Abstract
Introduction:
Postpartum hemorrhage (PPH) has remained the leading cause of maternal mortality. While anemia is a leading contributor to maternal morbidity, molecular, cellular and anemia-induced hypoxia, clinical studies of the relationship between prenatal-anemia and PPH have reported conflicting results. Therefore, our objective was to investigate the outcomes of studies on the relationships between prenatal anemia and PPH-related mortality.
Materials and Methods:
Electronic databases (MEDLINE, Scopus, ClinicalTrials.gov, PROSPERO, EMBASE, and the Cochrane Central Register of Controlled Trials) were searched for studies published before August 2019. Keywords included “anemia,” “hemoglobin,” “postpartum hemorrhage,” and “postpartum bleeding.” Only studies involving the association between anemia and PPH were included in the meta-analysis. Our primary analysis used random effects models to synthesize odds-ratios (ORs) extracted from the studies. Heterogeneity was formally assessed with the Higgins’ I2 statistics, and explored using meta-regression and subgroup analysis.
Results:
We found 13 eligible studies investigating the relationship between prenatal anemia and PPH. Our findings suggest that severe prenatal anemia increases PPH risk (OR = 3.54; 95% CI: 1.20, 10.4, p-value = 0.020). There was no statistical association with mild (OR = 0.60; 95% CI: 0.31, 1.17, p-value = 0.130), or moderate anemia (OR = 2.09; 95% CI: 0.40, 11.1, p-value = 0.390) and the risk of PPH.
Conclusion:
Severe prenatal anemia is an important predictive factor of adverse outcomes, warranting intensive management during pregnancy. PROSPERO Registration Number: CRD42020149184; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=149184.
Keywords: maternal mortality, meta-analysis, obstetric emergency, postpartum hemorrhage risk factors, postpartum hemorrhage-related mortality, prenatal anemia, systematic review
Introduction
Postpartum hemorrhage (PPH) is a critical and significant public health problem, and a leading cause of maternal mortality in developing countries.1 In 2017, an estimated 38 000 deaths were due to PPH (19% of all maternal deaths).2 Disturbingly, the incidence of PPH has been increasing in the United States. In the United States, a nationwide study reported that the incidence of PPH increased by 100% between 1998 and 2008.3
The management of PPH starts in the prenatal period, with identification of women who are at the highest risk for PPH from placental abnormalities, trauma to vaginal/cervical tissue, anemia, uterine atony, scarred uterus, and coagulation disorders. However, during labor, the mainstay of global PPH control is the active management of the third stage of labor with prophylactic use of uterotonics (AMSTL).4–6 While AMSTL is estimated to prevent over half of PPH cases, there are many cases of pregnant women who lose potentially deleterious amounts of blood despite AMSTL, warranting therapeutic administration of uterotonics.7 In a subset of women with PPH, uterine atony and bleeding remains refractory despite uterotonic administration, warranting surgical or other invasive interventions.8 It is plausible that the underlying cause and pathogenesis of PPH in this subset of women might differ from those due to atony, responsive to uterotonics. In addition, mechanistic studies suggest that anemia through its effect on nitric oxide synthesis might play a role in uterine atony.9–18
Anemia, one of the most frequent complications of pregnancy, is a leading contributor to maternal morbidity globally. Mechanistic, clinical and population studies suggest that preexisting anemia might also be a risk factor for PPH incidence.14–19 Although few clinical studies have compared the risk of PPH among pregnant women with anemia to pregnant women without anemia, findings from these studies have been conflicting, and there have been no systematic review and/or meta-analysis summarizing findings of these clinical studies that have examined the relationship between anemia in pregnancy and PPH risk. Therefore, the objective of this study was to synthesize the evidence regarding the relationship of prenatal anemia and the risk of PPH. We also sought to summarize the clinical studies that have examined the relationship of prenatal anemia and the risk of mortality among patients with PPH.
Methods
Database search, study criteria eligibility, and search strategy
The protocol for this review was registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42020149184), and we followed the PRISMA guidelines for protocols (PRISMA-P; Supporting Information, Appendix S1)20 for data extraction. We searched the literature for retrospective cohort studies, prospective cohort studies, and randomized clinical trials, and identified studies examining the association of prenatal anemia and PPH risk. Original peer-reviewed research articles published up to August 2019 in PUBMED/Medline (US National Library of Medicine), ClinicalTrials.Gov, EMBASE (Elsevier), PROSPERO and Web of Science (Clarivate Analytics) were examined. Our search included controlled vocabulary terms (MeSH and free text keywords) for anemia (exposure), PPH (outcome), pregnancy and postpartum (study population), and cohort/ randomized clinical trial (study design). Relevant synonyms and alternative spellings were identified via EMBASE’s controlled vocabulary (Emtree). Hand searching of references was also done. The search strategy for the association between anemia and PPH included Boolean operators “OR” (for related terms) and “AND” (for a combination of different concepts). Our ClinicalTrials.Gov search strategy incorporated a very similar search strategy, without including the “cohort” and “randomized controlled trial” terms in the search. In finalth analysis, we included studies of pregnant women any gestational age or parity, in any trimester of pregnancy, of any maternal age, and from any country that met our inclusion criteria. No restrictions by age, year of publication, or language were implemented.
Outcome measures
Our primary outcome of interest was the proportion of women with anemia who developed PPH. We assessed the following secondary outcomes: the proportion of women with developed mild anemia, moderate anemia, and severe anemia, and the associations between these classes of anemia severity with PPH. All primary and secondary outcomes were assessed as odds ratios (ORs) and adjusted odds ratios.
Data extraction, exposure, and outcome definitions and descriptions
The title and abstract of each study were screened and full-texts examined in duplicate by AIA and MK (Table 1). Discrepancies were resolved by a third author (Moshood O. Omotayo). The final inclusion–exclusion decisions were made after the articles were reviewed in full. Studies were excluded if they did not examine exposure-outcome relationship of interest, were nonhuman studies, cross-sectional studies or systematic reviews. Data extraction from full-text articles was done using a comprehensive extraction sheet. Information on study design, population, intervention, covariates, and findings were extracted. Anemia, defined as a decrease in red-cell mass below 11 g/dL, was diagnosed using hemoglobin testing. A number of different cutoffs were used by the included studies: 7,21,26,34 9,27 10,29 and 11 g/dL.21,23,31,33 In the main analysis, we restricted to studies that defined anemia based on the World Health Organization’s (WHO) classification.35 In sensitivity analysis, we included the most appropriate estimate from each study, and compared the impact of that approach on the results. We employed the definition of PPH used by the primary studies (>500 or >1000 mL—with or without modification based on clinical scenario). Severe PPH was regarded as estimated blood loss >1500 mL or requiring transfusion.21,27
Table 1.
Description of included studies
| Study | Study type | Sample size | Mean age, years | Country | Baseline anemia, % | Exposure cutoff for hemoglobin, g/L | Outcome, definition | Lindings, Odds ratio (95% CI) | p-Value | Confounders adjusted | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Butwick et al.21 | Cohort | 1789 | NA | United States | 43 | <110 ≥110 <100 ≥100 ≥110 100—<110 <100 |
PPH, estimated blood loss >1000 mL | 0.40 (0.33, 0.48) Ref 0.92 (0.71, 1.17) Ref Ref 0.50 (0.39, 0.64) 0.25 (0.19, 0.32) |
<0.0001 0.48 <0.0001 <0.0001 |
None | Poor |
| <110 ≥110 |
Severe PPH, estimated blood loss >1500 mL | Ref 0.35 (0.27, 0.45) |
<0.0001 | ||||||||
| <100 ≥100 |
Ref 0.28 (0.19, 0.41) |
<0.0001 | |||||||||
| ≥110 100—<110 <100 |
Ref 0.46 (0.34, 0.63) 0.23 (0.15, 0.34) |
<0.0001 <0.0001 |
|||||||||
| Geethoed et al.22 | Cohort | 309 | 22 | Ghana | 51 | <80 ≥110 |
PPH, estimated blood loss >500 mL | 0.96 (0.46, 2.01) Ref |
0.93 | None | Poor |
| Gonzales et al.23 | Cohort | 379, 816 | NA | Peru | 18 | <110 ≥110 <70 70 to <90 90 to <110 110 to 145 >145 |
PPH, estimated blood loss >500 mL | 1.21 (1.08, 1.36) Ref 6.15 (3.86, 9.78) 2.62 (2.03, 3.38) 1.01 (0.88, 1.15) Ref 0.84 (0.67, 1.05) |
0.0008 0.002 0.002 >0.05 - >0.05 |
None Age, education, marital status, BMI, prenatal care, parity, gestational diabetes mellitus, cardiopathy in current pregnancy, gestational age at which hemoglobin was first measured, and recent migration |
Poor |
| Kebede et al.24 | Cohort | 422 | NA | Ethiopia | 14 | <110 ≥110 |
PPH, based on clinician diagnosis | 7.4 (3.6, 15.3) Ref |
<0.05 | Confounders adjusted for but list not provided | Moderate |
| Luis et al.25 | Case–control | 212 | 27 | UK | 50 | <80 ≥110 |
PPH, estimated blood loss >500 mL if vaginal delivery and >1000 mL if cesarean | 2.64 (1.26, 5.55) Ref |
0.01 | None | Moderate |
| Malhotra et al.26 | Cohort | 447 | 27 | India | 7 | <110 ≥110 ≥110 100—<110 70—<100 <70 |
PPH, definition not specified | 1.53 (0.17, 13.8) Ref Ref 0.29 (0.01, 8.56) 4.82 (0.49, 47.1) 4.07 (0.25, 66.9) |
0.70 0.47 0.18 0.33 |
None | Poor |
| Nyflot et al.27 | Case–control | 3123 | 33 | Norway | 4 | <90 ≥90 |
Severe PPH, estimated blood loss >1500 mL or requiring blood transfusion | 4.27 (2.79, 6.54) Ref |
<0.001 | Previous PPH, anticoagulant medication, severe preeclampsia, uterine fibromas, multiple pregnancy, mode of delivery, IVF, fever, induction of labor, birthweight >4500 g, parity | Moderate |
| Parks et al28 | Cohort | 92 247 | NA | India and Pakistan | 88 | <110 ≥110 ≥110 100—<110 70—<100 <70 |
PPH, based on clinician diagnosis | 0.78 (0.63, 0.96) Ref Ref 0.65 (0.52, 0.82) 0.82 (0.66, 1.02) 5.31 (3.51, 8.02) |
0.018 0.0003 0.07 <0.0001 |
None | Moderate |
| Rukuni et al.29 | Cohort | 80 422 | 29 | Scotland | 9 | <100 ≥100 |
PPH, estimated blood loss >500 mL | 0.92 (0.86, 0.98) Ref |
0.007 | Age, parity, smoking status, ethnicity, socioeconomic status, BMI and CKD | Moderate |
| Selo-Ojeme et al.30 | Case–control | 208 | NA | Nigeria | 5 | <110 ≥110 |
PPH, estimated blood loss >500 mL | 1.29 (0.38, 4.36) Ref |
0.68 | None | Poor |
| Soltan et al.31 | Cohort | 152 | 25 | Egypt | 76 | <110 ≥110 ≥110 100—<110 70—<100 <70 |
PPH, estimated blood loss >500 mL | 7.78 (0.45, 134) Ref Ref 0.38 (0.01, 19.2) 80 (4.36, 1468) 384 (5.95, 24 784) |
0.16 0.63 0.003 0.005 |
None None |
Poor |
| Tort et al32 | Cohort | 3278 | NA | Senegal and Mali | NA | <70 ≥70 |
Mortality among women with PPH; PPH defined based on clinician diagnosis | 6.65 (3.77, 11.7) Ref |
NA | Country, location in relation to hospital, age, number of prenatal visits, preexisting disease, gestational hypertensive disorders, referral, prolonged labor, mode of delivery and birthweight | Moderate |
| Wandabwa et al.33 | Case–control | 606 | 24 | Uganda | 8 | <110 ≥110 |
PPH, estimated blood loss >500 mL, or any vaginal bleeding with hemodynamic deterioration | 6.10 (1.10, 35.4) Ref |
0.05 | Age, distance from home to hospital, marital status, educational level, job, spouse job, type of house, need to request permission to visit hospital, who pays for treatment, previous CS, PPH, number of pregnancies | Poor |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; IVF, in vitro fertilization; NA, not available.
Risk of bias assessment
We assessed the risk of bias of individual studies using the Newcastle Ottawa Scale which permits distinct quality scales for case–control and cohort studies, and assess the quality of nonrandomized studies with particular focus on study content, study design, and applicability.36 Using the Newcastle Ottawa Scale, the quality of the individual studies were assessed and interpreted, before they are incorporated into the meta-analysis. Two investigators (Ajibola I. Abioye and Moshood Kuyebi) independently assessed the studies and disagreements were resolved by consensus with a third author (Moshood O. Omotayo). This ranking did not influence decisions concerning exclusion of studies or analytic approach.
Statistical analysis
The primary analysis included OR estimates from each included study evaluating the relationship of anemia and PPH. For studies that reported more than one estimate, the estimate that incorporated the largest possible sample was included. If studies reported separate estimates for anemia categories or other subgroups, they were included separately if the individual participants in the subgroups were distinct. For studies reporting no OR estimates, we calculated them if sufficient information was provided. 21,23,25,26 In some cases, there were no exposed or nonexposed cases or noncases, and we imputed 0.5 to permit the estimation of the ORs.26,31
Random effects models based on the restricted maximum likelihood (REML), which explicitly model the between study variation, were selected a priori for the meta-analyses.37 The REML is superior to the more commonly used Der-Simonian Laird method, and allows inclusion of study-level covariates.38 Heterogeneity was formally assessed with the Higgins’ I2 statistics, a measure of the total variability that is due to between study variations. I2 was regarded as low if <50%, substantial if 50%–90% and considerable is >90%, in accordance with the general guidelines for Cochrane reviews, and p-values for Q-statistic reported.39 Heterogeneity was further assessed using meta-regression approaches and analysis within subgroups defined by age, baseline iron status, country income classification, and timing of prenatal hemoglobin assessment. The impact of an individual study on the findings was evaluated by removing one or more studies sequentially and comparing the pooled estimates obtained. Possible publication bias was visualized using funnel plots, quantitatively evaluated with the Egger’s tests based on mixed effects regression model, and explored with trim and fill analysis.40,41
p-Values are two sided and significance set at p < 0.05. Statistical analyses were conducted using Stata version 15.0 (College Station, TX) and R-Studio (1.0.153). Values presented in the text are means (±SD), means (95% CI), and means (±SE).
Results
Description of included studies
We identified 13 papers from an initial set of 2012 unique titles and abstracts reporting on the relationship of anemia and PPH (Appendix S2), and the PRISMA flowchart of the study selection is as described in Appendix S3. There were nine cohort studies and four case–controls studies. The studies were conducted in multiple different countries spanning Africa,22,24,30,31,33 Asia,26,28 Europe,25,27,29 North America,21 and South America.23 The prevalence of prenatal anemia varied widely, from 4% to 88%. The individual studies defined PPH differently: as estimated blood loss >500 mL,22,23,29–31 > 1000 mL21 or used different cutoffs depending on whether delivery was vaginal or by cesarean section,25 or whether vaginal bleeding was associated with features of hemodynamic deterioration such as hypotension or shock, or need for blood transfusion.33 Other studies defined PPH based on clinician diagnosis, without specifying how it was made. 24,28,32 One study only reported on severe PPH based on estimated blood loss >1500 mL. 27 Another study did not specify how PPH was defined.26 Only one study from Senegal and Mali 32 reported on PPH-related mortality. The studies were of poor 21,22,26,30,31,33 or moderate 24,25,27–29,32 quality based on the Newcastle-Ottawa Scale.
Anemia and PPH
Observational studies evaluating the relationship of anemia and PPH have reported inconsistent findings. We pooled estimates from eight studies that defined anemia as hemoglobin <11 g/dL per WHO recommendations,21,23,24,26,30,31,33 and found that anemia was not associated with PPH (OR: 1.39; 95% confidence interval [CI]: 0.64–3.01; p-value = 0.400, Figure 1). Visual inspection of the funnel plot did not suggest publication bias (Figure SS1), and the p-value for the Egger’s test of publication bias was 0.24. The OR did not considerably change on excluding any of the studies. The Higgins’ I2 statistic was 97%, suggestive of considerable heterogeneity (p-heterogeneity <0.0001). Heterogeneity was not influenced by year of study publication (p-value = 0.970), mean age (p-value = 0.240), or country income classification (p-value = 0.180). Heterogeneity did not also differ by whether PPH was defined using a 1000 mL cutoff (p-value = 0.100) or other criteria (p-value = 0.570), compared to 500 mL cutoff. The pooled OR estimate among cohort studies was 0.88 (95% CI: 0.52–1.49). Only one study 30 had a case–control design and its OR was 1.29 (95% CI: 0.39–4.36).
Figure 1.
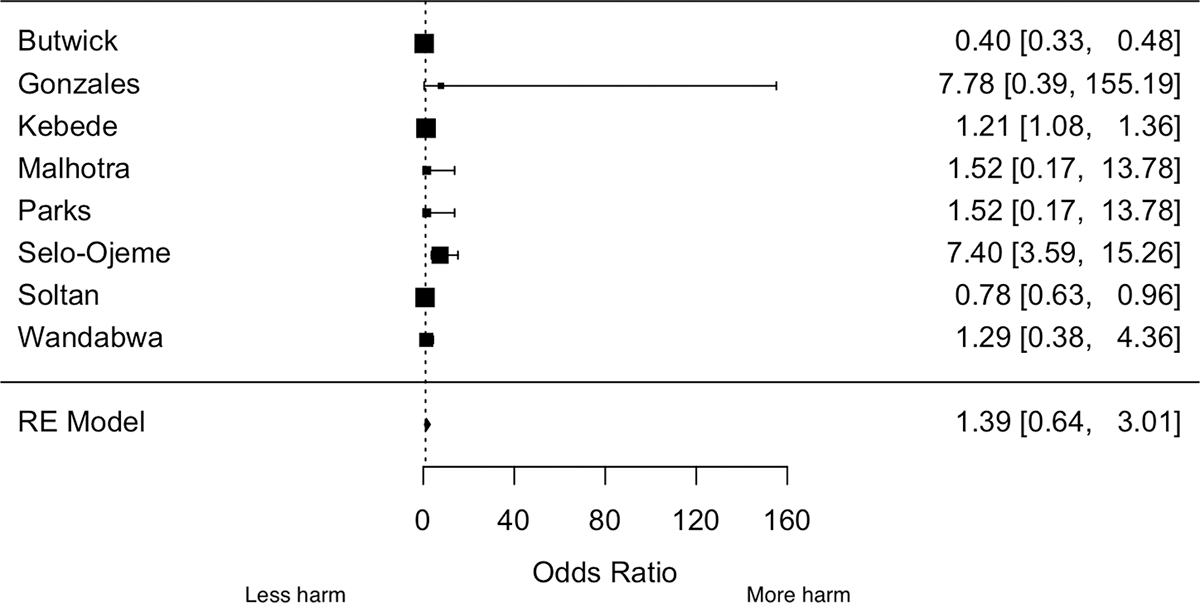
Forest plot of the association between anemia and postpartum hemorrhage
In sensitivity analysis, we pooled estimates from 12 studies regardless of how anemia was defined, but the magnitude and direction of the association did not substantially change (OR: 1.63, 95% CI: 0.93–2.86).
Anemia severity and PPH
Five cohort studies from Egypt, India, Pakistan Peru, and United States reported estimates across multiple categories of anemia severity in relation to the odds of PPH, allowing visualization (Figure 2) of the possible nonlinear relationship of anemia severity and PPH risk.21,23,26,28,31 The estimates from one study 31 were extreme and are not shown—though the direction of its estimates was consistent with the other studies. Overall, the scatter plot shows a possible inverted J-shape relationship of prenatal hemoglobin concentration and the risk of PPH. The plot suggests an increased risk of PPH when anemia is severe, but no increased risk at other hemoglobin levels.
Figure 2.
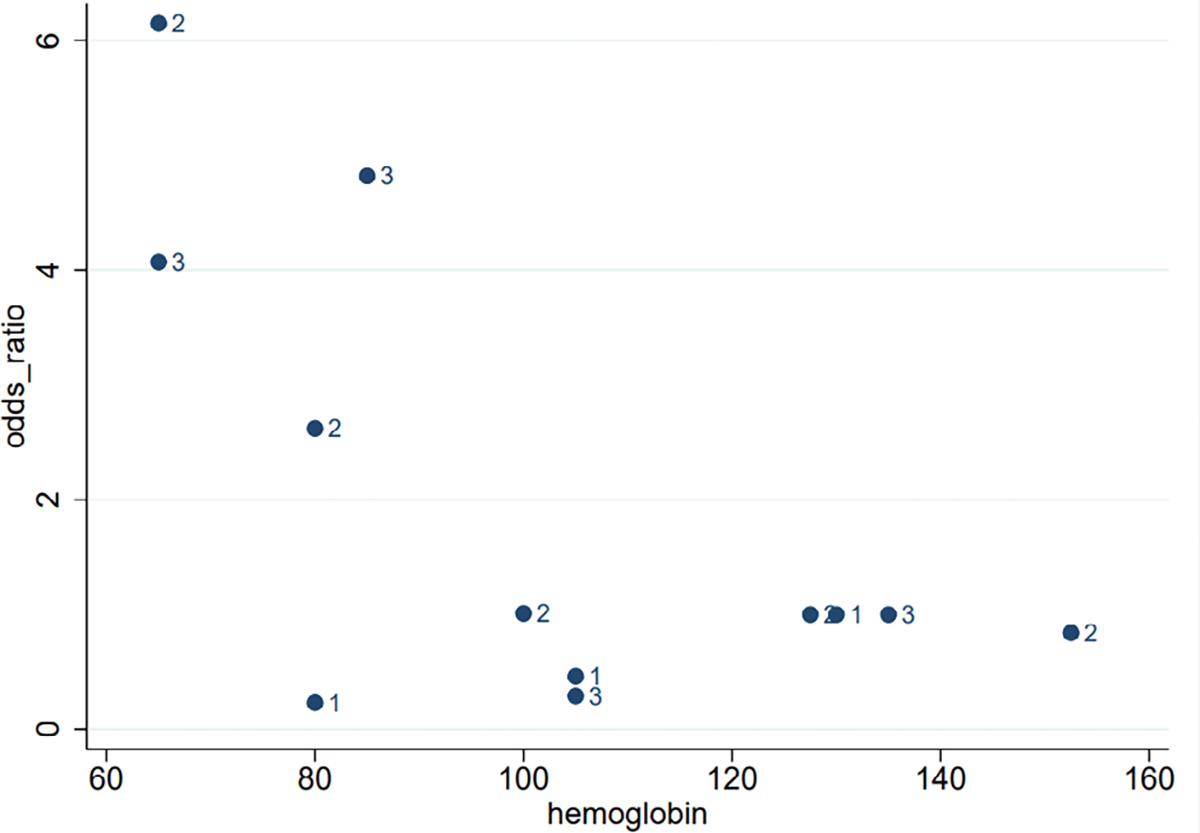
Scatterplot of the association between categories of anemia severity and PPH
Neither mild nor moderate anemia was significantly associated with the PPH. When we pooled the estimates for the relationship of mild anemia and PPH from four studies 21,23,26,31, we obtained a pooled OR of 0.60; (95% CI: 0.31, 1.17, p-value = 0.130, Figure SS2). There was low heterogeneity (I2 = 46%, p-heterogeneity = 0.240) and visual inspection of the funnel plot did not suggest publication bias (Figure SS3). The pooled OR for moderate anemia from five studies was 2.09 (95% CI: 0.40, 11.1, p-value = 0.390, Figure SS4). There was considerable heterogeneity (I2 = 97%, p-heterogeneity <0.0001). Visual inspection of the funnel plot suggested possible publication bias (Figure SS5), and the p-value for funnel plot asymmetry (Egger’s test) was 0.04. Imputing one study using trim and fill reduced the magnitude of the OR considerably (OR: 1.18; 95% CI: 0.16–8.68) but the direction did not change.
Severe anemia was associated with higher odds of PPH, unlike mild and moderate anemia. The pooled OR for severe anemia from five studies 22,23,25,26,31 was 3.54 (95% CI: 1.20, 10.4, p-value = 0.020, Figure 3). There was substantial heterogeneity (I2 = 83%, p-heterogeneity = 0.0001). Visual inspection of the funnel plot did not suggest possible publication bias (Figure SS6). The confidence intervals (CIs) were, however, much wider when the large Peruvian study 23 was excluded (OR: 3.37; 95% CI: 0.69–16.6).
Figure 3.
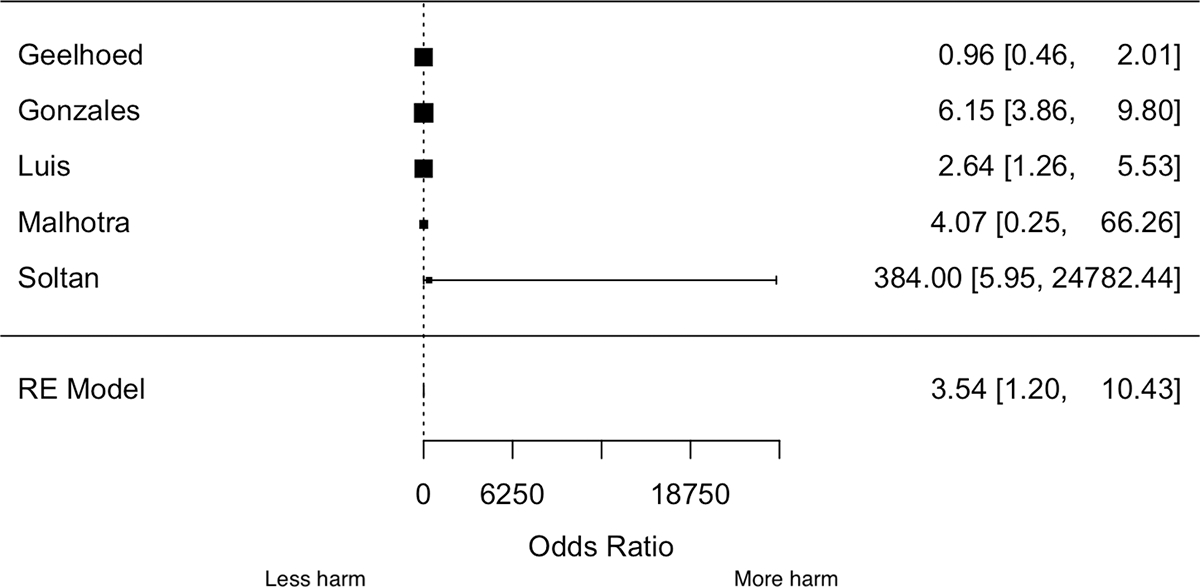
Forest plot of the association between severe anemia and PPH
Severe PPH
Two studies reported estimates for severe PPH and their findings were contradictory. A case–control study in Norway27 only reported estimates for severe PPH. It considered moderate anemia (<90 g/L) as a risk factor for severe PPH and found considerably higher odds (OR = 4.27; 95% CI: 2.79–6.54). This estimate was adjusted for multiple covariates including previous PPH, uterine fibromas, birthweight, parity, mode of delivery, and induction of labor. The second study was a US cohort study. The reported estimates for severe PPH were unadjusted for any confounders, and restricted to women with severe postpartum anemia.21 They found that anemia is associated with a considerably lower odds of PPH (OR: 0.35; 95% CI: 0.27, 0.47).
PPH-related mortality
One study from Senegal and Mali32 evaluated the predictors of PPH-related mortality in 46 referral hospitals in a 1-year period and found that severe prenatal anemia was associated with a considerably higher odds of mortality (OR: 6.65; 95% CI: 3.77–11.7). Estimates were adjusted for country, location in relation to hospital, age, number of prenatal visits, preexisting disease, gestational hypertensive disorders, referral, prolonged labor, mode of delivery, and birthweight.
Discussion
In this systematic review, we pooled estimates from studies that evaluated the relationship between prenatal anemia and PPH risk. Our findings suggest that prenatal anemia might increase PPH risk, but only when anemia is severe. Only one study investigated the relationship between anemia and PPH-related mortality and it indicated that severe prenatal anemia increases the risk of PPH-related mortality. However, the body of studies evaluating this relationship remains weak. Nevertheless, the likelihood that severe anemia increases the risk of PPH is another important motivation to address the underlying drivers of anemia risk among women of reproductive age, particularly in low- and middle-income countries where there is high prevalence of anemia.
Molecular and cellular studies provide plausible scientific premise for a direct relationship between prenatal anemia and risk of PPH-related mortality. Among adolescents, iron deficiency anemia has been associated with a 700% increase in nitric oxide production compared to adolescents with normal hemoglobin range.18 During pregnancy, NO produced by the trophoblast and placenta could play a significant role in maintaining uterine quiescence by paracrine effect since there is marginal activity of NO synthase (NOS) in the myometrium.10 It is known that NO participates in responses to acute hypoxia via inducing HIF but contributes to a negative feedback process in chronic hypoxia.9 In accordance, eNOS expression was shown to be increased in the placental tissue of pregnancies complicated by IUGR or preeclampsia, which could be an adaptive response to the increased resistance and poor trophoblastic invasion.11,12 Moreover, NO production in IUGR placenta was reported to be increased and this increase was more pronounced in those with compromised fetal oxygenation.13 It has not been reported whether placental NOS activity and NO production is increased in pregnancies complicated by severe anemia. However, it is reasonable to assess PPH with reference to placental NO production in pregnancies complicated by severe anemia. The body of clinical studies directly examining this relationship does not provide conclusive evidence of this relationship, although this meta-analysis suggests that severe prenatal anemia is associated with increased risk of PPH.
The WHO estimates that approximately 27% of maternal deaths are due to obstetric hemorrhages.42 It is plausible that preexisting anemia and consequent hypoxia can complicate the cardio-vascular compromise resulting from postpartum hemorrhage. This review shows that there is limited empirical evaluation from clinical studies. We found only one study that has examined the influence of prenatal anemia on PPH-related mortality. Given the high prevalence of anemia and public health significance of PPH, further empirical testing of this relationship, and quantification of the association may be important to guide maternal health policy decisions. Thus, more studies are warranted.
Anemia during the prenatal care period is an important predictive factor of adverse outcomes, possibly for PPH and mortality, warranting intensive follow-up. One study reported 6.7-fold higher odds of mortality among individuals with PPH if they had severe prenatal anemia.32 Preventive and therapeutic interventions for prenatal anemia might prevent PPH or PPH-related mortality although more studies are needed to evaluate effectiveness. There are different causes underlying prenatal anemia. Thus, the appropriate preventative and therapeutic interventions should be personalized, taking into consideration the hemoglobin electrophoresis, dietary history, infections, and inflammatory conditions. Potential interventions for averting deleterious consequences of anemia in pregnancy include early and routine screening with complete blood counts and hemoglobin electrophoresis during the initial prenatal visit, iron supplementation in women with iron deficiency anemia, and multiple micronutrient supplementation and transfusion of blood and blood products in women with severe or symptomatic anemia during pregnancy. At the population level, nutritional and educational interventions for improving dietary intake and reducing recurrent infections and inflammation among women of reproductive age can reduce the prevalence of severe anemia in low- and middle-income countries.43
This systematic review has multiple strengths. Anemia has high global prevalence and PPH is the leading cause of maternal mortality. The rarity of studies that have synthesized evidence on the relationship of these two conditions despite their importance to global maternal health, adds to the significance of this work. Second, we followed the PRISMA guidelines, registered the review in PROSPERO and followed rigorous methodological standards in the synthesis and reporting of evidence on this important question. Third, our analytical plan and interpretation focused on the clinical and public health implications of the body of work that has examined this relationship.
The implications for future research are enormous. There is need to design and implement prospective studies to examine the direct association between mild, moderate, and severe anemia and the risk and severity of PPH in diverse settings. Since PPH and PPH-related mortality are relatively rare outcomes, large cohort studies or well-designed case–control studies would be appropriate study designs.
This systematic review is limited by the scarcity and low quality of the primary studies, as well as inconsistency of measurement approaches across studies. Few studies have considered the relationship of anemia and PPH, and it was not possible to comprehensively evaluate the relationship of anemia severity and PPH using statistical techniques such as generalized least squares for trend estimation.44 Many studies did not report the mean gestational age at which anemia testing was done, especially in developing countries where third trimester labs are not done routinely, making it impossible to comprehensively explore heterogeneity using meta-regression. Some of the included studies were also restricted to patients who delivered via cesarean section or who developed postpartum anemia, likely limiting the external validity of the studies, or introducing selection bias.
The studies were of low or moderate quality as assessed by the Newcastle-Ottawa Scale. For many of the studies included, PPH was a secondary outcome and no adjustment for confounding was considered. There was also considerable unmeasured confounding due to factors such as gestational age for some of the studies that controlled for any confounders. The threat of unmeasured confounding is particularly great due to the wide CIs seen in the various analyses. For instance, the point estimates and CIs could be explained away by unmeasured confounding related to the exposure and the outcome by an E-value of 6.54, and a lower confidence limit of 1.69, but weaker confounding could not do so.45 An E-value greater than 3.0 tends to suggest that the identified association is more likely to represent a true causal relationship, and unlikely to be spurious. Nonetheless, future studies should carefully control for important confounding variables that may reasonably exert substantial influence on the association between anemia and PPH, including socioeconomic status, nutrition, and health insurance coverage.
Conclusion
Severe anemia during prenatal care is an important predictive factor of PPH, warranting early diagnosis and treatment. Published studies did not comprehensively control for confounders. Therefore, larger, well-designed prospective cohort studies with careful control for confounding are warranted.
Supplementary Material
Footnotes
Conflict of interest
The authors have nothing to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. As this is a systematic review and meta-analysis of already published studies, we do not have any stored data.
References
- 1.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018; 392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449.e1–7. [DOI] [PubMed] [Google Scholar]
- 4.Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;11: Cd007412. 10.1002/14651858.CD007412.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet. 1998;351:693–9. [DOI] [PubMed] [Google Scholar]
- 6.International Federation of Midwives, International Confederation of Gynaecologists. Joint statement: management of the third stage of labour to prevent post-partum haemorrhage. J Midwifery Womens Health. 2004;49:76–7. [DOI] [PubMed] [Google Scholar]
- 7.Sheldon WR, Durocher J, Winikoff B, Blum J, Trussell J. How effective are the components of active management of the third stage of labor? BMC Pregnancy Childbirth. 2013; 13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widmer M, Piaggio G, Hofmeyr GJ, et al. Maternal characteristics and causes associated with refractory postpartum haemorrhage after vaginal birth: a secondary analysis of the WHO CHAMPION trial data. BJOG. 2020;127:628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause B, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011; 32:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hijji J, Andolf E, Laurini R, Batra S. Nitric oxide synthase activity in human trophoblast, term placenta and pregnant myometrium. Reprod Biol Endocrinol. 2003;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myatt L, Eis A, Brockman D, Kossenjans W, Greer I, Lyall F. Inducible (type II) nitric oxide synthase in human placental villous tissue of normotensive, pre-eclamptic and intrauterine growth-restricted pregnancies. Placenta. 1997;18:261–8. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L, Eis A, Brockman D, Greer I, Lyall F. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod. 1997;12:167–72. [DOI] [PubMed] [Google Scholar]
- 13.Tikvica A, Kučan Jukić M, Pintarić I, et al. Nitric oxide synthesis in placenta is increased in intrauterine growth restriction and fetal hypoxia. Coll Antropol. 2008;32:565–70. [PubMed] [Google Scholar]
- 14.Node K, Kitakaze M, Kosaka H, Komamura K, Minamino T, Inoue M, et al. Increased release of NO during ischemia reduces myocardial contractility and improves metabolic dysfunction. Circulation. 1996;93:356–64. [DOI] [PubMed] [Google Scholar]
- 15.Kitakaze M, Node K, Komamura K, Minamino T, Inoue M, Hori M, et al. Evidence for nitric oxide generation in the cardiomyocytes: its augmentation by hypoxia. J Mol Cell Cardiol. 1995;27:2149–54. [DOI] [PubMed] [Google Scholar]
- 16.Grilli A, De Lutiis MA, Patruno A, et al. Effect of chronic hypoxia on inducible nitric oxide synthase expression in rat myocardial tissue. Exp Biol Med (Maywood). 2003;228:935–42. [DOI] [PubMed] [Google Scholar]
- 17.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007; 293:H2565–72. [DOI] [PubMed] [Google Scholar]
- 18.Choi JW, Pai SH, Kim SK, Ito M, Park CS, Cha YN. Iron deficiency anemia increases nitric oxide production in healthy adolescents. Ann Hematol. 2002;81:1–6. [DOI] [PubMed] [Google Scholar]
- 19.Surks HK. cGMP-dependent protein kinase I and smooth muscle relaxation: a tale of two isoforms. Circ Res. 2007;101: 1078–80. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butwick AJ, Walsh EM, Kuzniewicz M, Li SX, Escobar GJ. Patterns and predictors of severe postpartum anemia after cesarean section. Transfusion. 2017;57:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geelhoed D, Agadzi F, Visser L, Ablordeppey E, Asare K, O’Rourke P, et al. Maternal and fetal outcome after severe anemia in pregnancy in rural Ghana. Acta Obstet Gynecol Scand. 2006;85:49–55. [DOI] [PubMed] [Google Scholar]
- 23.Gonzales GF, Tapia V, Gasco M, Carrillo CE, Fort AL. Association of hemoglobin values at booking with adverse maternal outcomes among Peruvian populations living at different altitudes. Int J Gynaecol Obstet. 2012;117:134–9. [DOI] [PubMed] [Google Scholar]
- 24.Kebede BA, Abdo RA, Anshebo AA, Gebremariam BM. Prevalence and predictors of primary postpartum hemorrhage: an implication for designing effective intervention at selected hospitals, Southern Ethiopia. PLoS One. 2019;14: e0224579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luis J, Fadel MG, Lau GY, Houssein S, Ravikumar N, Yoong W. The effects of severe iron-deficiency anaemia on maternal and neonatal outcomes: a case-control study in an inner-city London hospital. J Obstet Gynaecol. 2016;36:473–5. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet. 2002;79:93–100. [DOI] [PubMed] [Google Scholar]
- 27.Nyflot LT, Sandven I, Stray-Pedersen B, et al. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth. 2017;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks S, Hoffman MK, Goudar SS, Patel A, Saleem S, Ali SA, et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand. 2016;95:555–64. [DOI] [PubMed] [Google Scholar]
- 30.Selo-Ojeme DO, Okonofua FE. Risk factors for primary postpartum haemorrhage. A case control study. Arch Gynecol Obstet. 1997;259:179–87. [DOI] [PubMed] [Google Scholar]
- 31.Soltan MH, Ibrahim EM, Tawfek M, Hassan H, Farag F. Raised nitric oxide levels may cause atonic postpartum hemorrhage in women with anemia during pregnancy. Int J Gynaecol Obstet. 2012;116:143–7. [DOI] [PubMed] [Google Scholar]
- 32.Tort J, Rozenberg P, Traore M, Fournier P, Dumont A. Factors associated with postpartum hemorrhage maternal death in referral hospitals in Senegal and Mali: a cross-sectional epidemiological survey. BMC Pregnancy Childbirth. 2015;15:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wandabwa J, Doyle P, Todd J, Ononge S, Kiondo P. Risk factors for severe post partum haemorrhage in Mulago hospital, Kampala, Uganda. East Afr Med J. 2008;85:64–71. [DOI] [PubMed] [Google Scholar]
- 34.Kavle JA, Stoltzfus RJ, Witter F, Tielsch JM, Khalfan SS, Caulfield LE. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr. 2008;26:232–40. [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO). Haemoglobin concentration for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO; 2011. [Google Scholar]
- 36.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 38.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 39.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duval S, Tweedie R. Trim and fill: a simple funnel-plot– based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheldon W, Blum J, Vogel J, Souza JP, Gülmezoglu AM, Winikoff B, et al. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization multicountry survey on maternal and newborn health. BJOG. 2014;121:5–13. [DOI] [PubMed] [Google Scholar]
- 43.Weze K, Abioye AI, Obiajunwa C, Omotayo MO. Spatial and temporal patterns and determinants of anemia among pregnant women, adolescents and children in sub-Saharan Africa. Public Health Nutr. 2020;23:1–14. 10.1017/S1368980020004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40–57. [Google Scholar]
- 45.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. As this is a systematic review and meta-analysis of already published studies, we do not have any stored data.


