Abstract
Background:
Recent modifications in organ allocation policies and increases in chronic liver diseases may have resulted in important changes in living donor liver transplantation (LDLT) in the US. We examined the trends, outcomes and factors associated with outcomes in adult LDLT.
Methods:
UNOS data on 2566 adult LDLT recipients from 01/01/2010 through 12/31/2019 were analyzed. LDLT graft and patient survivals were compared with propensity score-matched deceased donor liver transplant (DDLT) recipients by the Kaplan-Meier estimator. The association between preceding LDLT frequency and subsequent outcomes were assessed by Cox proportional-hazards mixed effects modelling.
Results:
After a stable annual frequency of LDLTs from 2010-2014 (~200 per year), the number of LDLTs doubled to 440 in 2019. One- and 5-year graft survivals for LDLT recipients were 88.4% and 78.1%, respectively, compared with 92.5% and 80.7% in propensity-score matched DBD recipients (p=0.005), respectively. Older donor age, recipient diabetes and life support requirement were significantly associated with graft failure among LDLT recipients (p values<0.05). Average preceding LDLT frequencies of <3/year, 3-20/year, and >20/year resulted in one-year graft survivals of 82%, 88-89%, and 93%, respectively (p values<0.05). There were 3 living donor deaths (0.12%).
Conclusions:
The frequency of LDLTs has doubled over the past decade, with good outcomes and acceptable donor safety profile. However, there appear to be varying threshold transplant frequencies (volume/unit time) associated with acceptable (88-89%) and aspirational (93%) one-year graft survivals. These data should be reassuring and encourage LDLT practice as efforts continue to expand the donor pool.
Keywords: living-donor liver transplantation, outcomes, graft failure, propensity-score matched analysis
INTRODUCTION
Living-donor liver transplantation (LDLT) increases the likelihood of waitlisted patients receiving a prime donor liver, who would be unlikely to be allocated a deceased donor organ in an expeditious fashion from the model for end-stage-liver-disease (MELD) score organ allocation system.1,2 There have been over 4,600 adult LDLTs performed in the U.S. through 2015, representing <5% of annual liver transplantations (LTs).3
In early 2019 the organ allocation policy was modified, with MELD exception scores capping at three points less than the median MELD score at transplant (MMaT-3) in the recipient’s geographic region after a mandatory six-month waiting period. Additionally, acuity circles were introduced in lieu of UNOS regions for organ allocation.4 These combined changes aimed to decrease disparity in access to deceased donor organs between geographic areas by undoing the advantage HCC patients had over patients with physiologic MELD scores. As a result, HCC patients may now have inadvertent increases in LT wait times. In tandem, the number of waitlisted patients has also been increasing disproportionately compared to the number of LTs performed which has been driven in part by the ongoing nonalcoholic steatohepatitis (NASH) and alcohol-associated liver disease (ALD) epidemics. 3,5-7 Furthermore, more LT candidates may have static uncompetitive MELD scores owing to the efficacy of direct-acting antivirals (DAAs) or alcohol cessation. The net impact of these factors is likely to include an increasing appeal of LDLT, with many centers expected to embrace LDLT in order to expand the donor pool.
The survival benefit of LDLT rather than waiting for a DDLT has been demonstrated,8 although the most recent study using the US national transplant database suggests that the survival benefit for LDLT had gradually disappeared by 2015.3 How LDLT compare to optimal DDLT grafts is unknown given the conflicting results and heterogenous analysis used in the literature. A single center has reported a statistically superior graft survival for LDLT,9 however, this was a largely unadjusted analysis. There have been no prior studies comparing LDLT outcomes with optimally-matched DDLTs for attributes affecting graft survival (e.g. cold ischemia time and donor age).
There are significant resource utilization and surgical expertise needed for LDLT.2,10 The landmark multicenter Adult-to-Adult Living Donor Liver Transplant Cohort Study (A2ALL) showed an overall one-year graft survival rate of 81% and identified a significant learning curve for adult LDLTs of 15–20 LDLTs.11,12 This was also demonstrated by a other studies whereby the learning curve leveled off after 20 LDLTs.13-15 Given that the number of LDLTs in the US has markedly increased, in conjunction with improved surgical techniques and peri-operative management, an updated assessment of how center experience affects outcomes is needed to guide new centers both from programmatic planning and patient safety standpoints. While the aforementioned studies showed a threshold for cumulative total number of LDLTs, it is not known how frequency (volume/unit of time) of LDLTs affects outcomes (e.g. the association of graft survival with number of LDLTs performed over the preceding 24 months).
The ethical principle of minimizing the risk of harm to the donors also needs consideration.16 While the adoption of the MELD score organ allocation system in 2002 may have resulted in the subsequent decrease observed in LDLTs, a widely publicized donor death in New York may also have contributed.17 Updated donor safety is needed to reassure LDLT centers and the public that living donation continues to be safe despite the likely increased practice which could conceivably lead to variability in outcomes. In addition, there also appears to be a rise in Good Samaritan/altruistic donors who are unrelated to the recipient.18 Unrelated donors reduce the likelihood of overly close HLA types and associated complications, such as graft-versus-host-disease, potentially resulting in improved outcomes (vs. related donors).19 It remains to be elucidated whether the potential advantage of unrelated donation has translated to improved LDLT outcomes.
Given the expected increase in LDLT practice and the shortcomings in the existing literature, our study objectives were to (1) examine the trends and outcomes in donors and recipients of adult LDLT, using optimally-matched DDLT controls; (2) study the effects of LDLT frequency on transplant center outcomes; (3) analyze for factors associated with graft failure among LDLT recipients.
MATERIALS & METHODS
Study Population & Data Management
National data was obtained from the United Network for Sharing Organs (UNOS) on adult patients (18 years of age or older) in the US who underwent a living donor liver transplant (LDLT) from January 1, 2010, through December 31, 2019. These data are prospectively collected by the Organ Procurement and Transplantation Network (OPTN), under contract from UNOS, from transplant programs, organ procurement organizations, and histocompatibility laboratories, supplemented by the Centers for Medicare & Medicaid Services and the National Technical Information Service's Death Master File.20 In order to ensure that donor quality of DDLT recipients were as similar to LDLT recipients as possible, deceased after cardiac death (DCD) donors, cold ischemic times of 6 hours or greater, donor liver with 30% or greater of steatosis, and hepatitis C virus (HCV)-antibody or nucleic acid testing (NAT) positive donors were excluded from the DDLT control group.21,22 Moreover, acute liver failures, recipients who had previous LT, and recipients who underwent multi-organ transplants or domino LT were also excluded.
Data from both the liver donor transplant recipient and donor datasets were merged and analyzed concurrently. Clinical data analyzed included donor and recipient demographic, anthropometric measurements, co-morbidities, laboratory values including lab MELD score, and diagnoses. Follow-up data included graft failure and mortality among transplant recipients, and mortality and morbidity among the living donors. As per OPTN, graft failure was defined as the occurrence of either recipient death or removal of the transplanted organ. Transplant recipients were assigned primary listing indications in a step-wise approach, starting from the transplant recipient registration (TRR) form, and next from the transplant candidate registration (TCR) form, if no diagnosis had been assigned from the TRR. HCC diagnoses were also assigned independent of chronic liver diseases.
Cohort analysis
Continuous variables were summarized by means and standard deviations (SD) or by medians and interquartile ranges (IQR), and frequencies and percentages were used for categorical variables. A comparative analysis between LDLT and DDLT recipients was performed. Comparison of continuous variables was based on the two-sample t-test for data with normal distribution, otherwise the two-sample Wilcoxon rank test was used. The two-sided chi-square test was used to compare categorical variables. Cox proportional-hazards regression modeling was used to assess for variables associated with graft failure among LDLT recipients only. Multiple forms of the model were explored incorporating all available variables which have been associated with post-transplant graft failure in the medical literature. Schoenfeld residuals were examined to ensure there was no violation of the proportional hazard assumption.
Propensity-score matched analysis
Propensity score matching can be used to reduce bias in retrospective studies, including selection bias and other potential confounders. Propensity score matching simulates a randomized controlled trial-like situation where the treatment and the control groups are matched in terms of selected confounders.23 The propensity score for each subject was estimated utilizing a logistic regression model for LDLT recipients as a function of variables which are associated with graft failure in the literature. These variables that were utilized to generate the propensity score for each subject were as follows:
- Donor variables: age, race, gender
- Recipient variables: age, race, gender, diabetes, body mass index (BMI), MELD score, HCC, etiology of liver disease (grouped as 1) alcohol-associated liver disease, 2) nonalcoholic fatty liver disease, 3) HCV, 4) cholestatic/autoimmune, and 5) other), and transplant year. After estimation of the propensity score for each subject, we performed one-to-one matching utilizing the nearest neighbor method with a caliper width of 0.15 of the standard deviation of the logit of the propensity score. All 2566 LDLT cases were matched. The balance of characteristics between LDLT and DDLT groups in the matched sample was checked by examining standardized % bias (<10% was desirable) and performing formal comparative analysis between covariates. Kaplan-Meier survival analysis with log-rank testing was used to estimate graft and patient survivals of the LDLT group compared with propensity-score matched donation-after brain death (DBD) controls. These results are provided in hazard ratios (HR) with confidence intervals (CIs).
Transplant volume metric analysis
In order to assess how LDLT volume affected outcomes, individual patients were assigned the number of LDLTs performed at their respective transplant center in the preceding 2 years before their transplant date. Preceding transplant volume assessment was adjudged to be more optimal means for assessing the true association between transplant volume and subsequent outcomes, rather than assessing total center volume whereby outcomes are analyzed with respect to future volume (e.g. a patient who was transplanted in 2011 in a low-volume LDLT center could assigned to a high-volume LDLT center if an increase in LDLT practice occurred in later years). The study investigators chose 2 years rather than 1 year as we believed this would be a more accurate reflection of a transplant center’s stability. Cox proportional-hazards regression modeling was used to assess frequency of preceding LDLT volume and one-year graft failure. Possible clustering by individual transplant centers was assessed for using a shared frailty random effects Cox model. Additional analyses was performed on ‘new’ LDLT centers during the study period, defined as no LDLT performed within the preceding two years.
The proportion of missing data was extremely low (<1%). A p value <0.05 was considered significant for the results. The statistical analyses were performed using the Stata statistical package (Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
RESULTS
Frequency and Comparative Demographics
After merging of the LDLT recipient and donor datasets, there were 2,644 LDLTs observed during the study period. Of these, 78 were domino LTs and were excluded, leaving 2,566 LDLTs in our final study cohort. Of the initial 64,041 adult DDLT recipients during the study period, 39,264 met the exclusion criteria, leaving 24,777 DBD LT recipients in the final control group. The study flow diagram detailing out cohort selection is shown in Supplemental Figure 1.
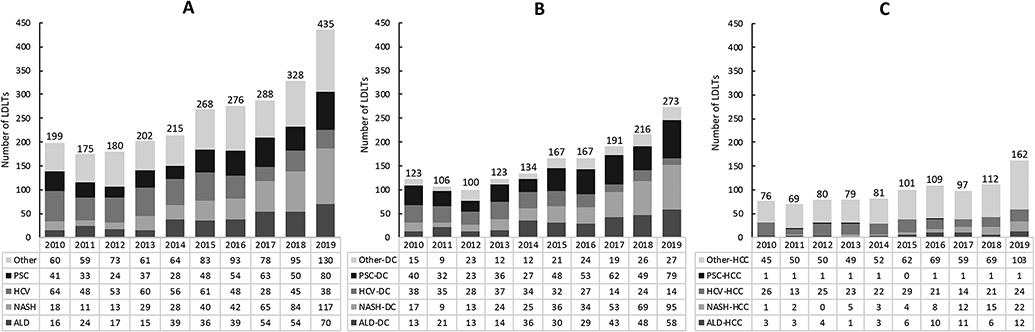
After a stable annual frequency of LDLTs from 2010-2014 (~200 per year), the number of LDLTs doubled to 440 in 2019 (Figure 1). Overall, listing indications for nonalcoholic steatohepatitis (NASH) and alcohol-associated liver disease (ALD) increased by over 6-fold and 4-fold, respectively. Conversely, listing indications for hepatitis C virus (HCV) decreased by almost half, although HCV was still the number 1 chronic liver disease listing indication during the study period accounting for 501/2566 (19.5%) of LDLTs, while PSC (17.8%) was the second most common. Listing indications for HCC also increased, from 76 in 2010 to 162 in 2019 and accounted for 966/2566 (37.6%) of LDLTs (Figure 1). Of note, the increase in LDLT occurred in the background of an increase in the DBD LT control group during the study time period, with the annual number of DBD LTs increasing from 1979 in 2010 to 3165 in 2019, using the study’s patient selection criteria outlined in Supplemental Figure 1. LDLT now represents ~7% of all adult LTs in the USA.
Figure 1. Number of living donor liver transplantations in the USA 2010 – 2019 stratified by etiology of liver disease and sub-stratified by (A) overall, (B) decompensated cirrhosis, and (C) hepatocellular carcinoma.
LDLTs, living donor liver transplantations; PSC, primary sclerosing cholangitis; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; ALD, alcohol-associated cirrhosis; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma.
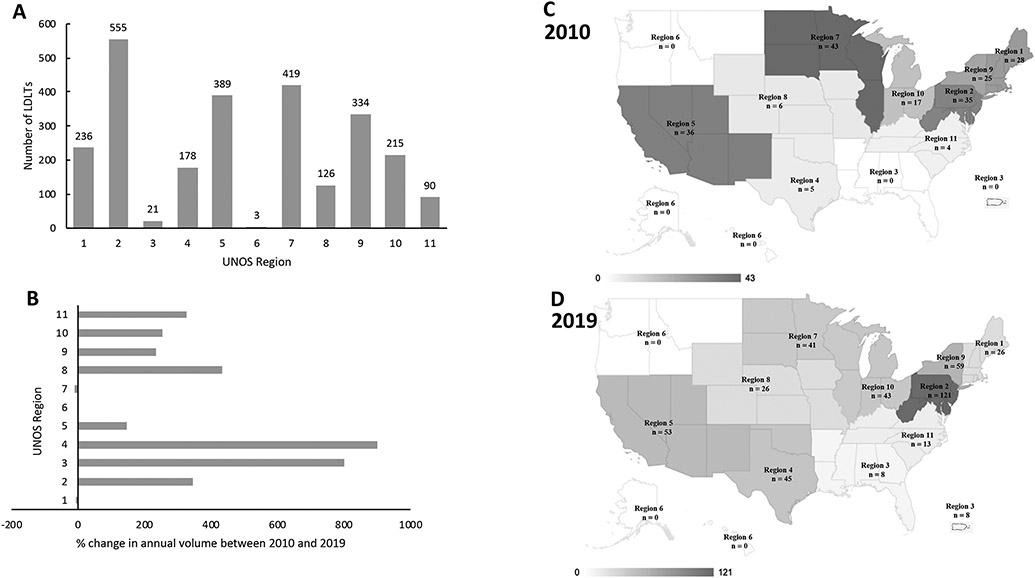
There were 56 transplant centers who performed at least one LDLT over the past decade. The number of transplant centers performing LDLTs has increased from 28 in 2010 to 43 in 2019 (Supplemental Table 1). All 11 UNOS regions (as designated prior to the implementation of acuity circles) performed LDLTs in 2019, except region 6 (Figure 2). There was a 13-fold difference in the frequency of LDLTs between the highest and lowest volume UNOS regions. LDLT recipients were younger (median = 56 years), received younger donors (median = 36 years), had lower MELD scores (median = 15) and longer waitlist time (median = 150 days), compared to recipients of DBD donors (all p values<0.001) (Table 1).
Figure 2. Geographic variation of living donor liver transplantations in the USA 2010 – 2019 stratified by UNOS region.
The bar charts display the (A) overall number and (B) % change between 2010 and 2019, while the maps show the number of LDLTs performed in (C) 2010 and (D) 2019 in respective UNOS regions. LDLTs, living donor liver transplantations; UNOS, United Network for Organ Sharing.
Table 1.
Baseline characteristics of liver transplant recipients in the USA between January 1, 2010 and December 31, 2019
| All patients (N=27,343) | Propensity score-matched patients (n=5124) | |||||
|---|---|---|---|---|---|---|
| LDLT (n=2566) | DBD LT (n=24777) | p- value |
LDLT* (n=2566) | DBD LT (n=2566) | p- value |
|
| Donor: | ||||||
| Age (years) | 36.0 (28.0, 45.0) | 44.0 (29.0, 56.0) | <0.001 | 36.0 (28.0, 45.0) | 33.0 (22.0, 48.0) | <0.001 |
| BMI (kg/m2) | 26.2 (23.5, 28.7) | 27.0 (23.5, 31.3) | <0.001 | 26.2 (23.5, 28.7) | 26.1 (22.5, 30.6) | 0.120 |
| Male gender | 1214 (47.3) | 14447 (58.3) | <0.001 | 1214 (47.3) | 1276 (49.7) | 0.083 |
| Caucasian race | 2088 (81.4) | 15780 (63.7) | <0.001 | 2088 (81.4) | 2031 (79.2) | 0.050 |
| Cold ischemia (hours)* | 1.5 (1.0, 2.1) | 4.7 (4.0, 5.4) | <0.001 | 1.5 (1.0, 2.1) | 4.7 (3.9, 5.5) | <0.001 |
| Recipient: | ||||||
| Age (years) | 56.0 (46.0, 63.0) | 58.0 (51.0, 63.0) | <0.001 | 56.0 (46.0, 63.0) | 56.0 (47.0, 63.0) | 0.850 |
| Male gender | 1401 (54.6) | 16275 (65.7) | <0.001 | 1401 (54.6) | 1425 (55.5) | 0.500 |
| Caucasian race | 2087 (81.3) | 17965 (72.5) | <0.001 | 2087 (81.3) | 2005 (78.1) | 0.004 |
| BMI (kg/m2) | 26.3 (23.3, 30.0) | 28.1 (24.6, 32.3) | <0.001 | 26.3 (23.3, 30.0) | 26.0 (23.2, 29.9) | 0.440 |
| Post-transplant LOS (days) | 10.0 (8.0, 16.0) | 9.0 (6.0, 15.0) | <0.001 | 10.0 (8.0, 16.5) | 8.0 (6.0, 12.0) | <0.001 |
| Waiting list time (days) | 150.0 (78.0, 302.0) | 96.0 (18.0, 284.0) | <0.001 | 150.0 (78.0, 302.0) | 163.5 (48.0, 351.0) | 0.079 |
| Diabetes | 607 (23.7) | 6627 (26.7) | <0.001 | 608 (23.7) | 621 (24.2) | 0.920 |
| Serum creatinine (mg/dL) | 0.8 (0.7, 1.1) | 1.0 (0.8, 1.6) | <0.001 | 0.8 (0.7, 1.1) | 0.9 (0.7, 1.1) | <0.001 |
| Serum total bilirubin (mg/dL) | 2.7 (1.4, 4.8) | 3.9 (1.7, 10.9) | <0.001 | 2.7 (1.4, 4.8) | 2.0 (1.0, 4.3) | <0.001 |
| MELD score | 15.0 (11.0, 20.0) | 21.0 (13.0, 30.0) | <0.001 | 15.0 (11.0, 20.0) | 14.0 (1.0, 20.0) | 0.004 |
| Life support requirement | 20 (0.8) | 1515 (6.1) | <0.001 | 20 (0.8) | 42 (1.6) | 0.005 |
| ICU | 32 (1.2) | 2739 (11.1) | <0.001 | 32 (1.2) | 70 (2.7) | <0.001 |
| Dialysis requirement | 16 (0.6) | 2374 (9.6) | <0.001 | 16 (0.6) | 40 (1.6) | 0.001 |
| Ascites (mild or worse) | 1628 (63.4) | 18518 (74.7) | <0.001 | 1628 (63.4) | 1607 (62.6) | 0.540 |
| Hepatic encephalopathy (grade 1 or worse) | 1285 (50.1) | 15565 (62.8) | <0.001 | 1285 (50.1) | 1306 (50.9) | 0.560 |
| Portal vein thrombosis | 295 (11.5) | 3092 (12.5) | 0.150 | 295 (11.5) | 295 (11.5) | 0.280 |
| HCC | 450 (17.5) | 8326 (33.6) | <0.001 | 450 (17.5) | 520 (20.3) | 0.013 |
Values are n (%) or median (interquartile range).
There are 10 propensity-score matched variables presented in italics in this table. In addition, recipients were also matched on transplant year and etiology of liver disease
BMI = body mass index; DBD = donation after brain death; LT = liver transplantation; ICU = intensive care unit; LDLT = living-donor liver transplantation; LOS = length of stay; MELD = model for end-stage liver disease.
Patient and graft survivals
There were 2566 DBD controls propensity-score matched to the 2566 LDLTs (Table 1). The covariate balancing is presented in Supplemental Figure 2. One- and 5-year patient survivals for LDLT recipients were 93.4% and 83.8%, respectively, compared with 94.6% and 83.0% in propensity-score matched DBD recipients (p=0.973), respectively (Figure 3). One- and 5-year graft survivals for LDLT recipients were 88.4% and 78.1%, respectively, compared with 92.5% and 80.7% in propensity-score matched DBD recipients (p=0.005), respectively (Figure 3). The 1-year and 5-year cumulative probabilities of liver re-transplantation were 5.3% and 7.3% in the LDLT group compared to 2.3% and 3.4% in the DBD LT control group, respectively (p<0.001). In order to assess how re-transplantation rates have changed over time the cohorts were divided into two periods: 2010-2014 (n=1941) and 2015-2019 (n=3191). LDLT recipient retransplantation risk (vs. the DBD controls) decreased from a HR of 2.26 [95% CI 1.54, 3.30] during the 2010-2014 period to a HR of 1.91 [95% CI 1.27, 2.85] during the 2015-2019 period. In addition, among LDLT recipients, those who were transplanted in the 2015-2019 period had reduced risk of being retransplanted compared to those transplanted in the 2010-2014 period (HR = 0.70 [95% CI 0.53, 0.92]).
Figure 3.
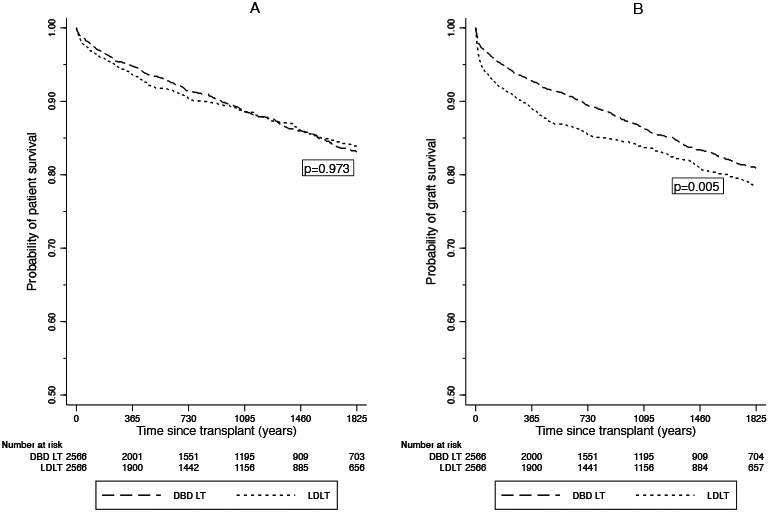
(A) Patient & (B) Graft survival for living-donor liver transplant (LDLT) recipients in the U.S. 2010-2019 vs. propensity-score matched donation after brain death liver transplant (DBD LT) recipients
Among LDLT recipients, graft failure was associated with increased donor age (HR = 1.02 [95% CI 1.01, 1.03]), recipient diabetes (HR = 1.38 [95% CI 1.10, 1.69]), lower recipient BMI (HR = 0.97 [95% CI 0.95, 0.99]), and recipient life support requirement (HR = 3.08 [95% CI 1.50, 6.30]) (Table 2). Recipient age was not associated with graft failure (HR = 1.00 (95% CI 1.00, 1.01). MELD score was also not associated with graft failure during the Cox model building stages and was omitted from the final model as it did not improve the model. Within the same Cox model, donor age was also incorporated as an ordinal variable (18-29 years, 30-39 years, 40-49 years, ≥50 years) instead of a continuous variable (Table 2). The donor age of 18-29 years had the best graft outcomes.
Table 2.
Multivariate Cox Proportional Hazards Model of Associations with Graft Failure in Living-Donor Liver Transplant Recipients 2010 - 2019 (N=2566)
| Covariate | HR (95% CI) | p-value |
|---|---|---|
| Donor: | ||
| Age (18-29 years as reference) | ||
| 30-39 | 1.35 (1.05-1.73) | 0.016 |
| 40-49 | 1.49 (1.15-1.92) | 0.002 |
| ≥50 | 1.70 (1.27-2.27) | <0.001 |
| Recipient: | ||
| Age | 1.00 (1.00-1.01) | 0.258 |
| Diabetes | 1.38 (1.10-1.69) | 0.003 |
| BMI | 0.97 (0.95-0.99) | 0.003 |
| Life support requirement | 3.08 (1.50-6.30) | 0.002 |
HR = hazards ratio; CI = confidence interval; BMI = body mass index.
At 1-year, there were 266 (10.4%) graft failures among the LDLT group compared with 227 (8.9%) in the DDLT control group. Among the graft failures, there was a higher rate of vascular thrombosis (1.9% vs 0.9%) (p = 0.350) and biliary complications (0.8% vs 0.4%) (p = 0.658) in the LDLT group, however, other/unknown comprised of the most graft failures in both groups (61.7% in LDLTs vs 48.5% in DDLTs) (p = 0.002).
Association of preceding transplant center volume on graft survival
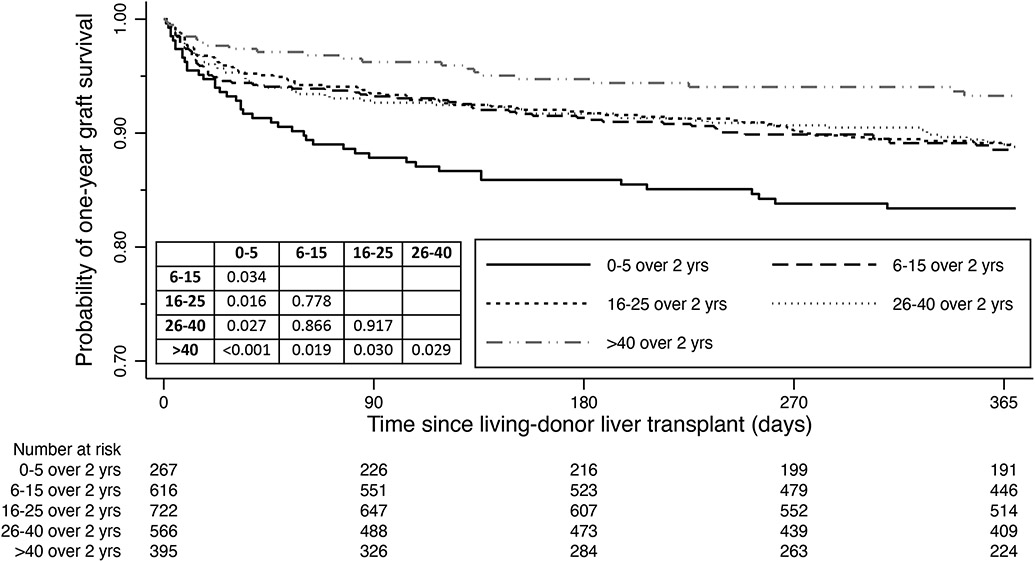
The 2566 LDLT recipients were divided into 5 groups on the basis of the preceding two years of transplant volume: 0-5 (n=267); 6-15 (n=616); 16-25 (n=722); 26-40 (n=566); and >40 (n=395). The one-year graft survivals were 82.7%, 88.1%, 88.5%, 88.5%, and 92.7% in the 0-5, 6-15, 16-25, 26-40 and >40 volume groups, respectively (Figure 4). The lowest volume group (0-5 in preceding 2 years, or an average or less than 3 annually) had statistically inferior outcomes to all other groups (all p values<0.05), while the highest volume group (>40 in prior 2 years, or an average of >20 LDLTs annually) had statistically superior outcomes to all other groups (all p values<0.05). A similar trend in outcomes was observed in the Cox proportional hazards mixed-effects model which adjusted for age of donor and recipient, recipient gender, diabetes and life support requirement, with individual transplant centers included as a random effect in order to account for possible clustering by individual transplant center (Table 3). The random effect was not statistically significant (p=0.073).
Figure 4.
One-year graft survival for living-donor liver transplants (LDLTs) in the U.S. 2010-2019, stratified by the number of LDLTs performed in the combined two years preceding the transplant date for each individual recipient at respective transplant centers. A volume effect was observed, with the lowest transplant volume having the most inferior outcomes, while the highest transplant volume having the best outcomes. The pairwise comparisons are also provided in the form of p values.
Table 3.
Multivariate* Cox Proportional Hazards Regression of One-Year Graft Failure in LDLT Recipients stratified by preceding transplant volume (N=2566)
| Covariate | HR (95% CI) | p-value |
|---|---|---|
| Transplant volume** (ref: 0-5 group): | ||
| 6-15 | 0.69 (0.47-1.02) | 0.063 |
| 16-25 | 0.62 (0.42-0.92) | 0.018 |
| 26-40 | 0.61 (0.41-0.94) | 0.024 |
| >40 | 0.36 (0.21-0.62) | <0.001 |
HR = hazards ratio; CI = confidence interval; LDLT = living-donor liver transplant.
adjusted for donor age, recipient age, gender diabetes and life support requirement, and includes individual transplant centers as a random effect (theta = 0.04, likelihood-ratio test of theta=0: p=0.073) in a shared frailty model.
in the preceding 2 years at each respective transplant center for individual patients.
Outcomes of ‘new’ LDLT transplant centers
There were 37 ‘new’ LDLT transplant centers during the study period (Supplemental Table 2). The one-year graft survival outcomes were analyzed among these centers in incremental blocks of 5 LDLTs. After the cumulative transplant numbers reached 25, outcomes appeared to plateau (Supplemental Table 3).
Total transplant volume and outcomes
The relationship between total transplant volume and one-year graft survival was also assessed. Low-volume centers who performed 1 to 25 LDLTs over 10 years, had significantly inferior outcomes to larger total volume centers (p<0.05) (Supplemental Table 4).
Relationship status of donors to recipients
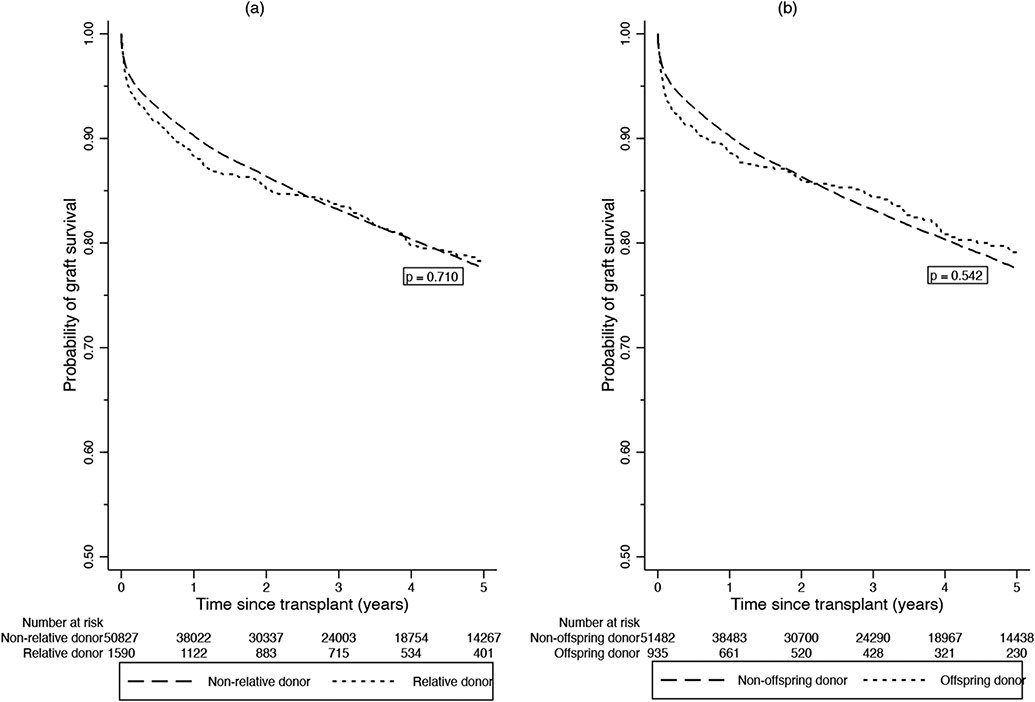
Among the 2,566 LDLT donors, 1590 (62%) were relatives of the recipients with 935/1590 (62%) being offspring. Unrelated liver donation tripled during the study time period from 70 in 2010 (representing 35.2% of 2010 LDLTs) to 205 in 2019 (representing 47% of 2019 LDLTs). The unadjusted 1- and 5-year graft survivals for LDLT recipients with related donors were 88.0% and 78.1% respectively, compared with 89.0% and 76.7%, respectively, in those who received unrelated donor livers (p=0.710) (Figure 5). There was no difference in graft survival rates between related and unrelated LDLTs when adjusted for donor age, cold ischemic time, and recipient age, diabetes, BMI, MELD score and life support requirement (HR = 1.04 (95% CI 0.85, 1.27), p = 0.726).The unadjusted 1- and 5-year graft survival rates for LDLT recipients whose donors were offspring were 88.2% and 78.7%, respectively, compared with 88.5% and 77.0%, respectively, in those who received non-offspring donor livers (p=0.542) (Figure 4). There was no difference in graft failure rates between offspring and non-offspring LDLT recipients when adjusted for the same variables described above (HR = 1.03 (95% CI 0.81, 1.31), p = 0.825).
Figure 5.
Graft survival rates for LDLTs stratified by (A) relative versus nonrelative donors and (B) offspring versus nonoffspring donors in the United States.
Donor outcomes
Among the 2566 living donors during the ten-year study period, there were 3 donor deaths (0.12%, 1 per 833). In 2010, two donors, a 56-year-old man and a 34-year-old man, died from cardiorespiratory arrests within 4 days of surgery. In 2016, a 50-year-old woman died due to a cardiac arrest secondary to a venous air embolism. In addition, there was one donor who developed acute liver failure requiring LT. 159/2566 (6%) of the living donors were re-hospitalized. 318/2566 (12.4%) had a documented post-surgical complication, with biliary complications (81/2566, 3.2%) being the most common (Supplemental Table 5).
DISCUSSION
LDLT has been shown to have comparable outcomes to optimal DDLTs, with implied but only partly fulfilled potential to reduce waitlist mortality by expanding the donor pool.9,12 Our study has several important findings. First, while LDLT remains comparatively underutilized in the U.S., the practice has undergone notable expansion in recent years, with maintenance of outcomes. An important novel finding of our study is that not only is center LDLT experience (total number of LDLT procedures performed) an important predictor of outcomes but that the frequency of LDLT volume is also important.
Recent changes in organ allocation policies and an increasingly-competitive transplant environment may have resulted in increased appeal and advantage of LDLT.24 The acuity circles allocation system has facilitated a national distribution of organs to the patients with the highest MELD scores, while consequently reducing organ access for patients with lower MELD scores prompting centers to look for ways to increase organ accessibility for patients relatively disadvantaged in a MELD-based organ allocation system. We observed a doubling of the annual rate of LDLT in 2019 to 440 cases from the baseline annual rate of ~200 earlier in this decade, and a 45% increase in the proportion of transplant centers performing LDLTs. Both decompensated cirrhosis and HCC have increased substantially in 2019, coinciding with the organ allocation policy change (26% for decompensated cirrhosis and 45% for HCC between 2018-2019). The main listing indications which are driving this change are PSC, HCC and NASH. LDLT now comprises of 7% of all LT practice in the US.
Some of the increase in LDLT practice has no doubt been driven by an expansion in Good Samaritan/altruistic donors, whereby we observed a tripling of unrelated donors between 2010 and 2019. There are potential practical implications to this, including decreasing the need for suboptimal (e.g. graft to weight ratio or age at the edge of acceptable) donor selection by increasing donor pool. Although we failed to confirm our hypothesis that unrelated donor LDLT recipients may have superior outcomes compared to related donor recipients,19 a trend towards improved outcomes was observed at 1- and 2- years post-transplant.19 If this practice continues to expand in the future, it is possible that a statistically significant difference may be observed with improved outcomes in LDLT recipients who receive unrelated donors.
The one-year graft survival among LDLT recipients over the past decade has been acceptable at 88.4%, and illustrates the overall improvement over time when compared to the 81% one-year graft survival in the seminal A2ALL report.11 There was a 4% higher one-year graft survival among propensity score-matched DBD LTs at 92.5%, likely reflective of the increased surgical complexity of LDLT and highlighting the careful planning needed when initiating a LDLT program. Interestingly, LDLT recipients had a higher rate of early graft failure, with LDLT recipients having a 3% higher rate of re-transplantation at one-year post transplant compared to DBD LT controls. To this end, we observed relatively higher proportions of vascular and biliary complications in the LDLT recipients, consistent with findings in the A2ALL cohort and in a meta-analysis,11,25,26 emphasizing the technical challenges inherent to LDLT that programs need to focus on when striving to optimize outcomes. Promisingly, there was a trend towards decreased re-transplantation rates among LDLT recipients between 2015-2019 compared to 2010-2014, possibly demonstrating early signals of improved outcomes as LDLT programs increase their experience.
As more transplant centers embrace LDLT practice, a clear understanding of the experience needed to achieve desired outcomes is important. It also allows patients to be fully informed when choosing their transplant program. A significant learning curve for adult LDLTs has been reported, with outcomes leveling off after 20 LDLTs.11,13-15 In our updated study on LDLTs, we have similarly shown that one-year graft survival continues to improve at ‘new’ transplant centers with increasing center volume until a slightly higher cumulative threshold is reached (after 25 LDLTs). Importantly, once LDLT centers achieve technical competency, regular LDLT practice is needed to maintain outcomes. An average preceding LDLT frequency of less than 3 per year is associated with subsequent inferior outcomes and a one-year graft survival of 82%. There were acceptable outcomes (88-89% one-year graft survival) observed at an average preceding frequency of 3 to 20 LDLTs per year. While there were excellent outcomes (93% one-year graft survival) observed at an average preceding frequency of 21 or more LDLTs per year. This could be considered as the aspirational goal of LDLT centers in the U.S. While >20 LDLTs per year may seem difficulty to achieve, five LDLT centers in the U.S. achieved this number in 2019 and Asian LDLT centers regularly greatly surpass this metric.27 This observation suggests that recent frequency of LDLT be added to total number of procedures as an important predictor of post-LDLT outcomes. Of note, an increased risk of graft failure was associated with older donors (a 2% increased risk of graft failure for each increase in year, with recipients of donors aged 18-29 having the best outcomes), recipient diabetes (38% increased risk) and recipient life support requirement (308% increased risk). These donor and recipient factors should be considered when assessing LDLT candidates, particularly in centers with a recent LDLT frequency of less than 3 per year (e.g. new LDLT transplant centers, whereby a more conservative approach appears prudent.
LDLT has long been a source of immense ethical debate, beginning prior to the first LDLT were performed,28,29 largely centered on the principle of minimizing harm to the donors.16 To this end, it is imperative that donor safety is considered in LDLT outcomes. A prior worldwide survey of LT centers estimated a low 0.2% donor mortality.30 Donor hepatectomies have also been shown to be safe in all age groups, including the elderly ≥ 60 years old.31 Our results show a slightly lower living donor death rate (0.12%, 1 per 833 LDLTs) over the past decade. With advances in minimally invasive surgery, laparoscopic donor hepatectomies for LDLT have now been safely performed and should make LDLT donation an even more attractive option for potential donors.32 Six percent (159/2566) of the living donors in our study were re-hospitalized, with almost half having biliary complications, confirming low recent donor morbidity in LDLT in the U.S.
We feel our method of assessing the association of preceding LDLT frequency and subsequent volume is robust, when compared to assessing total center volume whereby some outcomes are analyzed with respect to future volume. There may be unmeasured confounders such as individual surgeon experience and institutional resources which may affect the measurement of preceding LDLT frequency and subsequent outcomes. Surgical experience and skill will to some degree be transferable between institutions when one or more members of a surgical team from a higher volume/frequency LDLT center relocate to a lower volume/frequency LDLT center. While the UNOS database did permit the analysis of a large number of LDLTs, it does lack some granularity of data, such as morbidity among LDLT recipients, apart from graft failure etiologies, which would be important in considering the association of LDLT on quality of life. A large proportion of graft failures were recorded as “other” or “unknown”, which precluded any strong conclusions being inferred from the analysis of early graft failure among LDLT recipients. The UNOS database also contains missing data, however, this missing data was minimal.
In conclusion, our results showed an ongoing expansion of LDLT in the US in the setting of recent donor allocation policy changes and increased transplant center competition. Consistent volume is an important predictor of outcomes close to the norm, with acceptable outcomes maintained at a preceding LDLT frequency of 3-20 LDLTs per year, and aspirational outcomes at a LDLT frequency of >20 LDLTs per year. LDLT will undoubtedly remain an important option as recipient indications continue to expand.6,33,34 Future surgical innovations including increased use of left lobes and laparoscopic or robotic approaches, as well as efforts to expand the living donor candidate pool such as non-directed living liver donors, paired exchanges and donor champion programs may foster the advancement of LDLT in the US in the near future, while always following the double equipoise concept in balancing the donor risk and recipient benefit.2
Supplementary Material
Acknowledgements:
The authors would like to acknowledge the contribution of Dr. Kerollos N. Wanis (Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA) to the data analysis.
Footnotes
Conflicts of Interest/Financial Disclosures:
TGC, MM, JW, TP, FA, RHA, DdS have no relevant disclosures. AP has received grant/research support from TARGET Pharma Solutions and Exact Sciences; is on the speakers’ bureau for Simply Speaking Hepatitis; and is on an Medical Advisory Board for Exelixis, Eisai Inc and Genentech. MC has received grant/research support from Gilead, Conatus, Galectin; consultant fees from Gilead, Metacrine, Enterome, Novartis, AbbVie, Intercept, NGM Bio; and has been on an Advisory Committee for Gilead.
REFERENCES
- 1.Goldberg DS, French B, Thomasson A, Reddy KR, Halpern SD. Current trends in living donor liver transplantation for primary sclerosing cholangitis. Transplantation. 2011;91(10): 1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Gazala S, Olthoff KM. Current Status of Living Donor Liver Transplantation in the United States. Annu Rev Med. 2019;70: 225–238. [DOI] [PubMed] [Google Scholar]
- 3.Gruessner RWG, Gruessner AC. Solid-organ Transplants From Living Donors: Cumulative United States Experience on 140,156 Living Donor Transplants Over 28 Years. Transplant Proc. 2018;50(10): 3025–3035. [DOI] [PubMed] [Google Scholar]
- 4.Organ Procurement and Transplant Network (OPTN). Policies. Effective Date September/10/2020. Published 2020. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed 09/10/2020.
- 5.Cotter TG, Sandıkçı B, Paul S, et al. Liver Transplantation for Alcoholic Hepatitis in the US: Excellent Outcomes with Profound Temporal and Geographic Variation in Frequency. Am J Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Lo CM. Expanding living donor liver transplantation. Liver Transpl. 2016;22(S1): 37–39. [DOI] [PubMed] [Google Scholar]
- 7.Cotter TG, Paul S, Sandıkçı B, et al. Improved Graft Survival After Liver Transplantation for Recipients With Hepatitis C Virus in the Direct-Acting Antiviral Era. Liver Transpl. 2019;25(4): 598–609. [DOI] [PubMed] [Google Scholar]
- 8.Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54(4): 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humar A, Ganesh S, Jorgensen D, et al. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann Surg. 2019;270(3): 444–451. [DOI] [PubMed] [Google Scholar]
- 10.Lieber SR, Schiano TD, Rhodes R. Should living donor liver transplantation be an option when deceased donation is not? J Hepatol. 2018;68(5): 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242(3): 314–323, discussion 323-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olthoff KM, Abecassis MM, Emond JC, et al. Outcomes of adult living donor liver transplantation: comparison of the Adult-to-adult Living Donor Liver Transplantation Cohort Study and the national experience. Liver Transpl. 2011;17(7): 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6(1): 3–20. [DOI] [PubMed] [Google Scholar]
- 14.Bak T, Wachs M, Trotter J, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7(8): 680–686. [DOI] [PubMed] [Google Scholar]
- 15.Lo CM, Fan ST, Liu CL, et al. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg. 2004;240(1): 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer PA, Siegler M, Whitington PF, et al. Ethics of liver transplantation with living donors. N Engl J Med. 1989;321(9): 620–622. [DOI] [PubMed] [Google Scholar]
- 17.Brown RS. Live donors in liver transplantation. Gastroenterology. 2008;134(6): 1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raza MH, Aziz H, Kaur N, et al. Global experience and perspective on anonymous nondirected live donation in living donor liver transplantation. Clin Transplant. 2020;34(4): e13836. [DOI] [PubMed] [Google Scholar]
- 19.Kamei H, Oike F, Fujimoto Y, Yamamoto H, Tanaka K, Kiuchi T. Fatal graft-versus-host disease after living donor liver transplantation: differential impact of donor-dominant one-way HLA matching. Liver Transpl. 2006;12(1): 140–145. [DOI] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplant Network (OPTN). Scientific Recipients Transplant Recipient's (SRTR) Database: Overview. Available at: https://www.srtr.org/about-the-data/the-srtr-database/. Accessed 07/25/2019.
- 21.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4): 783–790. [DOI] [PubMed] [Google Scholar]
- 22.Rana A, Petrowsky H, Kaplan B, et al. Early liver retransplantation in adults. Transpl Int. 2014;27(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 23.D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19): 2265–2281. [DOI] [PubMed] [Google Scholar]
- 24.Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1: 193–299. [DOI] [PubMed] [Google Scholar]
- 25.Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2014;20(4): 425–436. [DOI] [PubMed] [Google Scholar]
- 26.Olthoff KM, Smith AR, Abecassis M, et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann Surg. 2015;262(3): 465–475; discussion 473-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibi T, Wei Chieh AK, Chi-Yan Chan A, Bhangui P. Current status of liver transplantation in Asia. Int J Surg. 2020;82S: 4–8. [DOI] [PubMed] [Google Scholar]
- 28.Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322(21): 1505–1507. [DOI] [PubMed] [Google Scholar]
- 29.Chan SC, Fan ST. Historical perspective of living donor liver transplantation. World J Gastroenterol. 2008;14(1): 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl. 2013;19(5): 499–506. [DOI] [PubMed] [Google Scholar]
- 31.Kadohisa M, Inomata Y, Uto K, et al. Impact of Donor Age on the Outcome of Living-donor Liver Transplantation: Special Consideration to the Feasibility of Using Elderly Donors. Transplantation. 2020. [DOI] [PubMed] [Google Scholar]
- 32.Soubrane O, Eguchi S, Uemoto S, et al. Minimally Invasive Donor Hepatectomy for Adult Living Donor Liver Transplantation: An International, Multi-Institutional Evaluation of Safety, Efficacy and Early Outcomes. Ann Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 33.Dueland S, Syversveen T, Solheim JM, et al. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann Surg. 2020;271(2): 212–218. [DOI] [PubMed] [Google Scholar]
- 34.Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162(3): 525–536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.