Abstract
Base editors (BEs) have opened new avenues for the treatment of genetic diseases. However, advances in delivery approaches are needed to enable disease targeting of a broad range of tissues and cell types. Adeno-associated virus (AAV) vectors remain one of the most promising delivery vehicles for gene therapies. Currently, most BE/guide combinations and their promoters exceed the packaging limit (∼5 kb) of AAVs. Dual-AAV delivery strategies often require high viral doses that impose safety concerns. In this study, we engineered an adenine base editor (ABE) using a compact Cas9 from Neisseria meningitidis (Nme2Cas9). Compared with the well-characterized Streptococcus pyogenes Cas9-containing ABEs, ABEs using Nme2Cas9 (Nme2-ABE) possess a distinct protospacer adjacent motif (N4CC) and editing window, exhibit fewer off-target effects, and can efficiently install therapeutically relevant mutations in both human and mouse genomes. Importantly, we show that in vivo delivery of Nme2-ABE and its guide RNA by a single AAV vector can efficiently edit mouse genomic loci and revert the disease mutation and phenotype in an adult mouse model of tyrosinemia. We anticipate that Nme2-ABE, by virtue of its compact size and broad targeting range, will enable a range of therapeutic applications with improved safety and efficacy due in part to packaging in a single-vector system.
Introduction
Point mutations represent the largest class of known human pathogenic genetic variants.1,2 Base editors (BEs), which comprise a single-guide RNA (sgRNA) loaded onto a Cas9 (nuclease-inactivated or nickase form) fused to a deaminase enzyme, enable precise installation of A•T to G•C (in the case of adenine base editors [ABEs])3 or C•G to T•A (in the case of cytidine base editors [CBEs])4 substitutions. In contrast to traditional nuclease-dependent genome editing approaches, BEs do not generate double-stranded DNA breaks, do not require a DNA donor template, and are more efficient in editing nondividing cells, making them attractive agents for in vivo therapeutic genome editing.
Although robust editing has been achieved in many cultured mammalian cell systems, safe and effective in vivo delivery of BEs remains a major challenge. To date, both nonviral and viral delivery methods have shown great promise for delivering BEs for in vivo therapeutic purposes in rodents and primates.5,6 For example, in vivo delivery through adeno-associated viruses (AAVs) has achieved efficient editing in a wide range of tissue and cell types including liver,5–10 heart,11 muscle,12,13 retina,14,15 inner ear,16 and central nervous system (CNS).17,18 However, the large coding sizes (5.2 kb) of the best characterized Streptococcus pyogenes Cas9 (SpyCas9)-containing BEs exceed the packaging limit of AAV (5 kb).19,20
Currently, in vivo delivery of BEs by AAV has been approached by splitting the SpyCas9 BEs between two AAVs and relying on the use of intein trans splicing for the assembly of the full-length effector.13,16–18,21,22 Although effective, this approach requires transduction of the target cell by both AAVs and successful in trans splicing of the two intein halves. The requirement to deliver two AAV vectors also increases the viral dosage needed for a treatment, which raises safety concerns and adds burdens to AAV manufacturing.23–25
Compact Cas9 orthologs are ideal candidates for engineering BEs suitable for single AAV delivery.26–28 For example, single AAV delivery of a domain-inlaid Staphylococcus aureus Cas9 (SauCas9) ABE has been reported in cultured HEK293 cells.29 Previously, we developed a Neisseria meningitidis Cas9 (Nme2Cas9) as an in vivo genome editing platform.27,30 Nme2Cas9 is a compact naturally accurate genome editor with a distinct N4CC protospacer adjacent motif (PAM) specificity. Recently, two other groups have successfully implemented Nme2-CBEs in cultured mammalian cells and in rabbits,31 as well as ABEs using Nme2Cas9 (Nme2-ABEs) in rice.32
In this study, we develop Nme2-ABEs and define their editing efficiencies, editing windows, and off-target activities in comparison with those of the widely applied Spy-ABEs in cultured mammalian cells. Next, we show that Nme2-ABE can edit multiple therapeutically significant loci, including one of the most common mutations occurring in Rett syndrome patients that cannot be targeted by other compact ABEs (e.g., ABEs derived from SauCas9 and SauCas9-KKH33–36) due to PAM restrictions.
Lastly, by optimizing the promoter and the nuclear localization signals, we show that Nme2-ABE and its guide can be packaged into a single AAV vector genome for in vivo delivery. One systematic administration of the single AAV vector encoding both Nme2-ABE and sgRNA readily corrects the disease-causing mutation and phenotype in an adult mouse model of hereditary tyrosinemia type 1 (HT1).
Results
Development of Nme2-ABE and comparison of editing windows and off-target effects with those of Spy-ABE
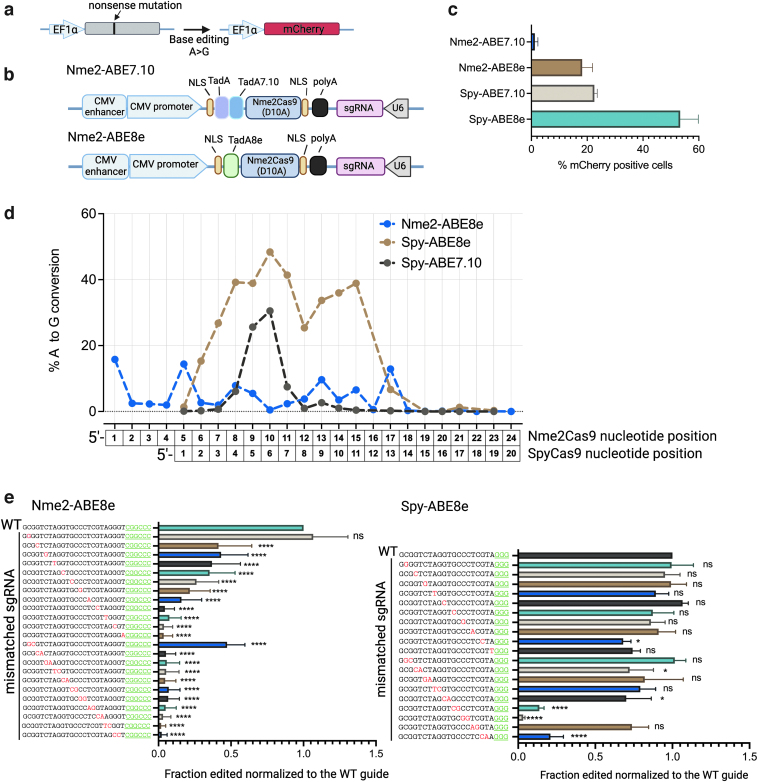
First, to evaluate base editing efficiency in a streamlined manner, we developed an ABE reporter cell line in which a G-to-A mutation in the mCherry coding sequence generates a nonsense mutation. Adenine base editing can reverse the mutation and recover red fluorescence, and the editing efficiency can be readily measured by fluorescence-activated cell sorting (FACS) (Fig. 1a and Supplementary Fig. S1a). Initially, we constructed Nme2-ABE7.10 by linking a TadA-TadA7.10 dimer from the Spy-ABE7.10 to the N-terminus of the Nme2Cas9 HNH nickase.3 However, by plasmid transient transfection, Nme2-ABE7.10 showed poor activity in the ABE reporter cell line.
FIG. 1.
Comparing the activity, editing window, and off-target effects of Nme2-ABE to those of Spy-ABE.
(a) Schematic representation of the ABE reporter HEK293T cell line.
(b) Schematic representation of the Nme2-ABE constructs.
(c) Comparison of editing efficiencies of Nme2-ABEs with those of Spy-ABEs in the ABE reporter cell line by plasmid transient transfection (n = 3 biological replicates).
(d) Summary of editing windows and comparison of editing efficiencies for the ABEs at endogenous genomic loci. Each data point represents the mean A-to-G editing efficiency measured by amplicon deep sequencing at the indicated position of the spacer across 12 Nme2Cas9 target sites and 8 SpyCas9 target sites, respectively (n = 3 biological replicates). Summaries of the individual A-to-G conversion and indel efficiencies at each target site are shown in Supplementary Figure S2.
(e) Comparison of Nme2-ABE8e mismatch tolerance with that of Spy-ABE8e in the ABE reporter cell line. The activities were measured by quantifying mCherry-positive cells using FACS. The activities of the effectors with the mismatched guides are normalized to those of the perfectly complementary (WT) guide. Red, mismatched nucleotides; green, PAM sequence (n = 3 biological replicates). Data represent mean ± SD; ns, p > 0.05; *p < 0.05; ****p < 0.0001 (one-way ANOVA). ABE, adenine base editor; ANOVA, analysis of variance; FACS, fluorescence-activated cell sorting; PAM, protospacer adjacent motif; SD, standard deviation; WT, wild type.
Because the evolved TadA8e is highly active and compatible with a wide range of Cas9s,35 we next engineered Nme2-ABE8e by linking a TadA8e monomer to the N-terminus of the Nme2Cas9 HNH nickase (Fig. 1b). We found that Nme2-ABE8e supports robust editing activity in the ABE reporter cell line (Fig. 1c).
Next, to define the editing window and editing efficiency of Nme2-ABE8e, and to compare these parameters with those of Spy-ABE7.10 and Spy-ABE8e, we transfected HEK293T cells with plasmids expressing each ABE along with sgRNAs targeting 12 human genomic loci for Nme2-ABE8e (including eight dual-target sites [target sites followed by NGGNCC PAMs for both SpyCas9 and Nme2Cas930] and four Nme2Cas9-specific target sites). We found that this first-generation Nme2-ABE8e has a broad (∼17 nt) but shallow editing window that, due to modest activity, was difficult to define clearly (Fig. 1d).
We next sought to understand the potential extent of off-target editing by Nme2-ABE8e. It has been shown that the major source of DNA off-target base editing is Cas9 dependent,37,38 caused by Cas9 binding and unwinding at near-cognate sequences. Because Nme2Cas9 is highly accurate during nuclease-driven editing in cells and in vivo,27,30,39 we hypothesized that Nme2-ABE8e would exhibit similar accuracy advantages relative to Spy-ABE8e. As an initial test, we systematically investigated the tolerance for nucleotide mismatches between the guide and the target sequence for the two effectors.
We designed a panel of guides targeting the ABE reporter with single- and di-nucleotide mismatches with the target sequence for both Nme2-ABE8e and Spy-ABE8e and measured their activities by plasmid transfection and FACS (Fig. 1e). Considering the differences in on-target efficiencies between the two effectors, we further normalized the activities of the mismatched guides to those of the perfectly complementary guides for each effector.
We found that Nme2-ABE8e exhibited significantly lower off-target editing propensity than Spy-ABE8e: although single-nucleotide mismatches in the seed region (guide nucleotide positions 17–24 for Nme2Cas9, and 10–20 for SpyCas9) and the majority of dinucleotide mismatches significantly compromised the editing efficiency of Nme2-ABE8e, these near-cognate sequences were mostly efficiently edited by Spy-ABE8e (Fig. 1e).
Inhibition of base editing by anti-CRISPR proteins that limit DNA binding activity
The development of Nme2Cas9 base editing platforms raises the possibility that regulation strategies developed for nuclease-based editing could be similarly implemented in the case of base editing. One such strategy is the use of anti-CRISPR (Acr) proteins that limit Cas9 DNA-binding activity,40–43 which have been deployed to reduce both off-target44 and off-tissue editing.45 AcrIIC3 and AcrIIC4 have been reported to reduce Nme2Cas9 DNA binding activity41,43,46 but have no effect on SpyCas9.41,43
To test whether such Acr proteins can function as off-switches for Nme2-ABE8e base editing, we cotransfected the ABE reporter cell line already described with plasmids expressing Nme2-ABE8e, sgRNA, and Acr proteins. Spy-ABE7.10 and AcrIIA4 (an anti-CRISPR that prevents SpyCas9 DNA binding,40 nuclease editing,40,44 and base editing47) were used as a positive control. Conversely, AcrE2, which is a Type I-E Acr43 that has no effect on SpyCas9 or Nme2Cas9 activity, was used as a negative control.
As expected, Spy-ABE7.10 base editing was reduced to background levels by AcrIIA4, but AcrE2, AcrIIC3, and AcrIIC4 had no effect (Supplementary Fig. S3a). By contrast, Nme2-ABE8e editing was strongly inhibited by AcrIIC3 and AcrIIC4, but AcrE2 and AcrIIA4 had no effect. These results confirm that these anti-CRISPRs can be effective off-switches for Nme2-ABE8e editing.
Tissue-specific miRNAs, in combination with miRNA response elements (MREs) in the 3′UTR of an Acr construct, have been used to restrict editing to cell types that express such miRNAs, both in cultured cells41,48 and in vivo.45 To determine whether such a strategy could be used to control Nme2-ABE8e, we inserted miR-122 MREs into the 3′UTRs of our AcrIIC3 and AcrIIC4 constructs. Mir-122 is a hepatocyte-specific miRNA that is expressed in Huh7 cells but not in HEK293 cells and has been used to validate this strategy for nuclease-based editing.
Again, AcrIIC3 and AcrIIC4 inhibited Nme2-ABE8e activity at an endogenous site in HEK293T cells, and inhibition largely persisted even with the MREs (Supplementary Fig. S3b), as expected since miR-122 is not present to silence Acr expression. In Huh7 cells, which are transfected with lower efficiencies, AcrIIC3 and AcrIIC4 again inhibited Nme2-ABE8e, but this inhibition of editing activity was largely relieved by the insertion of the MRE 122 sites. These results indicate that miRNA-repressible anti-CRISPRs can be used to enforce the cell-type specificity of base editing, as it can for nuclease editing.
Installation of therapeutically relevant edits with Nme2-ABE8e
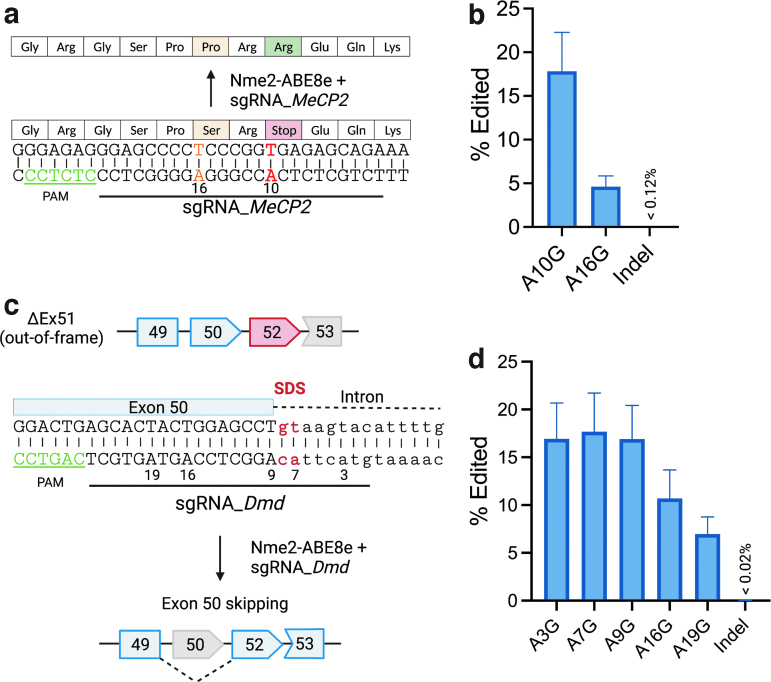
We next tested the potential of Nme2-ABE8e to correct pathogenic mutations or to introduce disease-suppressing mutations. One of the most common mutations that cause Rett syndrome is a C•G to T•A base transition that produces a nonsense mutation in the human MeCP2 gene (c.502 C>T; p.R168X).49–51 Because the target adenine is within a cytidine-rich region, this mutation is not readily accessible for ABEs based on other compact Cas9s such as SauCas9 and SauCas9-KKH.33–36 To test whether Nme2-ABE8e can correct this pathogenic mutation, we electroporated Nme2-ABE8e mRNA with a synthetic sgRNA into a Rett syndrome patient-derived fibroblast cell line that possesses this mutation.
By amplicon deep sequencing, we found that Nme2-ABE8e successfully edited the target adenine (A10) (Fig. 2a, b). A bystander edit at an upstream adenine (A16) causes a missense mutation (c.496 T>C; p.S166P), although this occurs with only one-fourth the frequency of intended edit at A10. Because S166 has been shown to be subject to phosphorylation in mice and is conserved from Xenopus laevis to humans,52 further investigation will be needed to determine whether bystander editing at A16 impairs functional rescue of edited cells. However, as with other BEs,3,29,53–58 future protein engineering efforts adjusting the editing window promise to provide greater control over Nme2-ABE editing outcomes.
FIG. 2.
Installation of therapeutically relevant edits with Nme2-ABE8e.
(a) Schematic representation of a nonsense mutation in the human MeCP2 gene (c.502 C>T; p.R168X) that causes Rett syndrome. The black underline denotes the target sequence of an Nme2-ABE8e guide for reverting the mutant A to G (wildtype) at position 10 (red, bold). The PAM region is underlined in green. A bystander edit at position 16 (orange) can generate a missense mutation (c.496 T>C; p.S166P).
(b) Amplicon deep sequencing quantifying the editing efficiency in Rett patient-derived fibroblasts transfected with the Nme2-ABE8e mRNA and the synthetic sgRNA_MeCP2 noted in (a) (n = 3 biological replicates).
(c) Schematic representation of the exon skipping strategy that restores the reading frame of the mouse Dmd transcript. Deletion of exon 51 (ΔEx51) can alter the reading frame and generate a premature stop codon in exon 52 (red). Adenine base editing at the splice site of exon 50 (red) by Nme2-ABE8e can cause exon 50 skipping (gray) and restore the Dmd reading frame. The PAM region is underlined in green.
(d) Amplicon deep sequencing quantifies the editing efficiency at the target site in mouse N2a cells transfected with the Nme2-ABE8e and sgRNA_Dmd expression plasmid (n = 3 biological replicates). SDS, splicing donor site.
Next, we sought to generate a disease-suppressing mutation that has been shown to reverse phenotypes of a validated Duchenne muscular dystrophy (DMD) mouse model (ΔEx51).59 The ΔEx51 mouse model was generated by deletion of the exon 51 in the Dmd gene, resulting in a downstream premature stop codon in exon 52, causing the production of a nonfunctional truncated dystrophin protein. Previously, it has been shown that the Dmd reading frame can be restored by skipping exon 50 by adenine base editing (Fig. 2c).13
However, in vivo base editing using ABEmax-SpyCas9-NG was delivered by dual-AAV vectors and required high viral doses. We identified a guide design for Nme2-ABE8e to target the adenine (A7) within the splice donor site downstream of exon 50 (Fig. 2c). By plasmid transfection in the mouse N2a cell line and amplicon deep sequencing, we found that Nme2-ABE8e can generate 17.67% ± 4.57% editing at A7 (Fig. 2d). The efficient editing at multiple bystander adenines is not a concern in this case as those adenines are within the skipped exon 50 or the intron.
Optimization of an Nme2-ABE8e construct for single AAV delivery
Previously, we showed that Nme2Cas9 with one or two sgRNAs can be packaged into a single AAV vector and support efficient editing in vivo.27,30 We reasoned, based upon the compact sizes of Nme2Cas9 and TadA8e, that Nme2-ABE8e with an sgRNA could be packaged into a single AAV for in vivo delivery. To achieve this, we first replaced NmeCas9 with Nme2-ABE8e in the minimized all-in-one AAV vector reported previously.27 We attached one cMyc nuclear localization signal (NLS) sequence on each terminus of Nme2-ABE8e while retaining the original promoters for effector and sgRNA expression (Fig. 3a, 2x cMyc).
FIG. 3.
Optimization of Nme2-ABE8e constructs for single-AAV delivery.
(a) Schematic representation of single AAV constructs with different NLS configurations.
(b) Comparison of different NLS configurations by plasmid transfection in the ABE reporter cell line.
(c) Comparison of Nme2-ABE activity when sgRNA expression is driven by the U6 or miniU6 promoters in the 2 × BP_SV40 NLS construct targeting the ABE reporter site (left) or endogenous human (middle) and mouse (right) genomic sites by plasmid transfection in cultured cells followed by amplicon deep sequencing (n = 3 biological replicates). Data represent mean ± SD; ns, p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (one-way ANOVA). AAV, adeno-associated virus; NLS, nuclear localization signal; sgRNA, single-guide RNA.
By transient transfection of vector backbone plasmids, the single AAV construct successfully edited the ABE reporter cell line. To further improve Nme2-ABE editing efficiency, we then tested three different NLS configurations: (1) one cMyc NLS on the N-terminus and two cMyc NLSs on the C-terminus (3x cMyc); (2) one Ty1 NLS, which derived from the yeast Ty1 retrotransposon that supports robust nuclear localization in dPspCas13b fusion proteins, on the N-terminus (Ty1)60; and (3) one bipartite SV40 NLS (BP_SV40) on each terminus (2 × BP_SV40) (Fig. 3a).61 When transfecting the vector plasmid into the ABE reporter cell line, the 2 × BP_SV40 construct showed the highest editing efficiency (Fig. 3b).
The total length of the vector constructed with the 2 × BP_SV40 NLS, hereafter Nme2-ABE8e-U6, is 4998 bp, very close to the packaging limit of AAV. To test whether we could further reduce the vector size without significantly compromising editing efficiency, we turned to a recently reported “miniU6” promoter that has been shown to support sgRNA expression and achieve comparable editing efficiencies as with the complete U6 promoter.62 Upon replacement of the U6 promoter with miniU6 promoter, the vector, hereafter Nme2-ABE8e-miniU6, was shortened to 4860 bp, within the packaging limit of AAV (Fig. 3c). Both constructs induced robust editing in the ABE reporter cell line through transient transfection of the vector plasmids (Fig. 3c).
To avoid potential ABE reporter-specific effects, we further tested both single AAV vector backbone plasmids at two endogenous target sites: (1) one of the human dual-target sites, DS12, and (2) a previously reported Nme2Cas9 target site in the mouse Rosa26 gene.45 By plasmid transfection in human HEK293T or mouse N2a cells, we observed significant editing at these loci by both vectors, although the Nme2-ABE8e-miniU6 vector was somewhat less efficient (Fig. 3c). We thus chose both vector designs for the subsequent in vivo study.
Hydrodynamic injection of single AAV vector plasmids corrects the disease mutation and phenotype in an adult mouse model of HT1
To test the in vivo editing efficiency and therapeutic potential of the single AAV constructs, we chose to target a pathogenic mutation associated with the liver disease HT1. HT1 is caused by mutations in fumarylacetoacetate hydrolase (FAH), which catalyzes one step of the tyrosine catabolic pathway. FAH deficiency leads to accumulations of toxic fumarylacetoacetate and succinyl acetoacetate, causing liver, kidney, and CNS damage.63 The FahPM/PM mouse model possesses a G•C to A•T point mutation in the last nucleotide of exon 8, which causes skipping of exon 8 and FAH deficiency (Fig. 4a).
FIG. 4.
In vivo validation of the single AAV vectors by hydrodynamic tail vein injection of plasmids in the FahPM/PM mice. (a) Illustration of the pathogenic point mutation in the FahPM/PM mouse model that causes exon 8 skipping of the Fah gene, and the guide design for Nme2-ABE8e to correct the point mutation. Red and bold, target adenine; orange, other bystander adenines; green and underlined, PAM. The black rectangle highlights the guide sequence used for Spy-RA6.3. (b) Illustration of constructs of the single AAV vector plasmids used in in vivo studies. (c) Editing efficiencies at the Fah mutant site by AAV plasmid electroporation in MEF cells derived from the FahPM/PM mouse. Data are from amplicon deep sequencing (n = 2 biological replicates). (d) Anti-FAH IHC staining showing FAH+ hepatocytes, before NTBC withdrawal, in the FahPM/PM mouse hydrodynamically injected with the indicated plasmid. The bar graph quantifies the percentage of FAH+ hepatocytes detected by IHC (n = 2 mice per group). Scale bars, 500 μm. (e) Body weight plot of mice injected with the single AAV vector plasmid showing gradual weight gain over a month after NTBC withdrawal. (f) RT-PCR analysis of the plasmid- or PBS-injected mouse livers using primers that span exons 5 and 9. The wild-type amplicon is 405 bp and exon 8 skipped amplicon is 305 bp. (g) Representative Sanger sequencing trace of the 405 bp RT-PCR band. (h) Anti-FAH IHC staining showing expansion of FAH+ hepatocytes 40 days post-NTBC withdrawal. Scale bars, 500 μm. (i) Quantification of the editing efficiency by amplicon deep sequencing of genomic DNA of the treated mouse livers harvested 40 days post-NTBC withdrawal. (d–i) (n = 2 mice per group). Data represent mean ± SD; ns, p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (one-way ANOVA). FAH, fumarylacetoacetate hydrolase; IHC, immunohistochemistry; MEF, mouse embryonic fibroblast; NTBC, 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione; PBS, phosphate-buffered saline; RT-PCR, reverse transcription PCR.
Without treatment, FAH-deficient mice will rapidly lose weight and eventually die. The FahPM/PM mouse can be treated with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), an inhibitor of an enzyme upstream within the tyrosine degradation pathway, which prevents toxin accumulation.64 Previously, we and others have tested various in vivo gene-editing tools to treat the FahPM/PM mouse model, including Cas9-directed homology-directed repair (HDR),27,65,66 base editing,67,68 microhomology-directed end joining,69 and prime editing.70 Among these, multiple approaches including AAV, lipid nanoparticle, and plasmid hydrodynamic tail vein injection have been used to deliver the gene-editing agents into this mouse model.
However, caveats should be considered when comparing the efficiencies of different gene-editing strategies: only the initial editing efficiency (measured before NTBC withdrawal) reflects the activity of the gene-editing agents, because after NTBC withdrawal, the hepatocytes in which Fah has been repaired will show clonal expansion over time due to their survival advantage.
We first validated a guide targeting the point mutation by electroporation of the single AAV vector plasmids, either Nme2-ABE8e-U6 or Nme2-ABE8e-miniU6 into mouse embryonic fibroblasts (MEFs) isolated from an FahPM/PM mouse (Fig. 4b). We also included a positive control using Spy-RA6.3, which is a codon-optimized Spy-ABE used previously to edit this mutation.67 We detected modest but significant editing by both Nme2-ABE vectors at the target adenine at position 13 (A13), despite low (∼12%) plasmid electroporation efficiencies.
We also observed higher levels of bystander editing at A16 with both vectors, and a lower level of bystander editing at A10 for Nme2-ABE8e-U6. Bystander editing at A10 (which changes an active-site-proximal serine into an alanine) was observed with Spy-RA6.3 as well, consistent with the previously published data.67 The effect (if any) of the intronic A16 edit on intron 8 splice donor activity has not been defined (Fig. 4c).
To test our single AAV vectors of Nme2-ABE8e in vivo, we first performed hydrodynamic tail vein injections of the AAV-vector plasmids into 10-week-old HT1 mice. We also injected Spy-RA6.3 plasmid or phosphate-buffered saline (PBS) as positive and negative controls, respectively.71 The experimental outline is shown in Supplementary Figure S4. Seven days postinjection, we sacrificed two mice from each experimental group, and one mouse from each control group, to measure the editing efficiency before hepatocyte expansion.
We then withdrew NTBC for the rest of the mice for long-term phenotypic study. Before NTBC withdrawal, anti-FAH immunohistochemistry (IHC) staining showed 4.58% ± 1.1% FAH+ hepatocytes from the group that was injected with the Nme2-ABE8e-U6 plasmid, and 1.71% ± 0.49% from the group injected with the Nme2-ABE8e-miniU6 plasmid (Fig. 4d). After NTBC withdrawal, we monitored body weight changes. The PBS-injected mice rapidly lost body weight after NTBC withdrawal and were euthanized. By contrast, mice injected with either the Nme2-ABE8e-U6 or Nme2-ABE8e-miniU6 plasmid gradually gained body weight, suggesting rescue of the pathological phenotype (Fig. 4e).
Forty days after NTBC withdrawal, we sacrificed mice from all surviving groups. To determine whether Nme2-ABE8e successfully corrects the Fah splicing defect, we extracted total RNA from the livers and performed reverse transcription PCR (RT-PCR) using primers that spanned exons 5 and 9. By contrast to the PBS-injected mice, which only showed a 305 bp PCR product corresponding to the truncated mRNA lacking exon 8, we observed that the 405 bp PCR product (containing exon 8) predominated in the Nme2-ABE-treated mice (Fig. 4f). Sanger sequencing of the 405 bp bands further confirmed the presence of the corrected G residue at the end of exon 8 (Fig. 4g).
When performing anti-FAH IHC staining, we observed expansion of FAH+ hepatocytes in the groups that were injected with either of the single AAV vector plasmids (Fig. 4h). Amplicon deep sequencing of genomic DNA from the livers of treated mice again provided evidence for Nme2-ABE activity (Fig. 4i). By contrast to the efficiencies achieved in the MEF cells, we observed a lower editing at A16 and no significant editing at A10, likely due to partial (A16) or complete (A10) selection against mice harboring bystander edits at those positions. The mouse injected with Spy-RA6.3 plasmid showed results consistent with previously reported data (Fig. 4d–i).67 These data indicate that our Nme2-ABE8e single AAV vector plasmids can correct the disease genotype and phenotype of the FahPM/PM mice in vivo.
In vivo base editing by single AAV delivered Nme2-ABE8e in the FahPM/PM mice
Encouraged by the initial results, we packaged AAV9 with the Nme2-ABE8e-U6 construct, as well as the Nme2-ABE8e-miniU6 construct, considering that the relatively smaller size of the latter may potentially benefit packaging efficiency (Fig. 5a). However, both constructs yielded similar vector titers. Next, to confirm AAV genome integrity, we performed AAV genomic DNA extraction and alkaline gel electrophoresis. We did not observe any sign of genome truncation (Supplementary Fig. S1b). We then tail vein injected 8-week-old FahPM/PM mice at a dosage of 4 × 1011 vg per mouse. We kept the mice on NTBC for 1 month before analyzing the editing efficiency.
FIG. 5.
In vivo base editing by single-AAV-delivered Nme2-ABE8e in FahPM/PM mice.
(a) Schematic representation of the AAV constructs.
(b) Anti-FAH IHC staining showing FAH+ hepatocytes, before NTBC withdrawal, in the FahPM/PM mouse injected with AAV9 expressing Nme2-ABE8e with a sgRNA targeting either the Fah gene or the Rosa26 gene that serves as a negative control. Scale bar, 500 μm. The bar graph quantifies the percentage of FAH+ hepatocytes detected by IHC (n = 4 mice in the Fah targeting group, n = 3 mice in the Rosa26 targeting group).
(c, d) Quantification of the editing efficiency by amplicon deep sequencing of genomic DNA from the AAV9-injected mouse livers harvested before NTBC withdrawal (n = 4 mice in the Fah targeting group, n = 3 mice in the Rosa26 targeting group and the PBS control group). Data represent mean ± SD; ns, p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA).
One month after AAV injection and before NTBC withdrawal, we sacrificed the mice and performed IHC staining using an anti-FAH antibody. The negative control groups injected with AAV9 expressing Nme2-ABE and an sgRNA targeting the Rosa26 gene did not show any FAH+ hepatocytes. By contrast, we observed 6.49% ± 2.08% FAH+ hepatocytes in the AAV9-Nme2-ABE8e-U6-Fah-treated group, and 1.62% ± 0.49% FAH+ hepatocytes in AAV9-Nme2-ABE8e-miniU6-Fah-treated group (Fig. 5b). Because repair of 1/100,000 hepatocytes was reported to rescue the phenotype, both AAV constructs achieved efficiencies that were well above the therapeutic threshold.72
Moreover, the percentage of edited hepatocytes by AAV9-Nme2-ABE8e-U6-Fah was higher than what has been reported previously by other genome editing strategies.65–67 By targeted deep sequencing, the editing efficiency at the target adenine (A13) in the AAV9-Nme2-ABE8e-U6-treated group is 0.34% ± 0.14%, whereas no significant editing was observed in the AAV9-Nme2-ABE8e-miniU6-treated group, possibly due to low efficiency that was below the detection limit of amplicon deep sequencing (Fig. 5c). The reason for the higher frequency of FAH+ hepatocytes than the frequency of editing at the DNA level is likely due to hepatocyte polyploidy,73 as well as the presence of genomic DNA from nonparenchymal cells.
Similar distinctions in FAH+ frequencies and genomic readouts were also observed in previous studies using this mouse model.67,70 We also measured the editing efficiency at the Rosa26 locus and observed ∼5% editing efficiency at the target site, indicating that the efficiency of AAV9-delivered Nme2-ABE8e is target site dependent, and higher efficiency sites can be identified (Fig. 5d). We did not detect any above-background level of indel, indicating that single AAV delivered Nme2-ABE8e can install precise editing in vivo without generating unwanted indels (Fig. 5c, d).
Optimizing effector and sgRNA arrangement improves editing efficiency by AAV delivery
Previous studies have shown that effector and sgRNA placement and orientation within the AAV genome can affect transgene expression levels and editing efficiencies.18,26,74 To test whether different arrangements of sgRNA and effector cassettes in the Nme2-ABE8e all-in-one AAV construct can further increase in vivo editing efficiency, we moved the U6-sgRNA cassette to the 3′ end of the AAV genome and reversed its orientation, similar to the optimal arrangement reported by Fry et al.74 (Fig. 6a). Using the same Rosa26 guide already described (Fig. 5d), we packaged the rearranged construct in AAV9 capsids and performed tail vein injections in 8-week-old mice at a dosage of 4 × 1011 vg per mouse.
FIG. 6.
Optimizing effector and sgRNA arrangement improves editing efficiency by AAV delivery.
(a) Schematic representation of the original (left) and the optimized AAV constructs (AAV9-Nme2-ABE8e-U6_V2, right).
(b) Quantification of the editing efficiency at the Rosa26 locus by amplicon deep sequencing using liver genomic DNA from mice tail vein injected with the indicated AAV (n = 3 mice in the AAV-injected group, n = 1 mouse in the PBS-injected group). Data from the original AAV configuration (a, left) are the same as that from the experiment shown in Figure 5d.
(c) Quantification of the editing efficiency at the Rosa26 locus by amplicon deep sequencing using striatum genomic DNA from mice intrastriatally injected with the indicated AAV samples (n = 3 mice in the AAV-injected group, n = 1 mouse in the PBS-injected group). Data represent mean ± SD, ns, p > 0.05; *p < 0.1; **p < 0.01 (one-way ANOVA).
We observed significantly improved editing efficiency (34% ± 11.6%) with the optimized construct (Fig. 6b), which is significantly greater than the 4.7% ± 0.94% efficiency with the original configuration, and comparable with the editing efficiency achieved previously by dual-AAV delivered split-intein SpyCas9-ABEmax targeting the Dnmt1 gene in adult mouse liver (38% ± 2.9%).18
Beyond the liver, AAV delivery possesses advantages in other tissue types, especially the CNS. To test whether our Nme2-ABE8e all-in-one AAV can generate efficient editing in the CNS, we performed intrastriatal injection in 8-week-old mice at a dosage of 1 × 1010 vg per mouse. We observed higher editing efficiency with the optimized construct (28.7% ± 8.95%) compared with the original configuration (10.68% ± 4.71%) (Fig. 6c). We conclude that efficient adenine base editing can be achieved in vivo through single AAV delivery of the Nme2-ABE8e system.
Nme2Cas9 is known to be highly accurate in nuclease-based editing in cells,27,30,39 and our mismatch scanning experiments (Fig. 1e) strongly suggest that the same will be true for Nme2-ABE8e in vivo. To evaluate potential Cas9-dependent off-target effects in the AAV9-injected mice, we performed amplicon deep sequencing at a Nme2Cas9 off-target site previously validated for the Rosa26 guide,30 using genomic DNA from livers of mice injected with the Nme2-ABE8e-U6-Rosa26_V2. This site has a perfect NNNNCC PAM, a perfect match in the first 10 positions of the seed region, only 4 mismatches overall, and potentially editable adenines at positions 2, 3, 6, 9, and 14 of the protospacer.
Off-target editing efficiency was at background levels at all adenines except A9, which (at 0.2% A-to-G conversion) was barely above the detection threshold (Supplementary Fig. S5), and >100x lower than the efficiency of on-target editing (Fig. 6b). In addition, although the TadA8e has been shown to modestly increase Cas9-independent DNA off-targeting and transcriptome-wide RNA off-target editing relative to those of other TadA variants, these off-target effects can be greatly ameliorated by introducing mutations in the TadA8e domain (e.g., V106W), without compromising the on-target efficiency.35 We conclude that Nme2-ABE8e is a highly accurate BE when delivered to the liver through a single AAV vector.
Discussion
Rapidly evolving and precise genome editing tools such as BEs and prime editors possess great potential to address the root causes of human genetic diseases.2,75,76 However, the safe and effective delivery of genome editors remains a major challenge. Viral delivery using AAV, which is the only FDA-approved in vivo gene therapy vector to date, has a limited packaging capacity. Previous studies using AAV to deliver BEs or prime editors have been reported to be effective in rodents,7,8,10–13,15–18,21 but all of them required multiple vectors and most of them required high viral dosage.7,10–13,17,18
To date, AAV administered at high dose has been reported to relate to severe toxicity or even death in nonhuman primates, piglets, and humans.23,24,77–79 Engineering single AAV deliverable genome editing tools has the potential to achieve therapeutic benefits at lower dosage, which would not only ease manufacturing burdens but also reduce likelihoods of serious adverse events.80,81
Engineering new genome editors based on compact Cas9s is an alternative approach to address this issue. Previously, single AAV delivery of Cas9 nucleases has been reported to generate non-homologous end joining-based editing in vivo.26,30,82 Precision editing through HDR has also been achieved with single AAV systems, although with low efficiencies.27,83 The only single AAV BE system reported previously (which was not tested in vivo) was based on SauCas9, which has limited targeting range due to its PAM constraints. In this study, we constructed and characterized a compact accurate ABE, Nme2-ABE8e, which can target many sites that are inaccessible to SauCas9 BEs due to their distinct PAMs.
We showed that Nme2-ABE8e with an sgRNA can be packaged into a single AAV vector, and a single intravenous injection in an adult disease mouse model of tyrosinemia reversed both the disease mutation and phenotypes. Furthermore, the dosage used in this study (4 × 1011 per mouse or 2 × 1013 vg/kg) is well below the 1 × 1014 vg/kg systemic doses that have been tolerated in clinical trials.77,78
Although the editing efficiency was modest at the Fah disease locus, we reached therapeutic thresholds for HT1, and the initial editing efficiency of the AAV9-delivered Nme2-ABE8e-U6 construct exceeded that reported previously at this locus using other precision genome editing tools and delivery approaches.65–67,70 Moreover, by optimizing transgene orientation in the AAV construct, we achieved significantly improved editing efficiency (approximately fivefold increase) by AAV9-delivered Nme2-ABE8e targeting the Rosa26 gene, comparable with that achieved previously by dual-AAV delivered split-intein SpyCas9-ABEmax in adult mouse liver targeting another genomic site.18
Future optimization of this system promises to further improve efficiency. There are multiple potential explanations for the inconsistent editing efficiencies we achieved with our first-generation Nme2Cas9 ABE that suggest directions for future improvement. First, structural analyses of Nme2Cas984 indicate that the position of the N-terminally fused TadA8e domain relative to the predicted path of the displaced strand is not optimal. With other effectors, domain-inlaid deaminase fusions have proven to be advantageous in some contexts,29,54 and the same is likely to be true with Nme2-ABEs.
Second, the current Nme2-ABE8e has a wide editing window that could result in increased bystander editing, consistent with previous observations of Nme2Cas9-ABE8e editing in plants.32 For example, we observed higher editing at a bystander adenine (A16) at the Fah locus compared with the on-target adenine at A13. Optimizations of linker composition and length,3,53 as well as domain-inlaid systems,29,54–58 may confer greater control over the editing window and improved efficiency in editing the intended position.
Our studies also suggest factors that must be considered for optimal guide expression. In contrast to a previous study that showed no significant difference between the U6 and miniU6 promoters in supporting Cas9 editing efficiency,62 we found that the construct with the miniU6 promoter was consistently less efficient than that with the U6 promoter, including through AAV delivery. Because the previous study compared these promoters in T cells by lentivirus transduction, our observations may only apply to certain delivery strategies and cellular contexts.
Conclusion
In this study, we have engineered and characterized Nme2-ABE8e editing in mammalian cell culture and achieved efficient in vivo editing by delivery of a single AAV vector. To our knowledge, this is the first single AAV-delivered in vivo base editing reported to date.29 We anticipate that Nme2-ABE8e, with its distinct PAM specificity, editing window, and high accuracy, will provide additional targetability, safety, and therapeutic potential for genome engineering applications.
The Bigger Picture
AAV vectors are important delivery tools for genome editing in vivo in multiple organs and cell types, especially extrahepatic tissues. However, high vector doses impose safety concerns, especially hepatotoxicity. Our study validates a compact BE that allows delivery through a single AAV vector, which has the potential to achieve therapeutic benefits at a lower dose than delivery strategies that require multiple vectors. Furthermore, our study validates and enables adenine base editing in mammals at sites adjacent to a CC PAM.
With further optimization, our validated single AAV-ABE vector construct can be readily applied to multiple target tissues and disease-associated loci. Moreover, our study also encourages the development of other compact BEs with distinct PAMs. We anticipate that our studies will accelerate precision somatic genome editing applications with enhanced safety in vivo.
Materials and Methods
Cell culture
HEK293T cells (ATCC CRL-3216), ABE reporter cells, MEF cells, and mouse N2a cells (ATCC CCL-131) were cultured in Dulbecco's modified Eagle media (DMEM; Cat. No.: 25-500; Genesee Scientific) supplemented with 10% fetal bovine serum (FBS; Cat. No.: 26140079; Gibco). Rett syndrome human patient-derived fibroblasts (hPDFs) were obtained from the Rett Syndrome Research Trust and cultured with DMEM supplemented with 15% FBS and 1 × nonessential amino acids (Cat. No.: 11140050; Gibco). All cells were incubated in a 37°C incubator with 5% CO2.
Molecular cloning
To generate the CMV-Nme2-ABE8e and the CMV-Nme2-ABE7.10 plasmids used in Figure 1, the Nme2-ABE8e, Nme2-ABE7.10, Spy-ABE8e, and Spy-ABE7.10 constructs were cloned into the pCMV-PE2 vector backbone (#132775; Addgene) by Gibson assembly. Brief, the pCMV-PE2 plasmid was digested with NotI and PmeI restriction enzymes, and the plasmid backbone was then Gibson assembled with five fragments: N-terminal NLS, TadA8e (for Nme2-ABE8e or Spy-ABE8e), or TadA-TadA*7.10 (for Nme2-ABE7.10 or Spy-ABE7.10), the linker, Nme2Cas9-D16A or SpyCas9-D10A nickase, and the C-terminal NLS.
The ABE reporter construct was cloned by site-directed mutagenesis to change the 47th amino acid (glutamine, CAG) of the mCherry coding sequence to a stop codon (TAG). The ABE reporter was further cloned into a lentiviral transfer vector backbone (#99373; Addgene) by Gibson assembly. The sgRNA expression plasmids shown in Figure 1 were cloned from pBluescriptSKII (#74705; Addene) in two steps. First, pBluescriptSKII was digested by NotI and XbaI, and a gene fragment that contains a U6 promoter, a type-IIS restriction cloning site (BfuAI), and a tracrRNA was assembled into the backbone by Gibson assembly. The plasmids were further digested with BfuAI and ligated to the annealed oligos to insert the guide sequences.
The single AAV vector plasmids shown in Figure 3a were cloned from the #119924 Addgene plasmid by replacing the Nme2Cas9 sequence with the Nme2-ABE8e sequence, and then subsequently recloned to encode different NLS configurations and the miniU6 promoter by restriction enzyme digestion and Gibson assembly. The NLS configuration of Nme2-ABE8e mRNA shown in Figure 2b was 2 × BP_SV40, and the plasmid shown in Figure 2d was the single AAV Nme2-ABE8e-U6 plasmid shown in Figure 3a (2 × BP_SV40).
To clone the AAV-Nme2-ABE8e_V2 plasmid shown in Figure 6, first, the 2 × BP_SV40 plasmid shown in Figure 3a was digested by PmeI and NotI, the AAV backbone fragment was then Gibson assembled with the fragment containing U1a-Nme2-ABE8e, and the fragment containing U6-sgRNA with homology sequences by overhang PCR. Most of the plasmids shown in Supplementary Figure S3a were previously deposited in Addgene (AcrIIC3, #85713; Addgene; AcrIIC4, #113434; Addgene; Acr-E2, #85677; Addgene). To clone the plasmid expressing AcrIIA4, #85713; Addgene plasmid was digested with XhoI and BamHI and Gibson assembled with a gene fragment containing AcrIIA4 coding sequence.46
As shown in Supplementary Figure S3b, the AcrIIC3-MRE122 plasmid was cloned from #129531; Addgene plasmid by replacing the Rosa26-targeting guide with the DS12-targeting guide through restriction enzyme cloning. Subsequent replacement of the coding sequence of AcrIIC3 with AcrIIC4 generated the AcrIIC4-MRE122 plasmid. Sequences of plasmids first described in this article are given in the Supplementary Note S1 and will be made available from Addgene.
ABE reporter HEK293T cell line
Lentivirus was produced following instructions from Addgene (https://www.addgene.org/protocols/lentivirus-production/). In brief, HEK293T cells were transfected with the transfer vector and the packaging plasmids psPAX2 (#12260; Addgene) and pMD2.G (#12259; Addgene), using Lipofectamine 3000 (Cat. No.: L3000015; ThermoFisher). Two days later, the medium was collected and filtered through a 0.45 μm filter (Cat. No.: 6780-25040; Cytiva) to remove cell debris.
The viral titer was determined using Lenti-X™GoStix™ (Cat. No.: 631280; Takara Bio). HEK293T cells were transduced with lentivirus encoding the ABE reporter at varying dilutions (1:10, 1:100, 1:500, and 1:1000) in the presence of 8 μg/mL polybrene (Cat. No.: TR-1003-G; Millipore Sigma). Three days after transduction, the medium was removed, and fresh medium was supplemented with 2 μg/mL puromycin to select cells expressing the full-length reporter construct. Seven days after selection, the puromycin-resistant cells were collected. Single-cell clones were established by serial dilution in 96-well plates.
Fluorescent reporter assay
Forty-eight hours after transfection, cells were trypsinized and harvested into microcentrifuge tubes. After centrifuging at 300 g for 3 min, cells were resuspended into 150 μL 1 × PBS for flow cytometry analysis (MACSQuant VYB). For each sample, 10,000 events were counted for FACS analysis. Data were analyzed using Flowjo v10. Representative gating strategy is shown in Supplementary Figure S2.
In vitro transcription of Nme2-ABE8e mRNA
Nme2-ABE8e mRNA was in vitro transcribed using the NEB HiScribe T7 RNA synthesis kit, from 500 ng of a linearized template. Uridine was fully substituted with 1-methylpseudouridine, and mRNA was capped cotranscriptionally using CleanCap AG analog (Cat. Nos. N-1081 and N-7113; TriLink Biotechnologies, respectively). All enzymes were purchased from New England Biolabs. Transcription was conducted according to the manufacturer's protocol with the following amendments: transcriptions were completed in 1 × NEB HiScribe transcription buffer, and 4 mM CleanCap AG was used during transcription.
Transcription reactions were incubated at 37°C for 2 h then treated with 0.4 U/μL DNase I (final concentration) for 15 min at 37°C. mRNAs were purified with NEB Monarch RNA purification columns and treated with pyrophosphatase for 1 h with 0.25 U/μg Antarctic phosphatase (final concentration) in 1 × Antarctic phosphatase buffer. The final product was further purified with a NEB Monarch RNA column and eluted in water.
Transfection and electroporation
For plasmid transfection, cells were seeded in 24-well plates at 80,000 cells per well in culture medium and incubated overnight. In brief, plasmids were transfected at 400 ng per well when targeting the ABE reporter, and 1 μg per well for the endogenous target sites. Specifically, as shown in Figures 1c, e, 2d, and 3b, c; Supplementary Figure S3, plasmids were transfected using Lipofectamine 3000 (Cat. No. L3000001; ThermoFisher), whereas as shown in Figure 1d and Supplementary Figure S2, plasmids were transfected using Lipofectamine 2000 (Cat. No. 11668030; ThermoFisher), following the manufacturer's protocols.
Electroporation was performed using Neon Transfection System 10 μL kit (Cat. No.: MPK 1096; ThermoFisher), with the following electroporation parameters: pulse voltage (1650 V), pulse width (20 ms), and pulse number (1). Specifically, as shown in Figure 2a, 264 ng Nme2-ABE8e mRNA and 100 pmole of sgRNA were electroporated into 50,000 Rett syndrome hPDFs, whereas as shown in Figure 4c, 1 μg plasmid DNA was electroporated into 100,000 MEF cells.
AAV production
AAV vector packaging was done at the Viral Vector Core of the Horae Gene Therapy Center at the UMass Chan Medical School as previously described.85 Constructs were packaged in AAV9 capsids and viral titers were determined by digital droplet PCR and gel electrophoresis followed by silver staining.
AAV genomic DNA extraction and alkaline agarose gel electrophoresis
Genomic DNAs were extracted from 1011 vg AAV by incubating with 20 U of DNase I (Cat. No.: EN0521; ThermoFisher) at 37°C for 30 min and then with an equal volume of 2 × Pronase solution (Cat. No. 10165921001; Sigma-Aldrich) for 4 h. The genomic DNA was subsequently purified by phenol–chloroform (Cat. No.:15593-049; ThermoFisher) extraction and ethanol precipitation. DNA pellets were resuspended in water and analyzed by alkaline agarose gel electrophoresis and SYBR Gold staining.
Mouse tail vein injection
All animal study protocols were approved by the institutional animal care and use committee (IACUC) at UMass Chan Medical School. The FahPM/PM mice were kept on water supplemented with 10 mg/L 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC; Cat. No.: PHR1731-1G; Sigma-Aldrich). Mice with >20% weight loss were humanely euthanized according to IACUC guidelines. For hydrodynamic tail vein injections, plasmids were prepared by EndoFree Plasmid Maxi Kit (Cat. No. 12362; Qiagen).
In brief, 30 μg SpyCas9-RA6.3 and 30 μg sgRNA plasmids, or 60 μg Nme2-ABE8e single AAV plasmids were suspended in 2 mL saline and injected through tail vein within 5–7 s into 10-week-old FahPM/PM mice. Mice were euthanized 7 days after injection and livers were collected for analysis. For AAV injection, a dosage of 4 × 1011 vg per mouse (in 200 μL saline) was tail vein injected into 8-week-old FahPM/PM mice.
Stereotactic intrastriatal injection
Eight- to nine-week-old mice were weighed and anesthetized by intraperitoneal injection of a 0.1 mg/kg fentanyl, 5 mg/kg midazolam, and 0.25 mg/kg dexmedetomidine mixture. Once pedal reflex ceased, mice were shaved and a total dose of 1 × 1010 vg of AAV was administered through bilateral intrastriatal injection (2 μL/side) performed as previously described86 at the following coordinates from bregma: +1.0 mm anterior–posterior, ±2.0 mm mediolateral, and −3.0 mm dorsoventral.
Once the injection was completed, mice were intraperitoneally injected with 0.5 mg/kg flumazenil and 5.0 mg/kg atipamezole and subcutaneously injected with 0.3 mg/kg buprenorphine. Mice were euthanized at 5 weeks postinjection and perfused with PBS. Brains were harvested and biopsies at the striatum were taken for genomic DNA extraction.
Genomic DNA extraction from cultured cells and mouse tissues
For cultured cells, genomic DNAs were extracted 72 h after plasmid transfection, or 48 h after mRNA and sgRNA electroporation. In brief, cell culture media were aspirated, and cells were washed with 1 × PBS. Genomic DNAs were prepared using QuickExtract DNA extraction solution (Lucigen) following the manufacturer's protocols.
All five lobes of mouse liver were combined and pulverized in liquid nitrogen, and 15 mg of the tissue from each mouse liver was used for genomic DNA extraction. Genomic DNA from mouse liver or striatum was extracted using GenElute Mammalian Genomic DNA Miniprep Kit (Cat. No.: G1N350; Millipore Sigma).
Amplicon deep sequencing and data analysis
Genomic DNA was amplified by PCR using Q5 High-Fidelity 2X Master Mix (Cat. No.: M0492; NEB) for 20 cycles. One microliter of the unpurified PCR product was used as a template for 20 cycles of barcoding PCR. The barcoding PCRs were further pooled and gel extracted using Zymo gel extraction kit and DNA clean and concentrator (Cat. Nos.: 11-301 and 11-303; Zymo Research ) and quantified by Qubit 1 × dsDNA HS assay kits (Cat. No.: Q32851; Thermo Fisher Scientific).
Sequencing of the pooled amplicons was performed using an Illumina MiniSeq system (300-cycles, FC-420-1004) following the manufacturer's protocol. The raw MiniSeq output was demultiplexed using bcl2fastq2 (Illumina, version 2.20.0) with the flag—barcode mismatches 0. To align the generated fastq files and to quantify editing efficiency, CRISPResso2 (Ref.87; version 2.0.40) was used in batch mode with BEs output and the following flags: -w 15, -q 30. Indel frequency = (insertions reads + deletions reads)/all aligned reads × 100%.
Immunohistochemistry
Mice were euthanized by CO2 asphyxiation and livers were fixed with 10% neutral buffered formalin (Cat. No.: 5735; Epredia), sectioned at 10 μm, and stained with hematoxylin and eosin for pathology analysis. For IHC, liver sections were dewaxed, rehydrated, and stained using an anti-FAH antibody (Cat. No.: ab83770; Abcam) at 1:400 dilution as described previously.67 The percentage of FAH-positive cells was quantified using the following equation: FAH+ hepatocytes % = N/280 (N = the average number of FAH-positive cells in five randomly chosen fields under a Leica microscope, bright field, 20 × magnification).
Reverse transcription PCR
All five lobes of each mouse liver were combined and pulverized in liquid nitrogen. Total RNA was extracted from 50 mg of liver tissue using TRIzol reagent (Cat. No.: 15596026; ThermoFisher) and reverse transcribed using SuperScript III First-Strand Synthesis System (Cat. No.:18080051; ThermoFisher). PCR was performed using primers previously described.67
This study was approved by the IRB of UMass Chan Medical School, and all the animal study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at UMass Chan Medical School.
Supplementary Material
Acknowledgments
We thank Yueying Cao and Greg Cottle for their assistance with the mouse colony maintenance and tail vein injections. We thank Zexiang Chen for his help with the in vitro mRNA transcription, Nadia Amrani for providing the AcrIIA4 plasmid, Tingting Jiang for providing FahPM/PM MEF cells and Spy-RA6.3 plasmid, and Suk Namkung for his assistance with the alkaline gel electrophoresis. We also thank the UMCMS Viral Vector Core for AAV packaging service, the UMCMS Morphology Core for tissue sectioning and IHC staining, the UMCMS Flow Cytometry Core for FACS support, and the Rett Syndrome Research Trust for patient-derived fibroblasts. We are grateful to all members of the Watts, Gao, Wolfe, Xue, and Sontheimer laboratories for their valuable discussions, advice, and helpful feedback.
Authors' Contributions
H.Z., X.D.G., and E.J.S. conceived the study. H.Z. designed, performed, and analyzed the in vivo experiments. H.Z., N.B., O.O., P.L., and X.D.G. designed, performed, and analyzed the in vitro experiments. T.R. and H.C. analyzed the deep sequencing data. K.K. performed intrastriatal injections. E.J.S., W.X., S.A.W., and J.K.W. supervised research. H.Z. and E.J.S. wrote the article with contributions from N.B., P.L., H.C., and K.K. All authors edited the article.
Author Disclosure Statement
H.Z., N.B., P.L., X.D.G., S.A.W., W.X., and E.J.S. are coinventors on patent filings related to this study. G.G. is scientific cofounder, scientific advisor, and equity holder of Voyager Therapeutics, Adrenas Therapeutics, and Aspa Therapeutics. E.J.S. is a cofounder, scientific advisor, and equity holder of Intellia Therapeutics, and a member of the scientific advisory board of Tessera Therapeutics.
Funding Information
This study was supported by the National Institutes of Health (Grant Nos. R01GM125797 to E.J.S., F31GM143879 to N.B., R01HL150669 to S.A.W., and S10OD028576 to the UMCMS Flow Cytometry Core); the Rett Syndrome Research Trust (to J.K.W., S.A.W., and E.J.S.); and the Leducq Foundation (Grant No. 20CVD04 to E.J.S.). G.G. was supported by grants from National Institutes of Health (R01NS076991-01, P01AI100263-01, P01HL131471-02, R01AI121135, UG3HL147367-01, R01HL097088, and U19AI149646-01). W.X. was supported by grants from the National Institutes of Health (DP2HL137167, P01HL131471 and UH3HL147367), American Cancer Society (129056-RSG-16-093), and the Cystic Fibrosis Foundation (XUE19XX0).
Supplementary Material
References
- 1. Landrum MJ, Lee JM, Benson M, et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862–D868. DOI: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rees HA, Liu DR. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19:770–788. DOI: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. DOI: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komor AC, Kim YB, Packer MS, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. DOI: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothgangl T, Dennis MK, Lin PJC, et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat Biotechnol. 2021;39:949–957. DOI: 10.1038/s41587-021-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593:429–434. DOI: 10.1038/s41586-021-03534-y. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Xue W, Zhang H, et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat Cell Biol. 2021;23:552–563. DOI: 10.1038/s41556-021-00671-4. [DOI] [PubMed] [Google Scholar]
- 8. Liu P, Liang S-Q, Zheng C, et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat Commun. 2021;12:2121.. DOI: 10.1038/s41467-021-22295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villiger L, Rothgangl T, Witzigmann D, et al. In vivo cytidine base editing of hepatocytes without detectable off-target mutations in RNA and DNA. Nat Biomed Eng. 2021;5:179–189. DOI: 10.1038/s41551-020-00671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Böck D, Rothgangl T, Villiger L, et al. In vivo prime editing of a metabolic liver disease in mice. Sci Transl Med. 2022;14(636):eabl9238.. DOI: 10.1126/scitranslmed.abl9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koblan LW, Erdos MR, Wilson C, et al. In vivo base editing rescues Hutchinson-Gilford progeria syndrome in mice. Nature. 2021;589:608–614. DOI: 10.1038/s41586-020-03086-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu S-M, Koo T, Kim K, et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat Biotechnol. 2018;36:536–539. DOI: 10.1038/nbt.4148. [DOI] [PubMed] [Google Scholar]
- 13. Chemello F, Chai AC, Li H, et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci Adv. 2021;7. [Epub ahead of print]; DOI: 10..1126/sciadv.abg4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suh S, Choi EH, Leinonen H, et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat Biomed Eng. 2021;5:169–178. DOI: 10.1038/s41551-020-00632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhi S, Chen Y, Wu G, et al. Dual-AAV delivering split prime editor system for in vivo genome editing. Mol Ther. 2021. [Epub ahead of print]; DOI: 10.1016/j.ymthe.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh W-H, Shubina-Oleinik O, Levy JM, et al. In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci Transl Med. 2020;12:eaay9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim CKW, Gapinske M, Brooks AK, et al. Treatment of a mouse model of ALS by in vivo base editing. Mol Ther. 2020;28:1177–1189. DOI: 10.1016/j.ymthe.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy JM, Yeh W-H, Pendse N, et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat Biomed Eng. 2020;4:97–110. DOI: 10.1038/s41551-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. DOI: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. DOI: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 21. Villiger L, Grisch-Chan HM, Lindsay H, et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat Med 2018;24:1519–1525. DOI: 10.1038/s41591-018-0209-1. [DOI] [PubMed] [Google Scholar]
- 22. Winter J, Luu A, Gapinske M, et al. Targeted exon skipping with AAV-mediated split adenine base editors. Cell Discov. 2019;5:41.. DOI: 10.1038/s41421-019-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinderer C, Katz N, Buza EL, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–298. DOI: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum Gene Ther. 2020;31:695–696. DOI: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Smith J, Breton C, et al. Meganuclease targeting of PCSK9 in macaque liver leads to stable reduction in serum cholesterol. Nat Biotechnol. 2018;36:717–725. DOI: 10.1038/nbt.4182. [DOI] [PubMed] [Google Scholar]
- 26. Ran FA, Cong L, Yan WX, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. DOI: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibraheim R, Tai PWL, Mir A, et al. Self-inactivating, all-in-one AAV vectors for precision Cas9 genome editing via homology-directed repair in vivo. Nat Commun. 2021;12:6267.. DOI: 10.1038/s41467-021-26518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maeder ML, Stefanidakis M, Wilson CJ, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 2019;25:229–233. DOI: 10.1038/s41591-018-0327-9. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen Tran MT, Mohd Khalid MKN, Wang Q, et al. Engineering domain-inlaid SaCas9 adenine base editors with reduced RNA off-targets and increased on-target DNA editing. Nat Commun. 2020;11:4871.. DOI: 10.1038/s41467-020-18715-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edraki A, Mir A, Ibraheim R, et al. A compact, high-accuracy Cas9 with a dinucleotide PAM for in vivo genome editing. Mol Cell. 2019;73:714–726.e4. DOI: 10.1016/j.molcel.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Z, Chen S, Jia Y, et al. Efficient and high-fidelity base editor with expanded PAM compatibility for cytidine dinucleotide. Sci China Life Sci. 2021;64:1355–1367. DOI: 10.1007/s11427-020-1775-2. [DOI] [PubMed] [Google Scholar]
- 32. Xu R, Qin R, Xie H, et al. Genome editing with type II-C CRISPR-Cas9 systems from Neisseria meningitidis in rice. Plant Biotechnol J. 2022;20:350–359. DOI: 10.1111/pbi.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hua K, Tao X, Zhu J-K. Expanding the base editing scope in rice by using Cas9 variants. Plant Biotechnol J. 2019;17:499–504. DOI: 10.1111/pbi.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, Zhang X, Wang L, et al. Correction to: Increasing targeting scope of adenosine base editors in mouse and rat embryos through fusion of TadA deaminase with Cas9 variants. Protein Cell. 2019;10:700.. DOI: 10.1007/s13238-019-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richter MF, Zhao KT, Eton E, et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol. 2020;38:883–891. DOI: 10.1038/s41587-020-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaudelli NM, Lam DK, Rees HA, et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat Biotechnol 2020;38:892–900. DOI: 10.1038/s41587-020-0491-6. [DOI] [PubMed] [Google Scholar]
- 37. Rees HA, Komor AC, Yeh W-H, et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat Commun. 2017;8:15790.. DOI: 10.1038/ncomms15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim D, Lim K, Kim S-T, et al. Genome-wide target specificities of CRISPR RNA-guided programmable deaminases. Nat Biotechnol. 2017;35:475–480. DOI: 10.1038/nbt.3852. [DOI] [PubMed] [Google Scholar]
- 39. Wen J, Cao T, Wu J, et al. Single AAV-mediated CRISPR-Nme2Cas9 efficiently reduces mutant hTTR expression in a transgenic mouse model of transthyretin amyloidosis. Mol Ther. 2022;30:164–174. DOI: 10.1016/j.ymthe.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rauch BJ, Silvis MR, Hultquist JF, et al. Inhibition of CRISPR-Cas9 with bacteriophage proteins. Cell. 2017;168:150–158.e10. DOI: 10.1016/j.cell.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J, Mir A, Edraki A, et al. Potent Cas9 inhibition in bacterial and human cells by AcrIIC4 and AcrIIC5 anti-CRISPR proteins. MBio. 2018;9:e02321-18. DOI: 10.1128/mBio.02321-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hwang S, Maxwell KL. Meet the Anti-CRISPRs: Widespread protein inhibitors of CRISPR-Cas Systems. CRISPR J. 2019;2:23–30. DOI: 10.1089/crispr.2018.0052. [DOI] [PubMed] [Google Scholar]
- 43. Pawluk A, Amrani N, Zhang Y, et al. Naturally occurring off-switches for CRISPR-Cas9. Cell. 2016;167:1829–1838.e9. DOI: 10.1016/j.cell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shin J, Jiang F, Liu J-J, et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv. 2017;3:e1701620.. DOI: 10.1126/sciadv.1701620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee J, Mou H, Ibraheim R, et al. Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins. RNA. 2019;25:1421–1431. DOI: 10.1261/rna.071704.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harrington LB, Doxzen KW, Ma E, et al. A broad-spectrum inhibitor of CRISPR-Cas9. Cell. 2017;170:1224–1233.e15. DOI: 10.1016/j.cell.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liang M, Sui T, Liu Z, et al. acriia5 suppresses base editors and reduces their off-target effects. Cells. 2020;9:1786.. DOI: 10.3390/cells9081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoffmann MD, Aschenbrenner S, Grosse S, et al. Cell-specific CRISPR–Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 2019;47:e75.. DOI: 10.1093/nar/gkz271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bienvenu T, Carrié A, de Roux N, et al. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9:1377–1384. DOI: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- 50. Lyst MJ, Bird A. Rett syndrome: A complex disorder with simple roots. Nat Rev Genet. 2015;16:261–275. DOI: 10.1038/nrg3897. [DOI] [PubMed] [Google Scholar]
- 51. Krishnaraj R, Ho G, Christodoulou J. RettBASE: Rett syndrome database update. Hum Mutat. 2017;38:922–931. DOI: 10.1002/humu.23263. [DOI] [PubMed] [Google Scholar]
- 52. Stefanelli G, Gandaglia A, Costa M, et al. Brain phosphorylation of MeCP2 at serine 164 is developmentally regulated and globally alters its chromatin association. Sci Rep. 2016;6:28295.. DOI: 10.1038/srep28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tan J, Zhang F, Karcher D, et al. Engineering of high-precision base editors for site-specific single nucleotide replacement. Nat Commun. 2019;10:439.. DOI: 10.1038/s41467-018-08034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chu SH, Packer M, Rees H, et al. Rationally designed base editors for precise editing of the sickle cell disease mutation. CRISPR J. 2021;4:169–177. DOI: 10.1089/crispr.2020.0144. [DOI] [PubMed] [Google Scholar]
- 55. Huang TP, Zhao KT, Miller SM, et al. Circularly permuted and PAM-modified Cas9 variants broaden the targeting scope of base editors. Nat Biotechnol. 2019;37:626–631. DOI: 10.1038/s41587-019-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Zhou L, Liu N, et al. BE-PIGS: A base-editing tool with deaminases inlaid into Cas9 PI domain significantly expanded the editing scope. Signal Transduct Target Ther. 2019;4:36.. DOI: 10.1038/s41392-019-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li S, Yuan B, Cao J, et al. Docking sites inside Cas9 for adenine base editing diversification and RNA off-target elimination. Nat Commun. 2020;11:5827.. DOI: 10.1038/s41467-020-19730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Zhou C, Huang S, et al. A Cas-embedding strategy for minimizing off-target effects of DNA base editors. Nat Commun. 2020;11:6073.. DOI: 10.1038/s41467-020-19690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chemello F, Wang Z, Li H, et al. Degenerative and regenerative pathways underlying Duchenne muscular dystrophy revealed by single-nucleus RNA sequencing. Proc Natl Acad Sci U S A. 2020;117:29691–29701. DOI: 10.1073/pnas.2018391117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson KM, Poosala P, Lindley SR, et al. Targeted cleavage and polyadenylation of RNA by CRISPR-Cas13. bioRxiv. 2019;531111. DOI: 10..1101/531111. [Google Scholar]
- 61. Koblan LW, Doman JL, Wilson C, et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol. 2018;36:843–846. DOI: 10.1038/nbt.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Preece R, Georgiadis C, Gkazi SA, et al. “Mini” U6 Pol III promoter exhibits nucleosome redundancy and supports multiplexed coupling of CRISPR/Cas9 effects. Gene Ther. 2020;27:451–458. DOI: 10.1038/s41434-020-0142-z. [DOI] [PubMed] [Google Scholar]
- 63. Aponte JL, Sega GA, Hauser LJ, et al. Point mutations in the murine fumarylacetoacetate hydrolase gene: Animal models for the human genetic disorder hereditary tyrosinemia type 1. Proc Natl Acad Sci U S A. 2001;98:641–645. DOI: 10.1073/pnas.98.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lindstedt S. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. DOI: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 65. Yin H, Xue W, Chen S, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551–553. DOI: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yin H, Song C-Q, Dorkin JR, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34:328–333. DOI: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song C-Q, Jiang T, Richter M, et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat Biomed Eng. 2020;4:125–130. DOI: 10.1038/s41551-019-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiang T, Henderson JM, Coote K, et al. Chemical modifications of adenine base editor mRNA and guide RNA expand its application scope. Nat Commun. 2020;11:1979.. DOI: 10.1038/s41467-020-15892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shin JH, Jung S, Ramakrishna S, et al. In vivo gene correction with targeted sequence substitution through microhomology-mediated end joining. Biochem Biophys Res Commun. 2018;502:116–122. DOI: 10.1016/j.bbrc.2018.05.130. [DOI] [PubMed] [Google Scholar]
- 70. Jang H, Jo DH, Cho CS, et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat Biomed Eng. 2022;6:181–194. DOI: 10.1038/s41551-021-00788-9. [DOI] [PubMed] [Google Scholar]
- 71. Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. DOI: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 72. Paulk NK, Wursthorn K, Wang Z, et al. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology. 2010;51:1200–1208. DOI: 10.1002/hep.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang M-J, Chen F, Lau JTY, et al. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017;8:e2805.. DOI: 10.1038/cddis.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fry LE, Peddle CF, Stevanovic M, et al. Promoter orientation within an AAV-CRISPR vector affects Cas9 expression and gene editing efficiency. CRISPR J. 2020;3:276–283. DOI: 10.1089/crispr.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–844. DOI: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 76. Newby GA, Liu DR. In vivo somatic cell base editing and prime editing. Mol Ther. 2021;29:3107–3124. DOI: 10.1016/j.ymthe.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. High-dose AAV gene therapy deaths. Nat Biotechnol. 2020;38:910. DOI: 10.1038/s41587-020-0642-9. [DOI] [PubMed] [Google Scholar]
- 78. Morales L, Gambhir Y, Bennett J, et al. Broader implications of progressive liver dysfunction and lethal sepsis in two boys following systemic high-dose AAV. Mol Ther. 2020;28:1753–1755. DOI: 10.1016/j.ymthe.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Flotte TR. Revisiting the “new” inflammatory toxicities of adeno-associated virus vectors. Hum Gene Ther. 2020;31:398–399. DOI: 10.1089/hum.2020.29117.trf. [DOI] [PubMed] [Google Scholar]
- 80. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18:358–378. DOI: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: Strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–150. DOI: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ibraheim R, Song C-Q, Mir A, et al. All-in-one adeno-associated virus delivery and genome editing by Neisseria meningitidis Cas9 in vivo. Genome Biol. 2018;19:137.. DOI: 10.1186/s13059-018-1515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Krooss SA, Dai Z, Schmidt F, et al. Ex vivo/in vivo gene editing in hepatocytes using “All-in-One” CRISPR-adeno-associated virus vectors with a Self-Linearizing Repair Template. iScience. 2020;23:100764.. DOI: 10.1016/j.isci.2019.100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sun W, Yang J, Cheng Z, et al. Structures of Neisseria meningitidis Cas9 complexes in catalytically poised and anti-CRISPR-inhibited states. Mol Cell. 2019;76:938–952.e5. DOI: 10.1016/j.molcel.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sena-Esteves M, Gao G. Introducing genes into mammalian cells: Viral vectors. Cold Spring Harb Protoc. 2020;2020:095513.. DOI: 10.1101/pdb.top095513. [DOI] [PubMed] [Google Scholar]
- 86. Alterman JF, Godinho BMDC, Hassler MR, et al. A divalent siRNA chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous system. Nat Biotechnol. 2019;37:884–894. DOI: 10.1038/s41587-019-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clement K, Rees H, Canver MC, et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat Biotechnol. 2019;37:224–226. DOI: 10.1038/s41587-019-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.