ABSTRACT
EUS-guided radiofrequency ablation (RFA) and ethanol ablation (EA) for pancreatic neuroendocrine tumors (PNETs) have recently been reported with good outcomes. We performed a systematic review and meta-analysis to evaluate the comparative effectiveness and safety of EUS-RFA and EUS-EA in the treatment of PNETs. A comprehensive search of multiple databases (through October 2020) was performed to identify studies that reported outcomes of EUS-RFA and EUS-EA of PNETs. Outcomes assessed included clinical success, technical success, and adverse events (AEs). A total of 181 (100 EUS-RFA, 81 EUS-EA) patients (60.7 ± 9.2 years) with 204 (113 EUS-RFA, 91 EUS-EA) PNETs (mean size 15.1 ± 4.7 mm) were included from 20 studies. There was no significant difference in the rates of technical success (94.4% [95% confidence interval (CI): 88.5–97.3, I2 = 0] vs. 96.7% [95% CI: 90.8–98.8, I2 = 0]; P = 0.42), clinical success (85.2% (95% CI: 75.9–91.4, I2 = 0) vs. 82.2% [95% CI: 68.2–90.8, I2 = 10.1]; P = 0.65), and AEs (14.1% [95% CI: 7.1–26.3, I2 = 0] vs. 11.5% [95% CI: 4.7–25.4, I2 = 63.5]; P = 0.7) between EUS-RFA and EUS-EA, respectively. The most common AE was pancreatitis with the rate of 7.8% and 7.6% (P = 0.95) for EUS-RFA and EUS-EA, respectively. On meta-regression, the location of PNETs in head/neck of pancreas (P = 0.03) was a positive predictor of clinical success for EUS-RFA. EUS-RFA and EUS-EA have similar effectiveness and safety for PNETs ablation. Head/neck location of PNETs was a positive predictor for clinical success after EUS-RFA.
Keywords: EUS, ethanol, neuroendocrine tumor, pancreas, radiofrequency ablation
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) originate from islets of Langerhans, which are a part of the diffuse neuroendocrine system of the gastrointestinal tract.[1] PNETs are classified as functional (10%–30%) and nonfunctional (70%–90%) NETS based on whether they secrete hormones or vasoactive substances. The overall prevalence of PNETs is approximately 1 in 100,000.[2] However, the prevalence of PNETs in autopsy studies is 0.5%–1.5%, indicating that a majority remain undiagnosed.[3,4] The annual incidence is approximately 0.8/100,000.[5] Analysis of the surveillance, epidemiology, and end results database shows a rising incidence of PNETs, likely attributed to the increased identification of small-sized tumors (<2 cm) on cross-sectional imaging.[6]
Although observation is now generally indicated for most patients with small (<2 cm), incidental, and nonfunctional PNETs, surgical resection is the treatment of choice for larger and functional PNETs. Surgical therapy has a significant benefit in terms of survival compared to conservative care (114 months vs. 35 months; P < 0.0001) but is associated with significant short-term and long-term adverse events (AEs).[7,8]
During the last decade, advances in EUS-guided ablative techniques have enabled a possible alternative to surgical resection. The two most commonly described techniques are radiofrequency ablation (RFA) and ethanol ablation (EA).[9,10] Both techniques have been reported to have variable outcomes in recent small case series; however, there is a lack of data comparing their effectiveness and safety.[9,10] We performed a systematic review and meta-analysis to evaluate the comparative effectiveness and safety of EUS–radiofrequency RFA and EUS-EA in the treatment of PNETs.
METHODS
Search strategy
We conducted a comprehensive search of several databases from inception to October 5, 2020. The databases included Ovid MEDLINE® and Epub Ahead of Print, In-Process and other nonindexed citations, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. An experienced medical librarian using inputs from the study authors helped with the literature search. Controlled vocabulary supplemented with keywords was used to search for studies of interest. The full search strategy is available in Appendix 1. The MOOSE checklist and PRISMA checklist were followed and are provided in Appendixes 2 and 3.[11,12]
Study selection
In this meta-analysis, we included studies that reported the outcomes of EUS-guided RFA or EA of PNET. Studies were included irrespective of the study sample size, inpatient/outpatient setting, and geography as long as they provided data needed for the analysis.
Studies done in pediatric population (age <18 years), case reports, and studies not published in the English language were our only exclusion criteria. In case of multiple publications from the same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained.
Data abstraction and quality assessment
Data on study-related outcomes in the individual studies were abstracted onto a standardized form by at least two authors (RG, AM, or AS), and two authors (RG, AM) did the quality scoring independently. Primary study authors were contacted via e-mail as needed for further information and/or clarification on data.
The Newcastle–Ottawa scale for cohort studies was used to assess the quality of studies.[13] This quality score consisted of 8 questions, the details of which are provided in Supplementary Table 1.
Supplementary Table 1.
Study quality assessment of included studies
| Study | Selection | Comparability Factors comparable between the groups |
Outcome | Score Maximum=8 | Quality High >6, medium 4-6, low ≤3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Representativeness of the average adult in community | Cohort size | Information on clinical outcomes | Outcome not present at start | Adequate clinical assessment | Follow-up time | Adequacy of follow-up | ||||
| Pai et al., 2015 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Lakhtakia et al., 2016 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Choi et al., 2018 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| De La Sena et al., 2018 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Thosani et al., 2018 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 | 0.5 | 6.5 | High |
| Fathima et al., 2019 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| De Nucci et al., 2019 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Oleinikov et al., 2019 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Dancour et al., 2019 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Barthet et al., 2019 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Trosic-Ivanisevic et al., 2019 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Younis et al., 2019 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Malikowski et al., 2020 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6.5 | High |
| Levy et al., 2012 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Park et al., 2015 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0.5 | 5.5 | Medium |
| Yang et al., 2015 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Qin et al., 2016 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
| Paik et al., 2016 | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6.5 | Medium |
| Choi et al., 2018 | 0 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 6.5 | High |
| Matsumoto et al., 2020 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | Medium |
Outcomes assessed
Pooled rate of clinical success per lesion basis. Clinical success was defined by individual study authors and included patients with symptom resolution for functioning lesion, or complete ablation/disappearance or absence of enhanced area within the tumors based on follow-up contrast-enhanced imaging (cross-sectional or EUS) for nonfunctioning lesions on follow-up.
Pooled rate of technical success assessed per session basis. It was defined by EUS-guided access to PNETs along with the completion of planned ablation procedure
Pooled rate of overall AEs also assessed per session basis
Pooled rate of acute pancreatitis (AP) after ablation procedure.
These outcomes were compared between EUS-RFA and EUS-EA groups.
Meta-regression analysis based on several factors was used to assess predictors of technical success, clinical success, AEs, and AP for both techniques.
Statistical analysis
We used meta-analysis techniques to calculate the pooled estimates in each case, following the methods suggested by Der-Simonian and Laird using the random-effect model with logit transformed proprotion.[14] When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis.[15] We assessed heterogeneity between study-specific estimates by using Cochran Q statistical test for heterogeneity and the I2 statistics.[16,17] In this, values of <30%, 30%–60%, 61%–75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.[18] Publication bias was ascertained qualitatively by visual inspection of funnel plot and quantitatively by the Egger test.[19] When publication bias was present, further statistics using Duval and Tweedie's “Trim and Fill” test was used to ascertain the impact of the bias.[20] Three levels of impact were reported based on the concordance between the reported results and the actual estimate if there were no biases. The impact was reported as minimal if both versions were estimated to be the same, modest if effect size changed substantially, but the final finding would still remain the same, and severe if the basic final conclusion of the analysis is threatened by the bias.[21] A mixed-effect model was used to compare both techniques based on subgroup analysis. A Wald-type test was conducted to compare the summary effect sizes across subgroups: using either a Z-score or a Q-statistic (both yield the same P value), whether or not two groups have significantly different outcomes.[22] P ≥ 0.05 was used “a-priori” to define the significance of the difference between the groups compared as provided by the statistical software. Meta-regression was also performed to assess the predictive influence of various factors on each outcome.
All analyses were performed using R statistical software (metafor package).
Search results and population characteristics
From an initial 7872 studies, 4829 records were screened, and 49 full-length articles were assessed. Twenty studies[23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] were included in the final analysis, of which 13 studies reported on the outcomes of EUS-RFA[23,25,26,27,28,29,30,32,34,35,38,39,42] and 7 reported outcomes on EUS-EA.[24,31,33,36,37,40,41] The schematic diagram of study selection is illustrated in Supplementary Figure 1 (725.9KB, tif) .
A total of 204 lesions from 20 studies (13 EUS-RFA, 7 EUS-EA) were included in our meta-analysis. Among the 204 lesions, 113 patients were in EUS-RFA group and 91 patients underwent EUS-EA of PNETs. The mean age in EUS-RFA and EUS-EA groups was 63.1 ± 10.2 and 57.4 ± 6.8 years (P < 0.001), respectively. The mean size of PNETs was 16.4 ± 5.1 mm in EUS-RFA group which was significantly higher as compared to 12.2 ± 1.7 mm in EUS-EA group (P < 0.001). The functioning status of PNETs was reported in 11 studies in EUS-RFA group and all studies in EUS-EA group. There were 41.6% (n = 45) in EUS-RFA group as compared to 38.4% (n = 35) functioning lesions in EUS-EA group (P = 0.64). There were 39% and 50.5% of females in EUS-RFA and EUS-EA groups, respectively. A total of 256 ablation sessions (118 EUS-RFA, 138 EUS-EA) were performed in our study population. The mean number of ablation sessions per lesion with EUS-RFA was 1.2 ± 0.4 as compared to 1.5 ± 0.4 in EUS-EA group (P < 0.001). The population characteristics along with data on assessed outcomes are shown in Table 1.
Table 1.
Data on study, population characteristics, and assessed outcomes included in the meta-analysis
| Author, year | Type of study | Intervention | Age (years)a | Number of patients | Total number of lesions | Functioning | Nonfunctioning | Female | Mean size (mm)a | Total number of sessions |
|---|---|---|---|---|---|---|---|---|---|---|
| Pai et al., 2015 | Prospective | RFA | 69.5±12.5 | 2 | 2 | 2 | 27.5±17.7 | 3 | ||
| Lakhtakia et al., 2016 | Prospective | RFA | 45±4.96 | 3 | 3 | 3 | 0 | 0 | 13±5.5 | 3 |
| Choi et al., 2018 | Prospective | RFA | 55±30.6 | 8 | 8 | 1 | 7 | 4 | 19.3±6.7 | 14 |
| De La Serna et al., 2018 | Prospective | RFA | 67.3±7.7 | 3 | 3 | NR | NR | 0 | 16.1±6.3 | 6 |
| Thosani et al., 2018 | Retrospective | RFA | NR | 3 | 3 | 3 | 0 | NR | 23 | 5 |
| Fathima et al., 2019 | Prospective | RFA | NR | 15 | 18 | 13 | 5 | NR | 13.6 | 17 |
| De Nucci et al., 2019 | Prospective | RFA | 78.6 | 10 | 11 | 3 | 8 | 4 | 14.5 (9-24) | 11 |
| Oleinikov et al., 2019 | Prospective | RFA | 60.4±14.4 | 18 | 25 | 8 | 19 | 8 | 14.3±7.3 | 18 |
| Dancour et al., 2019 | Prospective | RFA | 53.5±11.5 | 8 | 8 | 8 | 0 | 4 | 15.31±1.94 | 8 |
| Barthet et al., 2019 | Prospective | RFA | 59.9 (45-47) | 12 | 14 | 0 | 14 | 5 | 13.1 (10-20) | 14 |
| Trosic-Ivanisevic et al., 2019 | Retrospective | RFA | NR | 7 | 7 | 3 | 4 | NR | 11.8 (7.4-18) | 7 |
| Younis et al., 2019 | Prospective | RFA | 73.5 | 3 | 3 | 0 | 3 | NR | 10 (7-16) | 3 |
| Malikowski et al., 2020 | Prospective | RFA | 68.4±8.7 | 8 | 8 | 3 | 5 | 5 | 21.9±15.5 | 9 |
| Levy et al., 2012 | Prospective | Ethanol | 66.3±14.9 | 5 | 5 | 5 | 5 | 4 | 15±4.17 | 11 |
| Park et al., 2015 | Prospective | Ethanol | 52.5±20.5 | 11 | 14 | 4 | 4 | 6 | 12.3±3.2 | 18 |
| Yang et al., 2015 | Prospective | Ethanol | 59 | 4 | 4 | 4 | 4 | NR | N/A | 5 |
| Qin et al., 2016 | Prospective | Ethanol | 45.3±10.5 | 17 | 17 | 17 | 17 | 9 | 13.2±6.2 | 27 |
| Paik et al., 2016 | Prospective | Ethanol | 60±27 | 6 | 6 | 4 | 4 | 2 | 11.3±3.7 | 6 |
| Choi et al., 2018 | Prospective | Ethanol | 56.5±12.7 | 33 | 40 | 1 | 1 | 20 | 11 (range 7-20) | 63 |
| Matsumoto et al., 2020 | Prospective | Ethanol | 62.4±7.9 | 5 | 5 | 0 | 0 | 3 | 10.2±2.5 | 8 |
|
| ||||||||||
|
| ||||||||||
| Author, year | Location | Ethanol volume per session (mL)a, concentration (%) | Technical success | Clinical success | Total follow-up duration (months)a | Adverse events | Acute pancreatitis | Abdominal pain | Others | |
|
| ||||||||||
| Pai et al., 2015 | 2 head | NA | 3 | 2 | 3-6 | 0 | 0 | 0 | 0 | |
| Lakhtakia et al., 2016 | 2 head, 1 body | NA | 3 | 3 | 11-12 | 0 | 0 | 0 | 0 | |
| Choi et al., 2018 | 3 head, 5 body | NA | 14 | 6 | 13 | 2 | 1 | 1 | 0 | |
| De La Serna et al., 2018 | 1 head, 2 body | NA | 6 | 1 | 3-16 | 0 | 0 | 0 | 0 | |
| Thosani et al., 2018 | NR | NA | 5 | 3 | 5 | NR | NR | NR | NR | |
| Fathima et al., 2019 | NR | NA | 17 | 15 | 6-60 | 2 | 1 | 1 | 0 | |
| De Nucci et al., 2019 | 3 head, 6 body, 2 tail | NA | 11 | 11 | 12 | 0 | 0 | 0 | 0 | |
| Oleinikov et al., 2019 | 5 uncinate, 10 head, 8 body, 2 tail | NA | 18 | 25 | 2-27 | 0 | 2 | 0 | 0 | |
| Dancour et al., 2019 | 3 uncinate, 2 head, 2 body, 1 tail | NA | 8 | 8 | 1.5-21 | 0 | 0 | 0 | 0 | |
| Barthet et al., 2019 | 3 head, 6 body, 5 tail | NA | 14 | 12 | 12 | 2 | 1 | 1 | 0 | |
| Trosic-Ivanisevic et al., 2019 | 2 uncinate, 3 neck, 2 body | NA | 7 | 6 | 43876 | 4 | 0 | 3 | 1 (pancreatic fistula) | |
| Younis et al., 2019 | NR | NA | 3 | 1 | 0 | 1 | 0 | |||
| Malikowski et al., 2020 | 6 head, 1 neck, 1 body | NA | 9 | 8 | 1 | 1 | 0 | 0 | 0 | |
| Levy et al., 2012 | 3 head, 1 body, 1 tail | 1.2±1.7, 95%-98% | 11 | 4 | 17.3±11.5 | 0 | 0 | 0 | 0 | |
| Park et al., 2015 | 1 uncinate, 6 head, 7 body/tail | median 1.6 (range, 0.5-3.8), 99% | 18 | 92 | 14.8±8.1 | 5 | 3 | 2 | 0 | |
| Yang et al., 2015 | NR | 3.1, 98% | 5 | 3 | 17.3 | 0 | 0 | 0 | 0 | |
| Qin et al., 2016 | 5 head, 2 neck, 4 body, 6 tail | Mean 1.0 (range, 0.4-1.05 mL), NR | 27 | 17 | 1-21 | 0 | 0 | 0 | 0 | |
| Paik et al., 2016 | 4 head, 2 body | 2.4±1.4, 99% | 6 | 5 | 16.5 (range, 5.4-55.3) | 3 | 1 | 1 | 1 Fever | |
| Choi et al., 2018 | 23 uncinate/head, 17 body/tail | 1.1 (0.8-1.9), 99% | 63 | 24 | 42 (median 39-46) | 2 | 2 | 0 | 0 | |
| Matsumoto et al., 2020 | 2 head, 1 body, 2 tail | 0.7±0.2, 100% | 8 | 4 | 12 | 0 | 0 | 0 | 0 | |
a Values are reported as mean±SD or median (range) or range; b1 patient lost to follow-up. RFA: Radiofrequency ablation; NR: Not reported; NA: Not applicable; SD: Standard deviation
Characteristics and quality of included studies
Eighteen studies were prospective, and two studies were retrospective in nature. Among the 20 observational studies, 3 were of high quality and 17 were of medium quality. The quality assessment is shown in Supplementary Table 1.
Meta-analysis outcomes
The follow-up period ranged from 1 to 60 months in the study population.
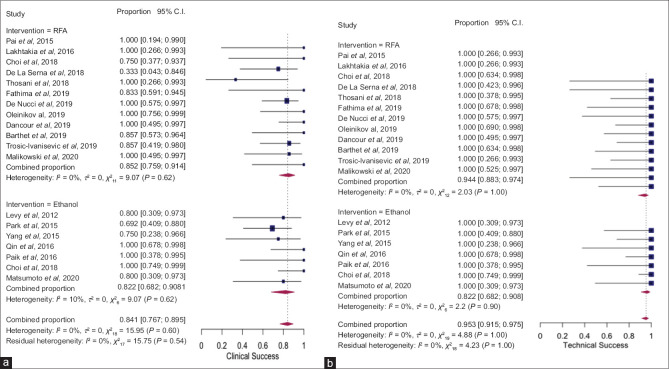
Clinical success for functioning lesions was defined by symptom resolution on follow-up. For nonfunctioning lesions, the definition of clinical success was more variable and included complete ablation/disappearance or absence of enhanced area within the tumor based on contrast-enhanced computed tomography or EUS examination on follow-up. The details of clinical success definition are shown in Supplementary Table 2. The pooled rate of clinical success after EUS-RFA and EUS-EA was 85.2 (95% confidence interval [CI], 75.9–91.4, I2 = 0) and clinical success rate of 82.2 (95% CI, 68.2–90.8, I2 = 10.1%) [Figure 1a]. There was no statistically significant difference between both techniques as evidenced by overlapping CI with P = 0.65. The pooled rate of technical success with EUS-RFA and EUS-EA was 94.4% (95% CI, 88.3–97.4, I2 = 0%) and 96.7% (95% CI, 90.8–98.8, I2 = 0%), respectively [Figure 1b] without any significant statistical difference (P = 0.42).
Supplementary Table 2.
Clinical success definition in each included study
| Author, year | Intervention | Total (n) | Clinical success | Clinical success definition |
|---|---|---|---|---|
| Pai et al., 2015 | EUS-RFA | 2 | 2 | Central area of necrosis on follow-up cross-sectional imaging and change in vascularity |
| Lakhtakia et al., 2016 | EUS-RFA | 3 | 3 | All functioning, symptom resolution |
| Choi et al., 2018 | EUS-RFA | 8 | 6 | Absence of enhancing tissue at tumor site on contrast-enhanced CT or EUS on follow-up |
| De La Sena et al., 2018 | EUS-RFA | 3 | 1 | CT shows well-defined nonenhancing area and EUS with hyperechogenic area with absence of malignant tissue after FNA |
| Thosani et al., 2018 | EUS-RFA | 3 | 3 | All functioning, symptom resolution |
| Fathima et al., 2019 | EUS-RFA | 18 | 15 | Symptom resolution for functioning and decrease in size for nonfunctioning |
| De Nucci et al., 2019 | EUS-RFA | 11 | 11 | Complete disappearance of lesions and symptom resolution |
| Oleinikov et al., 2019 | EUS-RFA | 25 | 25 | Presence of nonenhancing area (central necrosis) at the site of ablated lesion on CECT, fibrotic tissue on the site of ablated lesion on EUS, and loss of uptake on PET/CT |
| Dancour et al., 2019 | EUS-RFA | 8 | 8 | All functioning, symptom resolution |
| Barthet et al., 2019 | EUS-RFA | 14 | 12 | Disappearance of lesion |
| Trosic-Ivanisevic et al., 2019 | EUS-RFA | 7 | 6 | Disappearance of lesion and symptom resolution |
| Younis et al., 2019 | EUS-RFA | 3 | NR | Not applicable |
| Malikowski et al., 2020 | EUS-RFA | 8 | 8 | Good sonographic response and complete ablation |
| Levy et al., 2012 | EUS-EA | 5 | 3 | All functioning, symptom resolution |
| Park et al., 2015 | EUS-EA | 14 | 9/13 | Disappearance of enhanced area within the tumors based on contrast-enhanced CT or EUS on follow-up |
| Yang et al., 2015 | EUS-EA | 4 | 3 | All functioning, symptom resolution |
| Qin et al., 2016 | EUS-EA | 17 | 17 | All functioning, symptom resolution |
| Paik et al., 2016 | EUS-EA | 6 | 5 | Complete ablation on imaging or the absence of hormone-related symptoms |
| Choi et al., 2018 | EUS-EA | 40 | 24 | Absence of enhanced area within the tumors based on repeat imaging and negative cytology on EUS-FNB at 3-year follow-up |
| Matsumoto et al., 2020 | EUS-EA | 5 | 4 | Absence of enhanced area on follow-up CT every 3 months |
RFA: Radiofrequency ablation; EA: Ethanol ablation; CT: Computed tomography; CECT: Contrast-enhanced CT; FNB: Fine-needle biopsy; NR: Not reported, PET: Positron emission tomography
Figure 1.
Forest plot showing pooled rates of clinical success (a) and technical success (b) after EUS–radiofrequency ablation and EUS-EA of pancreatic neuroendocrine tumors
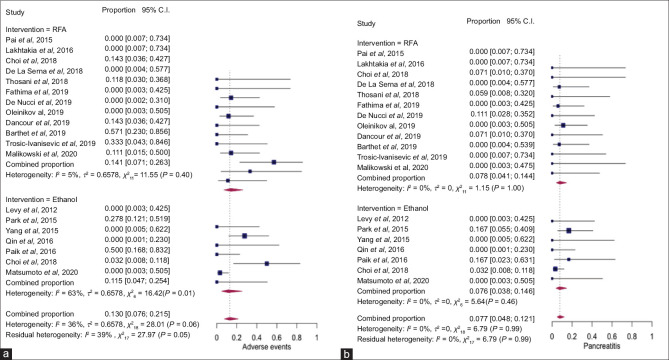
There were a total of 22 AEs in the study population, 12 in EUS-RFA and 10 in ethanol group. The most common AE was AP (50%) followed by abdominal pain (45.5%) and 1 case of pancreatic fistula (4.5%). The pooled rate of AEs after EUS-RFA and EUS-EA was 14.1% (95% CI, 7.1–26.3, I2 = 5%) and 11.5% (95% CI, 4.7–25.4, I2 = 63%), respectively, without any statistically significant difference (P = 0.7) [Figure 2a]. The pooled rate of pancreatitis with EUS-RFA and EUS-EA was 7.8% (95% CI, 4.1–14.4, I2 = 0%) and 7.6% (95% CI, 3.8–14.6, I2 = 0%) (P = 0.95), respectively [Figure 2b].
Figure 2.
Forest plot showing pooled rates of adverse events (a) and pancreatitis (b) after EUS–radiofrequency ablation and EUS-EA of pancreatic neuroendocrine tumors
The pooled results with their P values are summarized in Table 2.
Table 2.
Outcomes of EUS-radiofrequency and ethanol ablation of pancreatic neuroendocrine tumors
| Outcome | EUS-RFA | Ethanol | P |
|---|---|---|---|
| Clinical success | 85.2 (75.9-91.4), I2=0, 12 studies | 82.2 (68.2-90.8), I2=10.1, 7 studies | 0.65 |
| Technical success | 94.4 (88.3-97.4), I2=0, 13 studies | 96.7 (90.8-98.8), I2=0, 7 studies | 0.42 |
| Adverse events | 14.1 (7.1-26.3), I2=5, 12 studies | 11.5 (4.7-25.4), I2=63%, 7 studies | 0.7 |
| Acute pancreatitis | 7.8 (4.1-14.4), I2=0, 12 studies | 7.6 (3.8-14.6), I2=0, 7 studies | 0.95 |
Value are reported as pooled rate, 95% CI, I2 and number of studies. RFA: Radiofrequency; CI: Confidence interval
Meta-regression
Meta-regression was performed clinical success, technical success, AEs, and technical success of both techniques. The variables included were age, functioning PNETs, mean size (mm), location (head/neck or body/tail) for both techniques with addition of mean ethanol amount for EUS-EA. The only significant and positive predictor of clinical success was head/neck location of lesion for EUS-RFA ablation with regression coefficient of 0.24 (95% CI, 0.02–0.46, P = 0.032). There was a trend toward a higher rate of AEs with higher ethanol amount after EUS-EA with P = 0.09 but did not reach statistical significance. Age, functioning PNETs, size, and body/tail location did not have any significant predictive influence on assessed outcomes. Results of meta-regression are summarized in Supplementary Table 3. Scatter plot showing the relationship of head/neck location and clinical success with EUS-RFA is also shown in Figure 3.
Supplementary Table 3.
Predictors of EUS-radiofrequency ablation and ethanol ablation of pancreatic neuroendocrine tumors
| Factor | Technical success | Clinical success | Adverse events | Pancreatitis |
|---|---|---|---|---|
|
| ||||
| EUS-RFA | ||||
| Age | −0.003, P=0.94 | 0.009, P=0.86 | 0.0004, P=0.99 | −0.01, P=0.80 |
| Functioning | 0.063, P=0.55 | 0.008, P=0.88 | −0.071, P=0.35 | −0.001, P=0.98 |
| Size | −0.02, P=0.77 | −0.02, P=0.78 | −0.09, P=0.2149 | −0.001, P=0.98 |
| Head/neck | 0.076, P=0.52 | 0.24, P=0.032 | −0.08, P=0.51 | 0.03, P=0.65 |
| Body/tail | 0.086, P=0.38 | 0.04, P=0.53 | −0.054, P=0.46 | 0.006, P=0.92 |
|
| ||||
| Ethanol ablation | ||||
|
| ||||
| Age | −0.065, P=0.44 | −0.04, P=0.59 | 0.03, P=0.77 | −0.001, P=0.98 |
| Functioning | 0.03, P=0.77 | 0.09, P=0.36 | −0.08, P=0.56 | −0.04, P=0.66 |
| Size | −0.001, P=0.99 | −0.10, P=0.78 | −0.22, P=0.66 | −0.04, P=0.9 |
| Head/neck | 0.09, P=0.24 | 0.11, P=0.15 | −0.08, P=0.32 | −0.06, P=0.17 |
| Body/tail | 0.13, P=0.20 | 0.14, P=0.16 | −0.14, P=0.14 | −0.09, P=0.14 |
| Ethanol amount | −0.59, P=0.38 | −0.39, P=0.52 | 1.23, P=0.09 | 0.85, P=0.16 |
Values are regression coefficient with P value, Bold indicated significant P value. RFA: Radiofrequency ablation
Figure 3.
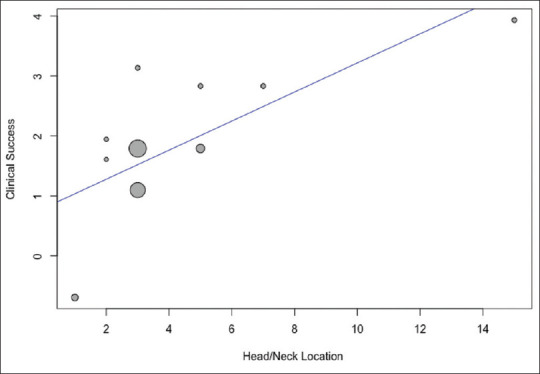
Scatter plot showing the relationship of head/neck location and clinical success after EUS-radiofrequency ablation of pancreatic neuroendocrine tumors
VALIDATION OF META-ANALYSIS RESULTS
Sensitivity analysis
To assess whether any one study had a dominant effect on the meta-analysis, we excluded one study at a time and analyzed its effect on the main summary estimate. The rate of clinical success, technical success, AEs, and pancreatitis ranged from 83.5 to 86.6, 94.8 to 95.5, 11.9 to 15.7, and 6.6 to 8.7, respectively, on sensitivity analysis. On this analysis, no single study significantly affected the outcome or the heterogeneity.
Heterogeneity
We assessed dispersion of the calculated rates using the I2 percentage values. I2 tells us what proportion of the dispersion is true vs. chance.[43] Overall, there was low heterogeneity in the assessed outcomes.
Publication bias
There was evidence of publication bias on visual inspection of the funnel plot as well as quantitative measurement using the Egger regression test (Egger's two-tailed P = 0.03) for clinical success. On further trim and fill analysis, seven missing studies were added which adjusted our primary outcome of clinical success to 78.3% (95% CI, 68.3–85.8) from 84.5% (95% CI, 77.3–89.7). Based on overlapping CI with our primary outcome, the impact of publication bias is considered modest. The funnel plot with added studies is shown in Figure 4.
Figure 4.
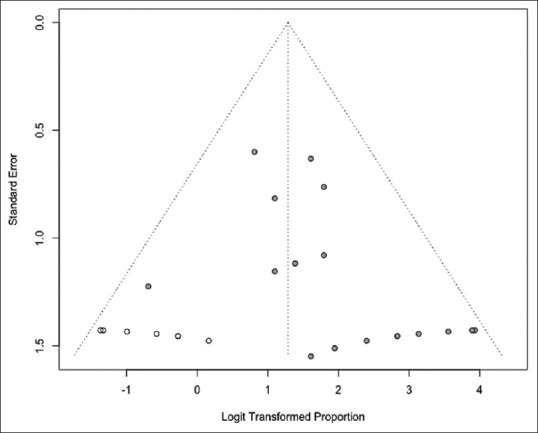
Funnel plot assessing publication bias with filled studies
DISCUSSION
Our study demonstrates that EUS-RFA and EUS-EA of PNETs are effective and safe with comparable outcomes. There was no significant difference in the rate of clinical success after EUS-RFA (85.2%) as compared to EUS-EA (82.2%) (P = 0.59). The technical success for EUS-RFA and EUS-EA was 94.3% and 96.7%, respectively (P = 0.41). The rate of AEs was 14.3% with EUS-RFA and 11.7% with EUS-EA with a P value of 0.69. On meta-regression, the location of PNETs in head/neck of pancreas (P = 0.03) was a positive predictor of clinical success for EUS-RFA ablation. Our study is the largest and first meta-analysis reporting and comparing outcomes of EUS-RFA and EUS-EA of PNETs.
The rates of clinical success after EUS-RFA and EUS-EA have been reported to range from 82.4% to 96% and 62.1% to 93.9%, respectively. In our study, the rate of clinical success after EUS-RFA was 85.2% as compared to EUS-EA (82.2%) (P = 0.59). The slight variability of our results is likely due to larger sample size and variable definition of clinical success by study authors along with the lack of standardized technique. Clinical success in both procedures is defined as a decrease in lesion size and appearance of a hypodense area of necrosis on imaging leading to decrease in size and/or improvement in symptoms (functioning PNETs). In addition, clinical success in nonfunctioning lesion is achieved by complete ablation of the lesion, whereas central ablation in functioning PNETs to abate symptoms is sufficient to achieve clinical success. A recent systematic review demonstrated that lesion size ≤18 mm had a very high positive predictive value of 97.1% predicting response to EUS-RFA of PNETs.[44] In our study, head/neck location of PNETs was associated with a positive predictor of clinical success after EUS-RFA, likely due to proximal location and relative ease of access leading to more complete ablation of lesions. However, we did not find a location to be a significant predictor after EUS-EA ablation, likely due to a smaller number of studies in EUS-EA group.
EUS-RFA and EUS-EA of pancreatic lesions were first studied in animal models.[45,46] Since then, RFA application has expanded as an adjunctive therapy for nonresectable pancreatic adenocarcinoma. The largest prospective study of EUS-RFA for PNETs included 12 patients and reported 100% technical success.[23] In our study, we recorded varied amounts of energy delivered per session from 10 W to 50W. The average duration of each session also ranged between 5 s and 120 s, and often, multiple sessions are required. In addition, the availability of two different RFA electrodes (Habib EUS-guided RFA probe [EndoHPB, EMcision UK, London, UK, recently purchased by Boston Scientific Corp., Marlborough, MA, USA] and EUSRA EUS-RFA system from Taewoong Medical (Taewoong Medical Co., Gimpo-si, Gyeonggi-do, South Korea]) also leads to nonuniform practice.[10] Most of our studies except Pai et al. used EUSRA system, so we were unable to do subgroup analysis based on RFA system. Similarly, the technical and clinical success of EUS-EA is dependent on the number of sessions and volume of ethanol which itself is dependent on the size of the lesion. The alcohol concentration ranged from 95% to 100%. In our study, both EUS-RFA and EUS-EA were associated with high technical success rates of 94.3% and 96.7%, respectively (P = 0.41). The high success rate is likely due to accurate lesion localization and targeting with EUS guidance. We did not find any significant predictor of technical success on meta-regression.
The rate of AEs and AP in EUS-RFA when compared with EUS-EA was not significantly different. When performing RFA, it is important to properly visualize the lesion under ultrasound guidance and avoid areas close to the gut wall, blood vessels, or ducts to avoid postprocedural AEs.[11] AP is the most common AE associated with EUS-RFA and EUS-EA. In a study, the authors recommended a minimum of 5 mm safety margin from a duct or vessel is necessary to avoid iatrogenic injuries with EUS-RFA.[29] Interestingly in our study, the amount of ethanol used for ablation showed a trend toward predicting AEs but did not reach statistical significance, likely due to small sample size and fewer studies. Paik et al. describe an episode of severe pancreatitis in one patient because of ethanol leakage into surrounding structures.[40] They determined that the presence of multiple side holes in the needle and excess amount of ethanol injection led to procedure-related AE. It is thus recommended to use small aliquots of ethanol injection using a single-hole needle and accurate targeting of lesion.[40]
Our study has several important clinical implications. First, we report that EUS-RFA and EUS-EA ablation of PNETs have similar clinical, technical success and safety profile. However, the size of lesions was significantly smaller, and the number of sessions was significantly higher in EUS-EA group as compared to EUS-RFA group. We, however, did not identify size or functioning lesion to be a significant predictor of success with both techniques. Although no direct comparison is available, the cost associated with RFA probe and RFA generator is quite high, whereas ethanol is relatively cheap. The cost-effectiveness needs to be further studied, especially if patients undergoing EUS-EA require more subsequent procedure. The greater number of procedures with EUS-EA potentially expose a patient to more procedural-related complications such as bleeding and perforation and patient compliance becomes even more important. Nevertheless, treatment should be chosen after multidisciplinary discussion based on available local expertise and informed discussion with the patient.
The strengths of this review are as follows: systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good-quality studies with detailed extraction of data, low heterogeneity, studies from throughout the world, and rigorous evaluation of study quality. There are limitations to this study, most of which are inherent to any meta-analysis. The included studies were not entirely representative of the general population, with most studies being performed in tertiary-care referral centers and by expert endoscopists. In addition, most lesions were small in size, nonstandardized techniques, variable follow–up, and definition of clinical success also added to the limitation of our study. Nevertheless, our study is the first and best available estimate in the literature thus far with respect to the reporting and comparing clinical outcomes of EUS-RFA and EUS-EA of PNETs.
In conclusion, our meta-analysis demonstrates that outcomes of EUS-RFA ablation for PNETs are similar to EUS-EA. Head/neck location of PNETs was a positive predictor for clinical success after EUS-RFA.
Supplementary materials
Supplementary information is linked to the online version of the paper on the endoscopic ultrasound website.
Financial support and sponsorship
Nil.
Conflicts of interest
TR is a consultant for Boston Scientific.
PRISMA Flow diagram showing search strategy for meta-analysis
APPENDIX
APPENDIX 1. LITERATURE SEARCH STRATEGY
Search strategy
In patients with neuroendocrine pancreatic tumors, compare ablation techniques, especially radiofrequency and ethanol ablation, for safety, efficacy, and other outcomes.
P = patients with neuroendocrine pancreatic tumors
I = radiofrequency ablation
C = Ethanol ablation
C = All other ablation techniques
O = Outcomes (open – safety, efficacy)
Ovid Embase (Ovid Interface) – 1974– February 10, 2020 (limited to English)
Exp ablation therapy/or (ablati* adj3 (therap or treat* or surg* or technique* or procedure)).mp. OR exp radiofrequency ablation/Or (radiofrequency surg*).mp/or RFA or (Ethanol ablat*).mp. or (OR exp ablation techniques/or cautery/or electrocoagulation/or argon plasma coagulation/or cryosurgery/or high-intensity focused ultrasound ablation/or ultrasound, high-intensity focused, transrectal/or laser therapy/or maze procedure/or radiofrequency ablation/or catheter ablation
AND exp pancreas islet cell tumor/or exp glucagonoma/or exp insulinoma/or exp pancreas islet cell/or exp somatostatinoma/or exp vipoma/OR (exp neuroendocrine tumor/AND exp pancreas cancer/) OR
(adenoma or alpha or beta or diaarheogenic or islet or langerhans or pancrea*) adj3 (adenoma or cell or tumor* or tumour* or islet) or glucagonoma/or insulinoma/or pancreatic islet cell carcinoma/or somatostatinoma/or vipoma/ or Zollinger Ellison syndrome/or (glucagonoma or insulinomor pancreatic islet cell carcinoma or gastrinoma or somatostatinoma or vipoma or Zollinger Ellison syndrome).mp.
Ovid Medline (Ovid Interface) – 1946–February 10, 2020 (limited to English)
Ethanol ablation.mp. OR exp Ablation Techniques/OR exp ablation techniques/or cautery/or electrocoagulation/or argon plasma coagulation/or cryosurgery/or high-intensity focused ultrasound ablation/or ultrasound, high-intensity focused, transrectal/or laser therapy/or maze procedure/or radiofrequency ablation/or catheter ablation/OR (ablati* adj3 (therap or treat* or surg* or technique* or procedure)).mp. OR exp radiofrequency ablation/Or (radiofrequency surg*).mp/or RFA or (Ethanol ablat*).mp.AND (exp Neuroendocrine Tumors/AND (exp Pancreatic Neoplasms/) or exp Carcinoma, Pancreatic Ductal/or pancreatic tumor.mp.) OR ((adenoma or alpha or beta or diaarheogenic or islet or langerhans or pancrea*) adj3 (adenoma or cell or tumor* or tumour* or islet)).mp. or glucagonoma/or insulinoma/ or pancreatic islet cell carcinoma/or somatostatinoma/or vipoma/or Zollinger Ellison syndrome/or (glucagonoma or insulinoma or gastrinoma or pancreatic islet cell carcinoma or somatostatinoma or vipoma or Zollinger Ellison syndrome).mp.
Cochrane Library (Wiley interface) – 1974–May 10, 2020
ethanol ablation.mp. OR exp Ablation Techniques/OR exp ablation techniques/or cautery/or electrocoagulation/or argon plasma coagulation/or cryosurgery/or high-intensity focused ultrasound ablation/or ultrasound, high-intensity focused, transrectal/or laser therapy/or maze procedure/or radiofrequency ablation/or catheter ablation/OR (ablati* adj (therap or treat*3 or surg* or technique* or procedure)).mp. OR exp radiofrequency ablation/Or (radiofrequency surg*).mp/or RFA or (Ethanol ablat*).mp.
AND
(exp Neuroendocrine Tumors/AND (exp Pancreatic Neoplasms/) or exp Carcinoma, Pancreatic Ductal/or pancreatic tumor.mp.) OR ((adenoma or alpha or beta or diaarheogenic or islet or langerhans or pancrea*) adj3 (adenoma or cell or tumor* or tumour* or islet)).mp. or glucagonoma/or insulinoma/or pancreatic islet cell carcinoma/or somatostatinoma/or vipoma/or Zollinger Ellison syndrome/or (glucagonoma or insulinoma or pancreatic islet cell carcinoma or somatostatinoma or vipoma or Zollinger Ellison syndrome).mp.
CINAHL (Ebsco interface) – 1965–May 10, 2020 (limited to English)
ethanol ablation OR (MH “Ablation Techniques+”) OR ablati* N3 (therap or treat* or surg* or technique* or procedure*) OR (radiofrequency surg*) or RFA or (Ethanol ablat*)
AND
((MH “Neuroendocrine Tumors+”) OR (MH “Carcinoid Tumor+”) OR (MH “Melanoma+”) OR (MH “Paraganglioma+”) OR (MH “Neurilemmoma+”) AND ((MH “Pancreatic Neoplasms+”) OR (MH “Adenoma, Islet Cell+”) OR (MH “Carcinoma, Islet Cell+”) OR (MH “Insulinoma”) OR (MH “Gastrinoma”) OR (MH “Glucagonoma”) OR (MH “Neoplasia, Pancreatic Intraepithelial”)) OR ((adenoma or alpha or beta or diaarheogenic or islet or langerhans or pancrea*) N3 (adenoma or cell or tumor* or tumour* or islet)) OR glucagonoma or insulinoma or pancreatic islet cell carcinoma or somatostatinoma or vipoma or Zollinger Ellison syndrome
Scopus (Elsevier interface) – 1974–June 10, 2020 (limited to English)
((TITLE-ABS-KEY (radiofrequency W/3 (ablati* OR surg*) OR rfa OR (ethanol AND ablati*))) OR (TITLE-ABS-KEY (ablati* W/3 (therap OR treat* OR surg* OR technique* OR procedure*))) OR (TITLE-ABS-KEY (cautery OR electrocoagulation OR “argon plasma coagulation” OR cryosurgery OR “high-intensity focused ultrasound ablation”/OR “laser therapy” OR “maze procedure” OR “catheter ablation”)))
AND
((TITLE-ABS-KEY ((neuroendocrine AND pancrea*) W/3 (tumor* OR tumour* OR adenoma* OR carcinoma* OR cancer OR neoplasm*))) OR (TITLE-ABS-KEY ((adenoma OR alpha OR beta OR diaarheogenic OR islet OR langerhans OR pancrea*) W/3 (adenoma OR cell OR tumor* OR tumour* OR islet))) OR (TITLE-ABS-KEY (glucagonoma OR insulinoma OR gastrinoma OR “pancreatic islet cell carcinoma” OR somatostatinoma OR vipoma OR “Zollinger Ellison syndrome”)))
Web of Science (Clarivate Analytics interface)- 1965-10/6/2020
TS=((“neuroendocrine pancrea*”) NEAR/3 (tumor* OR tumour* OR adenoma* OR carcinoma* OR cancer OR neoplasm*)) OR TS=((adenoma OR alpha OR beta OR diaarheogenic OR islet OR langerhans OR pancrea*) NEAR/3 (adenoma OR cell OR tumor* OR tumour* OR islet)) OR TS= (glucagonoma OR insulinoma OR gastrinoma OR “pancreatic islet cell carcinoma” OR somatostatinoma OR vipoma OR “Zollinger Ellison syndrome”
AND
TS=(cautery OR electrocoagulation OR “argon plasma coagulation” OR cryosurgery OR “high-intensity focused ultrasound ablation” OR “laser therapy” OR “maze procedure” OR “catheter ablation”) oR
TS=((radiofrequency NEAR/3 (ablati* OR surg*)) OR (ethanol AND ablati*)) OR
TS=(ablati* NEAR/3 (therap OR treat* OR surg* OR technique* OR procedure*)), exclude medline and Derwent innovations/patents
Appendix 2
Meta-analysis of observational studies in epidemiology checklist for meta-analyses of observational studies
| Item number | Recommendation | Reported on page number |
|---|---|---|
|
| ||
| Reporting of background should include | ||
| 1 | Problem definition | 5 |
| 2 | Hypothesis statement | - |
| 3 | Description of study outcome (s) | 7 |
| 4 | Type of exposure or intervention used | 6 |
| 5 | Type of study designs used | 6 |
| 6 | Study population | 6 |
| Reporting of search strategy should include | ||
| 7 | Qualifications of searchers (e.g., librarians and investigators) | 1 |
| 8 | Search strategy, including time period included in the synthesis and key words | 6, Appendix 1 |
| 9 | Effort to include all available studies, including contact with authors | 7 |
| 10 | Databases and registries searched | 6, Appendix 1 |
| 11 | Search software used, name and version, including special features used (e.g., explosion) | Appendix 1 |
| 12 | Use of hand searching (e.g., reference lists of obtained articles) | 6 |
| 13 | List of citations located and those excluded, including justification | 9, Supplementary Figure 1 (725.9KB, tif) |
| 14 | Method of addressing articles published in languages other than English | 6 |
| 15 | Method of handling abstracts and unpublished studies | 6 |
| 16 | Description of any contact with authors | 7 |
|
| ||
| Reporting of methods should include | ||
|
| ||
| 17 | Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested | 6-8 |
| 18 | Rationale for the selection and coding of data (e.g., sound clinical principles or convenience) | 6-8 |
| 19 | Documentation of how data were classified and coded (e.g., multiple raters, blinding, and interrater reliability) | 6-8 |
| 20 | Assessment of confounding (e.g., comparability of cases and controls in studies where appropriate) | 9 |
| 21 | Assessment of study quality, including blinding of quality assessors, stratification or regression on possible predictors of study results | 8-10 |
| 22 | Assessment of heterogeneity | 7-8, 12 |
| 23 | Description of statistical methods (e.g., complete description of fixed or random-effect models, justification of whether the chosen models account for predictors of study results, dose-response models, or cumulative meta-analysis) in sufficient detail to be replicated | 8 |
| 24 | Provision of appropriate tables and graphics | Tables 1-2, Figures 1-4 |
|
| ||
| Reporting of results should include | ||
|
| ||
| 25 | Graphic summarizing individual study estimates and overall estimate | Figures 1-3 |
| 26 | Table giving descriptive information for each study included | Tables 1 and 2 |
| 27 | Results of sensitivity testing (e.g., subgroup analysis) | 11 |
| 28 | Indication of statistical uncertainty of findings | 12-16 |
Appendix 3
PRISMA checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 3-4 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5-6 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | - |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 6, Appendix 1 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 6, Appendix 1 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6, Appendix 1 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 6-7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 6-7 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 9-10 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 8 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16)) | |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., health-care providers, users, and policymakers) | |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review level (e.g., incomplete retrieval of identified research, reporting bias) | |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009), Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement, PLoS Med 6 (7): e1000097. doi: 10.1371/journal.pmed1000097, For more information, visit: www.prisma-statement.org
REFERENCES
- 1.Klöppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol. 2007;18:150–5. doi: 10.1007/s12022-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 2.Mallinson CN, Bloom SR, Warin AP, et al. A glucagonoma syndrome. Lancet. 1974;2:1–5. doi: 10.1016/s0140-6736(74)91343-9. [DOI] [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–81. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 6.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aranha GV, Shoup M. Nonstandard pancreatic resections for unusual lesions. Am J Surg. 2005;189:223–8. doi: 10.1016/j.amjsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: The impact of surgical resection on survival. Cancer. 2009;115:741–51. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 9.Lakhtakia S, Seo DW. Endoscopic ultrasonography-guided tumor ablation. Dig Endosc. 2017;29:486–94. doi: 10.1111/den.12833. [DOI] [PubMed] [Google Scholar]
- 10.Rimbaş M, Horumbă M, Rizzatti G, et al. Interventional endoscopic ultrasound for pancreatic neuroendocrine neoplasms. Dig Endosc. 2020;32:1031–41. doi: 10.1111/den.13635. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. New York: John Wiley & Sons Ltd; 2000. pp. 205–28. [Google Scholar]
- 16.Kanwal F, White D. “ systematic reviews and meta-analyses” in<em>clinical gastroenterology and hepatology<em>. Clin Gastroenterol Hepatol. 2012;10(11):1184–6. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, UK: John Wiley & Sons, Ltd: John Wiley & Sons; 2006. [Google Scholar]
- 22.Subgroup Analyses. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009. pp. 149–86. [Google Scholar]
- 23.Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy. 2019;51:836–42. doi: 10.1055/a-0824-7067. [DOI] [PubMed] [Google Scholar]
- 24.Choi JH, Park DH, Kim MH, et al. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc. 2018;30:652–8. doi: 10.1111/den.13058. [DOI] [PubMed] [Google Scholar]
- 25.Dancour A, Benson A, Epshtein J, et al. Endoscopic ultrasound guided radiofrequency ablation of insulinomas is safe and effective. Gastrointest Endosc. 2019;89:AB323. [Google Scholar]
- 26.De la Serna C, Cimavilla M, Madrigal B, et al. EUS-guided radiofrequency ablation (EUS-RFA) for pancreatic neuroendocrine tumors and cystic mucinous neoplasms: A pilot study of safety, feasibility and efficacy. Gastrointest Endosc. 2018;87:AB332–3. [Google Scholar]
- 27.De Nucci G, Mandelli ED, Redaelli D, et al. Endoscopic ultrasound guided radiofrequency ablation for neuroendocrine tumors: A single center case series. Endoscopy. 2019;51:S57. [Google Scholar]
- 28.Fathima S, Lakhtakia S, Basha J, et al. Sustained response of EUS guided RFA of pancreatic neuro-endocrine tumor (PNET) J Gastroenterol Hepatol. 2019;34:315. [Google Scholar]
- 29.Choi JH, Seo DW, Song TJ, et al. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099–104. doi: 10.1055/a-0583-8387. [DOI] [PubMed] [Google Scholar]
- 30.Lakhtakia S, Ramchandani M, Galasso D, et al. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos) Gastrointest Endosc. 2016;83:234–9. doi: 10.1016/j.gie.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 31.Levy MJ, Thompson GB, Topazian MD, et al. US-guided ethanol ablation of insulinomas: A new treatment option. Gastrointest Endosc. 2012;75:200–6. doi: 10.1016/j.gie.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Malikowski T, Ganda T, Agarunov E, et al. Prospective assessment of the efficacy and safety of a newly designed endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) device for treating pancreatic neuroendocrine tumors and secondary pancreatic metastasis. Gastrointest Endosc. 2020;91:AB177–8. [Google Scholar]
- 33.Matsumoto K, Kato H, Kawano S, et al. Efficacy and safety of scheduled early endoscopic ultrasonography-guided ethanol reinjection for patients with pancreatic neuroendocrine tumors: Prospective pilot study. Dig Endosc. 2020;32:425–30. doi: 10.1111/den.13552. [DOI] [PubMed] [Google Scholar]
- 34.Oleinikov K, Dancour A, Epshtein J, et al. Endoscopic ultrasound-guided radiofrequency ablation: A new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104:2637–47. doi: 10.1210/jc.2019-00282. [DOI] [PubMed] [Google Scholar]
- 35.Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park DH, Choi JH, Oh D, et al. Endoscopic ultrasonography-guided ethanol ablation for small pancreatic neuroendocrine tumors: Results of a pilot study. Clin Endosc. 2015;48:158–64. doi: 10.5946/ce.2015.48.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin S, Jiang H, Luo W. Efficacy of EUS-guided ethanol injection in the treatment of benign and symptomatic insulinomas. Gastroenterology. 2016;150:S510. [Google Scholar]
- 38.Thosani N, Sharma NR, Raijman I, et al. Safety and efficacy of endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) in the treatment of pancreatic lesions: A multicenter experience. Gastrointest Endosc. 2018;87:AB84. [Google Scholar]
- 39.Trosic-Ivanisevic T, Robert M, David G, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors: A retrospective single center study. Swiss Med Wkly. 2019;149(SUPPL 240):5S. [Google Scholar]
- 40.Paik WH, Seo DW, Dhir V, et al. Safety and efficacy of EUS-guided ethanol ablation for treating small solid pancreatic neoplasm. Medicine (Baltimore) 2016;95:e2538. doi: 10.1097/MD.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Inabnet WB, 3rd, Sarpel U, et al. EUS-guided ethanol ablation of symptomatic pancreatic insulinomas. Gastrointest Endosc. 2015;82:1127. doi: 10.1016/j.gie.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Younis F, Shibolet O, Philips A, et al. Safety and efficacy of endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) of pancreatic cystic neoplasms (PCNS) and pancreatic neuroendocrine tumors (PNETS): Preliminary report of a prospective cohort. Endoscopy. 2019;51:S59. [Google Scholar]
- 43.Mohan BP, Adler DG. Heterogeneity in systematic review and meta-analysis: How to read between the numbers. Gastrointest Endosc. 2019;89:902–3. doi: 10.1016/j.gie.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Imperatore N, de Nucci G, Mandelli ED, et al. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: A systematic review of the literature. Endosc Int Open. 2020;8:E1759–64. doi: 10.1055/a-1261-9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aslanian H, Salem RR, Marginean C, et al. EUS-guided ethanol injection of normal porcine pancreas: A pilot study. Gastrointest Endosc. 2005;62:723–7. doi: 10.1016/j.gie.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: Results in a porcine model. Gastrointest Endosc. 1999;50:392–401. doi: 10.1053/ge.1999.v50.98847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Flow diagram showing search strategy for meta-analysis