Abstract
Background
The prognostic value of extravascular lung water (EVLW) measured by transpulmonary thermodilution (TPTD) in critically ill patients is debated. We performed a systematic review and meta-analysis of studies assessing the effects of TPTD-estimated EVLW on mortality in critically ill patients.
Methods
Cohort studies published in English from Embase, MEDLINE, and the Cochrane Database of Systematic Reviews from 1960 to 1 June 2021 were systematically searched. From eligible studies, the values of the odds ratio (OR) of EVLW as a risk factor for mortality, and the value of EVLW in survivors and non-survivors were extracted. Pooled OR were calculated from available studies. Mean differences and standard deviation of the EVLW between survivors and non-survivors were calculated. A random effects model was computed on the weighted mean differences across the two groups to estimate the pooled size effect. Subgroup analyses were performed to explore the possible sources of heterogeneity.
Results
Of the 18 studies included (1296 patients), OR could be extracted from 11 studies including 905 patients (464 survivors vs. 441 non-survivors), and 17 studies reported EVLW values of survivors and non-survivors, including 1246 patients (680 survivors vs. 566 non-survivors). The pooled OR of EVLW for mortality from eleven studies was 1.69 (95% confidence interval (CI) [1.22; 2.34], p < 0.0015). EVLW was significantly lower in survivors than non-survivors, with a mean difference of −4.97 mL/kg (95% CI [−6.54; −3.41], p < 0.001). The results regarding OR and mean differences were consistent in subgroup analyses.
Conclusions
The value of EVLW measured by TPTD is associated with mortality in critically ill patients and is significantly higher in non-survivors than in survivors. This finding may also be interpreted as an indirect confirmation of the reliability of TPTD for estimating EVLW at the bedside. Nevertheless, our results should be considered cautiously due to the high risk of bias of many studies included in the meta-analysis and the low rating of certainty of evidence.
Trial registration the study protocol was prospectively registered on PROSPERO: CRD42019126985.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04061-6.
Keywords: Lung edema, Transpulmonary thermodilution, Hemodynamic monitoring, Critically ill patients
Background
Extravascular lung water (EVLW) represents the amount of lung fluid outside the pulmonary vasculature, i.e. the cellular and extracellular fluid volume of the interstitial and alveolar spaces [1, 2]. As such, its elevation is an important pathophysiological pattern of hydrostatic pulmonary edema and acute respiratory distress syndrome (ARDS) [3]. The level of EVLW is correlated with the degree of diffuse alveolar damage in patients with ARDS [4].
Today, transpulmonary thermodilution (TPTD) is the only technique that allows the estimation of the total amount of EVLW [2]. This estimation has been validated against gravimetry, which is the reference method, in an autopsy study in humans [5]. It has been shown that TPTD is able to detect small and rapid increases in EVLW [6], contributing to the validation of the method.
Several studies have investigated the relationship between the amount of EVLW and mortality in septic patients [7], patients with ARDS [8] and critically ill patients in general [9]. Nevertheless, many of these studies were of small size [10, 11], some were retrospective [9, 12] and the link between EVLW and mortality reported by some of them was weak [13, 14]. A previous meta-analysis on the association of EVLW and mortality was performed ten years ago [15]. Nevertheless, it included studies in which EVLW had been evaluated through the double-indicator technique, which is not used anymore. Moreover, several other studies have since been performed. The relationship between the value of EVLW and outcome remains an important question. Confirming the prognostic value of EVLW may reinforce the clinical interest of the variable [16]. In addition, if it exists, it may indirectly contribute to confirming the reliability of its estimation by TPTD.
Methods
Clinical research question
The clinical research question was: What is the relationship between EVLW and mortality in critically ill patients?
PICO statement
The PICO statement was the following:
P-patient, problem or population: Critically ill adult patients.
I-intervention or exposure: Measurement of EVLW through the single indicator transpulmonary dilution method.
C-comparison, control or comparator: Comparison of EVLW between survivors and non-survivors patients, considering either the baseline value or maximal value reached during the intensive care unit (ICU) stay.
O-outcome: The primary outcome was the odds ratio (OR) of EVLW as a risk factor for mortality, defined either as in-hospital or 28-day or ICU mortality. The secondary outcome was mean differences between survivors and non-survivors in terms of EVLW value.
Identification of records
Our aim was to identify all studies evaluating the association between EVLW measured by TPTD, whatever the threshold used to define an elevated EVLW, and mortality in critically ill patients. We included in our analysis only studies that were published in full text or accepted for publication in indexed journals.
We searched the US National Library of Medicine’s MEDLINE database, the Embase database, and the Cochrane Database of Systematic Reviews for relevant studies published from 1960 to 1 June, 2021. We used the following medical subject headings and keywords: ‘‘EVLW”, “EVLWi”, “lung water”, “survival”, and “mortality”. The complete searching strategy is reported in Additional file 1: S1. We also looked for relevant articles cited in review articles, commentaries, editorials, and in the references of the original articles identified by our search. We excluded studies performed in children and in burned patients, studies published in languages other than English, and studies in which EVLW was estimated by methods different from TPTD. The search was performed by two authors (FG and RS) until no new records could be found. Conflicts regarding the inclusion or exclusion of studies were resolved by consensus with a third investigator (XM). The meta-analysis was performed according to the PRISMA statement [17] (Additional file 1: S2). The study protocol was prospectively registered in PROSPERO (CRD42019126985).
Data extraction
Using a standardized data form, we extracted several data elements from the included studies, including characteristics of the investigated population, the method used to measure EVLW, and the timing at which EVLW was measured. We collected the OR with its 95% confidence interval (95% CI) of EVLW as a risk factor for mortality, if available. If data needed for the analysis were not retrievable from the text, tables or figures, we systematically asked them to the authors of the studies.
Assessment of risk of bias in included studies
Two authors (FG and RS) independently assessed the overall quality of evidence at the outcome level according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system [18]. Moreover, they assessed the risk of bias of the included studies by following the criteria specified in the QUIPS tool [19]. It should be noted that this tool was not the one we initially planned to use for assessing the risk of bias (PROSPERO: CRD42019126985). For each criterion, the risk of bias was judged as high, moderate, or low. Disagreements between the reviewers were resolved by consensus with a third investigator (XM).
Statistical analysis
Pooled ORs were performed using continuity corrections [20]. Mean differences and standard deviation (SD) of the EVLW between survivors and non-survivors were considered. If a confidence interval of EVLW was reported, we converted it to SD for pooled analysis. The 95% CI was calculated using the Wilson method [21]. A random effects meta-analysis model was computed on the weighted mean differences (WMD) across the two groups to estimate the pooled size effect. A value of I2 ≥ 75% was considered as indicating a high heterogeneity [22].
To investigate the source of heterogeneity, pre-defined subgroup analyses were performed:
Timing of EVLW measurement: baseline (≤ 48 h) versus maximal value
EVLW indexation: actual versus predicted body weight
Study population: ARDS versus non-ARDS
Risk of bias: “moderate and low” versus “high”.
Publication bias was investigated using Deek’s test [23, 24]. The statistical significance was set at a p value < 0.05. The analyses were performed by using Review Manager version 5.3, R 3.3.5 with metafor packages [25].
Results
Characteristics of the included studies
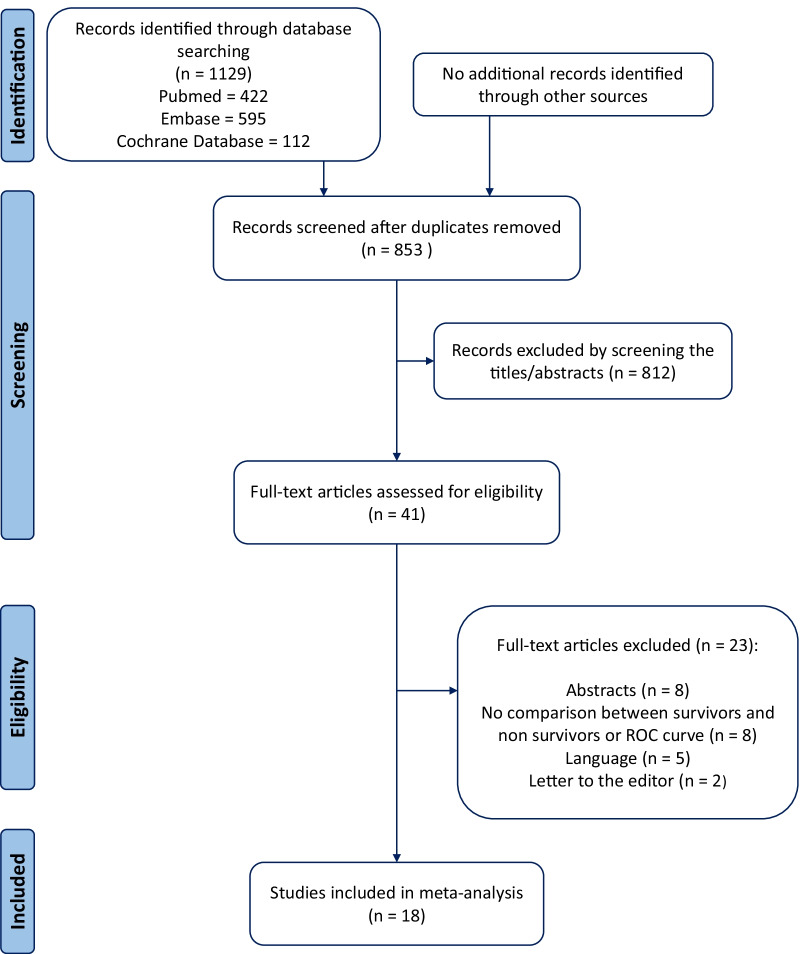
We included 18 studies that reported EVLW and mortality, with a total of 1296 patients enrolled [7, 8, 11–14, 26–37]. The flow chart is presented in Fig. 1. Data from nine studies [7, 8, 13, 14, 26, 31, 32, 36, 37] that were missing in the published articles were obtained by direct contact with authors, or retrieved in our database for studies performed by our group.
Fig. 1.
PRISMA flowchart
The main characteristics of the studies are reported in Table 1. Nine studies were performed specifically in ARDS patients [8, 11, 14, 30, 33–37], seven in septic shock patients [7, 12, 27–29, 31, 32], and two in unselected critically ill patients [13, 26]. All studies were performed in patients admitted to the ICU. Of them, the OR of EVLW as a risk factor for mortality could be extracted from 11 studies [8, 12, 13, 26, 28–30, 32, 33, 36, 37]. In 17 studies [7, 8, 11–14, 26–35, 37], the value of EVLW was provided at baseline, i.e. at the first time, it was measured (Table 1). The maximal value of EVLW observed during the study period was available in ten studies [7, 8, 12–14, 31, 32, 34, 36, 37], one in unselected critically ill patients [13], five in patients with ARDS [8, 14, 34, 36, 37] and four in patients with septic shock [7, 12, 31, 32] (Table 1).
Table 1.
Main characteristics of included studies
| Study ID | Year | No. of patients | Country | Type of study | Setting | Type of patient | EVLW indexation | Outcome |
|---|---|---|---|---|---|---|---|---|
| Martin et al. [27] | 2005 | 29 | USA | Prospective | Medical ICU | Severe sepsis/septic shock | ABW | 28-Day mortality |
| Kuzkov et al. [7] | 2006 | 38 | Russia | Prospective | Mixed ICU | Septic shock/ALI | ABW | 28-Day mortality |
| Chung et al. [28] | 2008 | 33 | Taiwan | Prospective | Medical ICU | Severe sepsis/septic shock | ABW | In-hospital mortality |
| Phillips et al. [11] | 2008 | 19 | USA | Prospective | ICU | ARDS | PBW/ABW | ICU mortality |
| Chung et al. [29] | 2010 | 67 | Taiwan | Prospective | Medical ICU | Severe sepsis/septic shock | PBW | ICU mortality |
| Craig et al. [30] | 2010 | 44 | UK | Prospective | ICU | ALI/ARDS | PBW/ABW | ICU mortality |
| Chew et al. [31] | 2012 | 51 | Sweden | Prospective | Mixed ICU | Severe sepsis/septic shock | PBW/ABW | ICU mortality |
| Cordemans et al. [13] | 2012 | 123 | Belgium | Retrospective | ICU | Critically ill | ABW | 28-Day mortality |
| Mallat et al. [32] | 2012 | 55 | France | Prospective | Mixed ICU | Septic shock | PBW/ABW | ICU mortality |
| Brown et al. [33] | 2013 | 59 | UK | Prospective | ICU | ALI/ARDS | PBW | ICU mortality |
| Jozwiak et al. [8] | 2013 | 200 | France | Retrospective | Medical ICU | ARDS | PBW | 28-Day mortality |
| Huber et al. [26] | 2014 | 50 | Germany | Prospective | ICU | Critically ill | PBW | ICU mortality |
| Tagami et al. [14] | 2014 | 192 | Japan | Post-hoc analysis | ICU | ARDS | PBW | 28-Day mortality |
| Zhao et al. [34] | 2015 | 21 | China | Prospective | ICU | ARDS | PBW | ICU mortality |
| Wang et al. [12] | 2016 | 105 | China | Retrospective | ICU | Septic shock | PBW | 28-Day mortality |
| Ma et al. [35] | 2019 | 41 | China | Retrospective | ICU | ARDS | PBW | In-hospital mortality |
| Huber et al. [36] | 2020 | 49 | Germany | Prospective | ICU | ARDS | PBW | 28-Day mortality |
| Shi et al. [37] | 2021 | 120 | France | Prospective | ICU | ARDS | PBW | ICU mortality |
ABW actual body weight, ALI acute lung injury, ARDS acute respiratory distress syndrome, EVLW extravascular lung water, ICU intensive care unit, PBW predicted body weight, UK United Kingdom, USA United States of America
Mortality was defined as the 28-day mortality in seven studies [7, 8, 12–14, 27, 36], as the ICU mortality in nine [11, 26, 29–34, 37], and as the in-hospital mortality in two studies [28, 35] (Table 1). The results of the GRADE and the QUIPS evaluation are provided in Table 2 and Additional file 1: S3.
Table 2.
The results of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) assessment of the evidence certainty on the association between the extravascular lung water and mortality
| Outcome | Relative effect (95% CI) |
No. of patients | No. of participants (studies) | Downgrade factors | Certainty of the evidence (GRADE) 18 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors | Non-survivors | Risk of bias | Inconsistency | Imprecision | Indirectness | Publication bias | ||||
| Mortality |
OR 1.69 (1.22–2.34) |
464/905 (51.3%) |
441/905 (48.7%) |
905 Adult patients (11 studies) |
Seriousa | Seriousb | Not serious | Seriousc | Not serious |
⊕○○○ VERY LOW |
|
WMD − 4.97 mL/kg (− 6.54; − 3.41) |
680/1246 (54.6%) |
566/1246 (45.4%) |
1246 Adult patients (17 studies) |
Seriousa | Seriousb | Not serious | Seriousc | Not serious |
⊕○○○ VERY LOW |
|
CI confidence interval, OR odds ratio, WMD weighted mean difference
aDowngraded by one level for the risk of bias because ten of 18 studies were evaluated as high risk of bias according to the QUIPS tool and four of 11 studies did not report adjusted OR. Nevertheless, no differences regarding the relative effects were observed between high and moderate and low risk of bias in subgroup analysis
bDowngraded by one level for inconsistency: substantial heterogeneity is seen between studies (I2 > 75%)
cDowngraded by one level for indirectness because different cut-off values for elevated EVLW definition (> 7 mL/kg/m2 in two studies, > 10 mL/kg/m2 in eight studies, not available in eight studies)
Association of EVLW with mortality
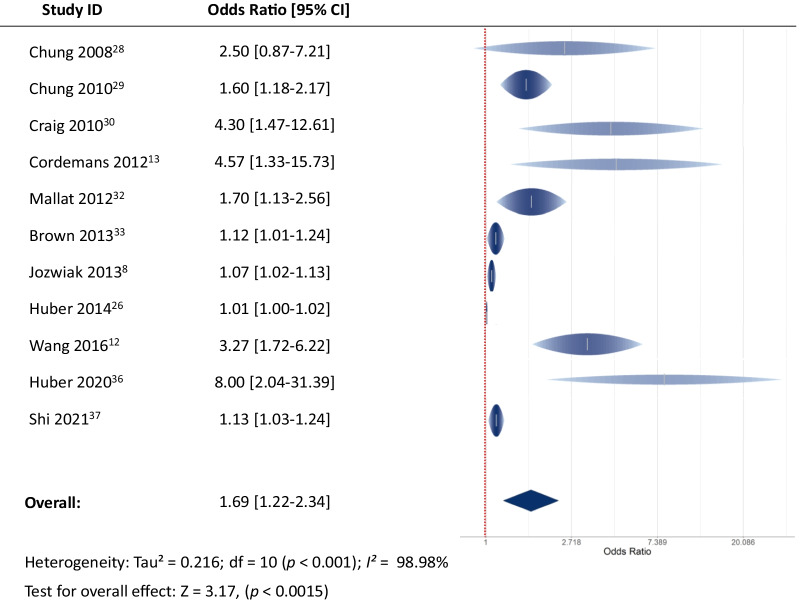
The pooled OR obtained from the 11 studies that reported OR [8, 12, 13, 26, 28–30, 32, 33, 36, 37] was 1.69 (95% CI [1.22; 2.34], I2 = 98.98%, p < 0.0015) (Fig. 2). Seventeen studies reported EVLW values of survivors and non-survivors, including 1 246 patients (680 survivors vs. 566 non-survivors) [7, 8, 11–14, 27–37] (Additional file 1: S4). The weighted mortality rates are presented in Additional file 1: Figure S5.
Fig. 2.
The pooled odds ratio of EVLW for mortality
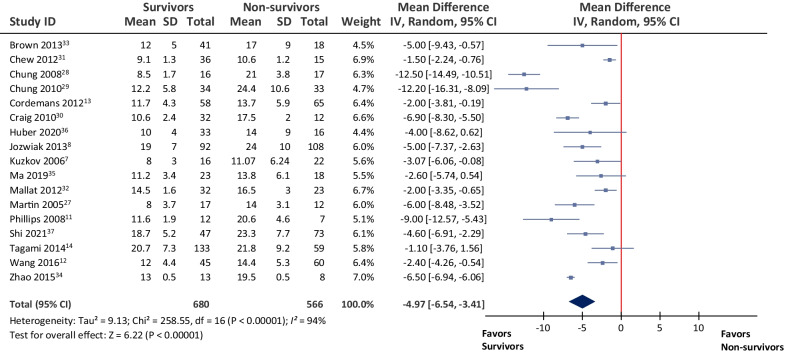
Overall, EVLW was significantly lower in survivors compared to non-survivors, with a mean difference of − 4.97 mL/kg (95% CI [− 6.54; − 3.41], p < 0.001) (Fig. 3). Since the statistical heterogeneity was significant (I2 = 93.8%, p < 0.001), the random-effects model was used to pool the data. The results in the prespecified subgroups were as follows.
Fig. 3.
Mean difference in extravascular lung water levels between survivors and non-survivors
Baseline EVLW versus maximal EVLW
When comparing OR of EVLW as a risk factor for mortality between studies in which EVLW at baseline was reported [28–30, 33, 36] to those in which maximal EVLW was reported [8, 12, 13, 26, 32, 37], the EVLW remained to be a risk factor in both groups (OR of group baseline EVLW: 2.22, 95% CI [1.17; 4.20] vs. OR of group maximal EVLW: 1.48, 95% CI [1.01; 2.17], p = 0.38) (Additional file 1: Figure S6).
In the eight studies in which the EVLW at baseline was reported [11, 27–31, 33, 35], it was lower in survivors than in non-survivors (WMD: − 6.90 mL/kg, 95% CI [− 10.27; − 3.53], p < 0.001). In the nine studies in which the maximal value of EVLW was reported [7, 8, 12–14, 32, 34, 36, 37], it was also lower in survivors than in non-survivors (WMD: − 3.43 mL/kg, 95% CI [− 5.28; − 1.59], p < 0.001). The WMD was not different between the two categories of studies (p = 0.08) (Additional file 1: Figure S7).
Actual versus predicted body weight for EVLW indexation
When comparing OR of EVLW as a risk factor for mortality between studies in which EVLW was indexed to actual body weight [13, 28, 32] to those in which it was indexed to predicted body weight [8, 12, 26, 29, 30, 32, 33, 36, 37], EVLW remained a risk factor in both groups (OR of actual body weight for EVLW indexation: 2.37, 95% CI [1.47; 3.83] vs. OR of predicted body weight for EVLW indexation: 1.54, 95% CI [1.13; 2.10], p = 0.16) (Additional file 1: Figure S8).
In the four studies that reported the EVLW indexed to actual body weight [7, 13, 27, 28], the survivors had significantly lower values of EVLW than non-survivors (WMD: − 5.92 mL/kg, 95% CI [− 11.09; − 0.75], p = 0.02). This was also the case in the 13 studies in which the EVLW was indexed to predicted body weight [8, 11, 12, 14, 29–37] (WMD: − 4.64 mL/kg, 95% CI [− 6.35; − 2.94], p < 0.001). The WMD was not different between the two groups (p = 0.65) (Additional file 1: Figure S9).
ARDS population versus non-ARDS population
When comparing OR of EVLW acting as a risk factor for mortality between studies that included ARDS patients [8, 30, 33, 36, 37] to those that included non-ARDS patients [12, 13, 26, 28, 29, 32], EVLW remained a risk factor in both groups (OR in ARDS patients: 1.09, 95% CI [1.05, 1.14] vs. OR in non-ARDS patients: 1.83, 95% CI [1.20, 2.79], p = 0.57) (Additional file 1: Figure S10).
In the nine studies dedicated to ARDS patients [8, 11, 14, 30, 33–37], the EVLW was lower in survivors than non-survivors (WMD: − 5.16 mL/kg, 95% CI [− 6.48; − 3.84], p < 0.001). This was also the case in the eight studies that included non-ARDS patients [7, 12, 13, 27–29, 31, 32] (WMD: − 5.00 mL/kg, 95% CI [− 7.65; − 2.35], p < 0.001). No significant difference in WMD was observed between the two groups (p = 0.92) (Additional file 1: Figure S11).
Risk of bias
When comparing studies according to the global risk of bias, there was no significant difference in OR between the studies with a high [26, 28, 30, 36] and moderate and low [8, 12, 13, 29, 32, 33, 37] risk of bias (OR of studies with low risk of bias: 1.46, 95% CI [1.10; 1.94] vs. OR in studies with a high risk of bias 2.62, 95% CI [1.04; 6.60], p = 0.37) (Additional file 1: Figure S12).
In studies with a moderate and low risk of bias [8, 12–14, 29, 32, 33, 37], the EVLW was lower in survivors than in non-survivors (WMD: − 3.80 mL/kg, 95% CI [− 5.49; − 2.11], p < 0.001). This was also the case in the studies with a high risk of bias [7, 11, 27, 28, 30, 31, 34–36] (WMD: − 5.83 mL/kg, 95% CI [− 8.12; − 3.54], p < 0.001) No significant difference in WMD was observed between the two groups, p = 0.16) (Additional file 1: Figure S13).
Publication bias
According to the results of Deek’s test, the funnel plot asymmetry test revealed the absence of publication bias within the studies considered (p = 0.31) (Additional file 1: Figure S14).
Discussion
Our systematic review and meta-analysis of 18 studies, involving 1296 patients, suggests that an increased value of EVLW is associated with increased mortality compared to less elevated values in ICU patients. The levels of EVLW were less increased in survivors compared to non-survivors. However, due to the high risk of bias of included studies and the low rating of certainty of evidence according to the GRADE assessment, these conclusions should be considered with caution.
A major advantage of TPTD, which is part of the advanced monitoring techniques in critically ill patients [38–41], is to provide a bedside estimation of EVLW [42]. EVLW measured by TPTD has been demonstrated to reliably detect diffuse alveolar damage (DAD), which is the histologic pattern of ARDS [4, 43–45]. The severity of DAD is heterogeneous among ARDS patients, and this is in accordance with the heterogeneity of EVLW in this population, as we have recently observed for instance in ARDS patients with Coronavirus disease 2019 (COVID-19) [37]. Since the presence of DAD is associated with a poorer outcome in ARDS [44, 45], EVLW may reflect the severity of pulmonary lesions in critically ill patients.
However, most of the conclusions regarding the prognostic value of EVLW come from heterogeneous studies, performed in different settings and with different methodologies. While some authors reported a close relationship between EVLW values and outcome [11], others did not [14]. Moreover, some studies included only a small series of patients [11, 34]. The present meta-analysis may thus clarify the relationship between EVLW and outcome in ICU patients. We found that an increased value of EVLW is one of the prognostic factors for mortality in ICU patients. The OR of EVLW as a risk factor for mortality was 1.69 [1.22; 2.34]. Also, mortality was significantly higher in patients with the highest EVLW values, either at baseline or at its maximum, compared to patients with the lowest EVLW values.
The heterogeneity of the included studies was significant. However, the subgroup analyses for OR and WMD were conducted to investigate the sources of heterogeneity. The association between EVLW and mortality has been described at different times, i.e. baseline, Day-3, or when it reached its maximal value, likely because these timings highly depend on the time when the TPTD device was set up. Nevertheless, our subgroup analyses showed that an increase in EVLW remains an unfavourable prognostic factor, regardless of the timing at which it is measured. In addition, we found no difference between studies in which EVLW was indexed to predicted body weight and those in which it was indexed to actual body weight, regarding OR for mortality as well as mean differences between survivors and non-survivors. Nonetheless, as the between-group difference between survivors and non-survivors was quite small and as the dimension of the lungs depends on the height of the patient rather than on actual weight fluctuations [26, 30], we still suggest indexing EVLW to the predicted body weight. EVLW was similarly associated with mortality in studies that specifically included ARDS patients and in studies with non-ARDS patients. This may suggest the value of EVLW for indicating disease severity not only in ARDS but also in other critically ill patients.
Since the risk of bias was estimated as high for many studies included and our results have “very low certainty of evidence” according to the GRADE assessment, our conclusions should be considered with caution. Obviously, EVLW should not be used to predict the outcome of ICU patients on an individual basis. There are many other prognostic factors in ICU patients. We rather believe that our results indirectly contribute to the recognition of TPTD for estimating EVLW. Indeed, EVLW measured by the technique would not be associated with the outcome if this estimation was unreliable. Although the estimation of EVLW by TPTD has been demonstrated to be correlated with the reference technique [46], reproducible [47], and able to detect small [48, 49] and rapid [6] variations, doubts may persist regarding its reliability [2, 3]. As the gold standard technique for measuring EVLW, namely gravimetry, can be performed only in cadavers, the validation of EVLW measurements in patients can only be indirect. The present meta-analysis may contribute to this indirect validation. Thus, our results suggest that clinicians may rely on the estimation of EVLW by TPTD. Besides, EVLW may help to identify patients with DAD and to grade the severity of ARDS [50, 51]. It may also be used in fluid management, as a marker indicating the risk of fluid administration, or as a guide for fluid removal [52]. Further studies should investigate the clinical interest of such strategies, describe the relationship between EVLW and respiratory mechanics, or evaluate the effect of some respiratory management such as prone position [53]. Studies testing the interest of integrating EVLW in the strategy of fluid management are also needed to better identify its clinical significance.
Our study suffers from many limitations. First, the OR of EVLW as a risk factor for mortality, which is the main factor to consider in meta-analyses on prognostic factors, was not provided in all the studies we included. Second, data for the comparison of the mean difference between survivors and non-survivors was not available in one of the included studies [26]. Nevertheless, this represents a minority (4%) of the whole cohort. Third, we did not obtain data regarding fluid balance since our principal objective was to confirm that EVLW measured by TPTD is associated with a worse outcome. Fourth, we did not investigate EVLW as an adjunctive variable to other techniques, such as ultrasonography and bioelectrical impedance, to evaluate the fluid status [54]. Finally, we limited our search to articles published in English language and did not expand our search to clinical trial registry databases.
Conclusion
In conclusion, although limited by the low rating of certainty of the evidence, this meta-analysis suggests that elevated levels of EVLW measured by TPTD are associated with mortality in ICU patients. This finding may be interpreted as an indirect confirmation of the reliability of TPTD for estimating EVLW.
Supplementary Information
Additional file 1. Supplementary information on further results.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- EVLW
Extravascular lung water
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ICU
Intensive care unit
- OR
Odds ratio
- TPTD
Transpulmonary thermodilution
- WMD
Weighted mean difference
Author contributions
FG acquired the data, performed data analysis and interpretation, and wrote the manuscript. RS acquired the data, performed data analysis and interpretation, and wrote the manuscript. J-LT conceived the study, participated in data analysis and interpretation, and contributed to writing the manuscript. DA performed data analysis and interpretation and contributed to writing the manuscript. PM acquired the data and contributed to data analysis. MJ acquired the data and contributed to data analysis. MSC contributed to data acquisition, provided original data, and contributed to writing the manuscript. WH performed studies integrated in the metaanalysis, and thus contributed to data acquisition. MK contributed to data acquisition, provided original data, and contributed to writing the manuscript. VVK contributed to data acquisition. TL contributed to data acquisition, provided original data, and contributed to writing the manuscript. MLNGM contributed to data acquisition, provided original data, and contributed to writing the manuscript. JM contributed to data acquisition, provided original data, and contributed to writing the manuscript. SGS contributed to data acquisition, provided original data, and contributed to writing the manuscript. TT contributed to data acquisition, provided original data, and contributed to writing the manuscript. TP participated in data analysis and interpretation, and contributed to writing the manuscript. XM conceived the study, performed data analysis and interpretation, and wrote the manuscript. FG, RS, J-LT, DA, PM, MJ, MSC, MK, VVK, TL, MLNGM, JM, SGS, TT, TP and XM reviewed the manuscript and approved its final version.
Funding
No funding.
Availability of data and materials
The datasets used and/or analysed in the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Monnet is member of the Medical Advisory Board of Pulsion Medical Systems, Getinge. He received fees for scientific lectures from Baxter and Philips, and restricted research grants from Baxter. Dr. Teboul is member of the Medical Advisory Board of Pulsion Medical Systems, Getinge. Dr. Chew has received travel reimbursements and honoraria from Pulsion Medical Systems and Edwards Lifesciences. Dr. Huber was member of the Medical Advisory Board of Pulsion Medical Systems. Dr. Kirov is member of the Medical Advisory Boards of Pulsion Medical Systems and Philips. Dr. Lahmer received travel grants from Gilead, Pfizer and MSD. Dr. Malbrain is founding President of WSACS (The Abdominal Compartment Society, http://www.wsacs.org) and current Treasurer, he is also member of the medical advisory Board of Pulsion Medical Systems (Getinge) and Serenno Medical, and consults for ConvaTec, Acelity, Spiegelberg, and Holtech Medical. He is co-founder of the International Fluid Academy (IFA). The IFA is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law (http://www.fluidacademy.org). Dr. Sakka is member of the Medical Advisory Board of Pulsion Medical Systems. Dr. Tagami is member of the Medical Advisory Board of Pulsion Medical Systems. The other authors declare that they have no conflict of interest regarding this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francesco Gavelli and Rui Shi contributed equally to this work
References
- 1.Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: recent advances and clinical applications. Ann Intensive Care. 2015;5:38. doi: 10.1186/s13613-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care. 2017;21:147. doi: 10.1186/s13054-017-1739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagami T, Ong MEH. Extravascular lung water measurements in acute respiratory distress syndrome: why, how, and when? Curr Opin Crit Care. 2018;24:209–215. doi: 10.1097/MCC.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagami T, Sawabe M, Kushimoto S, Marik PE, Mieno MN, Kawaguchi T, et al. Quantitative diagnosis of diffuse alveolar damage using extravascular lung water. Crit Care Med. 2013;41:2144–2150. doi: 10.1097/CCM.0b013e31828a4643. [DOI] [PubMed] [Google Scholar]
- 5.Tagami T, Kushimoto S, Yamamoto Y, Atsumi T, Tosa R, Matsuda K, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care. 2010;14:R162. doi: 10.1186/cc9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42:1882–1889. doi: 10.1097/CCM.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 7.Kuzkov VV, Kirov MY, Sovershaev MA, Kuklin VN, Suborov EV, Waerhaug K, et al. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med. 2006;34:1647–1653. doi: 10.1097/01.CCM.0000218817.24208.2E. [DOI] [PubMed] [Google Scholar]
- 8.Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;41:472–480. doi: 10.1097/CCM.0b013e31826ab377. [DOI] [PubMed] [Google Scholar]
- 9.Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;122:2080–2086. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- 10.Davey-Quinn A, Gedney JA, Whiteley SM, Bellamy MC. Extravascular lung water and acute respiratory distress syndrome–oxygenation and outcome. Anaesth Intensive Care. 1999;27:357–362. doi: 10.1177/0310057X9902700404. [DOI] [PubMed] [Google Scholar]
- 11.Phillips CR, Chesnutt MS, Smith SM. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med. 2008;36:69–73. doi: 10.1097/01.CCM.0000295314.01232.BE. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Cui N, Su L, Long Y, Wang X, Zhou X, et al. Prognostic value of extravascular lung water and its potential role in guiding fluid therapy in septic shock after initial resuscitation. J Crit Care. 2016;33:106–113. doi: 10.1016/j.jcrc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;2:S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagami T, Nakamura T, Kushimoto S, Tosa R, Watanabe A, Kaneko T, et al. Early-phase changes of extravascular lung water index as a prognostic indicator in acute respiratory distress syndrome patients. Ann Intensive Care. 2014;4:27. doi: 10.1186/s13613-014-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Lu B, Ni H. Prognostic value of extravascular lung water index in critically ill patients: a systematic review of the literature. J Crit Care. 2012;27(420):e1–8. doi: 10.1016/j.jcrc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Tagami T, Sakka SG, Monnet X. Diagnosis and treatment of acute respiratory distress syndrome. JAMA. 2018;320:305. doi: 10.1001/jama.2018.5924. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE Guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020;121:62–70. doi: 10.1016/j.jclinepi.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 21.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. J Am Stat Assoc. 1927;22:209–212. doi: 10.1080/01621459.1927.10502953. [DOI] [Google Scholar]
- 22.Hunter JE, Schmidt FL. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. 3. London: Sage; 2015. [Google Scholar]
- 23.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Crisp A, Miller S, Thompson D, Best N. Practical experiences of adopting assurance as a quantitative framework to support decision making in drug development. Pharm Stat. 2018;17:317–328. doi: 10.1002/pst.1856. [DOI] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 26.Huber W, Höllthaler J, Schuster T, Umgelter A, Franzen M, Saugel B, et al. Association between different indexations of extravascular lung water (EVLW) and PaO2/FiO2: a two-center study in 231 patients. PLoS ONE. 2014;9:e103854. doi: 10.1371/journal.pone.0103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin GS, Eaton S, Mealer M, Moss M. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Crit Care. 2005;9:R74–82. doi: 10.1186/cc3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung FT, Lin SM, Lin SY, Lin HC. Impact of extravascular lung water index on outcomes of severe sepsis patients in a medical intensive care unit. Respir Med. 2008;102:956–961. doi: 10.1016/j.rmed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Chung FT, Lin HC, Kuo CH, Yu CT, Chou CL, Lee KY, et al. Extravascular lung water correlates multiorgan dysfunction syndrome and mortality in sepsis. PLoS ONE. 2010;5:e15265. doi: 10.1371/journal.pone.0015265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, McLaughlin B, Elborn JS, et al. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med. 2010;38:114–120. doi: 10.1097/CCM.0b013e3181b43050. [DOI] [PubMed] [Google Scholar]
- 31.Chew MS, Ihrman L, During J, Bergenzaun L, Ersson A, Undén J, et al. Extravascular lung water index improves the diagnostic accuracy of lung injury in patients with shock. Crit Care. 2012;16:R1. doi: 10.1186/cc10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallat J, Pepy F, Lemyze M, Barrailler S, Gasan G, Tronchon L, et al. Extravascular lung water indexed or not to predicted body weight is a predictor of mortality in septic shock patients. J Crit Care. 2012;27:376–383. doi: 10.1016/j.jcrc.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Brown LM, Calfee CS, Howard JP, Craig TR, Matthay MA, McAuley DF. Comparison of thermodilution measured extravascular lung water with chest radiographic assessment of pulmonary oedema in patients with acute lung injury. Ann Intensive Care. 2013;3:25. doi: 10.1186/2110-5820-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B, Wang M, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15:98. doi: 10.1186/s12890-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma S, Zhao ML, Wang K, Yue YF, Sun RQ, Zhang RM, et al. Association of Ang-2, vWF, and EVLWI with risk of mortality in sepsis patients with concomitant ARDS: a retrospective study. J Formos Med Assoc. 2020;119:950–956. doi: 10.1016/j.jfma.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Huber W, Findeisen M, Lahmer T, Herner A, Rasch S, Mayr U, et al. Prediction of outcome in patients with ARDS: a prospective cohort study comparing ARDS-definitions and other ARDS-associated parameters, ratios and scores at intubation and over time. PLoS ONE. 2020;15:e0232720. doi: 10.1371/journal.pone.0232720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi R, Lai C, Teboul JL, Dres M, Moretto F, De Vita N, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care. 2021;25:186. doi: 10.1186/s13054-021-03594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernards J, Mekeirele M, Hoffmann B, Peeters Y, De Raes M, Malbrain ML. Hemodynamic monitoring: to calibrate or not to calibrate? Part 2–Non-calibrated techniques. Anaesthesiol Intensive Ther. 2015;47:501–516. doi: 10.5603/AIT.a2015.0076. [DOI] [PubMed] [Google Scholar]
- 39.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofkens PJ, Verrijcken A, Merveille K, Neirynck S, Van Regenmortel N, De Laet I, et al. Common pitfalls and tips and tricks to get the most out of your transpulmonary thermodilution device: results of a survey and state-of-the-art review. Anaesthesiol Intensive Ther. 2015;47:89–116. doi: 10.5603/AIT.a2014.0068. [DOI] [PubMed] [Google Scholar]
- 41.Peeters Y, Bernards J, Mekeirele M, Hoffmann B, De Raes M, Malbrain ML. Hemodynamic monitoring: to calibrate or not to calibrate? Part 1—Calibrated techniques. Anaesthesiol Intensive Ther. 2015;47:487–500. doi: 10.5603/AIT.a2015.0073. [DOI] [PubMed] [Google Scholar]
- 42.Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42:1350–1359. doi: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 43.Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute respiratory distress syndrome and diffuse alveolar damage. New insights on a complex relationship. Ann Am Thorac Soc. 2017;14:844–850. doi: 10.1513/AnnalsATS.201609-728PS. [DOI] [PubMed] [Google Scholar]
- 44.Cardinal-Fernández P, Bajwa EK, Dominguez-Calvo A, Menéndez JM, Papazian L, Thompson BT. The presence of diffuse alveolar damage on open lung biopsy is associated with mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Chest. 2016;149:1155–1164. doi: 10.1016/j.chest.2016.02.635. [DOI] [PubMed] [Google Scholar]
- 45.Cardinal-Fernandez P, Ortiz G, Chang CH, Kao KC, Bertreau E, Philipponnet C, et al. Predicting the impact of diffuse alveolar damage through open lung biopsy in acute respiratory distress syndrome-The PREDATOR Study. J Clin Med. 2019;8:829. doi: 10.3390/jcm8060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagami T, Kushimoto S, Tosa R, Omura M, Hagiwara J, Hirama H, et al. The precision of PiCCO® measurements in hypothermic post-cardiac arrest patients. Anaesthesia. 2012;67:236–243. doi: 10.1111/j.1365-2044.2011.06981.x. [DOI] [PubMed] [Google Scholar]
- 47.Monnet X, Persichini R, Ktari M, Jozwiak M, Richard C, Teboul JL. Precision of the transpulmonary thermodilution measurements. Crit Care. 2011;15:R204. doi: 10.1186/cc10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dres M, Teboul JL, Guerin L, Anguel N, Amilien V, Clair MP, et al. Transpulmonary thermodilution enables to detect small short-term changes in extravascular lung water induced by a bronchoalveolar lavage. Crit Care Med. 2014;42:1869–1873. doi: 10.1097/CCM.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 49.Gavelli F, Teboul JL, Azzolina D, Beurton A, Taccheri T, Adda I, et al. Transpulmonary thermodilution detects rapid and reversible increases in lung water induced by positive end-expiratory pressure in acute respiratory distress syndrome. Ann Intensive Care. 2020;10:28. doi: 10.1186/s13613-020-0644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perel A. Extravascular lung water and the pulmonary vascular permeability index may improve the definition of ARDS. Crit Care. 2013;17:108. doi: 10.1186/cc11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushimoto S, Endo T, Yamanouchi S, Sakamoto T, Ishikura H, Kitazawa Y, et al. Relationship between extravascular lung water and severity categories of acute respiratory distress syndrome by the Berlin definition. Crit Care. 2013;17:R132. doi: 10.1186/cc12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Compton F, Hoffmann C, Zidek W, Schmidt S, Schaefer JH. Volumetric hemodynamic parameters to guide fluid removal on hemodialysis in the intensive care unit. Hemodial Int. 2007;11:231–237. doi: 10.1111/j.1542-4758.2007.00174.x. [DOI] [PubMed] [Google Scholar]
- 53.McAuley DF, Giles S, Fichter H, Perkins GD, Gao F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002;28:414–418. doi: 10.1007/s00134-002-1248-z. [DOI] [PubMed] [Google Scholar]
- 54.Cleymaet R, Scheinok T, Maes H, Stas A, Malbrain L, De Laet I, et al. Prognostic value of bioelectrical impedance analysis for assessment of fluid overload in ICU patients: a pilot study. Anaesthesiol Intensive Ther. 2021;53:10–17. doi: 10.5114/ait.2021.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary information on further results.
Data Availability Statement
The datasets used and/or analysed in the present study are available from the corresponding author on reasonable request.