SUMMARY
Pathogenic enteric viruses are a major cause of morbidity and mortality, particularly among children in developing countries. The host response to enteric viruses occurs primarily within the mucosa, where the intestinal immune system must balance protection against pathogens with tissue protection and tolerance to harmless commensal bacteria and food. Here, we summarize current knowledge in natural immunity to enteric viruses, highlighting specialized features of the intestinal immune system. We further discuss how knowledge of intestinal anti-viral mechanisms can be translated into vaccine development with particular focus on immunization in the oral route. Research reveals that the intestine is a complex interface between enteric viruses and the host where environmental factors influence susceptibility and immunity to infection, while viral infections can have lasting implications for host health. A deeper mechanistic understanding of enteric anti-viral immunity with this broader context can ultimately lead to better vaccines for existing and emerging viruses.
INTRODUCTION
Pathogenic enteric viruses, characterized by fecal-oral transmission and intestinal replication, are a major cause of worldwide morbidity and mortality. Localized intestinal infection by enteric viruses such as adenovirus-A, -F, and -G (Adenoviridae), norovirus (Caliciviridae), astrovirus (Astroviridae), sapovirus (Caliciviridae), and rotavirus (Reoviridae) cause gastroenteritis characterized by tissue inflammation, disruption of the epithelial barrier, malabsorption, and diarrhea (Banyai et al., 2018; Liu et al., 2016). Rotavirus is the primary pathogen responsible for diarrheal mortality, causing >200,000 deaths and millions of hospitalizations each year, while adenovirus and norovirus are responsible for an additional 100,000 annual deaths (GBD Diarrhoeal Disease Collaborators, 2018; Hallowell et al., 2021). Hepatoviruses and enteroviruses (Picornaviridae), such as hepatitis A, poliovirus, and coxsackievirus, infect via the oral route but cause few gastrointestinal symptoms and result in clinical disease only after dissemination to another site. Enteric viruses have diverse genetic composition, cellular tropism, and pathophysiology, though all 16 human viruses known to be transmitted in oral route are non-enveloped, likely because a lipid membrane would be disrupted in the harsh environment of the digestive tract (Bushman et al., 2019). Key features of major enteric viruses are summarized in Figure 1.
Figure 1. Pathogenic enteric viruses.
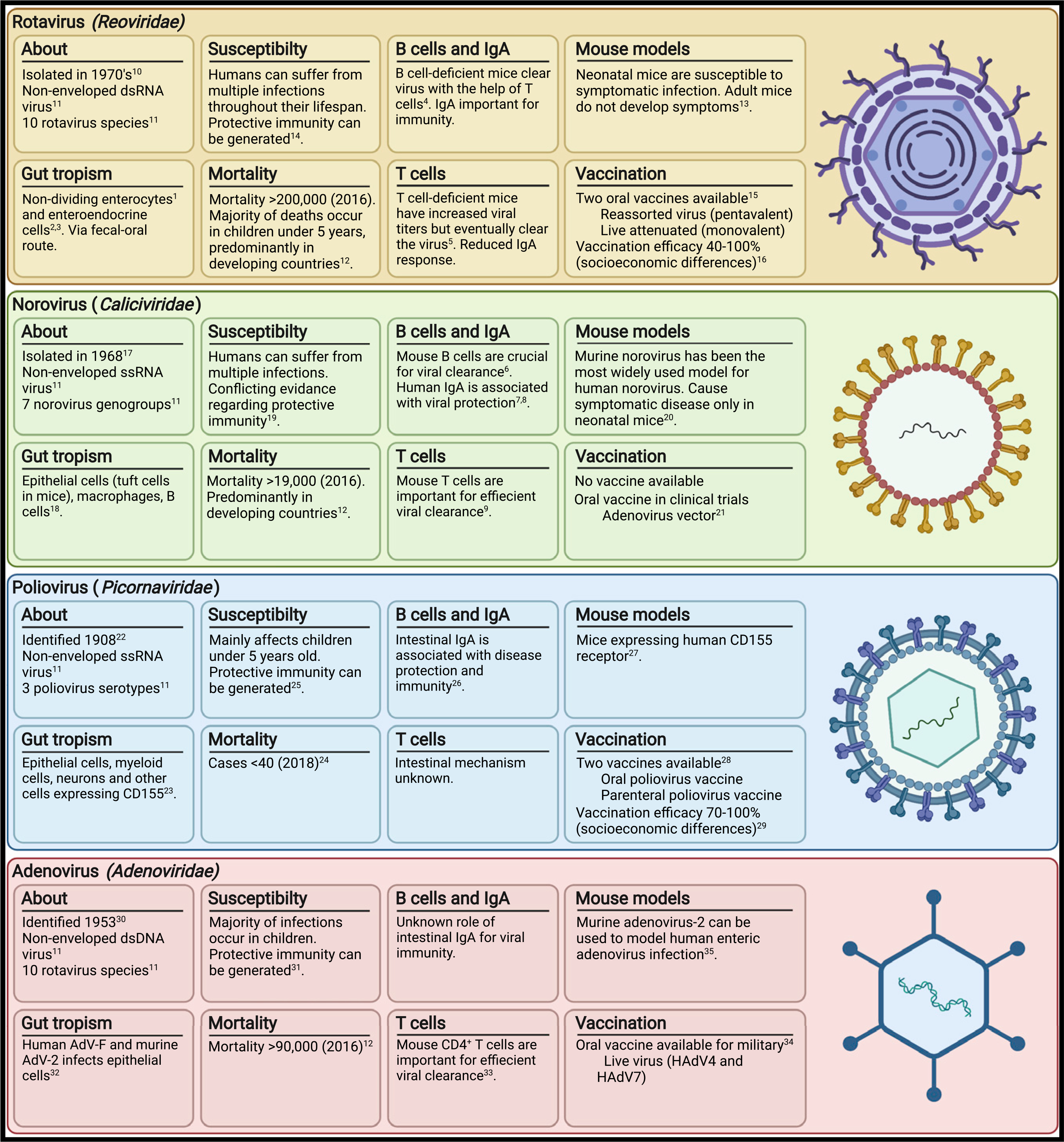
Key features of enteric viruses, natural immunity, and vaccines.
References: 1(Lundgren and Svensson, 2001); 2(Hagbom et al., 2011); 3(Saxena et al., 2016); 4(Franco and Greenberg, 1995); 5(Franco and Greenberg, 1997); 6(Chachu et al., 2008b); 7(Lindesmith et al., 2003); 8(Ramani et al., 2015); 9(Chachu et al., 2008a); 10(Bishop et al., 1973; Flewett et al., 1973); 11(Howley et al., 2020); 12(GBD Diarrheal Disease Collaborators, 2018); 13(Little and Shadduck, 1982; Sheridan et al., 1983); 14(Bhandari et al., 2014; Ruiz-Palacios et al., 2006; Velazquez et al., 1996); 15(Burnett et al., 2020); 16(Armah et al., 2010; Jiang et al., 2010; Madhi et al., 2010; O’Ryan and Linhares, 2009; Ruiz-Palacios et al., 2006; Vesikari et al., 2006, 2007; Zaman et al., 2010); 17(Kapikian et al., 1972); 18(Baldridge et al., 2016; Elftman et al., 2013; Gonzalez-Hernandez et al., 2013, 2014; Grau et al., 2017; Karst and Wobus, 2015); 19(Newman and Leon, 2015); 20(Roth et al., 2020); 21(Kim et al., 2018); 22(Eggers, 1999); 23(Mendelsohn et al., 1989; Nomoto, 2007); 24(Chard et al., 2020); 25(Nathanson, 2008); 26(Wright et al., 2016); 27(Khan et al., 2014; Koike et al., 1991); 28(Connor et al., 2022); 29(John, 1976; McBean et al., 1988); 30(Hilleman and Werner, 1954; Rowe et al., 1953); 31(Lion, 2014); 32(Greber and Flatt, 2019; Hashimoto et al., 1966); 33(Parsa et al., 2021); 34(Collins et al., 2020; Kuschner et al., 2013); 35(Hashimoto et al., 1966).
The host response to enteric viruses occurs primarily within the intestinal mucosa, which contains a single layer of epithelial cells, connective tissue beneath termed the lamina propria, and an underlying layer of muscle. Most immune cells are found in the epithelium and lamina propria, which each form distinct immune compartments. Induction of immune responses to intestinal antigen occurs in gut-draining lymph nodes and gut-associated lymphoid tissues such as Peyer’s patches (Mowat and Agace, 2014). The majority of immune stimulation in the intestine comes from commensal microbes and food. The intestinal immune system must balance immunological tolerance to these harmless entities with protection against pathogens. Dysregulated immune responses may lead to tissue damage, inflammatory bowel disease, or food allergy, and accordingly the intestinal immune system is set in a default state of tolerance. Although these immune-suppressive pathways are mainly targeted at extracellular antigen, there is a high threshold in general for immune activation in the gut. Enteric pathogens and vaccines that enter in the oral route must therefore provide a higher level of stimulation to overcome tolerance, providing important context for the nature of effective antiviral immunity in the gut (Faria et al., 2017; Zhou and Sonnenberg, 2018). Another key concept for antiviral immunity is that viruses have evolved strategies to evade every step of the immune response, while the immune system compensates with redundant mechanisms for viral detection and control. This tradeoff is made evident by the existence of multiple converging innate immune pathways for viral detection as well as the multi-faceted adaptive immune response.
During viral infection, epithelial and innate cells detect the breach via cytosolic and membrane pattern recognition receptors. A cascade of signaling pathways is activated within the cells that in turn activates the production of inflammatory mediators such as type I and III interferons (IFN-α/β/λ), interleukin (IL)-6, IL-1β, IL-18, and tumor necrosis factor alpha (TNF-α) (Figure 2A). These interferons and cytokines alarm the surrounding cells by inducing the activation of intrinsic anti-viral programs and the activation of endothelial cells to facilitate the recruitment of leukocytes. Dendritic cells (DCs) in the lamina propria that have taken up viral particles migrate to the mesenteric lymph nodes and present processed viral antigens to virus-specific T cells via major histocompatibility complex (MHC) class I and II molecules. Subsequently, virus-specific T cells differentiate into memory and effector subtypes with anti-viral properties and upregulate gut homing receptors such as α4β7 and CCR9 to migrate to the intestinal tissue (Figure 2B). The recruited intestinal virus-specific T cells can directly eliminate virus-infected cells via granzyme/perforin- or cytokine-mediated cytotoxicity (Figure 2C). Simultaneously, viral antigen capture and presentation within specialized immune structures in the intestinal tissue called Peyer’s patches results in the activation and differentiation of virus-specific B cells, generally in a T cell-dependent manner. The activated B cells differentiate into plasma cells that produce large amounts of virus-specific secretory immunoglobin A (IgA) antibodies that are translocated to the lumen where they can neutralize virus (Figure 2D). T cells and plasma cells activated during a primary viral infection can establish permanent tissue residence in the gut or migrate to the bone marrow and mount rapid responses upon secondary infection. Thus, the intestinal immune system can quickly detect and clear viruses through innate mechanisms, as well as establish long-term antigen-specific protective immunity through adaptive mechanisms.
Figure 2. Intestinal immunity during viral infections.
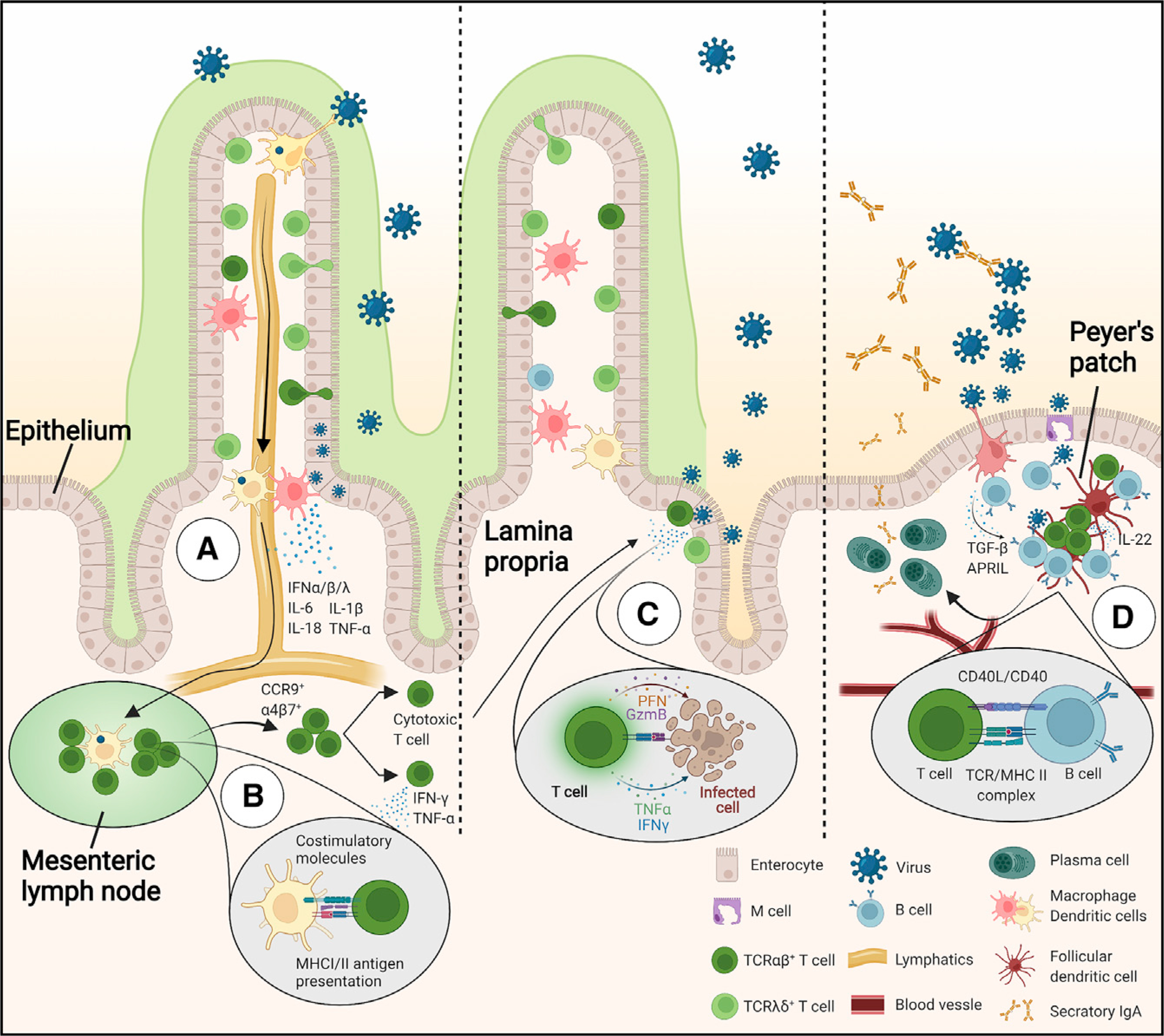
(A) Early recognition of viruses via pattern recognition receptors such as intracellular DNA and RNA sensors by innate immune cells results in an interferon and cytokine response. Virus and viral antigen uptake by dendritic cells, macrophages, and epithelial M cells leads to activation of the adaptive immune system.
(B) Dendritic cells in the lamina propria migrate to the draining mesenteric lymph nodes and present viral antigens to virus-specific T cells.
(C) Activated T cells migrate to the intestinal tissue and elicit antiviral immunity by cytokine secretion or cytotoxic activity.
(D) B cells activated in Peyer’s patches undergo IgA class switching and differentiate into plasma cells with the help of follicular T helper cells. Intestinal plasma cells produce secretory IgA that can neutralize viruses in the mucosa.
In this review, we will summarize current knowledge of natural immunity to enteric viruses and how this knowledge is being translated into vaccine development, highlighting specialized features of intestinal immunity. Human studies in this field have been hindered by difficulty in accessing mucosal tissues, necessitating the use of gut-homing immune responses in peripheral blood as the main readout for mucosal immunity. Accordingly, we will discuss findings from human studies where possible while also highlighting functional and mechanistic studies from mice.
INNATE ANTIVIRAL RESPONSES
The intestinal epithelium and associated mucus layer form a selective barrier that provides defense against enteric pathogens. Mucus, essentially a hydrogel of highly glycosylated linear proteins, is densely packed with anti-microbial peptides such as defensins that can disrupt viral envelopes: another reason enteric viruses that transmit via oral-fecal routes are non-enveloped (Daher et al., 1986; Linden et al., 2008). The mucus layer also contains secretory antibodies that can neutralize virus before it reaches the tissue (Mantis et al., 2011). The outer mucus layer is constantly shed and passed down the digestive tract to be excreted. The epithelium itself forms a structural barrier to viral invasion including a dense brush border (microvilli) on the apical side and multiprotein adhesion complexes between neighboring epithelial cells (tight junctions) (Delorme-Axford and Coyne, 2011). Enteric viruses have adapted numerous strategies to invade through the mucosa. For example, adenovirus and poliovirus can both use tight junction proteins as entry receptors (Bergelson et al., 1997; Mendelsohn et al., 1989). However, most luminal bacteria and viruses never breach the mucosal barrier and therefore may not directly interact with immune cells (Kuss et al., 2008; Mantis et al., 2011).
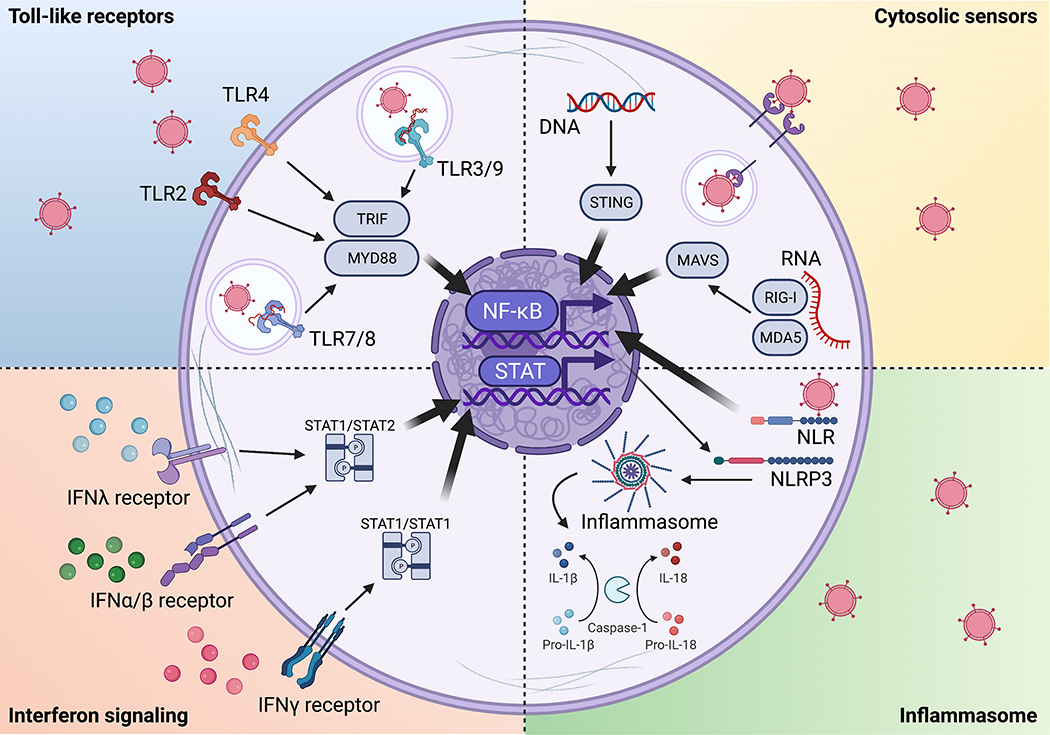
Viral detection occurs through pattern recognition receptors, either intrinsically by an infected cell through recognition of cytosolic nucleic acids or extrinsically by recognition of endosomal nucleic acids (Figure 3). Cytosolic sensors are universally expressed across cell types to recognize DNA (cGAS) or RNA (RIG-I and MDA5), leading to downstream activation of adaptor proteins STING or MAVS, respectively (Andrejeva et al., 2004; Ishikawa and Barber, 2008; Yoneyama et al., 2004). Endosomal sensing is mediated primarily by toll-like receptors (TLRs) that are expressed by intestinal epithelial cells, DCs, and macrophages, recognizing double-stranded RNA (TLR3), single-stranded RNA (TLR7/8), and CpG motifs in double-stranded DNA (TLR9) and recruiting downstream adaptor and signaling proteins MyD88 and TRIF (Blasius and Beutler, 2010). TLR sensing is important as MyD88-deficient mice have increased susceptibility for multiple enteric viruses (Fejer et al., 2008; Hartman et al., 2007; McCartney et al., 2008; Thackray et al., 2012; Uchiyama et al., 2015).
Figure 3. Detection of viruses by pattern recognition receptors.
Virus-derived RNA and DNA can be recognized by toll-like receptors (TLRs) and cytosolic sensors, resulting in the secretion of pro-inflammatory cytokines and IFNs. Inflammasome activation results in cytokine maturation and secretion and inflammation-induced cell death. IFNs activate gene expression programs that are important for downstream anti-viral immunity.
Viral detection pathways lead to downstream activation of the transcription factor nuclear factor κB, which leads to production of pro-inflammatory cytokines including IL-1β, IL-6, IL-12, TNF-α, and IFNs (Kawai and Akira, 2011). IFNs are critical in antiviral immunity, and mice lacking type I IFN receptor or the downstream adapter molecule STAT1 are highly susceptible to viral infections (Hwang et al., 1995; Ingle et al., 2018; Muller et al., 1994). Type I IFNs contribute to multi-pronged antiviral defense mechanisms that include promoting apoptosis of infected cells, antigen presentation pathways, and natural killer and cytotoxic T cell responses (Stetson and Medzhitov, 2006). While type I or II IFNs do not regulate viral load of chronic murine norovirus, these cytokines prevent the spread of virus from intestinal tuft cells to systemic extraintestinal sites such as lymph nodes and spleen (Baldridge et al., 2015; Nice et al., 2015). Most viruses have evolved mechanisms to evade the IFN response; for example, rotavirus targets IFN-regulatory factors (downstream of DNA/RNA sensors) to reduce the type I IFN response (Arnold and Patton, 2011). Type III interferon (IFNλ), which can be produced by epithelial and myeloid cells (Lauterbach et al., 2010), is also critical for control of enteric viruses in mice and induces long-lasting expression of IFN-stimulated genes relative to type I IFNs (Baldridge et al., 2017; Larsen et al., 2020; Ye et al., 2019). Consistently, mice lacking IFN-λ receptor exhibit high norovirus load in intestinal tissues and stool (Baldridge et al., 2017).
Nucleotide-binding oligomerization domain (NOD)-like receptors can be activated by enteric viruses though recognition of self-derived stress signals to induce the inflammasomes NLRP3 in immune cells or NLRP9 in IECs (Allen et al., 2009; Zhu et al., 2017). Inflammasomes are signaling complexes that activate caspase-1 or −11, resulting in inflammatory cell death, and secretion of the cytokines IL-1β and IL-18, which promote an adaptive antiviral response (Niu et al., 2019; Zhao and Zhao, 2020). The production of IL-18 and IL-22 by TLR5-stimulated DCs has been proposed to induce clearance of rotavirus (Zhang et al., 2014), while targeted deletion of NLRP9b in intestinal epithelial cells leads to diarrhea and increased rotavirus shedding in the feces (Zhu et al., 2017). The innate pathways described here enable broad detection of viruses that converge into a common pro-inflammatory response and conversely represent major targets for viral immune evasion strategies (Carty et al., 2021; Pichlmair and Reis e Sousa, 2007).
Innate responses occur rapidly, within hours of viral detection, and are critical for viral control during primary exposure. Additionally, innate cells contribute to induction of specific immunological memory through uptake and presentation of viral antigens to adaptive immune cells. Viral detection through pattern recognition receptors promotes antigen presentation through several mechanisms, including upregulation of costimulatory molecules, MHC class I and II, and CCR7-dependent migration to T cell zones in lymph nodes (van Montfoort et al., 2014). Antigen-presenting cells can also directly sample the intestinal lumen for antigens via trans-epithelial processes, though it is not known whether viruses can be sampled in this manner (Mazzini et al., 2014; Niess et al., 2005; Rescigno et al., 2001). Finally, specialized intestinal epithelial cells called M cells that overlay gut-associated lymphoid tissues such as Peyer’s patches can directly transport virus from the lumen through transcytosis, where they can be picked up for presentation (Dillon and Lo, 2019). However, several enteric viruses can also utilize M cells for entry and replication, and depletion of M cells in mice has been shown to limit infection with norovirus and reovirus (Dillon and Lo, 2019; Gonzalez-Hernandez et al., 2014). Initiation of adaptive immunity and formation of specific immune memory is key for protection against future infections.
INTESTINAL B CELLS AND IgA
B cells develop in the bone marrow, where they produce their B cell receptors by immunoglobulin gene rearrangements, which, following germinal center-mediated somatic hypermutation, provide virtually unlimited potential antigen recognition capacity. B cells are crucial players in mucosal immunity, especially as producers of secretory IgA, the most abundant antibody in mice and humans. Selective IgA deficiency in humans is associated with increased incidence of intestinal infections and more severe gastrointestinal symptoms, however, an estimated 85%–90% of IgA-deficient individuals are asymptomatic, suggesting alternate mechanisms such as increased IgM can compensate in absence of IgA (Yel, 2010). Following maturation of antigen-specific B cells at gut immune inductive sites, memory B cells and plasma cell precursors upregulate intestinal homing molecules α4β7 and CCR9 and migrate to the intestinal tissue (McNeal and Ward, 1995; Williams et al., 1998). Intestine-resident IgA plasma cells produce secretory dimeric IgA, which is transported to the lumen through the epithelial cells via polyIg-receptors (Mantis et al., 2011).
Secretory IgA is among the most important indicators of protection against enteric viral infection and can potentially neutralize virus in the mucus layer before it reaches host cells, preventing infection altogether. Indeed, clearance of enteric viruses has been positively correlated to the presence of specific intestinal IgA (Atmar et al., 2011; Blutt et al., 2012; Cheuvart et al., 2014; Lindesmith et al., 2003; Nishio et al., 1992; Ramani et al., 2015; Tacket et al., 2003; Unicomb et al., 1996). In contrast, circulating antibodies have limited access to the gut mucosa and hence are less capable of limiting the initial mucosal phase of enteric infections, though they have been shown to offer protection against the systemic phase of certain enteric pathogens (Faden et al., 1990; Hird and Grassly, 2012; Zeng et al., 2016). In many cases, serum-specific antibody titers correlate with protection against enteric viruses (Reeck et al., 2010; Velazquez et al., 2000), although it is unclear whether this is through direct control of infection or simply an indirect readout for control through secretory antibody induction. One study reported that 4 months after rotavirus infection, serum IgA levels could predict small intestine secretory IgA with 79% accuracy, whereas saliva (85%) and feces (92%) were more accurate predictors (Grimwood et al., 1988). Serum from adenovirus-infected mice has in vitro neutralizing activity but does not protect naive mice from enteric adenovirus infection, supporting the conclusion that serum antibodies cannot control mucosal infection (Lussier et al., 1987). Although an intestinal IgA response to SARS-CoV-2 has not been investigated, IgA antibodies dominate early specific antibody responses in the serum (monomeric, circulating), as well as in the saliva and lungs (secretory) (Sterlin et al., 2021). Furthermore, secretory IgA antibodies neutralize SARS-CoV-2 15-fold more effectively than their monomeric counterparts (Wang et al., 2021). Finally, mice lacking B cells fail to clear acute norovirus infection but are able to clear rotavirus infection, thus the absolute requirement for antibodies varies by virus (Chachu et al., 2008b; Franco and Greenberg, 1995; Karst et al., 2003). As most vaccines depend highly on humoral immunity, a better understanding of the role of circulating versus secretory antibodies as well as B cell-dependent and -independent pathways in the enteric anti-viral response is crucial.
Some evidence suggests that secretory antibodies generated by viral infections restricted to mucosal sites are short lived (<3 years) relative to serum antibodies generated by systemic infections (>10 years) (Slifka and Ahmed, 1996). Bacteria-specific intestinal IgA reportedly persists in monocolonized mice but declines rapidly in the presence of other microbiota, and additive increases in specific IgA were found after consecutive bacterial exposures, in contrast to prime-boost dynamics seen in peripheral antibody responses (Hapfelmeier et al., 2010). Mucosal IgA responses, therefore, may follow different dynamics than peripheral antibodies, and one possible mechanism for mucosal IgA degeneracy is continual replacement reflecting ongoing stimulation from the intestinal lumen. Rotavirus-specific IgA plasma cells can be detected in intestinal biopsies from the majority of human donors despite no recent history of rotavirus infection (Di Niro et al., 2010; Nair et al., 2016) and can be found in the lamina propria and bone marrow of mice 9 months after infection (Youngman et al., 2002). This suggests that despite potentially short-lived intestinal IgA, long-lived plasma cells may be able to quickly mount a humoral response upon re-infection. Studies on the longevity of intestinal humoral immunity are critical for understanding anti-viral mechanisms.
INTESTINE-RESIDENT T CELL RESPONSES
The majority of the immune cells found in the intestinal tissue are T cells, including peripherally recruited conventional TCRαβ T cells, which populate primarily the intestinal lamina propria, and tissue-resident innate-like T cells such as CD8αα TCRγδ, CD8αα, and CD4 CD8αα TCRαβ T cells primarily populating the intestinal epithelium (Cheroutre et al., 2011; Mayassi and Jabri, 2018). TCRαβ T cells primed in the gut-draining lymph nodes can migrate to the intestine where they functionally adapt, establishing permanent residency and memory characteristics (Bilate et al., 2020; Fonseca et al., 2020; Kiner et al., 2021; London et al., 2021).
Most mechanistic studies about the role of T cells in enteric virus infection derive from experiments in mice. While mice lacking both B and T cells become chronically infected with adenovirus or rotavirus (Dharakul et al., 1990; Franco and Greenberg, 1995; Umehara et al., 1984), animals lacking CD8+ T cells show delayed clearance of these enteric viruses (Chachu et al., 2008a; Franco et al., 1997; Zhu et al., 2016), which suggests an important, but not essential, role for cytotoxic T cells. Along the same lines, adoptive transfer of norovirus-specific CD8+ T cells can reduce the viral burden of Rag−/− mice persistently infected with norovirus (Tomov et al., 2013). Rotavirus-specific CD8+ T cells have been found in Peyer’s patches of infected mice expressing high levels of tissue homing receptors CCR9 and CXCR6 (Jiang et al., 2008). In humans, rotavirus-specific CD8+ T cells were found in the peripheral blood and intestinal epithelium by tetramer staining; these cells also concomitantly express the gut-homing receptors CD103 and integrin β7 and produce IFN-γ (Newell et al., 2013; Wei et al., 2006, 2009). However, studies in animal models suggest that the production of IFN-γ or perforin and expression of bβ integrin by CD8+ T cells are all dispensable for the control of rotavirus infection (Franco et al., 1997; Kuklin et al., 2000). On the other hand, perforin does have a role in protection against norovirus (Chachu et al., 2008a). The limited requirement for CD8+ T cells may be due to partially redundant function with other cytotoxic immune cells such as innate lymphoid cells and natural killer cells. For example, CD8+ T cells detect virus-infected cells through viral peptide presentation on MHC-I, but several viruses evade CD8+ T cell-mediated killing by downregulating MHC-I surface expression. In this case CD8+ T cells are dispensable for viral clearance while natural killer cells can detect “missing self” and initiate cytotoxicity (Hansen et al., 2010; Koutsakos et al., 2019). In some cases, persistence of viral infection can be linked to evasion of CD8+ T cell responses. For example, acute murine norovirus elicits effective CD8+ T responses and can be cleared, while chronic murine norovirus recruits fewer and less functional CD8+ T cells (Parsa et al., 2021; Tomov et al., 2013, 2017). While the mechanism of chronic murine norovirus evasion remains unclear, its selective infection of rare epithelial tuft cells may reduce antigen availability to CD8+ T cells, while acute murine norovirus that infects myeloid cells may be more accessible. Additional studies are needed to define mechanisms of CD8+ T cell-mediated viral control, how these pathways can be evaded by enteric viruses, and whether similar mechanisms apply during human infections.
Murine studies have also provided some understanding about the role of CD4+ T cells in enteric viral clearance. CD4+ T cell help is important for B cell class switching and affinity maturation, although some IgA is still found in CD4+ T cell-deficient mice, indicating that IgA class switching can occur via both T cell-dependent and -independent pathways (Fagarasan et al., 2010; Hornquist et al., 1995; Snider et al., 1999). Accordingly, CD4+ T cell-depleted mice show reduction in the generation of rotavirus-specific intestinal IgA, although they could still clear virus (Franco and Greenberg, 1997). Comparably, mice lacking CD4+ T cells show higher norovirus load but eventually clear infection (Chachu et al., 2008a; Zhu et al., 2013, 2016). To gain more insights into the role of peripherally-recruited T cells in enteric viral infections, we fate-mapped naive T cells (Merkenschlager et al., 2021) in mice infected with several different enteric virus strains. We found that viruses that spread systemically, such as reovirus T1L (a virus in the same family as rotavirus) and acute norovirus CW3, preferentially recruit CD8 T cells to the intestine. In contrast, enteric adenovirus and chronic norovirus CR6, which do not spread systemically, induced the recruitment of activated CD4+ T cells to the intestinal epithelium expressing CCR9, Ly-6A, granzyme B, and IFN-γ. Although mice infected with chronic norovirus did not clear infection, we found a role for norovirus-recruited CD4+ T cells, but not reovirus-recruited CD4+ T cells, in the clearance of heterologous adenovirus infection. Removal of TCR from CD4+ T cells before adenovirus infection increased viral titers and delayed viral clearance, but removal of TCR post-infection did not impact viral clearance (Parsa et al., 2021). These findings suggest TCR-dependent differentiation but TCR-independent function of virus-recruited T cells, similar to what we previously proposed for other intestinal T cell subsets (Bilate et al., 2020). We also found that heterologous viral clearance by pre-existing virus-recruited CD4+ T cells was dependent on IFN-γ secretion, which could be induced by stimulation with IL-12 and IL-18 without TCR engagement (Parsa et al., 2021). This suggests a unique differentiation pathway and TCR-independent function of virus-recruited intestinal CD4+ T cells in cross-protective viral clearance.
In the epithelial layer, most T cells in mice and a large fraction in humans are TCRγδ T cells (Guy-Grand et al., 1991). Murine studies demonstrated that TCRγδ T cells actively monitor the intestinal epithelium and can quickly identify infected or stressed epithelial cells (Hoytema van Konijnenburg et al., 2017). Upon activation, TCRγδ T cells exhibit cytotoxic properties and produce cytokines such as IFN-γ, TNF-α, and antimicrobial proteins (Cheroutre et al., 2011; Olivares-Villagomez and Van Kaer, 2018). Activated TCRγδ T cells can produce IFN-α and IFN-λ but were found to be dispensable for rotavirus clearance (Franco and Greenberg, 1997; Swamy et al., 2015). While part of these results could potentially be explained by redundancy in intestinal T cell subset function, the specific role of TCRγδ T cells in controlling enteric virus infections, particularly those with tropism for the epithelium, remains unclear but is an intriguing topic for further research.
HOST IMMUNITY, SUSCEPTIBILITY, AND HEALTH
Humans can suffer from multiple rotavirus, norovirus, and adenovirus infections throughout their lifespan, although the severity of disease is often reduced after repeated exposure (Gray et al., 2007; Velazquez, 2009; Velazquez et al., 1996). Epidemiological and clinical studies have demonstrated that strain-specific protective immunity can be generated after a single adenovirus, rotavirus, or norovirus infection, though there is conflicting evidence regarding the extent and duration of this protection (Gladstone et al., 2011; Simmons et al., 2013; Velazquez et al., 1996; Zhu et al., 2013). However, there are at least 30 serotypes of norovirus and 50 serotypes of adenovirus in circulation, with new variants constantly emerging, creating complications for long-term immunity (Lion, 2014; Ramani et al., 2014). Humans can be sequentially and even concurrently infected with different adenovirus strains within a span of 2 years (Gray et al., 2007). Host genetic diversity and environmental factors, including diet and gut microbiome, likely also contribute to variable outcomes in enteric virus susceptibility and immunity (Tan and Jiang, 2014).
The majority of deaths and severe illnesses from enteric viruses occur in children under 5 years old, and only neonatal mice are susceptible to symptomatic rotavirus or norovirus infection, whereas adult mice without pre-existing immunity are asymptomatic (GBD Diarrheal Disease Collaborators, 2018; Little and Shadduck, 1982; Sheridan et al., 1983) This suggests that age-related susceptibility to enteric viruses depends on both pre-existing immunity and maturation of the intestinal immune system. For example, lower levels of TLR3 are found in duodenal tissue from humans under 5 years old when compared to humans 6–20 years old, and, accordingly, TLR3 contributes to rotavirus control in adult mice but did not impact susceptibility in neonatal mice (Pott et al., 2012). Unfortunately, the short time frame in which mice can be symptomatically infected with rotavirus or norovirus creates difficulties in meaningful evaluation of protective immunity in murine models. Age-dependent aspects of mucosal immunity are an important consideration for timing of immunizations (discussed below).
While a balanced gut microbiota is known to protect against opportunistic bacterial infections, studies suggest that gut bacteria can both facilitate and protect against enteric virus infections under different circumstances. For example, antibiotic-treated mice have decreased susceptibility to poliovirus and rotavirus; findings possibly related to a microbiota-mediated suppression of the IFN-λ pathway (Baldridge et al., 2015; Kuss et al., 2011; Uchiyama et al., 2014). In contrast, another study reported that microbiota stimulates a homeostatic IFN-λ response (Van Winkle et al., 2022). Further, colonization of segmented filamentous bacteria suppresses rotavirus infection in mice (Shi et al., 2019). In humans, gut microbiota composition has been correlated with susceptibility to rotavirus and norovirus (Rodriguez-Diaz et al., 2017), and the contrasting findings above suggest that different microbial species or compositions may either stimulate or suppress IFN-λ. Poliovirus binding of bacterial LPS was shown to enhance virion stability, suggesting a role for direct interactions between viruses and bacteria in addition to indirect stimulation of the immune system (Robinson et al., 2014). Preexisting viral infections may also impact viral susceptibility. For example, the presence of a specific strain of astrovirus in immunodeficient mice could protect against rotavirus and norovirus in an IFN-λ-dependent manner (Ingle et al., 2019). Together these findings suggest that diverse intestinal signals contribute to the overall state of IFN stimulation in the intestine, with consequences for susceptibility to viral infection.
Alongside commensal bacteria, the intestine is host to a diverse and abundant virome that is composed principally of bacteriophages and endogenous retroviruses (Neil and Cadwell, 2018). Intriguingly, while germ-free or antibiotic-treated mice have numerous intestinal abnormalities, including altered tissue morphology and reduced intestinal immune cells, norovirus infection was sufficient to reverse many of these phenotypes and protect against chemical tissue injury in a type-I-IFN-dependent manner, suggesting that in absence of a native microbiome or virome, typically pathogenic viruses can potentially promote intestinal homeostasis (Kernbauer et al., 2014). This emerging field of research has demonstrated that the concept of viruses as exclusively pathogenic is outdated, and that commensal viruses, like bacteria, may provide both benefits and detriments to the host (Neil and Cadwell, 2018).
Several studies have linked enteric viral infections to other intestinal diseases. Human norovirus infection and acute gastroenteritis have been associated with post-infectious irritable bowel syndrome and inflammatory bowel disease (Glass et al., 2009; Marshall et al., 2007). Chronic norovirus infection also triggers enhanced intestinal pathology in mice expressing a human Crohn disease susceptibility allele, suggesting that host gene-virus interactions can promote intestinal disease (Cadwell et al., 2010). Additionally, active infection with norovirus or HIV has been linked to changes in gut microbiota composition, suggesting that viral infection can lead to dysbiosis (Nelson et al., 2012; Vujkovic-Cvijin et al., 2013). Human studies have linked rotavirus and reovirus with Celiac disease, while enteric viral infections in mice were shown to impair the induction of regulatory T cells and promote inflammatory T cell responses to dietary antigen (Bouziat et al., 2017, 2018; Stene et al., 2006). These studies suggest that viral infections may disrupt homeostatic intestinal immune tolerance to microbiota and food in certain circumstances.
Finally, enteric viruses may impact health beyond the gut. A relationship between enteric infections and malnutrition, each increasing susceptibility to the other, has long been proposed (Scrimshaw et al., 1968; Tickell et al., 2020). Severe childhood diarrhea and malnutrition have in turn been correlated with stunted growth and increased risk of chronic adulthood conditions such as cardiovascular disease (Margolis, 2010; Schlaudecker et al., 2011). Rotavirus infection has also been implicated in increased risk of type I diabetes in humans and mice. One proposed mechanism is cross-reactivity between viral antigen and self-antigen leading to destruction of insulin-secreting cells, though only indirect evidence for this exists (Burke et al., 2020; Gomez-Rial et al., 2020). A lot remains to be learned about mechanisms connecting enteric viruses and human diseases, but these observations highlight the importance of developing effective vaccines to prevent enteric virus infections.
TARGETING INTESTINAL IMMUNITY BY VACCINATION
Vaccines have been approved for each of the main enteric viruses discussed in this review except norovirus and overall have been highly successful at preventing serious viral disease. Poliovirus has been nearly eradicated from human populations after a global vaccination campaign (Razum et al., 2019), and mortality rate from diarrhea decreased 30% from 2010 to 2019, partly due to expanded distribution of rotavirus vaccines (De Jesus et al., 2020; IHME, 2019; Shah et al., 2017). However, enteric viruses still cause substantial mortality and health burden, especially for children in developing countries (GBD Diarrheal Disease Collaborators, 2018). Further, as viruses continuously evolve and adapt to evade immune responses and infect new niches, we must also adapt our immunization strategies. The COVID-19 pandemic has illustrated both the need for new vaccine strategies and relevance of mucosal immunity for prevention of infection.
Mucosal immune responses are highly compartmentalized such that the tissue of primary immunogen encounter is the site where the majority of effector and memory cells will traffic. The intestine-specific immune pathways of viral detection and response discussed above therefore depend on encountering the virus within the intestinal tract. Parenteral vaccines, for example those given in the intramuscular route, elicit strong peripheral immune responses but poor tissue immunity, and the circulating antibodies they generate have very limited access to mucosal tissue (Hird and Grassly, 2012; Holmgren and Czerkinsky, 2005; Zhaori et al., 1988). Further, several human studies have demonstrated that immunization at different mucosal sites (e.g. oral, rectal, nasal, vaginal) elicits mucosal antibody responses in the targeted tissue but produces variable antibody responses at other mucosal sites or even within different regions of the same mucosal tissue, thus, there is no universal route of mucosal immunization (Eriksson et al., 1998; Jertborn et al., 2001; Johansen et al., 2005; Johansson et al., 2004; Kozlowski et al., 1997; Ogier et al., 2005; Quiding-Jarbrink et al., 1997; van Splunter et al., 2018). The best route of immunization to generate intestinal immunity is therefore oral or rectal, although there is evidence that intranasal vaccination can elicit protective humoral and T cell responses in the intestine in some cases (Ciabattini et al., 2010; Esplugues et al., 2011; Ruane et al., 2013, 2016). Oral vaccines are particularly attractive as they are easy to administer without the need for sterile devices or trained personnel, a particular advantage in low-income countries where the need is highest. Thus, intestinal immunity generated through vaccination in the oral route is ideal for viruses that are restricted primarily to the gastrointestinal tract.
For viruses that infect through the fecal-oral route and then spread systemically (e.g. poliovirus, hepatitis A), parenteral and mucosal vaccines can both be effective. Intramuscular polio and hepatitis A vaccines produce circulating antibodies that prevent viral spread to the central nervous system or liver, respectively, protecting against serious disease. By contrast, the oral polio vaccine promotes both systemic and mucosal immunity, including a robust IgA response that limits viral replication in the gastrointestinal tract (Nathanson, 2008; Wright et al., 2016) (Box 1).
Box 1. Oral polio vaccines: A success story and a cautionary tale.
The polio vaccination campaign has overall been highly successful, resulting in near-global eradication of polio. Oral polio vaccination (OPV) promotes both mucosal and systemic immunity, including a robust IgA response that limits viral replication in the gastrointestinal tract (Nathanson, 2008; Wright et al., 2016). Intramuscular inactivated polio vaccines (IPVs), by contrast, do not promote a mucosal immune response but do produce circulating antibodies that prevent viral spread to the central nervous system and subsequent paralysis (Hird and Grassly, 2012). Both vaccines are highly effective and promote long-lasting protection, but parenteral vaccination does not prevent viral replication or subsequent community transmission due to the lack of mucosal immunity (Herremans et al., 1999; Shulman et al., 2015). Further, the OPV is both cheaper and easier to administer (Zimmermann et al., 2020). The main drawback of OPV is that in rare cases (4.7 per 1 million) the live attenuated virus can revert to neurovirulence, causing paralysis, whereas IPVs use inactivated virus and are not associated with the same risk (Platt et al., 2014). For this reason, most high-income countries have transitioned to IPV (Bandyopadhyay et al., 2015). Vaccine schedules giving first IPV then OPV have been shown to promote mucosal immunity while limiting potential risks, because systemic immunity is already in place upon exposure to the live vaccine (Alexander et al., 2004; Domok, 1984; Modlin et al., 1997). This indicates that combination parenteral and mucosal vaccination may be an option for combatting viruses, particularly those where immunity in multiple tissue sites is needed. However, formulation of effective oral vaccines without live virus remains a critical next step for vaccine development.
For viruses that can infect multiple mucosal tissues including the intestine (e.g. adenovirus and HIV), ideal vaccines will promote broad immunity across mucosal and systemic sites, though this has proved evasive. Furthermore, there are some points of evidence that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can infect and replicate in the intestine, suggesting that induction of intestinal immunity may be an important consideration for vaccine design (Box 2). It has been suggested that oral vaccines can induce airway-homing memory B cells and nasopharyngeal IgA (van Splunter et al., 2018; Zhaori et al., 1988) and that nasal immunization can induce gut-homing B cells and intestinal immunity (Esplugues et al., 2011; Ruane et al., 2013, 2016). However, other studies have shown no intestinal immune response to nasal immunization (Ogier et al., 2005; Quiding-Jarbrink et al., 1997), suggesting that this cross-tissue protection may depend on immunization protocol. Oral immunization does not typically elicit protection in the genital tract (Eriksson et al., 1998; Kozlowski et al., 1997), providing a barrier to the development of oral vaccines for sexually transmitted pathogens. To protect multiple tissues, mucosal vaccines may require additional components to modulate immune cell trafficking. One option being investigated is the use of the vitamin A derivative retinoic acid (RA) to improve mucosal homing. RA has been shown to promote IgA class switching and to upregulate expression of CCR9 and integrin α4β7 on T and B cells, leading to enhanced gut homing (Iwata et al., 2004; Lee et al., 2016; Mora et al., 2006; Ruane et al., 2016). Studies in mice have indicated that inclusion of RA with parenteral immunizations can promote gut, vaginal, and lung homing of T cells and increase specific intestinal IgA, resulting in improved protection against both bacterial and viral mucosal challenges (Hammerschmidt et al., 2011; Tan et al., 2011). In human trials, supplementation of oral typhoid vaccine with RA led to increased specific intestinal IgA and T cell expression of gut homing markers, though this effect was not seen with oral rotavirus, polio, or cholera vaccines (Lisulo et al., 2014; Mwanza-Lisulo et al., 2018). Defining further mechanisms of immune compartmentalization and cross-protection in mucosal tissue sites will be of great importance for improving mucosal vaccines.
Box 2. Evidence for intestinal SARS-CoV-2 infection.
SARS-CoV-2, the virus behind the COVID-19 pandemic, which killed more than 3 million people in 2020 alone (Simonsen and Viboud, 2021), is thought to primarily spread through respiratory droplets and replicate in airway epithelial cells (Stadnytskyi et al., 2020). There is some evidence for SARS-CoV-2 infection in the gastrointestinal tract, though the origins of such infections are unclear. Fecal-oral transmission has been proposed (Guo et al., 2021), though unlike other known human enteric viruses, SARS-CoV-2 has an envelope and therefore likely does not survive well in the stomach. It is also possible that SARS-CoV-2 can disseminate to the gut from the respiratory tract via circulation. SARS-CoV-2 enters cells using primarily ACE2, which is highly expressed on intestinal epithelial cells (Hamming et al., 2004; Hoffmann et al., 2020; Hu et al., 2021), and SARS-CoV-2 can infect and replicate in human intestinal organoids and organ chips (Bein et al., 2021; Lamers et al., 2020). While SARS-CoV-2 RNA is readily found in stool samples of infected individuals, detection of live, replicative virus has been difficult, perhaps due to viral isolation methods or timing of sample isolation (Holshue et al., 2020; Pedersen et al., 2022; Wolfel et al., 2020). We did not find any clinical symptoms or immune activation in the intestine or lungs of mice orally infected with ten times the lethal intranasal dose of mouse-adapted SARS-CoV-2 (unpublished data). However, it should be noted that this virus was adapted for intranasal infection, which could affect the intestinal tropism of the virus (Leist et al., 2020). Nevertheless, intragastric inoculation of SARS-CoV-2 in non-human primates manifested in productive infection of both respiratory and intestinal tissue (Jiao et al., 2021). Interestingly, SARS-CoV-2 shedding in stool can persist even after respiratory symptoms subside (Wu et al., 2020). Additionally, one study detected SARS-CoV-2 N protein in intestinal enterocytes in 5 of 14 individuals an average of 4 months after COVID-19 diagnosis, indicating prolonged viral shedding of SARS-CoV-2 (Gaebler et al., 2021). Finally, some patients infected with SARS-CoV-2 also display acute gastrointestinal symptoms (Pan et al., 2020; Wang et al., 2020). However, direct evidence of gastrointestinal damage caused by SARS-CoV-2 is lacking and needs further investigation.
Finally, immune compartmentalization exists even within the digestive tract, which may be an important consideration for vaccine design. We recently reported that immune responses in the proximal intestine are biased toward tolerance while the distal intestine is predisposed to more pro-inflammatory responses. As a consequence of this compartmentalization, immune tolerance to food proteins generated in the proximal intestine was not disrupted by pathogens infecting the distal intestine, but was disrupted by helminth infection restricted to the proximal intestine (Esterhazy et al., 2019). These findings suggest that targeting the distal intestine for vaccination might promote better protective immunity without the risk of disrupting homeostatic tolerance responses. For oral vaccines, the largest response is typically observed in the proximal intestine, though recent progress in vaccine delivery systems can enable a more targeted approach (Coffey et al., 2021; Johansson et al., 2004; van Splunter et al., 2018). Indeed, an adenovirus-vectored vaccine was found to be more immunogenic when delivered in radio-controlled capsules to the distal small intestine compared to the mid-small intestine (Kim et al., 2016).
ORAL VACCINES
There are four licensed oral vaccines for enteric viruses: two for rotavirus and two for poliovirus. Additionally, an oral vaccine for adenovirus is approved for military use. All approved oral vaccines use live attenuated or reassorted virus (Figure 1). Immunization with live virus works well in the oral route as they are naturally evolved to withstand degradation in the digestive tract and elicit immune responses through the same pathways as wild-type viruses, overriding intestinal immune tolerance. However, live strains come with a risk of genetic instability and can revert to virulence, as has been seen in rare cases (4.7 per 1 million) with the live polio vaccine (Platt et al., 2014) (Box 1). In more recent decades, the focus in viral vaccine development has shifted away from whole virus vaccines and toward compo-nent vaccines using viral protein (e.g. hepatitis B vaccine) or DNA/RNA (e.g. Pfizer/Moderna SARS-CoV-2 vaccines). These formulations are non-replicating and can be safer and more tar-geted than whole virus vaccines; however, they have so far been unsuccessful in the oral route due to poor survivability in the highly acidic and enzymatic environment of the digestive tract, the default state of immunological tolerance toward oral antigen, and a lack of safe and effective mucosal adjuvants (Lavelle and Ward, 2021). Viral vectors (e.g. AstaZeneca/Johnson and John-son SARS-CoV-2 vaccines) or virus-like particles (e.g. human papillomavirus vaccine) are more promising for oral use because their delivery and immunogenicity are similar to native strains, but they offer more opportunity for engineering and rational design. Importantly, vaccines using whole virus or viral vectors have native adjuvant properties and do not necessarily require additional adjuvant to stimulate mucosal immunity.
The immune response generated by existing oral vaccines using attenuated viral strains is overall similar to natural infection because the majority of immune stimulatory components remain intact. The inductive phase of oral rotavirus or poliovirus vaccination is characterized by a robust IgA response (Faden et al., 1990; Groome et al., 2017; Nishio et al., 1990; Ogra, 1995). In most studies, serum level of virus-specific IgA measured shortly after oral immunization is the best available correlate of vaccine protection (Angel et al., 2012; Cheuvart et al., 2014; Ogra, 1995; Plotkin, 2020). As discussed above, this is likely due to a correlation between circulating and secretory IgA, indicating that immunity from both natural infection and oral vaccination with enteric viruses depends on induction of secretory IgA, which can neutralize virus before it infects host cells and replicates.
T cell responses to oral immunization are less well studied than humoral responses, though T cells support mucosal IgA induction and very likely contribute to long-term protection. Of note, live vaccines are generally better at inducing T cell responses than component vaccines, which are often engineered and selected based on their ability to elicit humoral immunity (Gilbert, 2012). Following OPV, antigen-specific T cells in peripheral blood are characterized by expression of IFN-γ and gut-homing integrins (Vekemans et al., 2002; Wahid et al., 2005). T cell responses following rotavirus vaccination are poorly characterized, though low numbers of specific CD4+ T cells expressing gut-homing integrins can be detected in peripheral blood 2 weeks after immunization (Parra et al., 2014).
The goal of oral vaccination is long-term protection against enteric infections, mediated by persistent mucosal antibodies as well as memory B and T cells that can be quickly activated upon infection to mount both local and systemic humoral and cellular responses. Polio-specific antibodies and T cells can be consistently detected in circulation decades after oral immunization, in line with the observation that OPV in childhood confers long lasting protection (Bianchi et al., 2021; Kelley et al., 1991; Sivak and Bobier, 1978; Wahid et al., 2005). Whether intestinal immunity from OPV is equally long-lasting has not been thoroughly assessed, though subjects that receive OPV in infancy show increased poliovirus-specific mucosal IgA after intramuscular boosting in adulthood (Herremans et al., 1999), possibly due to re-activation of long-lived memory cells. Longevity of rotavirus vaccine protection is less well characterized as vaccines have only been widely available in the last 15 years. Further, rotaviruses are still highly prevalent in human populations, unlike poliovirus, so detection of specific antibodies years after vaccination could be due to “boosting” by natural infection. However, because severe illness and mortality from enteric viruses primarily occurs in children under 5 years, decades-long vaccine protection may not always be required.
ADENOVIRAL VECTOR VACCINES
One strategy that has shown promise in recent years for mucosal vaccination is the use of adenoviral vectors, which display diverse tissue tropism depending on the subtype (Arnberg, 2009; Kang et al., 2020). An oral adenoviral vaccine used in the U.S. military that consists of live adenovirus-4 and −7 is well tolerated and generates long-lasting immunity against subsequent respiratory infection (Collins et al., 2020; Kuschner et al., 2013). This demonstrates that adenoviruses can generate immunity across mucosal tissues, including gastrointestinal and respiratory tracts. In addition, adenoviruses offer great potential as flexible vaccine delivery systems because strains with different characteristics can be selected or engineered to target appropriate immunity for a given pathogen, as was recently reviewed (Barry et al., 2020). Other practical advantages of adenoviral vectors are that they do not integrate into the host genome, making them relatively safe, and they can be stably stored at room temperature, making them easy to distribute.
Oral vaccines using adenoviral vectors are under investigation for several viruses. One notable oral platform is VXA, which is currently in clinical trial for vaccination against influenza, norovirus, and SARS-CoV-2 (Vaxart, USA). VXA is a replication-defective adenovirus-5 vector expressing a TLR3 adjuvant (Liebowitz et al., 2020; Scallan et al., 2013). Phase 1 and 2 clinical trials with VXA expressing influenza or norovirus proteins have indicated induction of mucosal IgA as well as a robust mucosal homing CD4+ and CD8+ T cell response. By contrast, reports on whether parenteral adenoviral vaccines such as VXA can elicit mucosal antibody responses have been mixed (DOI 10.1101/2021.08.22.21262168) (Declercq et al., 2022). Pre-clinical studies showed that oral immunization with VXA encoding SARS-CoV-2 spike protein (VXA-Cov2) protected hamsters against severe disease (Johnson et al., 2022). Phase 1 clinical tri-als with VXA-Cov2 found induction of specific IgA in saliva and nasal fluid (Johnson et al., 2022). These early reports provide promising evidence that oral vaccination using adenoviral vec-tors can generate mucosal immune responses in both the gastrointestinal tract and airway, and a room-temperature-sta-ble oral tablet formulation would certainly be valuable for wide-spread COVID-19 vaccine distribution. However, the efficacy of these vaccines remains unproven in humans and is not yet be-ing tested in developing countries.
GLOBAL EFFICACY OF ORAL VACCINES
Enteric viruses are ubiquitous across the globe, contributing to health burden in both low- and high-income countries. However, out of 1.6 million deaths from diarrhea in 2016, nearly 1.5 million occurred in south Asia and sub-Saharan Africa, while only 30,000 occurred in high-income countries (GBD Diarrheal Disease Collaborators, 2018). Unfortunately, there is growing evidence that oral vaccines are less effective in low-income settings. Oral rotavirus vaccines report 85%–100% efficacy at preventing severe disease in middle- and high-income countries, but only 40%–60% in low-income countries (Armah et al., 2010; Jiang et al., 2010; Madhi et al., 2010; O’Ryan and Linhares, 2009; Ruiz-Palacios et al., 2006; Vesikari et al., 2006, 2007; Zaman et al., 2010). 3 doses of OPV resulted in nearly 100% seroconversion of children in the USA but only 70%–90% seroconversion of children in India (John, 1976; McBean et al., 1988). Similar discrepancies have been described for oral vaccines against Vibrio cholera and enterotoxigenic E. coli (Hallander et al., 2002; Svennerholm et al., 2021), indicating that the problem is not confined to anti-viral immunity.
A number of correlates have been proposed to explain vaccine failures in low-income settings, though methods and results in these studies are highly variable, making it difficult to draw firm conclusions (Parker et al., 2018). Additionally, many factors that impact gut immunity (e.g. host genetics, infection history, diet, microbiota) have complex relationships with each other, making it difficult to pinpoint specific causative factors on a population scale. For example, diet and microbiota play substantial roles in shaping the intestinal immune system, vary highly across socioeconomic regions, and are linked to infection susceptibility and gut homeostasis. Additionally, concomitant infection with enteric pathogens in vaccinated individuals could impact the initiation of immune responses to oral vaccines in gut-draining lymph nodes. However, observational studies have not identified consistent links between nutrition or commensal microbiota composition and oral vaccine efficacy, possibly because of the complex nature of the variables involved (Parker et al., 2018; Savy et al., 2009), highlighting the need for comprehensive longitudinal studies.
Enteric infection and vaccination history of both children and their mothers have been linked to oral vaccine efficacy. Infants from low-income countries are more likely to have rotavirus-specific serum IgA prior to immunization than infants from high-income countries (Cunliffe et al., 2014). Presence of specific maternal antibodies has been negatively associated with oral vaccine immunogenicity, though there is conflicting evidence regarding a relationship to breastfeeding, likely due to the complex nature of maternal-derived immune factors in breastmilk (Becker-Dreps et al., 2015; Moon et al., 2016; Rennels, 1996; Rongsen-Chandola et al., 2014; Sabin et al., 1963). Intriguingly, maternal helminth infection in the third trimester correlated with increased IgA responses to oral rotavirus or poliovirus vaccine (Clark et al., 2016).
Several studies suggest that certain concomitant enteric immune responses may interfere with vaccination. Diarrhea concurrent with OPV reduces seroconversion, suggesting that either specific conflicting immune responses or general intestinal inflammation and dysfunction can disrupt oral immunization (Parker et al., 2014). Immunogenicity of oral rotavirus or poliovirus vaccines was reduced by concurrent infection with nonpolio enterovirus, although several detected enteric pathogens did not significantly impact oral immunization (Taniuchi et al., 2016). Further, giving oral rotavirus and poliovirus vaccines on the same day lowers rotavirus seroconversion but not poliovirus seroconversion (Emperador et al., 2016). Finally, intestinal IgA responses to OPV wane with age and are sometimes undetectable in adults, even if there is pre-existing circulating immunity due to prior intramuscular vaccination during infancy (Brickley et al., 2019). These findings demonstrate that immune responses in the intestine may interfere with each other, though it may depend on certain shared pathways. Detangling specific mechanisms of disruption will be critical for finding solutions. The majority of studies above have assessed factors related to failure in seroconversion after oral vaccination. However, IgA seroconversion itself is less predictive of oral vaccine protection in low-income countries, suggesting that even when an initial immune response is mounted there are more complex environmental factors governing long-term mucosal immunity (Angel et al., 2012). To our knowledge studies have not yet addressed environmental factors in longevity of mucosal antibody responses.
Altogether, it is unlikely that a single factor is responsible for vaccine failure in developing countries. Chronic enteric infections, diarrhea, and malnutrition lead to a cluster of related downstream effects including gut barrier dysfunction, dysbiosis, increased inflammatory stimulation, and immune dysregulation. One explanation for vaccine failure is that a state of bystander hyperactivation of the intestinal immune system, potentially in conjunction with pre-existing antibodies, leads to clearance of live attenuated oral vaccines before a specific mucosal response can be generated. Such mechanism might then be insufficient to clear non-attenuated virus, leading to illness. Another possible explanation is that conflict with other ongoing immune responses in the intestine leads to poor responsiveness even if the vaccine is delivered to immune inductive sites. There may be a critical window for oral immunization between the time that maternal antibodies wane and other conflicting intestinal immune responses accumulate. Variation in prevalent circulating viral serotypes across geographical regions is also an important consideration in vaccine design. Understanding the basis for oral vaccine failure will be critical for design of new strategies, and these studies also demonstrate the importance of testing oral vaccines in a variety of geographical and socioeconomic settings because gut immunity is highly impacted by environment. Finally, they show that although vaccines are critical in the fight against global disease there will be no silver bullet for enteric viruses in low-income settings without basic access to food, clean water, and medicine.
CONCLUDING REMARKS
Enteric viruses are a major cause of mortality and global health burden, though the relatively low impact of these viruses in high-income countries shows that many of these deaths could be prevented with access to existing medical interventions. Nevertheless, as new viruses will continually emerge, it is important to continue efforts to understand viral pathogenesis and immunity, especially at mucosal sites where the majority of viral infections begin. Human studies in this field are challenging, but much needed insight could be gained from directly studying intestinal immune responses to viral infection and vaccination through biopsies and sampling of fecal antibodies. The majority of our mechanistic understanding of intestinal immunity comes from mouse studies; however, lab mice fail to capture the complexity of the human intestinal immune landscape as they typically lack an infection history and have a stable diet and environmental niche. Use of pet shop or “wildling” mice will likely provide more accurate models for understanding intestinal immunity. Better understanding of human intestinal anti-viral immunity can be combined with advances in vaccine technology to target effective and long-lasting protection in a variety of environmental contexts. We see great promise in oral vaccination for existing and emerging viruses if this can be achieved.
ACKNOWLEDGMENTS
We thank Y. Alvarez, B. Aydin, A. Bilate, G. Donaldson, and B. Reis for critical reading and discussion of the manuscript. The unpublished SARS-CoV-2 experiment described in Box 2 was done by R. Parsa, H.H. Hoffmann and M.C. Campos Canesso. Figures and tables were created with BioRender.com.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, and Sutter RW (2004). Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 292, 1696–1701. 10.1001/jama.292.14.1696. [DOI] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, and Ting JPY (2009). The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565. 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, and Randall RE (2004). The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U S A 101, 17264–17269. 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel J, Franco MA, and Greenberg HB (2012). Rotavirus immune responses and correlates of protection. Curr. Opin. Virol. 2, 419–425. 10.1016/j.coviro.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. (2010). Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376, 606–614. 10.1016/s0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- Arnberg N (2009). Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 19, 165–178. 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- Arnold MM, and Patton JT (2011). Diversity of interferon antagonist activities mediated by NSP1 proteins of different rotavirus strains. J. Virol. 85, 1970–1979. 10.1128/jvi.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, and Mendelman PM (2011). Norovirus vaccine against experimental human Norwalk Virus illness. N. Engl. J. Med. 365, 2178–2187. 10.1056/nejmoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, Lee S, Brown JJ, McAllister N, Urbanek K, Dermody TS, Nice TJ, and Virgin HW (2017). Expression of Ifnlr1 on intestinal epithelial cells is critical to the antiviral effects of interferon lambda against norovirus and reovirus. J. Virol. 91. e02079–16. 10.1128/jvi.02079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, and Virgin HW (2015). Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347, 266–269. 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, Turula H, and Wobus CE (2016). Norovirus regulation by host and microbe. Trends Mol. Med. 22, 1047–1059. 10.1016/j.molmed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay AS, Garon J, Seib K, and Orenstein WA (2015). Polio vaccination: past, present and future. Future Microbiol. 10, 791–808. 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- Banyai K, Estes MK, Martella V, and Parashar UD (2018). Viral gastroenteritis. Lancet 392, 175–186. 10.1016/s0140-6736(18)31128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, Rubin JD, and Lu SC (2020). Retargeting adenoviruses for therapeutic applications and vaccines. FEBS Lett. 594, 1918–1946. 10.1002/1873-3468.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Dreps S, Vilchez S, Velasquez D, Moon SS, Hudgens MG, Zambrana LE, and Jiang B (2015). Rotavirus-specific IgG antibodies from mothers’ serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr. Infect. Dis. J. 34, 115–116. 10.1097/inf.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bein A, Kim S, Goyal G, Cao W, Fadel C, Naziripour A, Sharma S, Swenor B, LoGrande N, Nurani A, et al. (2021). Enteric coronavirus infection and treatment modeled with an immunocompetent human intestine-onA-chip. Front. Pharmacol. 12, 718484. 10.3389/fphar.2021.718484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, and Finberg RW (1997). Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323. 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. (2014). Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine 32, A110–A116. 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
- Bianchi FP, Larocca AMV, Bozzi A, Spinelli G, Germinario CA, Tafuri S, and Stefanizzi P (2021). Long-term persistence of poliovirus neutralizing antibodies in the era of polio elimination: an Italian retrospective cohort study. Vaccine 39, 2989–2994. 10.1016/j.vaccine.2021.04.005. [DOI] [PubMed] [Google Scholar]
- Bilate AM, London M, Castro TBR, Mesin L, Bortolatto J, Kongthong S, Harnagel A, Victora GD, and Mucida D (2020). T cell receptor is required for differentiation, but not maintenance, of intestinal CD4(+) intraepithelial lymphocytes. Immunity 53, 1001–1014.e20. 10.1016/j.immuni.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RF, Davidson GP, Holmes IH, and Ruck BJ (1973). Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 302, 1281–1283. 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Blasius AL, and Beutler B (2010). Intracellular toll-like receptors. Immunity 32, 305–315. 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Blutt SE, Miller AD, Salmon SL, Metzger DW, and Conner ME (2012). IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 5, 712–719. 10.1038/mi.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouziat R, Biering SB, Kouame E, Sangani KA, Kang S, Ernest JD, Varma M, Brown JJ, Urbanek K, Dermody TS, et al. (2018). Murine norovirus infection induces TH1 inflammatory responses to dietary antigens. Cell Host Microbe 24, 677–688.e5. 10.1016/j.chom.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, et al. (2017). Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50. 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley EB, Connor RI, Wieland-Alter WF, Collett MS, Hartford M, Van Der Avoort H, Boesch AW, Weiner JA, Ackerman ME, McKinlay MA, et al. (2019). Intestinal antibody responses to a live oral poliovirus vaccine challenge among adults previously immunized with inactivated polio vaccine in Sweden. BMJ Glob. Health 4, e001613. 10.1136/bmjgh2019-001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RM, Tate JE, Jiang B, and Parashar UD (2020). Rotavirus and type 1 diabetes-is there a connection? A synthesis of the evidence. J. Infect. Dis. 222, 1076–1083. 10.1093/infdis/jiaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett E, Parashar UD, and Tate JE (2020). Real-world effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Glob. Health 8, e1195–e1202. 10.1016/s2214-109x(20)30262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman FD, McCormick K, and Sherrill-Mix S (2019). Virus structures constrain transmission modes. Nat. Microbiol. 4, 1778–1780. 10.1038/s41564-019-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, and Virgin HW (2010). Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145. 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Guy C, and Bowie AG (2021). Detection of viral infections by innate immunity. Biochem. Pharmacol. 183, 114316. 10.1016/j.bcp.2020.114316. [DOI] [PubMed] [Google Scholar]
- Chachu KA, LoBue AD, Strong DW, Baric RS, and Virgin HW (2008a). Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4, e1000236. 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, and Virgin H.W.t. (2008b). Antibody is critical for the clearance of murine norovirus infection. J. Virol. 82, 6610–6617. 10.1128/jvi.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard AN, Datta SD, Tallis G, Burns CC, Wassilak SGF, Vertefeuille JF, and Zaffran M (2020). Progress toward polio eradication - worldwide, january 2018-march 2020. MMWR Morb. Mortal. Wkly. Rep 69, 784–789. 10.15585/mmwr.mm6925a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, and Mucida D (2011). The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456. 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Madhi SA, Karkada N, Han HH, and Vinals C (2014). Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum. Vaccin. Immunother. 10, 505–511. 10.4161/hv.27097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabattini A, Pettini E, Arsenijevic S, Pozzi G, and Medaglini D (2010). Intranasal immunization with vaccine vector Streptococcus gordonii elicits primed CD4+ and CD8+ T cells in the genital and intestinal tracts. Vaccine 28, 1226–1233. 10.1016/j.vaccine.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Clark CE, Fay MP, Chico ME, Sandoval CA, Vaca MG, Boyd A, Cooper PJ, and Nutman TB (2016). Maternal helminth infection is associated with higher infant immunoglobulin A titers to antigen in orally administered vaccines. J. Infect. Dis. 213, 1996–2004. 10.1093/infdis/jiw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey JW, Gaiha GD, and Traverso G (2021). Oral biologic delivery: advances toward oral subunit, DNA, and mRNA vaccines and the potential for mass vaccination during pandemics. Annu. Rev. Pharmacol. Toxicol. 61, 517–540. 10.1146/annurev-pharmtox-030320-092348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ND, Adhikari A, Yang Y, Kuschner RA, Karasavvas N, Binn LN, Walls SD, Graf PCF, Myers CA, Jarman RG, and Hang J (2020). Live oral adenovirus type 4 and type 7 vaccine induces durable antibody response. Vaccines (Basel) 8, 411. 10.3390/vaccines8030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Brickley EB, Wieland-Alter WF, Ackerman ME, Weiner JA, Modlin JF, Bandyopadhyay AS, and Wright PF (2022). Mucosal immunity to poliovirus. Mucosal Immunol. 15, 1–9. 10.1038/s41385-021-00428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe N, Zaman K, Rodrigo C, Debrus S, Benninghoff B, Pemmaraju Venkata S, and Han HH (2014). Early exposure of infants to natural rotavirus infection: a review of studies with human rotavirus vaccine RIX4414. BMC Pediatr. 14, 295. 10.1186/s12887-014-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher KA, Selsted ME, and Lehrer RI (1986). Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60, 1068–1074. 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus MCS, Santos VS, Storti-Melo LM, De Souza CDF, Barreto í.D.D.C., Paes MVC, Lima PAS, Bohland AK, Berezin EN, Machado RLD, et al. (2020). Impact of a twelve-year rotavirus vaccine program on acute diarrhea mortality and hospitalization in Brazil: 2006–2018. Expert Rev. Vaccines 19, 585–593. 10.1080/14760584.2020.1775081. [DOI] [PubMed] [Google Scholar]
- Declercq J, Tobback E, Vanhee S, De Ruyck N, Gerlo S, Gevaert P, and Vandekerckhove L (2022). COVID-19 vaccination with BNT162b2 and ChAdOx1 vaccines has the potential to induce nasal neutralizing antibodies. Allergy 77, 304–307. 10.1111/all.15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Axford E, and Coyne CB (2011). The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses 3, 2462–2477. 10.3390/v3122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharakul T, Rott L, and Greenberg HB (1990). Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J. Virol. 64, 4375–4382. 10.1128/jvi.64.9.4375-4382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Niro R, Mesin L, Raki M, Zheng NY, Lund-Johansen F, Lundin KEA, Charpilienne A, Poncet D, Wilson PC, and Sollid LM (2010). Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J. Immunol. 185, 5377–5383. 10.4049/jimmunol.1001587. [DOI] [PubMed] [Google Scholar]
- Dillon A, and Lo DD (2019). M cells: intelligent engineering of mucosal immune surveillance. Front. Immunol. 10, 1499. 10.3389/fimmu.2019.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domok I (1984). Experiences associated with the use of live poliovirus vaccine in Hungary, 1959–1982. Rev. Infect. Dis. 6, S413–S418. 10.1093/clinids/6.supplement_2.s413. [DOI] [PubMed] [Google Scholar]
- Eggers HJ (1999). Milestones in early poliomyelitis research (1840 to 1949). J. Virol. 73, 4533–4535. 10.1128/jvi.73.6.4533-4535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elftman MD, Gonzalez-Hernandez MB, Kamada N, Perkins C, Henderson KS, Nunez G, and Wobus CE (2013). Multiple effects of dendritic cell depletion on murine norovirus infection. J. Gen. Virol. 94, 1761–1768. 10.1099/vir.0.052134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, Anand A, and Zaman K (2016). Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin. Infect. Dis. 62, 150–156. 10.1093/cid/civ807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K, Quiding-Jarbrink M, Osek J, Mӧller A, Bjork S, Holmgren J, and Czerkinsky C (1998). Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect. Immun. 66, 5889–5896. 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W Jr., Rongvaux A, Van Rooijen N, Haberman AM, et al. (2011). Control of TH17 cells occurs in the small intestine. Nature 475, 514–518. 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterhazy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, and Mucida D (2019). Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130. 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, and Ogra PL (1990). Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J. Infect. Dis. 162, 1291–1297. 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, and Suzuki K (2010). Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273. 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Faria AMC, Reis BS, and Mucida D (2017). Tissue adaptation: implications for gut immunity and tolerance. J. Exp. Med. 214, 1211–1226. 10.1084/jem.20162014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, et al. (2008). Key role of splenic myeloid DCs in the IFN-αβ response to adenoviruses in vivo. PLoS Pathog 4, e1000208. 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett TH, Bryden AS, and Davies H (1973). Letter: virus particles in gastroenteritis. Lancet 302, 1497. 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Beura LK, Quarnstrom CF, Ghoneim HE, Fan Y, Zebley CC, Scott MC, Fares-Frederickson NJ, Wijeyesinghe S, Thompson EA, et al. (2020). Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421. 10.1038/s41590-020-0607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MA, and Greenberg HB (1995). Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69, 7800–7806. 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MA, and Greenberg HB (1997). Immunity to rotavirus in T cell deficient mice. Virology 238, 169–179. 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- Franco MA, Tin C, Rott LS, VanCott JL, McGhee JR, and Greenberg HB (1997). Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J. Virol. 71, 479–486. 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, et al. (2021). Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644. 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Diarrhoeal Disease Collaborators (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18, 1211–1228. 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SC (2012). T-cell-inducing vaccines - what’s the future. Immunology 135, 19–26. 10.1111/j.1365-2567.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, et al. (2011). Protective effect of natural rotavirus infection in an Indian birth cohort. N. Engl. J. Med. 365, 337–346. 10.1056/nejmoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, and Estes MK (2009). Norovirus gastroenteritis. N. Engl. J. Med. 361, 1776–1785. 10.1056/nejmra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rial J, Rivero-Calle I, Salas A, and Martinon-Torres F (2020). Rotavirus and autoimmunity. J. Infect. 81, 183–189. 10.1016/j.jinf.2020.04.041. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez MB, Liu T, Blanco LP, Auble H, Payne HC, and Wobus CE (2013). Murine norovirus transcytosis across an in vitro polarized murine intestinal epithelial monolayer is mediated by M-like cells. J. Virol. 87, 12685–12693. 10.1128/jvi.02378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Hernandez MB, Liu T, Payne HC, Stencel-Baerenwald JE, Ikizler M, Yagita H, Dermody TS, Williams IR, and Wobus CE (2014). Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells. J. Virol. 88, 6934–6943. 10.1128/jvi.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau KR, Roth AN, Zhu S, Hernandez A, Colliou N, DiVita BB, Philip DT, Riffe C, Giasson B, Wallet SM, et al. (2017). The major targets of acute norovirus infection are immune cells in the gut-associated lymphoid tissue. Nat. Microbiol. 2, 1586–1591. 10.1038/s41564-017-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GC, McCarthy T, Lebeck MG, Schnurr DP, Russell KL, Kajon AE, Landry ML, Leland DS, Storch GA, Ginocchio CC, et al. (2007). Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006. Clin. Infect. Dis. 45, 1120–1131. 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, and Flatt JW (2019). Adenovirus entry: from infection to immunity. Annu. Rev. Virol. 6, 177–197. 10.1146/annurev-virology-092818-015550. [DOI] [PubMed] [Google Scholar]
- Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, and Barnes GL (1988). Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J. Clin. Microbiol. 26, 732–738. 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, et al. (2017). Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 17, 843–853. 10.1016/s1473-3099(17)30242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Tao W, Flavell RA, and Zhu S (2021). Potential intestinal infection and faecal-oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 18, 269–283. 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, and Vassalli P (1991). Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173, 471–481. 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, et al. (2011). Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 7, e1002115. 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallander HO, Paniagua M, Espinoza F, Askelof P, Corrales E, Ringman M, and Storsaeter J (2002). Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 21, 138–145. 10.1016/s0264-410x(02)00348-1. [DOI] [PubMed] [Google Scholar]
- Hallowell BD, Chavers T, Parashar U, and Tate JE (2021). Global estimates of rotavirus hospitalizations among children below 5 Years in 2019 and current and projected impacts of rotavirus vaccination. J. Pediatr. Infect Dis Soc. piab 114. 10.1093/jpids/piab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, and Forster R (2011). Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 121, 3051–3061. 10.1172/jci44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, and van Goor H (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637. 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, and Fruh K (2010). Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328, 102–106. 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. (2010). Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709. 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, and Amalfitano A (2007). Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 81, 1796–1812. 10.1128/jvi.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sugiyama T, and Sasaki S (1966). An adenovirus isolated from the feces of mice I. Isolation and identification. Jpn. J. Microbiol. 10, 115–125. 10.1111/j.1348-0421.1966.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Herremans TM, Reimerink JH, Buisman AM, Kimman TG, and Koopmans MP (1999). Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 162, 5011–5018. [PubMed] [Google Scholar]
- Hllleman MR, and Werner JH (1954). Recovery of new agent from patients with acute respiratory illness. Proc. Soc. Exp. Biol. Med. 85, 183–188. 10.3181/00379727-85-20825. [DOI] [PubMed] [Google Scholar]
- Hird TR, and Grassly NC (2012). Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 8, e1002599. 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, and Czerkinsky C (2005). Mucosal immunity and vaccines. Nat. Med. 11, S45–S53. htt 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, et al. (2020). First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382, 929–936. 10.1056/nejmoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornquist CE, Ekman L, Grdic KD, Schon K, and Lycke NY (1995). Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J. Immunol. 155, 2877–2887. [PubMed] [Google Scholar]
- Howley PMK, David MK, and Whelan S (2020). Fields Virology, Seventh Edition.
- Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, and Mucida D (2017). Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 171, 783–794.e13. 10.1016/j.cell.2017.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Guo H, Zhou P, and Shi ZL (2021). Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19, 141–154. 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, and Kola I (1995). A null mutation in the gene encoding a type I interferon receptor component eliminates anti-proliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. U S A 92, 11284–11288. 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHME (2019). GBD 2019 Cause and Risk.
- Ingle H, Lee S, Ai T, Orvedahl A, Rodgers R, Zhao G, Sullender M, Peterson ST, Locke M, Liu TC, et al. (2019). Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-λ. Nat. Microbiol. 4, 1120–1128. 10.1038/s41564-019-0416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle H, Peterson ST, and Baldridge MT (2018). Distinct effects of type I and III interferons on enteric viruses. Viruses 10, 46. 10.3390/v10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, and Barber GN (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678. 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, and Song SY (2004). Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538. 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jertborn M, Nordstrom I, Kilander A, Czerkinsky C, and Holmgren J (2001). Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69, 4125–4128. 10.1128/iai.69.6.4125-4128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JQ, He XS, Feng N, and Greenberg HB (2008). Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection. J. Virol. 82, 6812–6819. 10.1128/jvi.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang V, Jiang B, Tate J, Parashar UD, and Patel MM (2010). Performance of rotavirus vaccines in developed and developing countries. Hum. Vaccin. 6, 532–542. 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L, Li H, Xu J, Yang M, Ma C, Li J, Zhao S, Wang H, Yang Y, Yu W, et al. (2021). The gastrointestinal tract is an alternative route for SARS-CoV-2 infection in a nonhuman primate model. Gastroenterology 160, 1647–1661. 10.1053/j.gastro.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, and Brandtzaeg P (2005). Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood 106, 593–600. 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- Johansson EL, Bergquist C, Edebo A, Johansson C, and Svennerholm AM (2004). Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine 22, 984–990. 10.1016/j.vaccine.2003.09.002. [DOI] [PubMed] [Google Scholar]
- John TJ (1976). Antibody response of infants in tropics to five doses of oral polio vaccine. Br. Med. J. 1, 812. 10.1136/bmj.1.6013.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Martinez CI, Tedjakusuma SN, Peinovich N, Dora EG, Birch SM, Kajon AE, Werts AD, and Tucker SN (2022). Oral vaccination protects against severe acute respiratory syndrome coronavirus 2 in a Syrian hamster challenge model. J. Infect. Dis. 225, 34–41. 10.1093/infdis/jiab561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Ismail AM, Dehghan S, Rajaiya J, Allard MW, Lim HC, Dyer DW, Chodosh J, and Seto D (2020). Genomics-based re-examination of the taxonomy and phylogeny of human and simian Mastadenoviruses: an evolving whole genomes approach, revealing putative zoonosis, anthroponosis, and amphizoonosis. Cladistics 36, 358–373. 10.1111/cla.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, and Chanock RM (1972). Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10, 1075–1081. 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM, and Wobus CE (2015). A working model of how noroviruses infect the intestine. PLoS Pathog. 11, e1004626. 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, and Virgin H.W.t. (2003). STAT1-dependent innate immunity to a Norwalk-like virus. Science 299, 1575–1578. 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Kawai T, and Akira S (2011). Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kelley PW, Petruccelli BP, Stehr-Green P, Erickson RL, and Mason CJ (1991). The susceptibility of young adult Americans to vaccine-preventable infections. A national serosurvey of US Army recruits. JAMA 266, 2724–2729. 10.1001/jama.1991.03470190072032. [DOI] [PubMed] [Google Scholar]
- Kernbauer E, Ding Y, and Cadwell K (2014). An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98. 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Toyoda H, Linehan M, Iwasaki A, Nomoto A, Bernhardt G, Cello J, and Wimmer E (2014). Poliomyelitis in transgenic mice expressing CD155 under the control of the Tage4 promoter after oral and parenteral poliovirus inoculation. J. Gen. Virol. 95, 1668–1676. 10.1099/vir.0.064535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Liebowitz D, Lin K, Kasparek K, Pasetti MF, Garg SJ, Gottlieb K, Trager G, and Tucker SN (2018). Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight 3, e121077. 10.1172/jci.insight.121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Martinez CJ, Hodgson KA, Trager GR, Brandl JR, Sandefer EP, Doll WJ, Liebowitz D, and Tucker SN (2016). Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Sci. Rep. 6, 37295. 10.1038/srep37295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiner E, Willie E, Vijaykumar B, Chowdhary K, Schmutz H, Chandler J, Schnell A, Thakore PI, LeGros G, Mostafavi S, et al. ; The Immunological Genome Project Consortium (2021). Gut CD4(+) T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nat. Immunol. 22, 216–228. 10.1038/s41590-020-00836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, and Nomoto A (1991). Transgenic mice susceptible to poliovirus. Proc. Natl. Acad. Sci. U S A 88, 951–955. 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos M, McWilliam HEG, Aktepe TE, Fritzlar S, Illing PT, Mifsud NA, Purcell AW, Rockman S, Reading PC, Vivian JP, et al. (2019). Downregulation of MHC class I expression by influenza A and B viruses. Front. Immunol. 10, 1158. 10.3389/fimmu.2019.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski PA, Cu-Uvin S, Neutra MR, and Flanigan TP (1997). Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65, 1387–1394. 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklin NA, Rott L, Darling J, Campbell JJ, Franco M, Feng N, Muller W, Wagner N, Altman J, Butcher EC, and Greenberg HB (2000). α4β7 independent pathway for CD8+ T cell–mediated intestinal immunity to rotavirus. J. Clin. Invest. 106, 1541–1552. 10.1172/jci10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschner RA, Russell KL, Abuja M, Bauer KM, Faix DJ, Hait H, Henrick J, Jacobs M, Liss A, Lynch JA, et al. (2013). A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine 31, 2963–2971. 10.1016/j.vaccine.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, and Pfeiffer JK (2011). Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252. 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Etheredge CA, and Pfeiffer JK (2008). Multiple host barriers restrict poliovirus trafficking in mice. PLoS Pathog. 4, e1000082. 10.1371/journal.ppat.1000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, et al. (2020). SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54. 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SB, Cowley CJ, and Fuchs E (2020). Epithelial cells: liaisons of immunity. Curr. Opin. Immunol. 62, 45–53. 10.1016/j.coi.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, et al. (2010). Mouse CD8α+ DCs and human BDCA3+ DCs are major producers of IFN-λ in response to poly IC. J. Exp. Med. 207, 2703–2717. 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle EC, and Ward RW (2021). Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250. 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Jang YS, Jin BR, Kim SJ, Kim HJ, Kwon BE, Ko HJ, Yoon SI, Lee GS, Kim WS, et al. (2016). Retinoic acid enhances lactoferrin-induced IgA responses by increasing betaglycan expression. Cell. Mol. Immunol. 13, 862–870. 10.1038/cmi.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist SR, Dinnon KH 3rd, Schafer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, et al. (2020). A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183, 1070–1085.e12. 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D, Gottlieb K, Kolhatkar NS, Garg SJ, Asher JM, Nazareno J, Kim K, McIlwain DR, and Tucker SN (2020). Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis 20, 435–444. 10.1016/s1473-3099(19)30584-5. [DOI] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, and McGuckin MA (2008). Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197. 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, and Baric R (2003). Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9, 548–553. 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Lion T (2014). Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 27, 441–462. 10.1128/cmr.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisulo MM, Kapulu MC, Banda R, Sinkala E, Kayamba V, Sianongo S, and Kelly P (2014). Adjuvant potential of low dose all-trans retinoic acid during oral typhoid vaccination in Zambian men. Clin. Exp. Immunol. 175, 468–475. 10.1111/cei.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little LM, and Shadduck JA (1982). Pathogenesis of rotavirus infection in mice. Infect. Immun. 38, 755–763. 10.1128/iai.38.2.755-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, et al. (2016). Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388, 1291–1301. 10.1016/s0140-6736(16)31529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Bilate AM, Castro TBR, Sujino T, and Mucida D (2021). Stepwise chromatin and transcriptional acquisition of an intraepithelial lymphocyte program. Nat. Immunol. 22, 449–459. 10.1038/s41590-021-00883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren O, and Svensson L (2001). Pathogenesis of rotavirus diarrhea. Microbes Infect. 3, 1145–1156. 10.1016/s1286-4579(01)01475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier G, Smith AL, Guenette D, and Descoteaux JP (1987). Serological relationship between mouse adenovirus strains FL and K87. Lab. Anim. Sci. 37, 55–57. [PubMed] [Google Scholar]
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. (2010). Effect of human rotavirus vaccine on severe diarrhea in African infants. N. Engl. J. Med. 362, 289–298. 10.1056/nejmoa0904797. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, and Corthesy B (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611. 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R (2010). Childhood morbidity and health in early adulthood: life course linkages in a high morbidity context. Adv. Life Course Res. 15, 132–146. 10.1016/j.alcr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JK, Thabane M, Borgaonkar MR, and James C (2007). Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin. Gastroenterol. Hepatol. 5, 457–460. 10.1016/j.cgh.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Mayassi T, and Jabri B (2018). Human intraepithelial lymphocytes. Mucosal Immunol. 11, 1281–1289. 10.1038/s41385-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, and Rescigno M (2014). Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 40, 248–261. 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- McBean AM, Thoms ML, Albrecht P, Cuthie JC, and Bernier R; The Field Staff and Coordinating Committee (1988). Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines. Am. J. Epidemiol. 128, 615–628. 10.1093/oxfordjournals.aje.a115009. [DOI] [PubMed] [Google Scholar]
- McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin IV HW, and Colonna M (2008). MDA-5 recognition of a murine norovirus. PLoS Pathog. 4, e1000108. 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, and Ward RL (1995). Long-term production of rotavirus antibody and protection against reinfection following a single infection of neonatal mice with murine rotavirus. Virology 211, 474–480. 10.1006/viro.1995.1429. [DOI] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, and Racaniello VR (1989). Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 56, 855–865. 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Merkenschlager J, Finkin S, Ramos V, Kraft J, Cipolla M, Nowosad CR, Hartweger H, Zhang W, Olinares PDB, Gazumyan A, et al. (2021). Dynamic regulation of TFH selection during the germinal centre reaction. Nature 591, 458–463. 10.1038/s41586-021-03187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, and Patriarca PA; the Baltimore Area Polio Vaccine Study Group (1997). Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine-live attenuated oral poliovirus vaccine immunization schedules. J. Infect. Dis. 175, S228–S234. 10.1093/infdis/175.supplement_1.s228. [DOI] [PubMed] [Google Scholar]
- Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, van Niekerk N, Jiang B, and Madhi SA (2016). Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin. Infect. Dis. 62, 157–165. 10.1093/cid/civ828. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. (2006). Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160. 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mowat AM, and Agace WW (2014). Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685. 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, and Aguet M (1994). Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921. 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Mwanza-Lisulo M, Chomba MS, Chama M, Besa EC, Funjika E, Zyambo K, Banda R, Imikendu M, Sianongo S, Hancock REW, et al. (2018). Retinoic acid elicits a coordinated expression of gut homing markers on T lymphocytes of Zambian men receiving oral Vivotif, but not Rotarix, Dukoral or OPVERO vaccines. Vaccine 36, 4134–4141. 10.1016/j.vaccine.2018.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N, Newell EW, Vollmers C, Quake SR, Morton JM, Davis MM, He XS, and Greenberg HB (2016). High-dimensional immune profiling of total and rotavirus VP6-specific intestinal and circulating B cells by mass cytometry. Mucosal Immunol. 9, 68–82. 10.1038/mi.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N (2008). Chapter 1 the pathogenesis of poliomyelitis: what we don’t know. Adv. Virus. Res. 71, 1–50. 10.1016/s0065-3527(08)00001-8. [DOI] [PubMed] [Google Scholar]
- Neil JA, and Cadwell K (2018). The intestinal virome and immunity. J. Immunol. 201, 1615–1624. 10.4049/jimmunol.1800631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Walk ST, Taube S, Taniuchi M, Houpt ER, Wobus CE, and Young VB (2012). Disruption of the human gut microbiota following Norovirus infection. PLoS One 7, e48224. 10.1371/journal.pone.0048224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Nair N, Kidd BA, Greenberg HB, and Davis MM (2013). Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 31, 623–629. 10.1038/nbt.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, and Leon JS (2015). Norovirus immunology: of mice and mechanisms. Eur. J. Immunol. 45, 2742–2757. 10.1002/eji.201545512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, and Virgin HW (2015). Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273. 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. (2005). CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258. 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Nishio O, Sakae K, Ishihara Y, Isomura S, and Inouye S (1992). Adenovirus infection and specific secretory IgA responses in the intestine of infants. Microbiol. Immunol. 36, 623–631. 10.1111/j.1348-0421.1992.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Nishio O, Sumi J, Sakae K, Ishihara Y, Isomura S, and Inouye S (1990). Fecal IgA antibody responses after oral poliovirus vaccination in infants and elder children. Microbiol. Immunol. 34, 683–689. 10.1111/j.1348-0421.1990.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Niu J, Wu S, Chen M, Xu K, Guo Q, Lu A, Zhao L, Sun B, and Meng G (2019). Hyperactivation of the NLRP3 inflammasome protects mice against influenza A virus infection via IL-1β mediated neutrophil recruitment. Cytokine 120, 115–124. 10.1016/j.cyto.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Nomoto A (2007). Molecular aspects of poliovirus pathogenesis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 83, 266–275. 10.2183/pjab.83.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Ryan M, and Linhares AC (2009). Update on Rotarix: an oral human rotavirus vaccine. Expert Rev. Vaccines 8, 1627–1641. 10.1586/erv.09.136. [DOI] [PubMed] [Google Scholar]
- Ogier A, Franco MA, Charpilienne A, Cohen J, Pothier P, and Kohli E (2005). Distribution and phenotype of murine rotavirus-specific B cells induced by intranasal immunization with 2/6 virus-like particles. Eur. J. Immunol. 35, 2122–2130. 10.1002/eji.200526059. [DOI] [PubMed] [Google Scholar]
- Ogra PL (1995). Comparative evaluation of immunization with live attenuated and inactivated poliovirus vaccines. Ann. N. Y. Acad. Sci. 754, 97–105. 10.1111/j.1749-6632.1995.tb44442.x. [DOI] [PubMed] [Google Scholar]
- Olivares-Villagomez D, and Van Kaer L (2018). Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. 39, 264–275. 10.1016/j.it.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, et al. (2020). Clinical characteristics of COVID-19 patients with digestive symptoms in hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 115, 766–773. 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EP, Kampmann B, Kang G, and Grassly NC (2014). Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis. J. Infect. Dis. 210, 853–864. 10.1093/infdis/jiu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker EP, Ramani S, Lopman BA, Church JA, Iturriza-Gomara M, Prendergast AJ, and Grassly NC (2018). Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol. 13, 97–118. 10.2217/fmb-2017-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra M, Herrera D, Calvo-Calle JM, Stern LJ, Parra-Lopez CA, Butcher E, Franco M, and Angel J (2014). Circulating human rotavirus specific CD4 T cells identified with a class II tetramer express the intestinal homing receptors α4β7 and CCR9. Virology 452–453, 191–201. 10.1016/j.virol.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, London M, Rezende de Castro TB, Reis B, des Amorie JB, Smith JG, and Mucida D (2021). Newly recruited intraepithelial Ly6A+CCR9+CD4+ T cells protect against enteric viral infection. Preprint at bioRxiv. 10.1101/2021.11.10.468106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RM, Tornby DS, Bang LL, Madsen LW, Skov MN, Sydenham TV, Steinke K, Jensen TG, Johansen IS, and Andersen TE (2022). Rectally shed SARS-CoV-2 in COVID-19 inpatients is consistently lower than respiratory shedding and lacks infectivity. Clin. Microbiol. Infect. 28. 304.e1. e301–304.e3. 10.1016/j.cmi.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, and Reis e Sousa C (2007). Innate recognition of viruses. Immunity 27, 370–383. 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Platt LR, Estivariz CF, and Sutter RW (2014). Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J. Infect. Dis. 210, S380–S389. 10.1093/infdis/jiu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA (2020). Updates on immunologic correlates of vaccine-induced protection. Vaccine 38, 2250–2257. 10.1016/j.vaccine.2019.10.046. [DOI] [PubMed] [Google Scholar]
- Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, Backhed F, Baumann U, Pabst O, Bleich A, and Hornef MW (2012). Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 8, e1002670. 10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiding-Jarbrink M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher EC, Lazarovits AI, Holmgren J, and Czerkinsky C (1997). Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Invest. 99, 1281–1286. 10.1172/jci119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Atmar RL, and Estes MK (2014). Epidemiology of human noroviruses and updates on vaccine development. Curr. Opin. Gastroenterol. 30, 25–33. 10.1097/mog.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Neill FH, Opekun AR, Gilger MA, Graham DY, Estes MK, and Atmar RL (2015). Mucosal and cellular immune responses to norwalk virus. J. Infect. Dis. 212, 397–405. 10.1093/infdis/jiv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razum O, Sridhar D, Jahn A, Zaidi S, Ooms G, and Muller O (2019). Polio: from eradication to systematic, sustained control. BMJ Glob. Health 4, e001633. 10.1136/bmjgh-2019-001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, and Atmar RL (2010). Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202, 1212–1218. 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels MB (1996). Influence of breast-feeding and oral poliovirus vaccine on the immunogenicity and efficacy of rotavirus vaccines. J. Infect. Dis. 174, S107–S111. 10.1093/infdis/174.supplement_1.s107. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, and Ricciardi-Castagnoli P (2001). Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367. 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Jesudhasan PR, and Pfeiffer JK (2014). Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15, 36–46. 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz J, Garcia-Mantrana I, Vila-Vicent S, Gozalbo-Rovira R, Buesa J, Monedero V, and Collado MC (2017). Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci. Rep. 7, 45559. 10.1038/srep45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, et al. (2014). Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 32, A134–A139. 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]
- Roth AN, Helm EW, Mirabelli C, Kirsche E, Smith JC, Eurell LB, Ghosh S, Altan-Bonnet N, Wobus CE, and Karst SM (2020). Norovirus infection causes acute self-resolving diarrhea in wild-type neonatal mice. Nat. Commun. 11, 2968. 10.1038/s41467-020-16798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, and Ward TG (1953). Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 84, 570–573. 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, Zhu H, Velinzon K, Choi JH, Studt N, et al. (2013). Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 210, 1871–1888. 10.1084/jem.20122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M, Simchoni N, Rahman A, Garg A, Weinstein EG, et al. (2016). Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med. 213, 53–73. 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. (2006). Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 354, 11–22. 10.1056/nejmoa052434. [DOI] [PubMed] [Google Scholar]
- Sabin ABM, Michaels RH, Krugman SK, Eiger ME, Berman PH, and Warren J (1963). Effect OF oral poliovirus vaccine IN newborn children : I. Excretion of virus after ingestion of large doses of type I or of mixture of all three types, in relation to level of placentally transmitted antibody. Pediatrics 31, 623–640. 10.1542/peds.31.4.623. [DOI] [Google Scholar]
- Savy M, Edmond K, Fine PEM, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, and Prentice AM (2009). Landscape analysis of interactions between nutrition and vaccine responses in children. J. Nutr. 139, 2154S–2218S. 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, et al. (2016). Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56. 10.1128/jvi.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan CD, Tingley DW, Lindbloom JD, Toomey JS, and Tucker SN (2013). An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin. Vaccin. Immunol 20, 85–94. 10.1128/cvi.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaudecker EP, Steinhoff MC, and Moore SR (2011). Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr. Opin. Infect. Dis. 24, 496–502. 10.1097/qco.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw NS, Taylor CE, and Gordon JE (1968). Interactions of nutrition and infection. Monogr. Ser. World Health Organ. 57, 3–329. [PubMed] [Google Scholar]
- Shah MP, Tate JE, Mwenda JM, Steele AD, and Parashar UD (2017). Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev. Vaccines 16, 987–995. 10.1080/14760584.2017.1371595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Eydelloth RS, Vonderfecht SL, and Aurelian L (1983). Virus-specific immunity in neonatal and adult mouse rotavirus infection. Infect. Immun. 39, 917–927. 10.1128/iai.39.2.917-927.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, et al. (2019). Segmented filamentous bacteria prevent and cure rotavirus infection. Cell 179, 644–658.e13. 10.1016/j.cell.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman LM, Martin J, Sofer D, Burns CC, Manor Y, Hindiyeh M, Gavrilin E, Wilton T, Moran-Gilad J, Gamzo R, et al. ; for the GPI Genotype–Phenotype Identification Group (2015). Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013. Clin. Infect. Dis. 60, 1057–1064. 10.1093/cid/ciu1136. [DOI] [PubMed] [Google Scholar]
- Simmons K, Gambhir M, Leon J, and Lopman B (2013). Duration of immunity to norovirus gastroenteritis. Emerg. Infect. Dis. 19, 1260–1267. 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, and Viboud C (2021). A comprehensive look at the COVID-19 pandemic death toll. Elife 10, e71974. 10.7554/elife.71974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak JG, and Bobier CW (1978). Accommodation and chromatic aberration in young children. Invest. Ophthalmol. Vis. Sci. 17, 705–709. [PubMed] [Google Scholar]
- Slifka MK, and Ahmed R (1996). Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 4, 394–400. 10.1016/0966-842x(96)10059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider DP, Liang H, Switzer I, and Underdown BJ (1999). IgA production in MHC class II-deficient mice is primarily a function of B-1a cells. Int. Immunol. 11, 191–198. 10.1093/intimm/11.2.191. [DOI] [PubMed] [Google Scholar]
- Stadnytskyi V, Bax CE, Bax A, and Anfinrud P (2020). The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U S A 117, 11875–11877. 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS, and Rewers M (2006). Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am. J. Gastroenterol. 101, 2333–2340. 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, et al. (2021). IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 13, abd2223. 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, and Medzhitov R (2006). Type I interferons in host defense. Immunity 25, 373–381. 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Svennerholm AM, Lundgren A, Leach S, Akhtar M, and Qadri F (2021). Mucosal immune responses against an oral enterotoxigenic Escherichia coli vaccine evaluated in clinical trials. J. Infect. Dis. 224, S821–S828. 10.1093/infdis/jiab475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M, Abeler-Dorner L, Chettle J, Mahlakoiv T, Goubau D, Chakravarty P, Ramsay G, Reis e Sousa C, Staeheli P, Blacklaws BA, et al. (2015). Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat. Commun. 6, 7090. 10.1038/ncomms8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, and Estes MK (2003). Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. 108, 241–247. 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Tan M, and Jiang X (2014). Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev. Mol. Med. 16, e5. 10.1017/erm.2014.2. [DOI] [PubMed] [Google Scholar]
- Tan X, Sande JL, Pufnock JS, Blattman JN, and Greenberg PD (2011). Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J. Virol. 85, 8316–8327. 10.1128/jvi.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Platts-Mills JA, Begum S, Uddin MJ, Sobuz SU, Liu J, Kirkpatrick BD, Colgate ER, Carmolli MP, Dickson DM, et al. (2016). Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine 34, 3068–3075. 10.1016/j.vaccine.2016.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Duan E, Lazear HM, Kambal A, Schreiber RD, Diamond MS, and Virgin HW (2012). Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication. J. Virol. 86, 13515–13523. 10.1128/jvi.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickell KD, Sharmin R, Deichsel EL, Lamberti LM, Walson JL, Faruque ASG, Pavlinac PB, Kotloff KL, and Chisti MJ (2020). The effect of acute malnutrition on enteric pathogens, moderate-to-severe diarrhoea, and associated mortality in the Global Enteric Multicenter Study cohort: a post-hoc analysis. Lancet Glob. Health 8, e215–e224. 10.1016/s2214-109x(19)30498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomov VT, Osborne LC, Dolfi DV, Sonnenberg GF, Monticelli LA, Mansfield K, Virgin HW, Artis D, and Wherry EJ (2013). Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 87, 7015–7031. 10.1128/jvi.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomov VT, Palko O, Lau CW, Pattekar A, Sun Y, Tacheva R, Bengsch B, Manne S, Cosma GL, Eisenlohr LC, et al. (2017). Differentiation and protective capacity of virus-specific CD8(+) T cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity 47, 723–738.e5. 10.1016/j.immuni.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama R, Chassaing B, Zhang B, and Gewirtz AT (2014). Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J. Infect. Dis. 210, 171–182. 10.1093/infdis/jiu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama R, Chassaing B, Zhang B, and Gewirtz AT (2015). MyD88-mediated TLR signaling protects against acute rotavirus infection while inflammasome cytokines direct Ab response. Innate Immun. 21, 416–428. 10.1177/1753425914547435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara K, Hirakawa M, and Hashimoto K (1984). Fluctuation of antiviral resistance in the intestinal tracts of nude mice infected with a mouse adenovirus. Microbiol. Immunol. 28, 679–690. 10.1111/j.1348-0421.1984.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Unicomb LE, Jarecki-Khan K, Hall A, and Podder G (1996). Previous enteric adenovirus infection does not protect against subsequent symptomatic infection: longitudinal follow-up of eight infants. Microbiol. Immunol. 40, 161–168. 10.1111/j.1348-0421.1996.tb03320.x. [DOI] [PubMed] [Google Scholar]
- van Montfoort N, van der Aa E, and Woltman AM (2014). Understanding MHC class I presentation of viral antigens by human dendritic cells as a basis for rational design of therapeutic vaccines. Front. Immunol. 5, 182. 10.3389/fimmu.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Splunter M, van Hoffen E, Floris-Vollenbroek EG, Timmerman H, de Bos EL, Meijer B, Ulfman LH, Witteman B, Wells JM, Brugman S, et al. (2018). Oral cholera vaccination promotes homing of IgA(+) memory B cells to the large intestine and the respiratory tract. Mucosal Immunol. 11, 1254–1264. 10.1038/s41385-018-0006-7. [DOI] [PubMed] [Google Scholar]
- Van Winkle JA, Peterson ST, Kennedy EA, Wheadon MJ, Ingle H, Desai C, Rodgers R, Constant DA, Wright AP, Li L, et al. (2022). Homeostatic interferon-lambda response to bacterial microbiota stimulates preemptive antiviral defense within discrete pockets of intestinal epithelium. Elife 11, e74072. 10.7554/elife.74072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekemans J, Ota MO, Wang EC, Kidd M, Borysiewicz LK, Whittle H, McAdam KP, Morgan G, and Marchant A (2002). T cell responses to vaccines in infants: defective IFNg production after oral polio vaccination. Clin. Exp. Immunol. 127, 495–498. 10.1046/j.1365-2249.2002.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez FR (2009). Protective effects of natural rotavirus infection. Pediatr. Infect. Dis. J. 28, S54–S56. 10.1097/inf.0b013e3181967c03. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Calva JJ, Guerrero ML, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, and Ruiz-Palacios GM (1996). Rotavirus infection in infants as protection against subsequent infections. N. Engl. J. Med. 335, 1022–1028. 10.1056/nejm199610033351404. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, Glass RI, Pickering LK, and Ruiz-Palacios GM (2000). Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis. 182, 1602–1609. 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, and Bouckenooghe A (2007). Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 370, 1757–1763. 10.1016/s0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. (2006). Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 354, 23–33. 10.1056/nejmoa052664. [DOI] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, et al. (2013). Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 5, 193ra191. 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid R, Cannon MJ, and Chow M (2005). Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J. Virol. 79, 5988–5995. 10.1128/jvi.79.10.5988-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA 323, 1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann HH, Oliveira TY, Oren DA, et al. (2021). Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl Med. 13, eabf1555. 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Li J, Zhang X, Tang Y, Wang J, and Wu Y (2009). A naturally processed epitope on rotavirus VP7 glycoprotein recognized by HLA-A2.1-restricted cytotoxic CD8+ T cells. Viral Immunol. 22, 189–194. 10.1089/vim.2008.0091. [DOI] [PubMed] [Google Scholar]
- Wei J, Li JT, Zhang XP, Tang Y, Wang JX, Zhang B, and Wu YZ (2006). Identification of an HLA-A*0201-restricted cytotoxic T-lymphocyte epitope in rotavirus VP6 protein. J. Gen. Virol. 87, 3393–3396. 10.1099/vir.0.82031-0. [DOI] [PubMed] [Google Scholar]
- Williams MB, Rose JR, Rott LS, Franco MA, Greenberg HB, and Butcher EC (1998). The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J. Immunol. 161, 4227–4235. [PubMed] [Google Scholar]
- Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wright PF, Connor RI, Wieland-Alter WF, Hoen AG, Boesch AW, Ackerman ME, Oberste MS, Gast C, Brickley EB, Asturias EJ, et al. (2016). Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect. Dis 16, 1377–1384. 10.1016/s1473-3099(16)30169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, et al. (2020). Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 5, 434–435. 10.1016/s2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Schnepf D, and Staeheli P (2019). Interferon-lambda orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 19, 614–625. 10.1038/s41577-019-0182-z. [DOI] [PubMed] [Google Scholar]
- Yel L (2010). Selective IgA deficiency. J. Clin. Immunol. 30, 10–16. 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, and Fujita T (2004). The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737. 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Youngman KR, Franco MA, Kuklin NA, Rott LS, Butcher EC, and Greenberg HB (2002). Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J. Immunol. 168, 2173–2181. 10.4049/jimmunol.168.5.2173. [DOI] [PubMed] [Google Scholar]
- Zaman K, Anh DD, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Thiem VD, Mai LTP, Luby SP, et al. (2010). Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 376, 615–623. 10.1016/s0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, and Nunez G (2016). Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44, 647–658. 10.1016/j.immuni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, et al. (2014). Prevention and cure of rotavirus infection via TLR5/NLRC4–mediated production of IL-22 and IL-18. Science 346, 861–865. 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, and Zhao W (2020). NLRP3 inflammasome-A key player in antiviral responses. Front. Immunol. 11, 211. 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaori G, Sun M, and Ogra PL (1988). Characteristics of the immune response to poliovirus virion polypeptides after immunization with live or inactivated polio vaccines. J. Infect. Dis. 158, 160–165. 10.1093/infdis/158.1.160. [DOI] [PubMed] [Google Scholar]
- Zhou L, and Sonnenberg GF (2018). Essential immunologic orchestrators of intestinal homeostasis. Sci. Immunol 3, eaao1605. 10.1126/sciimmunol.aao1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li HB, Wang G, et al. (2017). Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670. 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Jones MK, Hickman D, Han S, Reeves W, and Karst SM (2016). Norovirus antagonism of B-cell antigen presentation results in impaired control of acute infection. Mucosal Immunol. 9, 1559–1570. 10.1038/mi.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN 3rd, et al. (2013). Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 9, e1003592. 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Hagedorn B, and Lyons H (2020). Projection of costs of polio eradication compared to permanent control. J. Infect. Dis. 221, 561–565. 10.1093/infdis/jiz488. [DOI] [PubMed] [Google Scholar]