Abstract
At the vertebrate neuromuscular junction (NMJ), presynaptic homeostatic potentiation (PHP) refers to the upregulation of neurotransmitter release via an increase in quantal content (QC) when the postsynaptic nicotinic acetylcholine receptors (nAChRs) are partially blocked. The mechanism of PHP has not been completely worked out. In particular, the identity of the presumed retrograde signal is still a mystery. We investigated the role of acid-sensing ion channels (ASICs) and extracellular protons in mediating PHP at the mouse NMJ. We found that blocking AISCs using benzamil, psalmotoxin-1 (PcTx1), or mambalgin-3 (Mamb3) prevented PHP. Likewise, extracellular acidification from pH 7.4 to 7.2 triggered a significant, reversable increase in QC and this increase could be prevented by PcTx1. Interestingly, an acidic saline (pH 7.2) also precluded the subsequent induction of PHP. Using immunofluorescence we observed ASIC2a and ASIC1 subunits at the NMJ. Our results indicate that protons and ASIC channels are involved in activating PHP at the mouse NMJ. We speculate that the partial blockade of nAChRs leads to a modest decrease in the pH of the synaptic cleft (~ 0.2 pH units) and this activates ASIC channels on the presynaptic nerve terminal.
Keywords: synaptic, plasticity, homeostatic, proton, ASIC
Graphical Abstract

INTRODUCTION
Synaptic homeostasis, defined as a process that returns synaptic transmission to its original set point following a perturbation, is observed at a wide range of synapses and organisms (Rich and Wenner, 2007; Turrigiano, 2012; Frank, 2014; Davis and Müller, 2015; Fox and Stryker, 2017; Delvendahl and Müller, 2019). At the adult vertebrate neuromuscular junction (NMJ), synaptic homeostasis regulates the amount of acetylcholine (ACh) released by the presynaptic nerve terminal to maintain a set level of excitatory response in muscle cells. For example, partial block or loss of nicotinic acetylcholine receptors (nAChRs) triggers an upregulation of quantal content (QC) to restore a normal level of end-plate potentials (EPPs) in rat, mouse and human NMJs (Miledi et al., 1978; Molenaar et al., 1979, 1991; Cull-Candy et al., 1980; Wilson, 1982; Plomp et al., 1992; Wang et al., 2010, 2016; Orr et al., 2020). D-tubocurarine (d-TC) is one of many blockers of nAChR that triggers such upregulation of QC in vertebrate NMJs, a phenomenon termed presynaptic homeostatic potentiation (PHP) (Wilson, 1982; Wang et al., 2016, 2018b).
Numerous components of the synapse have been implicated in PHP (Davis and Müller, 2015; Delvendahl and Müller, 2019; Frank et al., 2020), including the novel suggestion that noncanonical signaling by the nAChRs ultimately leads to upregulation of vesicle release presynaptically (Wang et al., 2018b). However, the nature of the retrograde message that conveys the status of the postsynaptic nAChRs to the presynaptic nerve terminal is not known. We propose that protons in the synaptic cleft serve that role.
In certain cases it has been shown that upon neuronal stimulation, synaptic cleft pH decreases as vesicles (pH 5) that are more acidic than the larger extracellular pool (pH 7.4), are released into the synaptic cleft; such acidification may underlie synaptic plasticity in retinal reciprocal synapses and drosophila NMJs (Hirasawa and Kaneko, 2003; Caldwell et al., 2013; Wang et al., 2014; Beckwith-Cohen et al., 2019). The key players that respond to acidosis and upregulate presynaptic neurotransmitter release are acid-sensing ion channels (ASICs). As a subfamily of the epithelial sodium channel (ENaC)/ degenerin (DEG) channel, ASICs are activated by extracellular acidification, permeable to Na± and Ca2±, and highly expressed in the CNS and in touch-sensitive neurons in the PNS (Boscardin et al., 2016; Vullo and Kellenberger, 2019). Female mice lacking ASIC1a have impaired motor function compared to wild type, indicating that ASICs indeed help maintain synaptic transmission at NMJs (Urbano et al., 2014). We investigated the hypothesis that a decrease in extracellular pH in the synaptic cleft is responsible for PHP by activating ASICs. We showed that blocking ASICs abolished the upregulation of QC caused by either extracellular acidification or partial blockage of nAChRs. Furthermore, extracellular acidification precludes PHP induced by d-TC.
EXPERIMENTAL PROCEDURE
Ethical approval
Use and care of animals was approved and supervised by the Institutional Animal Use and Care Committee (IACUC) at Grinnell College.
Animals and solutions
C57B/6 mice (4- to 8-week-old; Jackson Laboratories, Bar Harbor, Maine, USA) were anesthetized using CO2 inhalation, followed by cervical dislocation. The epitrochleoanconeus (ETA) muscle was dissected and perfused with fresh mouse Ringers solution (109.5 mM NaCl, 3.45 mM KCl, 0.7 mM MgCl2·6H2O, 1.7 mM NaH2PO4·H2O, 1.7 mM NaH2PO4·7H2O, 30.7 mM NaHCO3, 1.8 mM CaCl2·2H2O, and 11.2 mM D-glucose; pH 7.4) and a gas mixture of 95% O2 and 5% CO2 at 3 ml/min. The experiments were all performed at 24±2°C.
Drugs
In all experiments, the ETA muscle was perfused with 0.8 μM μ-Conotoxin GIIIB (μ-Ctx; Alomone Labs, Jerusalem, Israel) for 20 mins to inhibit voltage-gated Na± channels in the muscle prior to recording. The ETA muscle was then perfused with 0.4 μM μ-Conotoxin GIIIB throughout the experiment. In Figs. 1 and 2, the muscle was constantly perfused with pH 7.4 ringer. In Figs. 3 and 4, the muscle was subsequently perfused with low pH mouse ringer (117 mM NaCl, 23.2 mM NaHCO3, [other components remain unchanged]; pH 7.2) (122.2 mM NaCl, 18.0 mM NaHCO3, [other components remain unchanged]; pH 7.0). The final pH of the ringer was verified before the start of each experiment. In Figs. 2 and 4, benzamil hydrochloride hydrate (Sigma-Aldrich, St. Louis, MO), psalmotoxin-1 (PcTx1; Alomone Labs), or mambalgin-3 (Mamb3; Alomone Labs), were applied (in the presence of μ-Ctx) for 30 minutes prior to recording. 75–100 nM D-tubocurarine (d-TC; Tocris, Bristol, United Kingdom) was applied for 10 minutes before measuring EPPs or mEPPs. Benzamil was prepared as a 50 mM stock solution with DMSO, and subsequently dissolved in the mouse ringer at 1:1000 or 1:500. When DMSO was applied by itself at 1:1000 or 1:500 dilution at the mouse NMJ, no changes of electrophysiological parameters were observed. PcTx1 and Mamb3 were prepared as 50 mM stock solutions in water and diluted into mouse ringers to produce the final concentrations.
Fig. 1.
D-Tubocurarine (d-TC) induces presynaptic homeostatic potentiation (PHP) and this is blocked by ASIC inhibitors. A. mEPPs and EPPs were measured before and after a 10 min incubation in d-TC (n = 75 NMJs, 15 mice). Quantal Content (QC) was estimated from corrected EPP and mEPP amplitudes. The data are presented as violin plots, which show the probability density of the data at different values. The mean differences are shown as estimation plots (see Experimental Procedure: Statistical Analysis.) The insets are sample traces of EPPs and mEPPs before (black line) and after (grey line) d-TC. Each trace is the average of 10 measurements. Calibration bars indicate 0.2 mv, 2 ms for the mEPPs and 5 mv, 2 ms for the EPPs. B. QC was calculated from corrected mEPP and EPP amplitudes in the presence of 50 μM benzamil (n=25, 5 mice), 100 μM benzamil (n=15, 3 mice), 50 nM PcTx1 (n=73, 10 mice), 100 nM PcTx1 (n=25, 5 mice), and 50 nM Mamb3 (n=20, 4 mice) before and after a 10 min incubation in d-TC. For each condition, the ratios of QC after vs. before dTC were calculated and plotted as violin plots. The mean differences are shown as estimation plots. In both A and B, means are depicted as black dots; 95% confidence intervals are indicated by the ends of vertical error bars. In all figures, p values are indicated and were calculated from an ordinary one-way ANOVA.
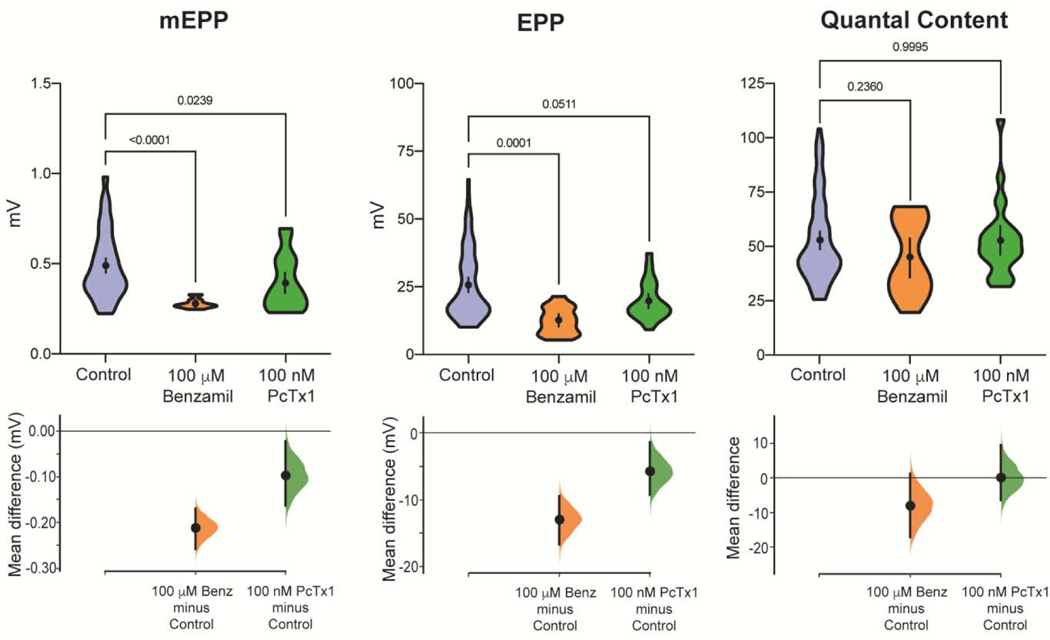
Fig. 2.
High concentrations of the ASIC inhibitors Benzamil and PcTx1 reduce mEPP and EPP amplitude. MEPPs, EPPs and Quantal Content are plotted from NMJs incubated in either control saline (n=75, same data show in 1A), 100 μM benzamil (n=15, 3 mice), and 100 nM PcTx1 (n=25, 5 mice). Data are plotted as in Fig. 1. P values are indicated and were calculated from Student’s t-test or ordinary one-way ANOVA.
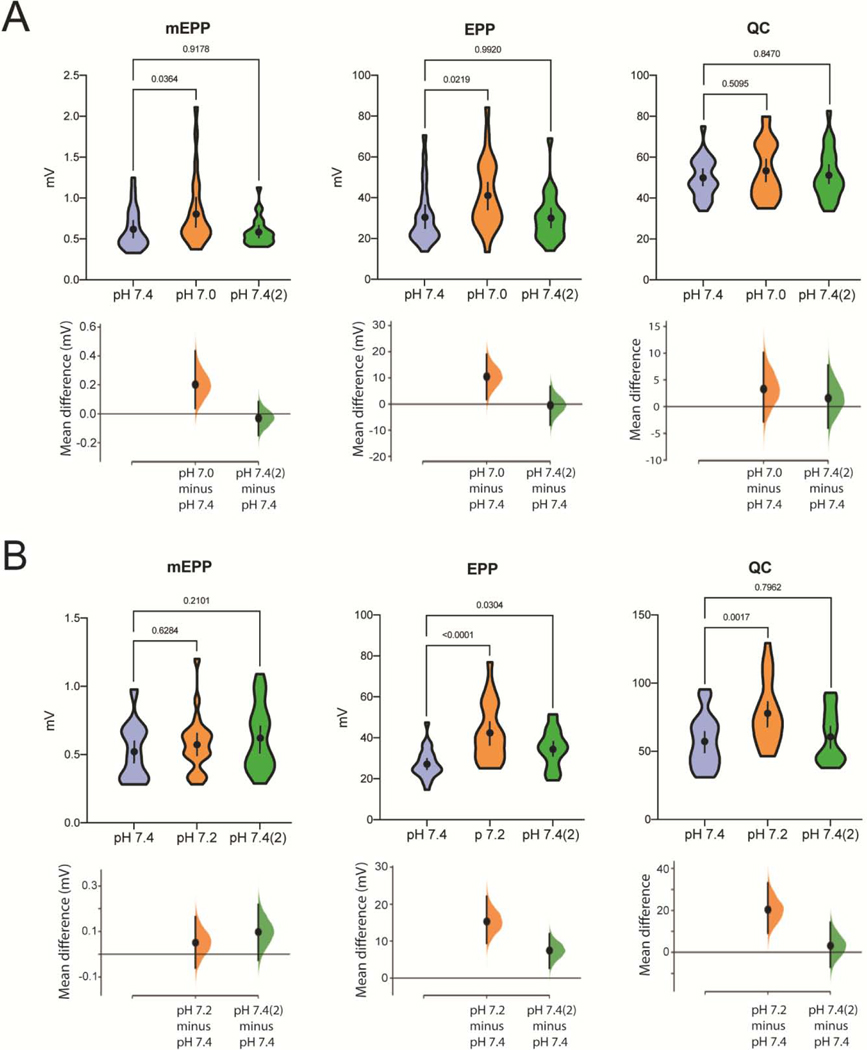
Fig. 3.
The Effect of Lowering extracellular pH on QC. A, mEPPs and EPPs were measured and QC estimated in pH 7.4 saline, after a 7 min incubation in pH 7.0 saline, and after a 20 min reapplication of pH 7.4 saline (n=25, 5 mice). The data are presented in the upper axes as violin plots, which show the probability density of the data at different values. Mean differences are plotted on the lower axes as bootstrap sampling distributions. Means are depicted as black dots; 95% confidence intervals are indicated by the ends of vertical error bars. B, mEPPs and EPPs were measured and QC estimated in pH 7.4 saline, after a 7 min incubation in pH 7.2 saline, and after a 20 min reapplication of pH 7.4 saline (n = 25, 5 mice). The data are presented as in 1A. In all figures, p values are indicated and were calculated from an ordinary one-way ANOVA.
Fig.4.
Lowering extracellular pH underlies the upregulation of QC triggered by d-TC. A. mEPPs and EPPs were measured and QC estimated before and after a 10 min incubation in pH 7.2 saline with 100nM PcTx1 present throughout (n=25, 5 mice). The data for mEPPs, EPPs and QC are presented as violin plots. The plot on the far right includes the data from Fig. 3B to depicts the effect of pH 7.2 saline under control conditions vs. in the presence of 100 nM PcTx1. The Mean differences are plotted as a bootstrap sampling distribution. Means are depicted as black dots; 95% confidence intervals are indicated by the ends of vertical error bars. In all figures, p values are indicated and were calculated from an ordinary one-way ANOVA. B. mEPPs and EPPs were measured and QC was estimated in pH 7.4 saline, after a 7 min incubation in pH 7.2 saline, and then after a 15 min incubation with d-TC, still in pH 7.2 saline (n = 5 mice). Although dTC had its normal effects on mEPP and EPP amplitude (compare to Fig. 1A), dTC did not alter QC when compared to pH 7.2 saline. The data is plotted as in panel A. C. The ratio of QC after application of dTC compared to before dTC was applied (post/pre dTC) is shown for pH 7.4 saline (this is the same data presented in Fig 1B, Control) and for pH 7.2 saline (the same data presented in Fig. 4B).
Immunofluorescence
After dissection, mouse (Mus musculus; strain: C57BL/6J) Epitrochleoanconeus (ETA) muscles were fixed in 4% paraformaldehyde for 15 minutes at 20°C. The muscles were then rinsed in pH 8 HEPES buffer, permeabilized (0.3% Triton X-100 buffer in pH 7.4 HEPES buffer) for 30 minutes at 37°C, and incubated in Blocking Solution (1% bovine serum albumin – Jackson Immuno Research Laboratories – supplemented with 0.1% Triton X-100 in pH 7.4 HEPES buffer) for 1 hour prior to the application of the primary antibody (1 hour at 37° C). The muscles were then incubated in Blocking Solution overnight at 4°C before the application of the secondary antibody (2 hours at 20°C). Muscles were rinsed with Blocking Solution for 1 hour following the application of the secondary antibody before applying α-bungarotoxin (30 minutes at 20°C). For ASIC1a staining, we paired the primary rabbit anti-ASIC1a antibody (ASC-014; Alomone Labs, Jerusalem, Israel) with the American Qualex Goat Anti-rabbit FITC IgG (H±L) secondary antibody (A102FN). For ASIC2a staining we paired the primary rabbit anti-ASIC2a antibody (ASC-012; Alomone Labs) with the American Qualex Goat Anti-rabbit FITC IgG (H±L) secondary antibody (A102FN). For ASIC1 staining we paired the guinea pig primary anti-ASIC1 antibody (AGP-053; Alomone Labs) with Goat Anti-Guinea pig Alexa Fluor 488 IgG (H±L) secondary antibody (A109FN). We co-stained all of the muscles with α-bungarotoxin conjugated to Alexa Fluor 555 (Invitrogen). Primary antibodies were diluted (1:200) in Blocking Solution, secondary antibodies were diluted (1:500) in Blocking Solution, and α-bungarotoxin was diluted (1:500) in pH 7.4 HEPES buffer. Following the staining procedure, muscles were severed from their tissue attachments and mounted on slides with Antifade Mounting Medium with DAPI (Vectashield).
Electrophysiology
The ETA muscle motor axon was stimulated through a glass suction electrode with single square pulses using an SD-9 Stimulator (Grass Instrument Co., Quincy, Massachusetts, USA) set at a frequency of 0.2 Hz, duration of 0.2 ms, and a suprathreshold voltage sufficient to elicit an action potential (Wang et al., 2018a). End-plate potentials (EPPs) and miniature end-plate potentials (mEPPs) were recorded using glass microelectrodes (3M KCl; resistance: 10—50 MΩ). Membrane potentials were amplified using an A-M Systems Model 1600 Amplifier (A-M Systems, Sequim, Washington, USA) and recorded with a PowerLab 4/25 paired with its LabChart software (AD-Instruments, Colorado Springs, Colorado, USA). Recordings were taken in randomly selected muscle fibers for each condition, stimulated at 0.2 Hz (~13 EPPs were measured and averaged per muscle fiber); only muscle fibers with resting potentials below −70mV were accepted. The measured resting potentials and their absolute differences from −75 mV are shown in Table 1S (supplemental data).
Data analysis
EPP and mEPP amplitudes were corrected according to the resting membrane potential (−75 mV). EPP amplitude was further adjusted to account for non-linear summation (McLachlan and Martin, 1981):
where is the EPP amplitude, is the corrected EPP amplitude, E is the reversal potential (we used 0 mV), 𝑉𝑚 is the resting potential, and f is the coefficient meant to correct the experimental voltage-current slope, which is 0.8. QC was calculated from the ratio of corrected EPP amplitude and an average of at least 20 mEPP amplitudes.
Statistical analysis
When comparisons are made between groups of data, we used estimation plots, which emphasize effect sizes and confidence intervals (Gardner and Altman, 1986; Ho et al., 2019a). We entered our raw data into the website https://www.estimationstats.com/#/ to estimate effect sizes (Ho et al., 2019b). The estimation plots are displayed on a separate axis and reveal the size of the difference between two conditions and the 95% confidence intervals. Probability distributions were generated from 5000 bootstrap samples; confidence intervals were bias corrected and accelerated. The P values reported are the likelihoods of observing the effect sizes, if the null hypothesis of zero difference is true. For each permutation P value, 5000 reshuffles of the control and text labels were performed.
In addition to estimating differences between groups of data as described above, we also directly compared groups of data using standard null-hypothesis significance testing. Statistical analysis and plotting were done in Prism 9 (GraphPad Software; San Diego, CA). Statistical significance was determined using Student’s t-test or ordinary one way ANOVA. All data are presented as mean ± SE unless indicated otherwise.
RESULTS
ASIC inhibitors block PHP
Partial block of nAChRs with d-TC induces the upregulation of QC in mouse NMJs (Wang et al., 2016). When we applied d-TC (75 – 100 nM) to decrease the mEPP amplitude by an average of 50.4±2.2%, QC increased by 51.4±6.1% (Fig. 1 A, Table 1). The time course of mEPPs remained unchanged. To test for possible roles of ASICs in PHP at the mouse NMJ, we examined the effects of various ASIC inhibitors (see Table 1). Similar to other epithelial Na channels (ENaCs), ASICs can be blocked by the amiloride analogue benzamil (Leng et al., 2016). We found that 50 μM benzamil reduced PHP by half; QC only increased by 28.0±9.0% after applying dTC (Fig. 1B, Table 1). The mean increase was significantly less that what we observed under normal conditions (Fig. 1B). When the concentration of benzamil was doubled to 100 μM, PHP was blocked completely (Fig. 1B, Table 1).
Table 1.
Effect of ASIC inhibitors on mEPP, EPP and Quantal Content. Mean values ± SEM are shown prior to and 10 minutes after applying dTC. The mean ratios ± SEM of each parameter measured after vs. before dTC are shown to the right of each bracket. P-values are calculated by an ordinary one-way ANOVA.

|
not significant,
p < 0.05,
p < 0.01,
p < 0.001,
p< 0.0001
Our next step was to test more specific ASIC inhibitors on PHP. We first experimented with psalmotoxin-1 (PcTx1), which antagonizes ASIC1a homomers with a very high affinity (IC50 of 1 nM) (Escoubas et al., 2000). When we added 50 nM PcTx1, PHP was reduced but not blocked completely (Fig. 1B, Table 1). Application of dTC increased QC by 20.3±5.7%, an increase significantly different from normal conditions (Fig. 1B, Table 1). It was only when we applied 100 nM PcTx1 that PHP was blocked completely (Fig. 1B, Table 1).
Following our experiments with PcTx1, we tested another high affinity inhibitor of ASIC channels, Mambalgin-3 (Mamb3) (Baron et al., 2013). Of all of the toxins we tested, Mamb3 had the most potent effects on PHP at the mouse NMJ. 50 nM Mamb3 completely blocked PHP (Fig. 1B, Table 1).
While carrying out the above experiments, we made the unexpected observation that at high concentrations the ASIC inhibitors also had direct effects on EPP and mEPP amplitudes. 100 μM benzamil by itself reduced EPPs and mEPPs by 51.0±5.0 and 43.3±1.3 %, respectively, when complared to control conditions (Fig. 2, Table 1). Similarly, 100 nM PcTx1 also significantly reduced EPP and mEPP amplitudes by 22.5±5.5 and 19.8±6.0%, respectively (Fig. 2, Table 1). Thus, these ASIC inhibitors appear to have multiple effects on synaptic transmission at the mouse NMJ.
A Decrease in Extracellular pH Upregulates QC
The discovery that ASICs are involved in PHP led us to ask whether a decrease in extracellular pH could increase neurotransmitter release in an ASIC dependent manner. Currently there are six known homologous ASIC subunits, ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4; these subunits can assemble into heterotrimeric channels with different pH dependencies and kinetics (Wemmie et al., 2013; Wu et al., 2016; Vullo and Kellenberger, 2019). Female mice lacking ASIC1a were shown to be weaker than wild type on grip strength and motor function, suggesting that ASIC1a is functional at the NMJ; additionally, ASIC1a and ASIC2a are highly expressed in the CNS, serving as primary sensors of acidosis and having been implicated in synaptic plasticity in several studies (Urbano et al., 2014; Huang et al., 2015; Liu et al., 2018).
Previous studies demonstrated that ASIC1a channels open at pH < 7 and have an activation threshold near pH 7 (Boscardin et al., 2016; Liu et al., 2018). To test if neurotransmitter release in the mouse NMJ has such pH dependency, we incubated ETA muscles in pH 6.6 and pH 6.8 saline. However, EPP and mEPP amplitudes increased and QC decreased progressively over time in pH < 7 saline. Since it was difficult to elicit stable recordings beyond 7 minutes, we discontinued studying the effects of saline less than pH 7 and focused on pH 7.0 saline. While the measurements remained stable, lowering pH from 7.4 to 7.0 failed to increase QC, though both EPP and mEPP amplitudes increased significantly (Fig. 3 A). There was no change to either the muscle resting potential (−72.2±0.6 mV compared to −74.4±0.8 mV) or MEPP frequency (0.55 ± 0.05 s−1 compared to 0.64 ± 0.05 s−1). After reapplying pH 7.4 saline, both EPP and mEPP amplitudes decreased and became comparable to those recorded before lowering pH, and the change in QC remained insignificant.
Since our results were not consistent with the involvement of homomeric ASIC1a channels in QC (Boscardin et al., 2016; Liu et al., 2018), we considered the possibility that heteromeric ASIC1a/2a channels, which represent a significant portion of ASICs in CNS neurons (Sherwood et al., 2009, 2012; Liu et al., 2018), were similarly involved at the mouse NMJ. Unlike homomeric ASIC1a or ASIC2a channels, ASIC1a/2a channels only experience significant desensitization at pH 7.0 or lower (Joeres et al., 2016a; Liu et al., 2018). According to studies on synaptic plasticity in the reciprocal synapse between photoreceptors and horizontal cells, the pH in the synaptic cleft is only slightly more acidic (about 0.2 unit) compared to the larger extracellular pool (pH 7.4). Thus, we tested the effect of reducing the pH of the extracellular saline to 7.2 (Hirasawa and Kaneko, 2003; Wang et al., 2014; Beckwith-Cohen et al., 2019). In contrast to what we observed at pH 7.0, lowering pH from 7.4 to 7.2 significantly increased QC (35.6±8.0 %; Fig. 3B), but had no effect on either the mEPP amplitude (0.52+0.06 mV at pH 7.4 compared to 0.58+0.04 mV at pH 7.2) or resting membrane potential (−73.2±0.7 at pH 7.4 compared to −76.9±0.7 mV at pH 7.2). These results suggest that ASIC1a/2a channels might drive PHP in the mouse NMJ given that the upregulation of QC is present at pH 7.2 but is abolished at pH 7.0, consistent with ASIC1a/2a pH dependencies, though we cannot exclude the possible role of other ASICs.
As mentioned previously, lowering pH to 7.0 induced a significant increase in mEPP amplitude, 33.1±8.6 %, an effect not observed when pH was lowered to 7.2 (Fig. 3 A, B). Also, in the pH 7.2 experiments, the change in QC was reversible as it returned to original values after the reapplication of pH 7.4 mouse saline, suggesting that the observed upregulation of QC after lowering pH is physiological rather than pathological (Fig. 3 A, B).
Extracellular acidosis underlies d-TC triggered PHP
To connect the observed increase in QC following application of pH 7.2 saline to the d-TC induced PHP, we performed the following two additional experiments. We first studied the effect of PcTx1, which blocked PHP (Fig. 1B), on the effect of pH 7.2 saline. When we applied 100nM PcTx1, the increase in QC normally seen when pH was lowered to 7.2 dissapeared (Fig. 4 A). This is shown most clearly in the panel on the far right in Fig. 4A, where the ratio of QC at pH 7.2 vs. pH 7.4 is compared under control conditions and in the presence of PcTx1.
We next asked if pre-exposing ETA muscles to an acidic mouse saline (pH 7.2) would preclude the normal upregulation of QC triggered by d-TC. We first measured the effect of lowering pH from 7.4 to 7.2, as we had done previously (Fig. 3B), and observed similar results; mEPP amplitude was unchanged, but EPP amplitude and QC increased by 39.4±7.2 and 23.9±4.7%, respectively (Fig. 4B). We then added dTC to the pH 7.2 saline and in this case, QC was unchanged although mEPP and EPP amplitude decreased by 46.4±2.9 and 47.9±3.2, respectively (Fig. 4B). This result is shown most clearly in Fig. 4C, where the ratio of QC before and after applying dTC is compared between pH 7.4 and 7.2 saline. The inability of d-TC to increase QC under an already acidic environment further suggests that PHP employs the same mechanism as the increase in QC induced by lowering extracellular pH to 7.2.
ASICs are present at the mouse NMJ
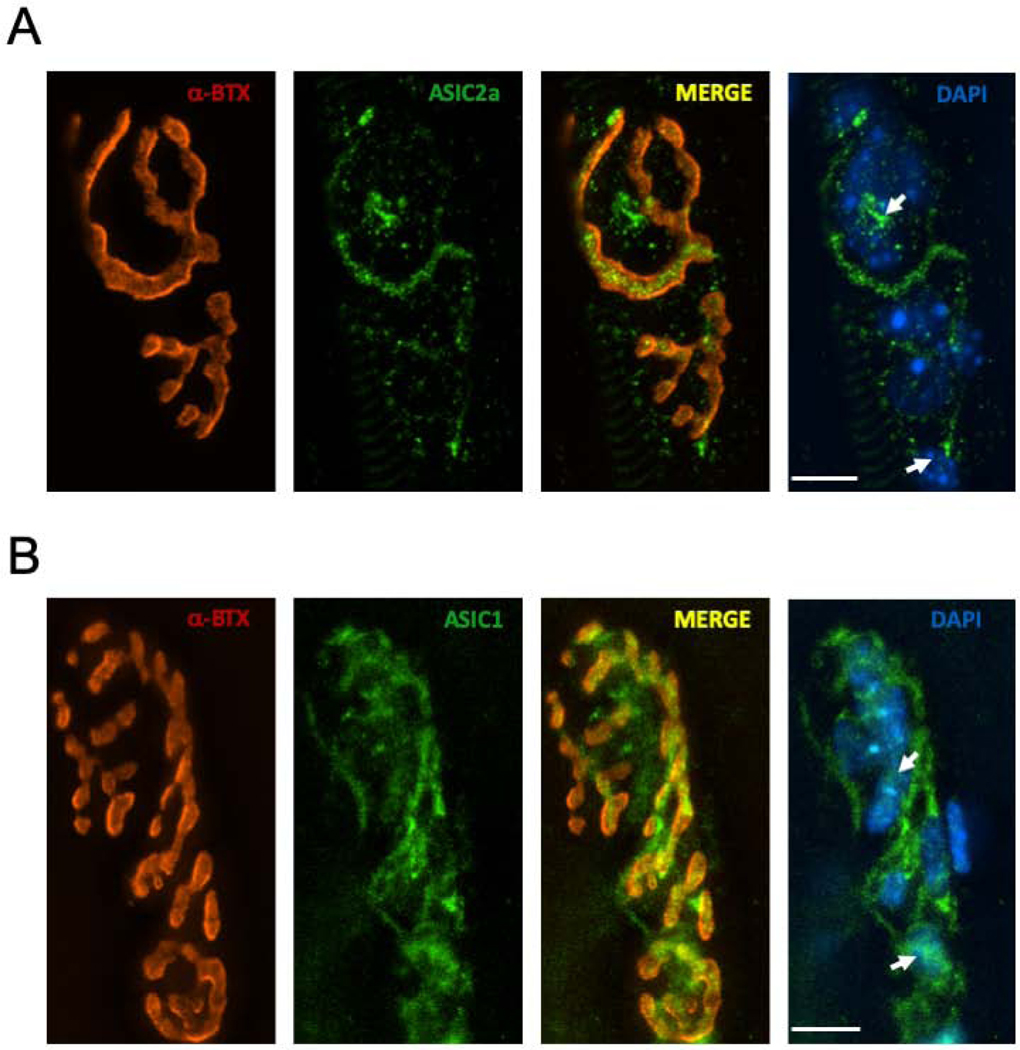
To see if ASIC receptors were present at the mouse NMJ, we performed immunofluorescence. When we applied antibodies to the ASIC2a subunit (ASC-012, Alomone Labs, Jerusalem) we observed clear staining at the NMJ. As shown in Fig. 5A, bright punctate staining was observed in close proximity to α-bungarotoxin which labels the nAChRs. Although light microscopy is not adequate for definitively identifying the component of the NMJ being stained by the ASIC2a antibodies, the pattern is consistent with the presynaptic nerve terminal. Most NMJs examined also contained clusters of staining that were not localized in the immediate vicinity of the nerve terminal. Instead, these clusters were in the area of the NMJ normally occupied by the perisynaptic Schwann cells (PSCs). This is made apparent in Fig. 5A by showing that these clusters typically appeared over the DAPI-stained nuclei of the PSCs.
Fig. 5.
ASIC1 and ASIC2a are located at the mouse NMJ. A. All of the images shown are maximum projections of 16 images collected at 0.5 μm increments vertically through the field containing the NMJ. α-bungarotoxin (α -BTX) is shown in Red. ASIC2a antibodies are in Green. Arrows point to clusters of ASIC2a staining that are outside the area defining the motor nerve terminal but near perisynaptic Schwann cell nuclei, indicated by the DAPI stain (Blue). Calibration bar, 10 μm. B. All of the images shown are maximum projections of 18 images collected at 0.5 μm increments vertically through the field containing the NMJ. α-bungarotoxin (α -BTX) is shown in Red. ASIC1 antibodies are in Green. Arrows point to clusters of ASIC2a staining that are outside the area defining the motor nerve terminal but near perisynaptic Schwann cell nuclei, indicated by the DAPI stain (Blue). Calibration bar, 10 μm.
In contrast to our results with ASIC2a, we did not detect staining by antibodies specific to the ASIC1a subunit (ASC-014; Alomone Labs, Jerusalem). However, we did observe staining with a general antibody to ASIC1 subunits (AGP-053, Alomone Labs, Jerusalem). The staining overall was less intense than what we observed with the ASIC2a antibody and in some NMJs staining was undetectable. Those NMJs that were stained with the ASIC1 antibody exhibited the same pattern we observed with the ASIC2a antibody (Fig. 5B). That is, the staining primarily lined up with α-BTX, with some staining appearing to associate with PSCs.
DISCUSSION
At the vertebrate NMJ, presynaptic homeostatic potentiation (PHP) refers to the upregulation of QC following partial blockage of nAChRs. The full mechanism responsible for PHP remains unknown, though previous studies have implicated several key components for such synaptic homeostasis (Davis and Müller, 2015; Simó et al., 2018; Wang et al., 2018b). We examined the role of extracellular acidification acting through ASICs on PHP at the mouse NMJ. We could prevent PHP with the ASIC inhibitors benzamil, PcTx1, and Mamb3. PcTx1 also blocked the increase in QC observed following mild extracellular acidification (pH 7.2). Finally, PHP was precluded by extracellular acidification. Our findings suggest that the upregulation of QC though ASICs is pH specific and underlies PHP at the mouse NMJ.
Which types of ASICs contribute to PHP at the mouse NMJ?
Our results with benzamil are similar to previous reports that benzamil blocks PHP at NMJs in both Drosophila larvae (Younger et al., 2013; Orr et al., 2017) and mouse diaphragm (Orr et al., 2020); however, in these studies 50 μM benzamil was sufficient to abolish PHP. In our case, 50 μM was only partially effective; only when we doubled the concentration to 100 μM uM did we observe complete inhibition. It remains to be determined whether this apparent difference in sensitivity to benzamil reflects a true pharmacological difference or investigational differences. Consistent with either possibility, we also had to apply a relatively high concentration of PcTx1 (100 nM) to block PHP since 50 nM was only partically effective. However, these differences notwithstanding, the involvement of ASIC1a homomers is unlikely since PcTx1 should have been more potent. This leaves open the possibility of other subunit arrangements, such as an ASIC1a/2a or 1a/2b heterotrimer. It is important to note that previous studies have reported that heteromultimeric assemblies of ASIC1a with other ASIC subunits are insensitive to PcTx1 (Escoubas et al., 2000) or are even enhanced by it (Chen et al., 2006; Sherwood et al., 2011; Liu et al., 2018); however, other studies have reported that high concentrations of PcTx1 (≥ 50 nM) do inhibit ASIC1a/2b (Sherwood et al., 2011) and ASIC1a/2a heteromers (Joeres et al., 2016b).
In addition to our results with benzamil and PcTx1, we also found that Mamb3 was highly effective at preventing PHP. The mambalgins have not been studied as extensively as PcTx1, but have been shown effective at blocking ASIC1a, 1b and 1a/2a multimers (Baron et al., 2013; Kellenberger and Schild, 2015). Thus, our results with Mamb3 provide further support for a role for heteromeric ASIC channels in PHP at the mouse NMJ.
Our immunofluorescence data only partially supports our hypothesis that ASIC1a/2a is the major ASIC at the mouse NMJ. Although antibodies to ASIC2a clearly stained the NMJ with a pattern consistent with the presynaptic nerve terminal and perisynaptic Schwann cells (Fig. 5A), our attempts to detect ASIC1a were unsuccessful. We did however, detect staining, albeit somewhat weaker, with a less specific antibody to ASIC1 subunits (Fig. 5B). The fact that we had an easier time detecting ASIC2a compared to ASIC1 may be because ASIC2a subunits are more abundant than ASIC1a subunits at the mouse NMJ. The antibody we used for ASIC1a may simply have failed to bind at sufficient levels to allow us to detect a signal. Relatively fewer ASIC1a subunits would also explain why we observed weaker staining with the generic ASIC1 antibody. Interestingly, these results are all consistent with an ASIC 1a-2a-2a subunit composition, in which the 2a subunits outnumber 1a subunits. Although such a heterotrimer was once thought to be insensitive to PcTx1 because it lacks a 1a-1a subunit interface, PcTx1 has more recently been shown to inhibit heteromers engineered to contain two 2a subunits (Joeres et al., 2016b).
The localization of ASICs
Studies of synaptic plasticity in the CNS showed that postsynaptic ASICs modulate synaptic transmission in response to extracellular acidification (Wemmie et al., 2002; González-Inchauspe et al., 2017). However, a recent study showed that ASICs can also be presynaptically localized in the mouse NMJ as spontaneous neurotransmitter release was reduced by ASIC1a activation in female mice (Urbano et al., 2014). Our electrophysiological data indicated that the number of ACh quanta released per presynaptic action potential significantly increased in response to partial block of nAChRs with dTC (Fig. 1A) or by extracellular acidification to pH 7.2 (Fig. 3B). Therefore, we propose that ASICs are present on the presynaptic nerve terminal to regulate vesicle release through increasing Na+ and/or Ca2+ influx. Yet, our results do not exclude the possibility of postsynaptic ASICs in the mouse NMJ. In fact, we found that high concentrations of benzamil and PcTx1 significantly decreased the amplitude of mEPPs (Fig. 2) and lowering the extracellular pH from 7.4 to 7.0 significantly increased mEPP amplitude (Fig. 3A). Both of these results might involve the activity of ASICs in the postsynaptic membrane, which alter the sensitivity of the nAChRs. Alternatively, this could reflect a change in the filling of synaptic vesicles presynaptically or a change in the activity of the acetylcholinesterase in the synaptic cleft (Poiana et al., 1985; Wessler et al., 2015).
Our immunofluorescence data is consistent with ASICs being present on the presynaptic terminal, the muscle or both. All we can state with certainty is that the majority of staining follows the pattern of the nerve terminal and underlying nAChRs (Fig. 5). There also appears to be staining associated with the PSCs. A possible function of ASICs on PSCs is an area for future study.
The pH dependencies of ASICs
Previous studies, mostly in Xenopus leavis oocytes, indicated that the pH50 of ASIC1a is approximately 6.5 (Gautschi et al., 2017; Vullo et al., 2017; Vullo and Kellenberger, 2019). Likewise, a study on ASIC1a in motor nerve terminals in female mice suggested that the ASIC1a is essential to maintain synaptic transmission and is activated by pH 6 saline (Urbano et al., 2014). However, we could not record stable EPPs or mEPPs when we applied pH < 7 saline solutions to ETA muscles. This suggested to us that a pH drop to < 7 is not physiologically relevant and might cause pathological damage to the mouse NMJ. In contrast, mild acidification produced electrophysiological changes that were readily reversed upon return to normal pH saline.
The discrepancy between our results and published studies can be explained by two possibilities. First, other ASICs, but not ASIC1a, predominate ETA muscle NMJs, such as ASIC1a/2a as we are suggesting. Second, we lowered and maintained the pH of the bathing solution for at least 7 min whereas a previous study only transiently dropped the pH by using a “puffer” pipette (Urbano et al., 2014). Our method caused a uniform decrease of pH in the extracellular environment, and the extent of decrease (from 7.4 to 7.2) might be comparable to that following brief application of pH 6 saline (Urbano et al., 2014). Further, the assumption that the pH drop in the mouse NMJ is about 0.2 (from 7.4 to 7.2) is supported by studies of the reciprocal synapse between photoreceptors and horizontal cells, which indicate that pH in synaptic cleft is only slightly more acidic (about 0.2 unit) compared to the larger extracellular pool (pH 7.4) (Hirasawa and Kaneko, 2003; Wang et al., 2014; Beckwith-Cohen et al., 2019).
How d-TC might lead to PHP
After confirming that ASICs, activated by extracellular acidification, produce the upregulation of QC observed in PHP, we now ask how block of nAChR by d-TC causes extracellular acidification in the synaptic cleft. This remains a mystery; however, one testable hypothesis is that the inhibition of nAChRs reduces the activity of the plasma membrane Ca2+ ATPase (PMCA) and this leads to acidification. PMCA is a Ca2+/H+ antiporter that pumps Ca2+ across membranes in electroneutral exchange for protons (Thomas, 2009; Ono et al., 2019; Stawarski et al., 2020). In the Drosophila larval NMJ, PMCA pumps out 1 Ca2+ in exchange for 2 H+, causing robust alkalinization of the synaptic cleft exceeding 1 pH unit (Stawarski et al., 2020). Though both pre- and postsynaptic PMCAs are present, the postsynaptic Ca2+/H+ exchange is the major contributor to the alkalinization of synaptic cleft in the Drosophila larval NMJ since the postsynaptic Ca2+ influx is reported to be as much as 100-fold greater than the presynaptic influx (Lu et al., 2016; Stawarski et al., 2020). At the mouse NMJ partial block of nAChRs by d-TC would reduce the influx of Ca2+ across the postsynaptic membrane since fewer nAChR channels are opened. Since the PMCA is stimulated by Ca2+-calmodulin (Enyedi et al., 1989) the decrease in postsynaptic Ca2+ might result in less activation of the PMCA and the removal of fewer protons from the synaptic cleft. The ensuing acidification, as previously proposed to be ~ 0.2 pH unit, activates ASICs and upregulates QC. Future experiments exclusively on the role of PMCA at the mouse NMJ are required to further explore the mechanism of PHP that leads to the acidification of synaptic cleft.
The physiological relevance of PHP
Block of nAChR using d-TC might not be physiologically relevant unless nAChRs have loss-of-function mutations or reduced expression on muscle cells. However, extracellular acidosis is physiologically present as muscle activity accumulates lactic acid and CO2, which leads to acid efflux from cellular compartments to extracellular spaces and reduces pH in the synaptic cleft (Street et al., 2001; Juel, 2008; Lühker et al., 2017). In a study where interstitial pH was measured in human skeletal muscles during graded exercises at different power outputs, it was found that the mean interstitial pH dropped from 7.38 to 7.27 (30 W), 7.16 (50 W), or 7.04 (70 W) (Street et al., 2001). Such results are consistent with our findings on ASIC pH dependencies in a mammalian NMJ, since an optimum output exercise (50 W) lowered the interstitial pH to 7.16 (activation pH) while an overloaded exercise lowered the pH to 7.04 (desensitizing pH) (Street et al., 2001).
Interestingly, in the same study, the peak acidification occurred 1 min after the cessation of exercise, and the interstitial pH became fully recovered after 20 mins (Street et al., 2001). Our results also show that the upregulation of QC in pH 7.2 saline is abolished after 20 mins of reapplying pH 7.4 saline (Fig. 3 B). Combining the extent of pH drop required to activate ASICs and the reversibility of QC upregulation in a mammalian NMJ, our study suggests that d-TC induced PHP shares the same mechanism used to respond to exercise induced acidosis.
Supplementary Material
Highlights:
Presynaptic homeostatic potentiation (PHP) refers to the ability of synapses to compensate for a decrease in the response of the postsynaptic receptors by increasing the evoked release of neurotransmitter.
The mammalian neuromuscular junction has been known for a long time to undergo PHP following the application of nicotinic acetylcholine receptor antagonists. Although many of the molecular details responsible for PHP have been worked out, the identity of the presumed retrograde signal remains a mystery.
In the present study, we performed experiments on Epitrochleoanconeus (ETA) muscles from mice that implicate extracellular protons and acid-sensing ion channels (ASICs) in PHP.
We propose a novel mechanism in which the blockade of nicotinic receptors leads to acidification of the synaptic cleft and this increases the quantal release of acetylcholine by activating ASIC channels on the presynaptic terminal.
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health [grant number. R15NS072735-02] and Grinnell College [intramural funding]. CL was responsible for the general conception and design of the research. YZ and CW performed the electrophysiology experiments. JF performed the immunofluorescence. CL performed the data analysis. CL and YZ wrote the paper.
ABBREVIATIONS
- ACh
acetylcholine
- ASIC
acid-sensing ion channel
- CHO
Chinese hamster ovary
- dTC
D-tubocurarine
- Deg
degenerin channel
- DMSA
dimethyl sulfoxide
- EPP
end-plate potential
- ENaC
epithelial sodium channel
- ETA
epitrochleoanconeus
- Mamb3
mambalgin-3
- mEPP
miniature end-plate potential
- μ-Ctx
μ-Conotoxin
- NMJ
neuromuscular junction
- nAChR
nicotinic acetylcholine receptor
- PMCA
plasma membrane Ca2+ ATPase
- PSC
perisynaptic Schwann cell
- PHP
presynaptic homeostatic potentiation
- PcTx1
psalmotoxin-1
- QC
quantal content
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baron A, Diochot S, Salinas M, Deval E, Noël J, Lingueglia E (2013) Venom toxins in the exploration of molecular, physiological and pathophysiological functions of acid-sensing ion channels. Toxicon 75:187–204. [DOI] [PubMed] [Google Scholar]
- Beckwith-Cohen B, Holzhausen LC, Wang T-M, Rajappa R, Kramer RH (2019) Localizing Proton-Mediated Inhibitory Feedback at the Retinal Horizontal Cell–Cone Synapse with Genetically-Encoded pH Probes. J Neurosci 39:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin E, Alijevic O, Hummler E, Frateschi S, Kellenberger S (2016) The function and regulation of acid‐sensing ion channels (ASICs) and the epithelial Na+ channel (ENaC): IUPHAR Review 19. Brit J Pharmacol 173:2671–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell L, Harries P, Sydlik S, Schwiening CJ (2013) Presynaptic pH and vesicle fusion in Drosophila larvae neurones. Synapse 67:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kalbacher H, Gründer S (2006) Interaction of Acid-sensing Ion Channel (ASIC) 1 with the Tarantula Toxin Psalmotoxin 1 is State Dependent. J Gen Physiology 127:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Miledi R, Trautmann A, Uchitel OD (1980) On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiology 299:621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Müller M (2015) Homeostatic Control of Presynaptic Neurotransmitter Release. Annu Rev Physiol 77:1–20. [DOI] [PubMed] [Google Scholar]
- Delvendahl I, Müller M (2019) Homeostatic plasticity—a presynaptic perspective. Curr Opin Neurobiol 54:155–162. [DOI] [PubMed] [Google Scholar]
- Enyedi A, Vorherr T, James P, McCormick DJ, Filoteo AG, Carafoli E, Penniston JT (1989) The calmodulin binding domain of the plasma membrane Ca2+ pump interacts both with calmodulin and with another part of the pump. J Biological Chem 264:12313–12321. [PubMed] [Google Scholar]
- Escoubas P, Weille JRD, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275:25116–25121. [DOI] [PubMed] [Google Scholar]
- Fox K, Stryker M (2017) Integrating Hebbian and homeostatic plasticity: introduction. Philosophical Transactions Royal Soc B Biological Sci 372:20160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA (2014) Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology 78:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, James TD, Müller M (2020) Homeostatic control of Drosophila neuromuscular junction function. Synapse 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Altman DG (1986) Confidence intervals rather than P values: estimation rather than hypothesis testing. British Medical Journal 292:746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi I, Bemmelen MX van, Schild L (2017) Proton and non-proton activation of ASIC channels. Plos One 12:e0175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Inchauspe C, Urbano FJ, Guilmi MND, Uchitel OD (2017) Acid-Sensing Ion Channels Activated by Evoked Released Protons Modulate Synaptic Transmission at the Mouse Calyx of Held Synapse. J Neurosci 37:2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A (2003) pH Changes in the Invaginating Synaptic Cleft Mediate Feedback from Horizontal Cells to Cone Photoreceptors by Modulating Ca2+ Channels. J Gen Physiology 122:657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019a) Moving beyond P values: Everyday data analysis with estimation plots. Biorxiv:377978. [DOI] [PubMed] [Google Scholar]
- Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019b) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16:565–566. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jiang N, Li J, Ji Y-H, Xiong Z-G, Zha X (2015) Two aspects of ASIC function: Synaptic plasticity and neuronal injury. Neuropharmacology 94:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeres N, Augustinowski K, Neuhof A, Assmann M, Gründer S (2016a) Functional and pharmacological characterization of two different ASIC1a/2a heteromers reveals their sensitivity to the spider toxin PcTx1. Sci Rep-uk 6:27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeres N, Augustinowski K, Neuhof A, Assmann M, Gründer S (2016b) Functional and pharmacological characterization of two different ASIC1a/2a heteromers reveals their sensitivity to the spider toxin PcTx1. Sci Rep-uk 6:27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C (2008) Regulation of pH in human skeletal muscle: adaptations to physical activity. Acta Physiol 193:17–24. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L (2015) International Union of Basic and Clinical Pharmacology. XCI. Structure, Function, and Pharmacology of Acid-Sensing Ion Channels and the Epithelial Na+ Channel. Pharmacol Rev 67:1–35. [DOI] [PubMed] [Google Scholar]
- Leng T, Si H, Li J, Yang T, Zhu M, Wang B, Simon RP, Xiong Z (2016) Amiloride Analogs as ASIC1a Inhibitors. Cns Neurosci Ther 22:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hagan R, Schoellerman J (2018) Dual actions of Psalmotoxin at ASIC1a and ASIC2a heteromeric channels (ASIC1a/2a). Sci Rep-uk 8:7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Chouhan AK, Borycz JA, Lu Z, Rossano AJ, Brain KL, Zhou Y, Meinertzhagen IA, Macleod GT (2016) High-Probability Neurotransmitter Release Sites Represent an Energy-Efficient Design. Curr Biol 26:2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühker O, Berger MM, Pohlmann A, Hotz L, Gruhlke T, Hochreiter M (2017) Changes in acid–base and ion balance during exercise in normoxia and normobaric hypoxia. Eur J Appl Physiol 117:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Martin AR (1981) Non-linear summation of end-plate potentials in the frog and mouse. J Physiology 311:307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Molenaar PC, Polak RL (1978) α-Bungarotoxin enhances transmitter ‘released’ at the neuromuscular junction. Nature 272:641–643. [DOI] [PubMed] [Google Scholar]
- Molenaar PC, Oen BS, Plomp JJ, Kempen GThHV, Jennekens FGI, Hesselmans LFGM (1991) A non-immunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol 196:93–101. [DOI] [PubMed] [Google Scholar]
- Molenaar PC, Polak RL, Miledi R, Alema S, Vincent A, Newsom-Davis J (1979) Acetylcholine in Intercostal Muscle from Myasthenia Gravis Patients and in Rat Diaphragm after Blockade of Acetylcholine Receptors. Prog Brain Res 49:449–458. [DOI] [PubMed] [Google Scholar]
- Ono Y, Mori Y, Egashira Y, Sumiyama K, Takamori S (2019) Expression of plasma membrane calcium ATPases confers Ca2+/H+ exchange in rodent synaptic vesicles. Sci Rep-uk 9:4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BO, Gorczyca D, Younger MA, Jan LY, Jan Y-N, Davis GW (2017) Composition and Control of a Deg/ENaC Channel during Presynaptic Homeostatic Plasticity. Cell Reports 20:1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BO, Hauswirth AG, Celona B, Fetter RD, Zunino G, Kvon EZ, Zhu Y, Pennacchio LA, Black BL, Davis GW (2020) Presynaptic Homeostasis Opposes Disease Progression in Mouse Models of ALS-Like Degeneration: Evidence for Homeostatic Neuroprotection. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, Kempen GT van, Molenaar PC (1992) Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiology 458:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiana G, Scarsella G, Biagioni S, Senni MI, Cossu G (1985) Membrane acetylcholinesterase in murine muscular dystrophy In vivo and in cultured myotubes. Int J Dev Neurosci 3:331–340. [DOI] [PubMed] [Google Scholar]
- Rich MM, Wenner P (2007) Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci 30:119–125. [DOI] [PubMed] [Google Scholar]
- Sherwood T, Franke R, Conneely S, Joyner J, Arumugan P, Askwith C (2009) Identification of Protein Domains That Control Proton and Calcium Sensitivity of ASIC1a. J Biol Chem 284:27899–27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood TW, Frey EN, Askwith CC (2012) Structure and activity of the acid-sensing ion channels. Am J Physiol-cell Ph 303:C699–C710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood TW, Lee KG, Gormley MG, Askwith CC (2011) Heteromeric Acid-Sensing Ion Channels (ASICs) Composed of ASIC2b and ASIC1a Display Novel Channel Properties and Contribute to Acidosis-Induced Neuronal Death. J Neurosci 31:9723–9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó A, Just-Borràs L, Cilleros-Mañé V, Hurtado E, Nadal L, Tomàs M, Garcia N, Lanuza MA, Tomàs J (2018) BDNF-TrkB Signaling Coupled to nPKCε and cPKCβI Modulate the Phosphorylation of the Exocytotic Protein Munc18–1 During Synaptic Activity at the Neuromuscular Junction. Front Mol Neurosci 11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarski M, Hernandez RX, Feghhi T, Borycz JA, Lu Z, Agarwal AB, Reihl KD, Tavora R, Lau AWC, Meinertzhagen IA, Renden R, Macleod GT (2020) Neuronal Glutamatergic Synaptic Clefts Alkalinize Rather Than Acidify during Neurotransmission. J Neurosci 40:1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D, Bangsbo J, Juel C (2001) Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiology 537:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC (2009) The plasma membrane calcium ATPase (PMCA) of neurones is electroneutral and exchanges 2 H+ for each Ca2+ or Ba2+ ion extruded. J Physiology 587:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G (2012) Homeostatic Synaptic Plasticity: Local and Global Mechanisms for Stabilizing Neuronal Function. Csh Perspect Biol 4:a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Lino NG, González-Inchauspe CMF, González LE, Colettis N, Vattino LG, Wunsch AM, Wemmie JA, Uchitel OD (2014) Acid-sensing ion channels 1a (ASIC1a) inhibit neuromuscular transmission in female mice. Am J Physiol-cell Ph 306:C396–C406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullo S, Bonifacio G, Roy S, Johner N, Bernèche S, Kellenberger S (2017) Conformational dynamics and role of the acidic pocket in ASIC pH-dependent gating. Proc National Acad Sci 114:3768–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullo S, Kellenberger S (2019) A molecular view of the function and pharmacology of acid-sensing ion channels. Pharmacol Res 154:104166. [DOI] [PubMed] [Google Scholar]
- Wang JS, Bojovic D, Chen Y, Lindgren CA (2018a) Homocysteine sensitizes the mouse neuromuscular junction to oxidative stress by nitric oxide. Neuroreport 29:1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-M, Holzhausen LC, Kramer RH (2014) Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci 17:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McIntosh JM, Rich MM (2018b) Muscle Nicotinic Acetylcholine Receptors May Mediate Trans-Synaptic Signaling at the Mouse Neuromuscular Junction. J Neurosci 38:1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pinter MJ, Rich MM (2016) Reversible Recruitment of a Homeostatic Reserve Pool of Synaptic Vesicles Underlies Rapid Homeostatic Plasticity of Quantal Content. J Neurosci 36:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Q, Engisch KL, Rich MM (2010) Activity-Dependent Regulation of the Binomial Parameters p and n at the Mouse Neuromuscular Junction In Vivo. J Neurophysiol 104:2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Welsh MJ (2002) The Acid-Activated Ion Channel ASIC Contributes to Synaptic Plasticity, Learning, and Memory. Neuron 34:463–477. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ (2013) Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 14:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Michel-Schmidt R, Kirkpatrick CJ (2015) pH-dependent hydrolysis of acetylcholine: Consequences for non-neuronal acetylcholine. Int Immunopharmacol 29:27–30. [DOI] [PubMed] [Google Scholar]
- Wilson DF (1982) Influence of presynaptic receptors on neuromuscular transmission in rat. Am J Physiolcell Ph 242:C366–C372. [DOI] [PubMed] [Google Scholar]
- Wu J, Xu Y, Jiang Y-Q, Xu J, Hu Y, Zha X (2016) ASIC subunit ratio and differential surface trafficking in the brain. Mol Brain 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger MA, Müller M, Tong A, Pym EC, Davis GW (2013) A Presynaptic ENaC Channel Drives Homeostatic Plasticity. Neuron 79:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.