ABSTRACT
Bckground and Objectives:
EUS-guided cystogastrostomy is a well-established advanced endoscopic technique with a steep-learning curve which necessitates an ex-vivo simulator that would allow for adequate training. The aim of this study is to evaluate the feasibility of the model in allowing training for EUS-guided cystogastrostomy using lumen-apposing metal stent (LAMS).
Subjects and Methods:
The model was created by ROEYA Training Center, Egypt, using native porcine tissue to create fluid collections simulating both cystic and solid lesions. It was designed and tested in advance while the hydrogel was added on-site. The simulator was evaluated prospectively in five training sessions involving 17 international experts. The task was to successfully deploy the LAMS to drain the created cyst. After using the simulator, the experts were asked to fill a questionnaire to assess their experience. The primary endpoint was overall satisfaction with the model as a training tool.
Results:
All of the experts were satisfied with the model as a tool to train endoscopists for the technique. 76.5% (n = 11) of the experts thought the model to be moderately realistic. Proper visualization was reported by 94.1% of the experts. All experts believed the lesions to be either slightly like or very similar to real lesions. The model was graded “easy” in difficulty by 11 of the experts.
Conclusions:
In all parameters assessed, the experts thought the model to be a useful tool for future training. This preliminary study suggests that the aforementioned simulator can be used to train endoscopists on using LAMS in a risk-free environment.
Keywords: cystogastrostomy, EUS-guided drainage, training model
INTRODUCTION
EUS-guided cystogastrostomy is a technique that allows for drainage of peripancreatic fluid collections, namely pancreatic pseudocysts and walled-off necrosis. It is performed by creating a fistulous tract either through the stomach or duodenum and the cavity of the peripancreatic fluid collection.[1]
Pancreatic pseudocysts are fluid collections that accumulate in peripancreatic tissues as a complication of both acute and chronic pancreatitis. While pancreatic pseudocysts may resolve spontaneously, symptoms such as epigastric pain, vomiting, and sepsis may develop.[2] Pancreatic pseudocysts occur in up to 20% of patients with acute pancreatitis and 40% of the cases of chronic pancreatitis.[3] In addition, walled-off necrosis is the development of partially liquefied necrotic collection that is contained within a well-defined inflammatory wall. This occurs with acute necrotic collections that persist for over 4 weeks after the onset of necrotizing pancreatitis, a complication of acute pancreatitis that develops in 15% of patients.[4,5]
Cyst/collection drainage is indicated in the case of symptomatic collections, enlarging cysts, infection, and walled-off pancreatic necrosis. Minimally invasive techniques include percutaneous drainage, endoscopic drainage, and minimally invasive surgery.[6]
Guidelines published by the American Society of Gastrointestinal Endoscopy and the European Society of Gastrointestinal Endoscopy recommend endoscopic drainage or percutaneous drainage of infected walled-off necrosis as a first-line intervention.[6,7]
New larger diameter lumen-apposing metal stents (LAMS) have simplified the process of drainage. In two retrospective, multicenter studies evaluating the safety and efficacy of endoscopic therapy using LAMS for the drainage of pancreatic pseudocysts and walled-off necrosis, LAMS proved to effectively create a large and sustained cystogastrostomy.[8,9]
Despite the wide range of indications, EUS-guided cystogastrostomy remains a challenging technique. Comprehensive knowledge of diagnostic and therapeutic EUS is necessary. Since patients with symptomatic fluid collections must be managed at highly qualified centers, ex-vivo simulators are a promising modality to be used by trainees to learn the targeted endoscopic skills in a safe, less stressful environment that does not subject patients to any risk.
This is a preliminary study that aims to evaluate the feasibility of the newly developed simulator in providing training for the EUS-guided cystogastrostomy using LAMS specifically, the Hot AXIOS™ system (Boston Scientific, Marlborough, MA).
SUBJECTS AND METHODS
Study setting
The present study took place between March 2018 and December 2019. ROEYA Training Center, Egypt, provided training simulators at five international endoscopy workshops with dedicated hands-on training sessions. The simulator was evaluated prospectively as 17 expert gastroenterologists were invited to evaluate the model. The group of experts included four from Europe, seven from the Middle East, and six from Asia.
Animal models
The models were designed and tested 2 weeks before each event. On the day of the event, the models were prepared with on-site injection of hydrogel. Injection of the gel into the cysts was done about 5–15 min before the training sessions.
Model preparation
The simulator was created by ROEYA Training Center using native porcine tissue to create the fluid collections. The model was derived and adapted from reports by Baron and DeSimio and Moryoussef et al.[10,11] Three segments from the colon were used to create three cysts. Next, the cysts were sutured to the anterior gastric wall. These cysts were sutured in one of two locations: either near the pylorus of the stomach or the fundus. The cyst near the pylorus simulated a cystic lesion while the cyst sutured near the fundus was covered by the liver to simulate a solid lesion.
The stomach was then fixed in a copper tray to ensure proper conductivity of the electrical current of the EUS [Figure 1]. Finally, the whole specimen was covered with hydrogel to provide the optimum conducting medium for ultrasound waves. The EUS simulator facilitates the simulation of fluoroscopy without the use of X-ray using simulated fluoroscopy camera [Figure 2]. Fluoroscopy simulation was achieved by proper lighting in combination with transparent and nontransparent objects and without the use of harmful radiation. The camera was installed inside the simulator in an effort to create a substitute for fluoroscopy.
Figure 1.
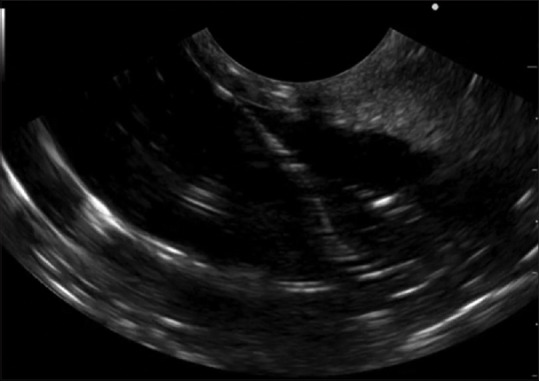
EUS view of the created cyst
Figure 2.
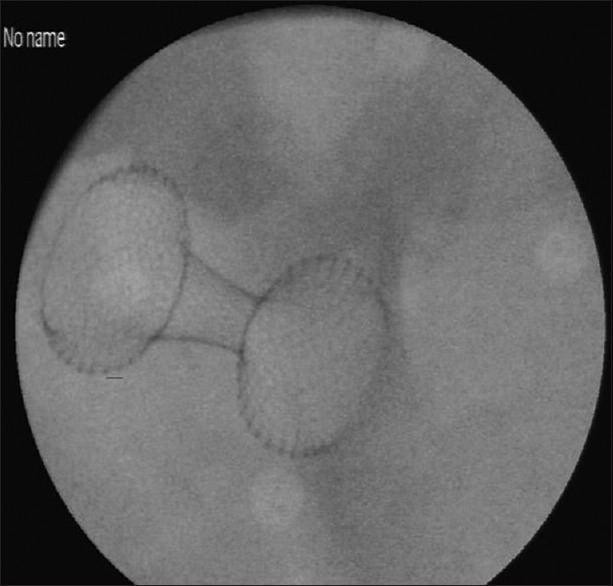
Black and white image for the released stent (representative of X-RAY view)
EUS-cystogastrostomy procedure
Equipment
A linear echoendoscope with an ultrasound processor (EG-580 UT and SU1-H processor, FUJIFILM Medical Systems, Japan) was used along with an electrosurgical generator (VIO300D, ERBE, Tübingen, Germany) and the 15, 20 mm Hot AXIOS Stent and Electrocautery-Enhanced Delivery System (Boston Scientific, Marlborough, MA, USA).
The Hot AXIOS is a novel double-flanged covered self-expanding metallic stent with a “dumbbell” configuration that holds the tissue walls in apposition. The barbell shape of the stent decreases the possibility of stent migration.[12]
Procedure
The Hot AXIOS catheter was passed through the biopsy channel of the echoendoscope and advanced through the gastric mucosa under EUS guidance until the catheter was visualized.
Next, the device was energized using the electrosurgical generator that was preadjusted on the pure-cut setting. The catheter was advanced until the penetration of the targeted cyst was achieved [Figure 3].
Figure 3.
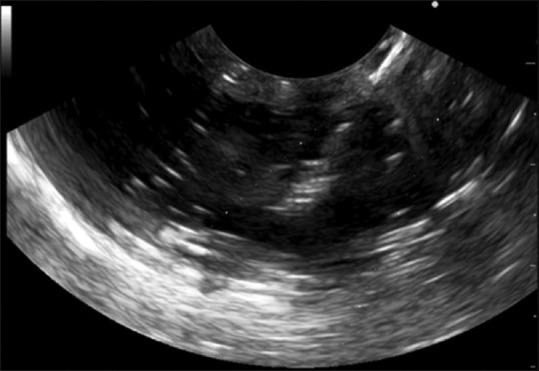
EUS-guided puncture of the created lesion
When the catheter was fully inside the targeted cyst, the distal flange of the stent was deployed under the guidance of EUS until it was visible inside the cyst which confirmed that the distal flange was fully opened [Figure 4]. The distal flange was pulled backward toward the gastric wall. When the shape of the flange changed from flat to oval, the proximal flange was deployed.
Figure 4.
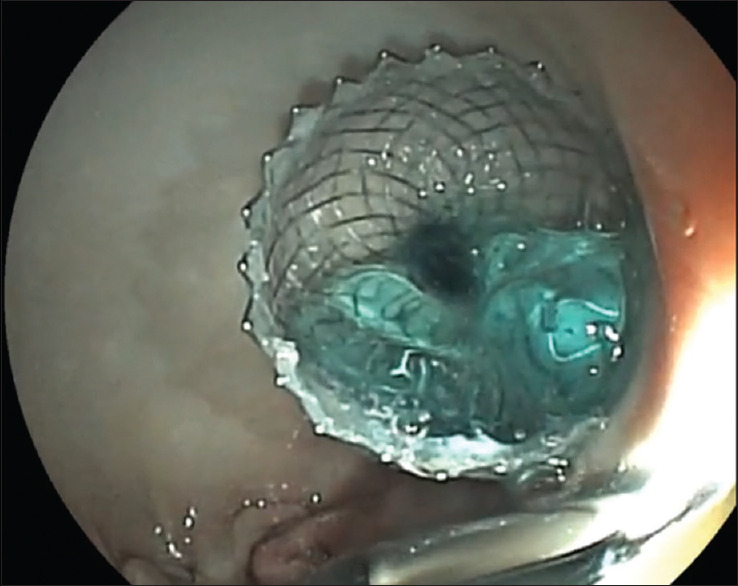
Endoscopic image of the cyst after puncture achieved with full deployment of the stent
Finally, the echoendoscope was gently withdrawn while the catheter control hub was simultaneously slowly pushed downward to allow for the release of the proximal flange from the working channel.
The simulator allows for multiple punctures of the created lesions; therefore, on each simulator, an average of 5–7 EUS cyst gastrostomy procedures using LAMS can be performed.
Study design
Once the model was designed, it was first tested by its designers for its ability to allow EUS-guided cystogastrostomy procedures. The task was to successfully deploy the Hot AXIOS™ system to drain the created cyst. Throughout the study duration, 17 international experts were asked to perform the procedure themselves using the training model. All of the experts have more than 10 years of endoscopic experience and perform a variety of EUS procedures including EUS cystogastrostomy along with other advanced techniques such as EUS-guided biliary drainage and EUS celiac plexus block.
Study end-points
The experts were asked to fill a questionnaire within a week after the use of the training model.
The primary endpoint was overall satisfaction with the simulator as a training tool for EUS-guided cystogastrostomy.
Secondary endpoints regarding the training model included:
Impression of realism of the model
Visualization of the target lesion
Anatomical accuracy of the model
Overall difficulty of the model
Impression of clinical benefit
Effect on performance time
Effect on postprocedure side effects.
RESULTS
Overall, the experts considered the model an adequate tool to train endoscopists on EUS-guided cystogastrostomy using LAMS.
Evaluation of secondary endpoints is included below [Figures 5-11].
Figure 5.
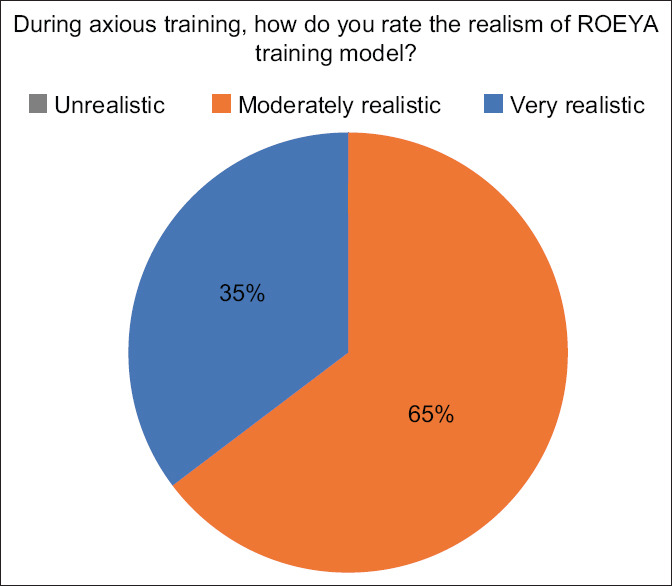
Results of expert questionnaire when asked about their impression of realism
Figure 11.
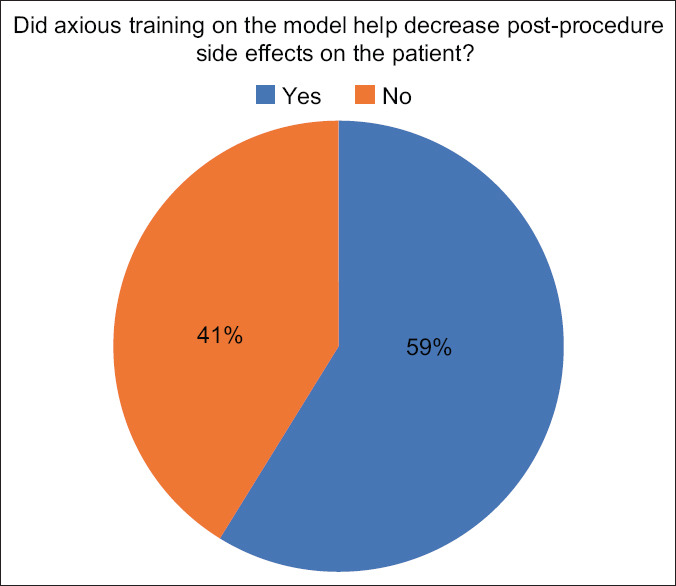
Results of expert questionnaire when asked about postprocedure side effects after training
The experts assessed the realism of the model in regards to emulating lesions found in real-life patients. 64.7% (n = 11) thought the lesions to be “moderately realistic” while 35.3% (n = 6) thought it to be “very realistic.” None of the experts thought the model to be “unrealistic” [Figure 5].
When asked about the visibility of the target lesion, 16 (94.1%) of the experts thought the lesion to be “properly visualized” using the provided equipment while only one expert (5.9%) believed it to be “hardly visualized.” None of the experts were unable to visualize the lesion [Figure 6].
Figure 6.
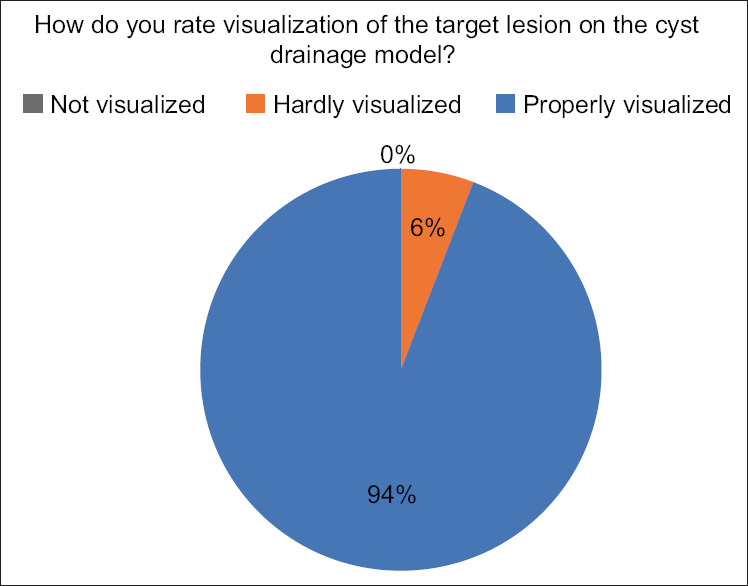
Results of expert questionnaire when asked about visualization of the target lesion
The experts compared the anatomical accuracy of the created lesions to actual, real lesions as would be seen in patients. 76.5% (n = 13) thought the lesions to be “slightly like the actual lesions” while 23.5% (n = 4) thought the lesions to be “very similar.” None of the experts thought the created lesions to be completely dissimilar to actual lesions [Figure 7].
Figure 7.
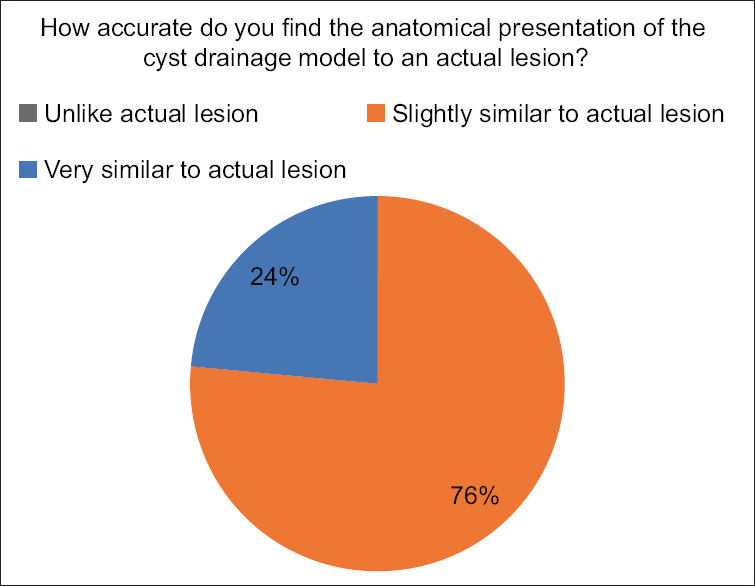
Results of expert questionnaire when asked about anatomical accuracy
The experts were asked to rate the overall difficulty of the cyst drainage model on a scale of easy, moderate difficulty, and very difficult. “Easy” was defined as the ability of the procedure to be performed by any trainee using the model. “Moderate difficulty” suggests that the model can only be performed by experienced trainees while “very difficult” indicates that only the experts can perform the procedure. Of 17, 11 of the experts (64.7%) found the model to be “easy” while 6 or 35.3% found it to be of “moderate difficulty” [Figure 8].
Figure 8.
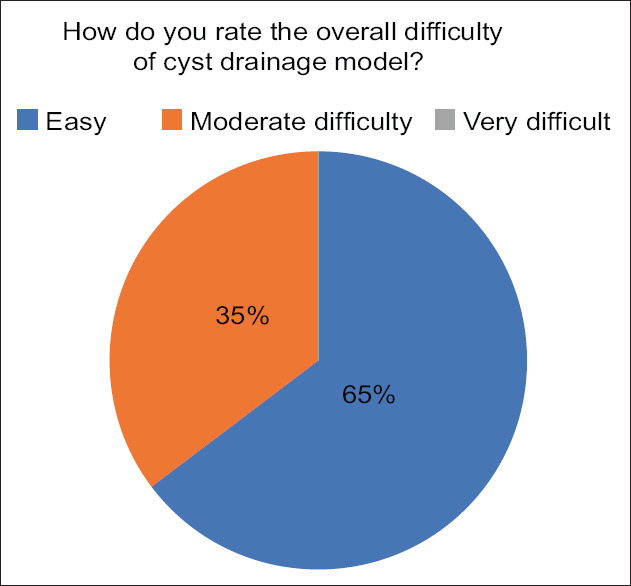
Results of expert questionnaire when asked about overall difficulty of the model
Three of the included survey questions were regarding the expert's perception of experience gained after using the model [Figures 9-11]. When asked if the model was helpful in the expert's clinical practice, 15 experts (88.2%) answered “yes” while two experts (11.8%) responded “no” [Figure 9].
Figure 9.
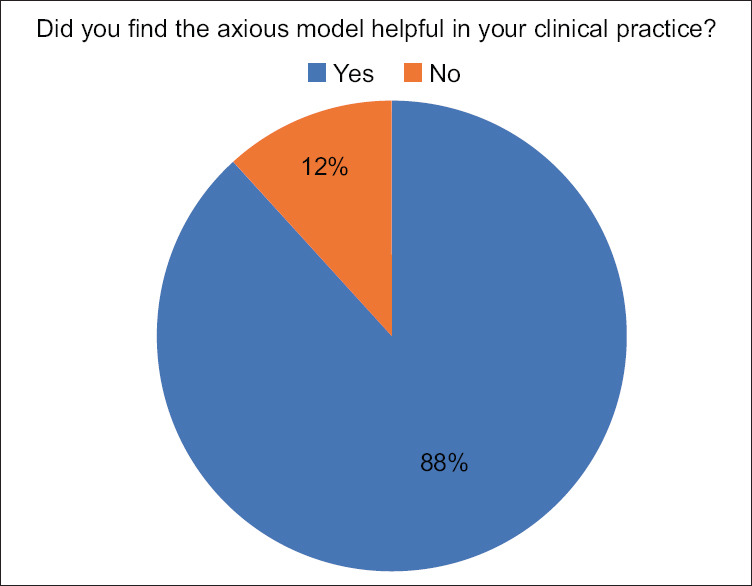
Results of expert questionnaire when asked if training on model helped their clinical practice
Regarding performance time, 13 experts (76.5%) reported that their performance time decreased after the training [Figure 10].
Figure 10.
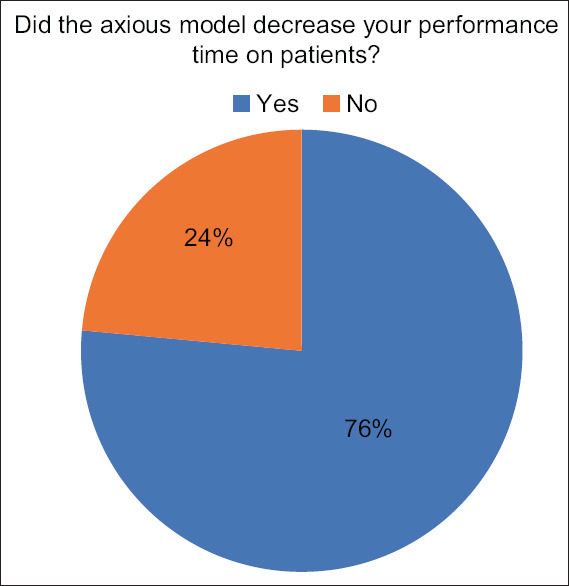
Results of expert questionnaire when asked about procedure time after training
When asked if the training decreased postprocedure side-effects, 10 experts (58.9%) reported to have decreased complications while seven experts (41.2%) found that the training had no effect [Figure 11].
DISCUSSION
EUS-guided drainage of peripancreatic fluid collections has become one of the most technically demanding procedures in gastrointestinal endoscopy. However, these advanced techniques, including EUS-cystogastrostomy using LAMS, require certain skills to be acquired over a learning curve. Hence, specific and validated models are necessary to provide safe and efficient training to endoscopists to achieve proficiency in a risk-free environment.
The results of this study demonstrated that expert opinion found the model favorable in terms of realism, visualization of the target lesion, and anatomical accuracy [Figures 5-7]. Most of the experts (67.4%) rated the model as “easy” which means that any trainee can use the model without the need of rich experience in EUS [Figure 8]. The purpose was to evaluate whether a trainee would be able to use the model with ease even with step-by-step instruction from a mentor or expert which is necessary during training. All of the experts believed the model to be an efficient tool to be used for endoscopy training in the evaluated technique.
Our study has limitations. Since we only included EUS experts in this preliminary study, the effect of the model on their clinical practice could not be fully assessed. However, we have included a few questions [Figures 9-11] that were asked in the 6-month period following the training. Most of the experts found the model to be helpful in their clinical practice. Overall, the model improved most of the expert's techniques by decreasing performance time, but there was not much effect on postprocedure side effects on the patients. While the purpose of the study was to act as a preliminary evaluation of the training model, less-experienced GI-fellow input is needed to further validate the model.
Our main finding is that the model is considered an adequate tool by experts, and therefore, this study serves as a preliminary validation of the training model.
CONCLUSIONS
In conclusion, we recommend the use of this model for the training of complicated EUS procedures, specifically, EUS-guided cystogastrostomy using LAMS. These procedures require adequate training and experience. While this study was focused on receiving feedback from endoscopists who are experienced in the technique, further work is needed to conduct a study focused on endoscopists with less experience.
Financial support and sponsorship
Nil.
Conflicts of interest
Marc Giovannini is a Founding Editors- in-Chief of the journal, and Hussein Okasha, Nonthalee Pausawasdi, Pradermchai Kongkam and Thawee Ratanachu-ek are Editorial Board Members. The article was subject to the journal's standard procedures, with peer review handled independently of these editors and their research groups.
REFERENCES
- 1.Renelus BD, Jamorabo DS, Gurm HK, et al. Comparative outcomes of endoscopic ultrasound-guided cystogastrostomy for peripancreatic fluid collections: A systematic review and meta-analysis. Ther Adv Gastrointest Endosc. 2019;12:1–11. doi: 10.1177/2631774519843400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol. 2009;15:38–47. doi: 10.3748/wjg.15.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerch MM, Stier A, Wahnschaffe U, et al. Pankreaspseudozysten: Abwarten, endoskopisch drainieren, resezieren? Dtsch Arztebl. 2009;106:614–21. [Google Scholar]
- 4.Sarr MG, Banks PA, Bollen TL, et al. The new revised classification of acute pancreatitis 2012. Surg Clin North Am. 2013;93:549–62. doi: 10.1016/j.suc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 5.da Costa DW, Boerma D, van Santvoort HC, et al. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg. 2014;101:e65–79. doi: 10.1002/bjs.9346. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Standards of Practice Committee. Muthusamy VR, Chandrasekhara V, et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc. 2016;83:481–8. doi: 10.1016/j.gie.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Arvanitakis M, Dumonceau JM, Albert J, et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524–46. doi: 10.1055/a-0588-5365. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AA, Adler DG, Nieto J, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large retrospective, multicenter U.S. experience (with videos) Gastrointest Endosc. 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Sharaiha RZ, Tyberg A, Khashab MA, et al. Endoscopic therapy with lumen-apposing metal stents is safe and effective for patients with pancreatic walled-off necrosis. Clin Gastroenterol Hepatol. 2016;14:1797–803. doi: 10.1016/j.cgh.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Moryoussef F, Leblanc S, Bertucat A, et al. Comparative evaluation of two porcine ex vivo models for training in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc Int Open. 2017;5:E1020–6. doi: 10.1055/s-0043-117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron TH, DeSimio TM. New ex-vivo porcine model for endoscopic ultrasoundguided training in transmural puncture and drainage of pancreatic cysts and fluid collections (with videos) Endosc Ultrasound. 2015;4:34–9. doi: 10.4103/2303-9027.151326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues-Pinto E, Baron TH. Evaluation of the AXIOS stent for the treatment of pancreatic fluid collections. Expert Rev Med Devices. 2016;13:793–805. doi: 10.1080/17434440.2016.1222898. [DOI] [PubMed] [Google Scholar]


