inferior vena cava, with multiple retroperitoneal lymph node metastases (Fig. 2A-1 and A-2). Considering the large size of the tumor and its invasion of important blood vessels, resection by operation was not suggested. Ultrasound-guided peritoneal biopsy was performed on 11 March, 2021. Pathological results suggested a diagnosis of neuroblastoma. Bone marrow biopsy indicated there was no metastasis. Due to the tumor crossed the midline of the abdomen and the patient's age at >18 months, she was assessed as ʻstage III, high riskʼ and three courses of chemotherapy were performed (Table I). CT re-examination on 22 June, 2021 showed a right adrenal tumor with a size of 5.5x3.8x3.1 cm, multiple enlarged lymph nodes retroperitoneally reduced compared with the previous states (Fig. 1C and D). Following preoperative evaluation, the conditions for surgical resection was achieved (it was determined the patient could undergo laparoendoscopic resection and surgery proceeded with). Laparoscopic right adrenal neuroblastoma resection was performed on 9 July, 2021.
Figure 1.
CT images of the same site before and after operation. (A and B) Abdominal CT scan on March 1, 2021 revealed a large tumor surrounding the right renal artery, vein and vena cava. (C and D) The volume of tumor was reduced after three times of chemotherapy. (E and F) Following surgery and five courses of chemotherapy, abdominal CT showed no tumor recurrence. ☆ indicates tumor tissue. CT, computed tomography scan.
Figure 2.
Comparison of PET images in the same location preoperative and postoperative. (A-1 and A-2) Preoperative PET-CT image. (A-3 and A-4) Postoperative PET-CT image. (B) Systemic PET-CT reexamination after six courses of postoperative chemotherapy showed no tumor recurrence or metastasis. ☆ indicates tumor tissue. PET, positron emission tomography; CT, computed tomography scan.
Table I.
Preoperative and postoperative chemotherapy schedule and time.
| Dates, year.month.day | Cycles | Plan | Evaluation |
|---|---|---|---|
| 2021.3.27-2021.4.3 | 1 | VCR + CDDP + VP16 + CTX | |
| 2021.4.18-2021.4.22 | 2 | IFOS + CBP + ADR | |
| 2021.5.09-2021.5.16 | 3 | VCR + CDDP + VP16 + CTX | Complete assessment |
| Surgery and postoperative evaluation | |||
| 2021.7.24-2021.7.31 | 4 | VCR + CDDP + VP16 + CTX | |
| 2021.8.23-2021.8.28 | 5 | IFOS + CBP + THP | |
| 2021.9.14-2021.9.21 | 6 | VCR + CDDP + VP16 + CTX | |
| 2021.10.11-2021.1016 | 7 | IFOS + CBP + THP | |
| 2021.11.02-2021.11.09 | 8 | VCR + CDDP + VP16 + CTX | |
| 2021.12.03-2021.12.07 | 9 | IFOS + CBP + THP | Complete assessment end of chemo |
Chemotherapy drug doses: VCR (vincristine) 1.5 mg/m2. days 1 and 8, CTX (cytoxan) 1.0 g/m2. days 1 and 2, CDDP (cisplatin) 25 mg/m2. days 1-5, VP16 (etoposide) 100 mg/m2. day 1-5, IFOS (ifosfamide) 1.5 g/m2. day 1-5, THP (pirarubicin) 30 mg/m2. day 1, CBP (carboplatin) 550 mg/m2. day 2.
The procedure was as follows (Fig. 3A-H): A 2 cm incision was made through the umbilicus, a TRIPORT was inserted and another 5 mm Trocar was inserted into the right upper abdomen as an auxiliary working port. The ascending colon ligament, hepatic colon ligament and duodenal collateral mesentery were released to fully expose the surgical visual field. Exploration revealed that the tumor originated from the right adrenal gland, crossed the midline and was located behind the inferior vena cava, encompassing the artery and vein of the right kidney and multiple enlarged lymph nodes adjacent to the abdominal aorta. The tumor was dissected at the right renal hilum and the tumor tissue surrounding the right renal vessel was carefully dissected and segmented with an ultrasound knife to bare the right renal vessel. Then the inferior vena cava was dissociated and gently pulled by traction wire to expose the tumor tissue located in the adrenal gland and paravertebral area. Finally, lymph nodes adjacent to the abdominal aorta and the right hilum were dissected. Abdominal drainage tube was indwelled and the incision was closed to complete the operation. Red blood cells (2 units) were transfused intraoperatively. Postoperative recovery was satisfactory. According to the postoperative pathology, ganglioneuroblastoma was diagnosed and metastasis was seen in 3/5 lymph nodes, with the DNA of tumor tissue being polyploid and the amplification of N-myc gene positive. Chemotherapy was introduced in the two weeks following the operation, with a total of six courses. Abdominal enhancement CT re-examination on 2 November, 2021 showed no clear signs of tumor recurrence and metastasis (Fig. 1E and F). On 19 January, 2022, PET-CT examination (Fig. 2B) showed no clear abnormal hypermetabolic foci in the whole body. After a comprehensive evaluation, the patient achieved clinical treatment and chemotherapy was ended. The treatment has been finished and follow-up is continuing.
Figure 3.
Operation process and postoperative abdominal appearance. (A) Duodenum dissociated to fully expose surgical field. (B) The tumor surrounded the right renal vein. (C) The tumor tissue was dissected, the tumor surrounding the right renal vein was segmented and the right renal vein was isolated. (D) After dissociating the inferior vena cava, the tumor tissue was exposed by gently pulling the inferior vena cava. (E) Tumor tissue behind the vena cava was dissected and the enlarged lymph nodes were dissected. (F) Tumor surrounding the right renal artery was cleaved and excised. (G) Tumor tissue located in the right adrenal gland was resected. (H) Inferior vena cava and bilateral renal veins after resection of tumor tissue. (I) Abdominal appearance on day 10 after surgery.  indicates tumor tissues,
indicates tumor tissues,  indicates abdominal aorta, □ indicates the artery of right renal,
indicates abdominal aorta, □ indicates the artery of right renal,  indicates the inferior vena cava,
indicates the inferior vena cava,  indicates the vein of the right renal,
indicates the vein of the right renal,  indicates the incision of drainage tube,
indicates the incision of drainage tube,  indicates the incision of TRIPORT and
indicates the incision of TRIPORT and  indicates the incision of 5 mm-trocar.
indicates the incision of 5 mm-trocar.
Discussion
Laparoscopic surgery for malignant solid tumors in children is still in the stage of clinical exploration. The adrenal gland is the most common site of neurogenic tumors in children. Due to the deep anatomical location and narrow space of adrenal tumors, traditional laparotomy requires a large incision and is truly difficult to expose. Laparoscopic technology has a broad surgical field which can accurately determine the location of the tumor and the relationship with the surrounding tissues; surgical trauma is small, postoperative recovery is fast and patients can also receive chemotherapy and radiotherapy earlier following surgery compared with conventional surgery and thus is gradually gaining favor among surgeons. At present, a series of problems related to neuroblastoma and the indications of laparoscopic surgery are still a hot topic.
The survival of patients with high risk neuroblastoma has improved significantly with the use of intensive multimodality treatment regimens including chemotherapy, surgery, radiation therapy, myeloablative chemotherapy followed by stem cell rescue and immunotherapy (2). Complete resection of the tumor is still the most important key to improve the prognosis. Several details regarding the role of aggressive surgery in high-risk neuroblastoma remain controversial (2). The present study reported the comprehensive treatment and operation process of this patient with high risk neuroblastoma in detail. The purpose of this paper was to introduce the successful experience of minimally invasive treatment of stage III neuroblastoma, in the hope that it might become a treatment scheme.
Abdominal neurogenic tumors are often concealed, large and easy to invade the surrounding tissues and blood vessels, resulting in the difficulty of laparoscopy. The indications and complications of laparoscopic surgery are still the focus of attention. With the development of laparoscopic technology, reports of successful laparoscopic neurogenic tumor resection in children have gradually increased and satisfactory surgical results have been achieved. However, there are few literature reports on the successful treatment of stage III-IV neuroblastoma by laparoscopy. Complications of laparoscopic surgery of neurogenic tumors mainly include intraoperative bleeding, conversion to laparotomy, renal atrophy or renal infarction, diaphragm injury and intestinal obstruction (3-5). There has been no reports of tumor recurrence at the puncture site of Trocar after laparoscopic neurogenic tumor resection (6). Leclair et al (7) report that laparoscopic minimally invasive technology is suitable for children with complete tumor capsule and negative image-defined risk factors (IDRFs). Tumors crossing the midline of abdomen and positive IDRFs should be contraindications for laparoscopic surgery, as due to the large size of the tumor, safe and effective complete resection cannot be achieved. Al-Shanafey and Habib (5) report that among 18 children with abdominal neurogenic tumors treated by laparoscopic surgery, two cases were converted to laparotomy due to renal vein tumor invasion and limited visual field and three cases with stage IV neuroblastoma had in situ recurrence following operation. Although there are no absolute contraindications for endoscopic neuroblastoma, tumor diameter >6 cm, blocked venous return and invasion of adjacent organs or blood vessels should be relative contraindications for laparoscopy. Owing to the lack of a large number of statistical analysis results of clinical data, the International Pediatric Endosurgery Group (IPEG) suggests that the indication of laparoscopic surgery should be tumor diameter <6 cm, IDRFs negative and no obvious surrounding tissue invasion adhesion (8). In the present case, the tumor size was >6 cm at the initial diagnosis, IDRFs was positive and the tumor surrounded the right renal artery, vein and inferior vena cava, which was not suitable for surgery. Although the tumor still involved the right renal artery and vein after three cycles of preoperative chemotherapy, laparoscopic surgery was performed for the child because the tumor size was significantly reduced to <6 cm, which provided sufficient procedure space for operation and the surgeon had professional skills in laparoscopic surgery.
In laparoscopic surgery, the tumor is removed by splitting it into small pieces. In the present case, the tumor was difficult to expose and the right kidney artery and vein passed through the tumor. Even if open surgery was performed, the tumor needed to be split and segmented. Depending on the surgeon's experience in laparoscopic technology, combined with preoperative IDRFs, an ultrasound knife was used to split the tumor along the blood vessels, remove the tumor in pieces and complete lymph node dissection, so as to achieve the same effect as open surgery. Meanwhile, the patient could suffer less trauma and experience fast recovery. Ultrasonic knife was used for precise dissection. When cutting tumor tissue with ultrasonic knife, a high enough temperature (controlled at 100˚C) inactivated the tumor cells in the cutting plane. At the same time, the tumor bed was washed with sterilized water. This can effectively prevent the spread of tumor cells during tumor resection. It was safe and effective to split and free the tumor along the blood vessels without spreading pollution and the wound surface was clean and dissected clearly. In addition, TRIPORT was used through the umbilical fossa and the protective sleeve was used to protect the incision to avoid tumor planting and, at the same time, it was convenient to remove the specimen while the tumor was cut into small pieces. The incision scar was not obvious (Fig. 3I) and the aesthetic effect was good. Moreover, local chemotherapy immersion irrigation after resection of tumor can effectively reduce tumor recurrence in situ. Postoperative chemotherapy was started two weeks after the operation, which benefited from the rapid recovery of minimally invasive technology of laparoscopic surgery, indicating that the timely continuation of postoperative chemotherapy is also an important factor for the good treatment effect of this case.
To sum up, for children suffering from neuroblastoma with large tumor volume and vascular invasion, preoperative chemotherapy can be given and minimally invasive laparoscopic surgery can be one of options to be considered when the tumor size is <6 cm. During the operation, the tumor tissue can be removed by segmental resection and the removal of as much tumor tissue as possible is an important factor to improve the prognosis. Laparoscopic minimally invasive surgery has little trauma and quick recovery and children can receive postoperative chemotherapy as early as possible, which is conducive to good recovery. Basically, the prerequisite and requirements for performing this operation are professional laparoscopic skills and an experienced team.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article
Authors' contributions
GH analysed the data and wrote the original draft. GY was responsible for data acquisition and participated in the writing of the original manuscript. GH an GY agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. WH and YS made substantial contributions to conception and acquisition of data. ML made substantial contributions to analysis and interpretation of data. SL made substantial contributions to conception and design of the study, replied to the reviewers' comments and took responsibility for communication with the journal. ML and WH confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki and the study protocol was approved by the ethics committee of Xiamen University.
Patient consent for publication
The data and pictures used in the present study were authorized by the parents of the child.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu KX, Joshi S. ʻRe-educatingʼ tumor associated macrophages as a novel immunotherapy strategy for neuroblastoma. Front Immunol. 2020;11(1947) doi: 10.3389/fimmu.2020.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung C, Boterberg T, Lucas J, Panoff J, Valteau-Couanet D, Hero B, Bagatell R, Hill-Kayser CE. Neuroblastma. Pediatr Blood Cancer. 2021;68 (Suppl 2)(e28473) doi: 10.1002/pbc.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acker SN, Bruny JL, Garrington TP, Partrick DA. Minimally invasive surgical techniques are safe in the diagnosis and treatment of pediatric malignancies. Surg Endosc. 2015;29:1203–1208. doi: 10.1007/s00464-014-3795-0. [DOI] [PubMed] [Google Scholar]
- 4.Fascetti-Leon F, Scotton G, Pio L, Beltrà R, Caione P, Esposito C, Mattioli G, Saxena AK, Sarnacki S, Gamba P. Minimally invasive resection of adrenal masses in infants and children: Results of a European multi-center survey. Surg Endosc. 2017;31:4505–4512. doi: 10.1007/s00464-017-5506-0. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shanafey S, Habib Z. Feasibility and safety of laparoscopic adrenalectomy in children: Special emphasis on neoplastic lesions. J Laparoendosc Adv Surg Tech A. 2008;18:306–309. doi: 10.1089/lap.2007.0166. [DOI] [PubMed] [Google Scholar]
- 6.Gurria JP, Malek MM, Heaton TE, Gehred A, Lautz TB, Rhee DS, Tracy ET, Grant CN, Baertshiger RM, Bruny J, et al. Minimally invasive surgery for abdominal and thoracic neuroblastic tumors: A systematic review by the APSA cancer committee. J Pediatr Surg. 2020;55:2260–2272. doi: 10.1016/j.jpedsurg.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Leclair MD, de Lagausie P, Becmeur F, Varlet F, Thomas C, Valla JS, Petit T, Philippe-Chomette P, Mure PY, Sarnacki S, et al. Laparoscopic resection of abdominal neuroblastoma. Ann Surg Oncol. 2008;15:117–124. doi: 10.1245/s10434-007-9499-0. [DOI] [PubMed] [Google Scholar]
- 8.IPEG guidelines for the surgical treatment of adrenal masses in children. J Laparoendosc Adv Surg Tech A. 2010;20:7–9. doi: 10.1089/lap.2010.9999. International Pediatric Endosurgery Group. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article