Abstract
Poly(l-lactic acid) (PLA)-degrading Amycolatopsis sp. strains K104-1 and K104-2 were isolated by screening 300 soil samples for the ability to form clear zones on the PLA-emulsified mineral agar plates. Both of the strains assimilated >90% of emulsified 0.1% (wt/vol) PLA within 8 days under aerobic conditions. A novel PLA depolymerase with a molecular weight of 24,000 was purified to homogeneity from the culture supernatant of strain K104-1. The purified enzyme degraded high-molecular-weight PLA in emulsion and in solid film, ultimately forming lactic acid. The optimum pH for the enzyme activity was 9.5, and the optimum temperature was 55 to 60°C. The PLA depolymerase also degraded casein and fibrin but did not hydrolyze collagen type I, triolein, tributyrin, poly(β-hydroxybutyrate), or poly(ε-caprolactone). The PLA-degrading and caseinolytic activities of the enzyme were inhibited by diisopropyl fluorophosphate and phenylmethylsulfonyl fluoride but were not significantly affected by soybean trypsin inhibitor, N-tosyl-l-lysyl chloromethyl ketone, N-tosyl-l-phenylalanyl chloromethyl ketone, and Streptomyces subtilisin inhibitor. Thus, Amycolatopsis sp. strain K104-1 excretes the unique PLA-degrading and fibrinolytic serine enzyme, utilizing extracellular polylactide as a sole carbon source.
Polylactide, or poly(l-lactic acid) (PLA), is a promising material to be used as a renewable and biodegradable plastic, based on the following observations. (i) Lactic acid can be efficiently produced by fermentation of renewable resources such as starchy materials, cane molasses, and cellulose. (ii) PLA can be synthesized by conventional chemical engineering, and it has a higher melting point (170°C) and a higher glass transition temperature (60°C) than the other aliphatic polyesters. Advanced polymer processing technology can furnish the fibers and the sheets of PLA with high tensile strength and high transparency comparable to those of polyethylene terephthalate. (iii) Although PLA is an artificial polymer, it is hydrolyzable by some hydrolases such as proteinase K from Tritirachium album (6, 23, 29), the lipase from Rhizopus delemer (29), and the polyester polyurethane-degrading enzyme from Comamonas acidovorans strain TB-35 (1). (iv) Microbial degradation of PLA was implied by the efficient degradation that occurs under composting conditions (11). In fact, Torres et al. found that several fungal strains from a culture collection degraded and assimilated PLA film (28). However, the PLA degradation by the fungi was very slow, so the fungal strains are not an appropriate source for isolation of a PLA-degrading enzyme(s) (28). Pranamuda et al. have succeeded in isolating a PLA-degrading Amycolatopsis sp. strain, HT-32, by screening soil samples for the ability to form clear zones on the PLA-emulsified mineral agar plates (21). PLA emulsion and PLA film were efficiently degraded in cultures of strain HT-32, and the PLA degradation was implied to be an enzymatic process (21). Recently, Ikura and Kudo also isolated Amycolatopsis sp. strain 3118 and identified it as a PLA-degrading bacterium (10). Further, Tomita et al. isolated a PLA-degrading thermophile, Bacillus brevis strain 93, by enrichment culture for soil samples at 60°C in a mineral medium with PLA film (27). However, PLA-degrading enzymes have not yet been isolated from the PLA-degrading microbes.
In this study, we isolated PLA-degrading actinomycetes by screening soil samples for the ability to degrade high-molecular-weight PLA and purified a novel PLA-degrading enzyme from the isolated bacterium, Amycolatopsis sp. strain K104-1. This is the first report describing purification and characterization of a PLA depolymerase from PLA-degrading microbes.
MATERIALS AND METHODS
Chemicals.
PLA with a number-average molecular weight (Mn) of 220,000 was from Shimadzu Co. (Kyoto, Japan). Poly(3-hydroxybutyrate) with an average molecular weight of 1,000,000 and poly(ε-caprolactone) with molecular weights of 70,000 to 100,000 were from Sigma Chemical Co. (St. Louis, Mo.). Unless otherwise stated, chemicals of analytical grade were used in this study.
Culture media.
A mineral medium containing 0.1% (wt/vol) emulsified PLA was prepared with or without 1.5% (wt/vol) agar for isolation and cultivation of PLA-degrading microbes. One gram of PLA was emulsified in 1 liter of the basal medium described by Nishida and Tokiwa (20): 250 mg of Difco yeast extract (Difco Laboratories, Detroit, Mich.), 1,000 mg of ammonium sulfate, 100 mg of NaCl, 200 mg of MgSO4·7H2O, 20 mg of CaCl2·2H2O, 10 mg of FeSO4·7H2O, 0.5 mg of Na2MoO4·2H2O, 0.5 mg of Na2WO4, 0.5 mg of MnSO4, and 100 mg of Plysurf A210G (a surfactant; Daiichi Kogyo Seiyaku, Tokyo, Japan) in 1 liter of 10.7 mM potassium phosphate buffer (pH 7.1). The isolated PLA-degrading microbes were purified on ISP medium 1 (5.0 g of Difco tryptone-peptone and 3.0 g of Difco yeast extract in 1 liter, pH 7.1) (3) containing 1.5% (wt/vol) agar. ISP medium 2 (10 g of Difco malt extract, 4 g of Difco yeast extract, and 4 g of glucose in 1 liter, pH 7.3) (3) was used for tests of chemical and biochemical properties of the isolated microbes unless otherwise stated.
Morphological, chemical, and biochemical characteristics of the PLA-degrading strains.
PLA-degrading colonies on the agar plate containing ISP medium 2 were fixed with 2% (vol/vol) glutaraldehyde, dehydrated in 50 to 100% (vol/vol) ethanol, and lyophilized in 2,2-dimethylpropanol by the critical-point method. The lyophilized colonies were coated with platinum-vanadium and were observed under a Hitachi S-4200 scanning electron microscope (Hitachi, Tokyo, Japan) at an acceleration voltage of 5 kV.
The diaminopimelic acid isomer and whole-cell sugar pattern were identified as described by Lechevalier and Lechevalier (15) and Staneck and Roberts (26). Menaquinones and phospholipids were extracted and analyzed as described by Minnikin et al. (18). The occurrence of mycolic acid was analyzed as described by Minnikin et al. (17). Acid production from sugars, decarboxylation of benzoate and citrate, decomposition of hypoxanthine and xanthine, and production of amylase and nitrate reductase by the isolates were assayed as described by Gordon et al. (7).
Nucleotide sequence of 16S rRNA gene.
The 16S rRNA gene was amplified by a PCR using chromosomal DNA from the isolates as the templates, essentially as described by Edwards et al. (5). The forward and reverse primers were AGAGTTTGATCCTGGCTCAG (primer A) and AAGGAGGTGATCCAGCCGCA (primer H), respectively, and Ex Taq polymerase (TaKaRa, Kyoto, Japan) was used. DNA sequencing was carried out in an ABI Prism 310 DNA sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.), using the ABI Prism dye terminator cycle-sequencing ready-reaction kit (Perkin-Elmer Applied Biosystems). A homology search for the nucleotide sequences was done using BLAST on the DDBJ/GenBank/EMBL nucleotide sequence databases.
Degradation and assimilation of emulsified PLA in the culture of Amycolatopsis sp. strains.
The PLA-degrading strains were cultured in ISP medium 1 at 37°C for 2 days and collected by centrifugation. The collected bacteria (wet weight, 1 g) were inoculated into the PLA-emulsified liquid mineral medium (150 ml) and were cultivated at 37°C with shaking at 125 strokes per min. Two milliliters of the cultures was withdrawn in triplicate once every 24 h and lyophilized. The lyophilized samples were hydrolyzed in 1 M NaOH at 100°C for 1 h. The hydrolysates were neutralized with 1 M HCl and filtered through cellulose acetate membranes (pore size, 0.2 μm; Advantec Co., Tokyo, Japan), and loaded onto a TSKgel ODS-120A column (diameter, 0.75 cm; length, 30 cm; Tosoh, Tokyo, Japan) equilibrated with 50 mM ammonium phosphate buffer (pH 2.4). Lactic acid was eluted with the same buffer, and the concentration of lactic acid was determined spectrophotometrically at 210 nm. Lithium lactate was used as a standard.
PLA degradation by the concentrated culture supernatant of strain K104-1.
After cultivation of strain K104-1 in PLA-emulsified liquid medium at 37°C for 5 to 7 days, the culture medium was centrifuged at 18,000 × g for 15 min. The culture supernatant obtained was concentrated 100-fold by ultrafiltration using an Amicon YM-10 membrane (Amicon Co., Danvers, Mass.), followed by filtration through a sterile cellulose acetate membrane (pore size, 0.2 μm). PLA (0.11%, wt/vol) was emulsified with 0.011% (wt/vol) Plysurf A210G in 10.7 mM potassium phosphate buffer (pH 7.1). The PLA emulsion (0.9 ml) was mixed with the 100-fold-concentrated culture supernatant (0.1 ml), and the mixture was incubated at 37°C for 24 h. A portion (0.1 ml) of the mixture was withdrawn, lyophilized, and solubilized in 10 μl of 1 M HCl. The solubilized sample was spotted onto a thin-layer plate (silica gel 1.05715; E. Merck, Darmstadt, Germany). The thin-layer plate was developed with a mixture of ethyl acetate, toluene, water, and formic acid (2/3/1.2/0.9, vol/vol) and was sprayed with 5% phosphomolybdate.
Assay for PLA depolymerase activity.
PLA (0.1%, wt/vol) was emulsified with Plysurf A210G (0.01%, wt/vol) in 10 mM potassium phosphate buffer (pH 7.0) and was used as a substrate. Mixtures of enzyme solutions (5 μl) and the PLA emulsion (45 μl) were put into the wells of a 96-well multiplate and were kept at 37°C for 30 min with continuous shaking at 500 rpm unless otherwise stated. The decrease in turbidity of the PLA emulsions was measured at a wavelength of 630 nm using a multiplate reader (MTP32; Corona Electric Co., Katsuda, Japan). One unit of the PLA-degrading activity was defined as a 1-U decrease in absorbance at 630 nm per min under the assay conditions described.
Purification of PLA-degrading enzyme.
The culture supernatant from 5 liters of the culture medium of K104-1 was concentrated 100-fold by ultrafiltration as described above. The concentrated culture supernatant was dialyzed against 20 mM potassium phosphate buffer (pH 6.0), and applied onto a TSKgel CM-Toyopearl 650M column (Tosoh, Tokyo, Japan; diameter, 1.3 cm; height, 2.0 cm) equilibrated with the same buffer. Adsorbed proteins were eluted with a linear gradient of NaCl (0 to 1 M). Active fractions were combined, dialyzed against 20 mM potassium phosphate buffer (pH 6.0), and put onto a TSKgel CM-5PW column (Tosoh; diameter, 0.75 cm; height, 7.5 cm) equilibrated with the same buffer. Adsorbed proteins were eluted with a linear gradient of NaCl (0 to 0.25 M), and active fractions were combined and dialyzed against 20 mM Tris HCl buffer (pH 7.5). The dialyzed fraction was mixed with the same volume of 2 M ammonium sulfate and put onto a TSKgel phenyl-5PW column (Tosoh; diameter, 0.75 cm; height, 7.5 cm) equilibrated with 20 mM Tris HCl buffer (pH 7.5) containing 1 M ammonium sulfate. Adsorbed proteins were eluted with a descending linear gradient of ammonium sulfate (1 to 0 M), and active fractions were combined and dialyzed against 10 mM potassium phosphate buffer (pH 7.1). The purified PLA depolymerase thus obtained was divided into small portions and was kept at −80°C until use.
Degradation of emulsified PLA and film PLA by the purified PLA depolymerase.
The emulsion of 0.1% (wt/vol) PLA was incubated with the purified PLA depolymerase (0 to 65 μg/ml) in 50 μl of 10 mM potassium phosphate buffer (pH 7.1) at 37°C for 30 min, and the degradation products were analyzed by thin-layer chromatography as described above, except for the use of a mixture of ethyl acetate, toluene, water, and formic acid (2/1.5/1/0.75, vol/vol) as the developing solvent.
A portion of 2.5% (wt/vol) PLA solution in dichloromethane was put into a Teflon dish and dried under air to prepare a PLA film (thickness, approximately 5 μm). A piece of the PLA film (5.0 to 5.5 mg) was treated with the purified PLA depolymerase (140 μg/ml) in 0.2 ml of 10 mM Tris HCl buffer (pH 8.6) at 37°C for 48 h or left untreated, and the film weight was measured. To avoid a pH drop, the reaction mixture was put in a dialysis tube with a cutoff size of 10 kDa and dialyzed against 200 ml of the same buffer during the incubation. Residual small pieces of the disintegrated film were collected and dried at 60°C, and the weight of the pieces was collectively measured. The pieces of the film were coated with platinum-vanadium and were observed under a scanning electron microscope (Hitachi S-4200) at an acceleration voltage of 5 kV.
Effects of pH and temperature on the PLA-degrading activity of the purified enzyme. (i) Optimal pH and optimal temperature.
PLA-degrading activity of the purified enzyme (0.1 μg) was assayed under standard conditions except for pH (i.e., pH 3.5 to 10) and temperature (30 to 100°C).
(ii) Stability of the enzyme activity at different pHs.
The purified enzyme (0.13 μg) was kept at pH 3.5 to 10 at 4°C for 24 h, and residual activity was assayed under standard conditions.
(iii) Thermostability of the enzyme.
The purified enzyme (0.13 μg) was kept at 30 to 100°C for 1 h, and residual activity was assayed under standard conditions. The PLA-degrading activity obtained under standard conditions was considered 100% activity.
Activities of the purified PLA depolymerase for various substrates.
The caseinolytic activity of the PLA depolymerase was assayed essentially as described by Hagihara et al. (8). The purified enzyme (0.25 μg of protein) was incubated with 1% (wt/vol) casein in 1 ml of 10 mM Tris HCl buffer (pH 7.5) at 37°C for 1 h, and absorbance at 275 nm was measured for the trichloroacetic acid-soluble fraction. One unit of the caseinolytic activity was defined as the enzyme activity releasing 1 μg of tyrosine equivalent per min. Fibrinolytic activity was assayed by the fibrin plate method (2). For preparation of fibrin plates, a 1% (wt/vol) agarose solution (3.7 ml), 1.2% (wt/vol) human fibrinogen (1.25 ml; Sigma), and 100 NIH units of human thrombin/ml (0.05 ml; Sigma) in 100 mM Tris HCl buffer (pH 8.0) were mixed in a petri dish (diameter, 60 mm). Serial dilutions of the enzyme sample (approximately 5 μl) were put into holes in the fibrin plate and incubated at 37°C for 1 h. Diameters of the clear zones surrounding the holes were compared with those formed by the standard fibrinolytic enzyme. Human plasmin (Sigma) was used as a standard fibrinolytic protease, and one unit of plasmin was defined as the enzyme activity hydrolyzing α-casein to produce an increase in absorbance at 275 nm of 1.0 at pH 7.5 at 37°C for 20 min. Collagenase activity was assayed as described by Sasagawa et al. (24), using 0.1% (wt/vol) insoluble collagen type I from bovine tendon (Sigma). Collagenase from Clostridium histolyticum (Sigma) was used as a standard collagenase.
Emulsions of 0.1% (wt/vol) triolein, tributyrin, poly(β-hydroxybutyrate), and poly(ε-caprolactone) were prepared with 0.01% (wt/vol) Plysurf A210. The emulsified triolein or tributyrin was incubated with the purified enzyme in 10 mM Tris-HCl buffer (pH 9.5) at 37°C for 30 min, and hydrolysis of the emulsified triolein or tributyrin was measured as the decrease in turbidity (absorbance at 630 nm) or as the acidification of the emulsion, respectively. Degradation of poly(β-hydroxybutyrate) and poly(ε-caprolactone) by the purified enzyme was measured at 37°C for 30 min as the decrease in turbidity of the emulsified substrates at 630 nm.
Protein chemistry.
Protein concentration was assayed essentially as described by Bradford, using bovine serum albumin as a standard (4). Isoelectric focusing was done by using a 5% polyacrylamide gel containing Ampholine of pH 7 to 11 (Pharmacia Biotech., Uppsala, Sweden) according to the protocol of the manufacturer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done essentially as described by Laemmli (13). Molecular mass markers (type III from Daiichi Pure Chemicals) were phosphorylase b from rabbit muscle (97.4 kDa), bovine serum albumin (66.3 kDa), aldolase from rabbit muscle (42.4 kDa), carbonic anhydrase (30.0 kDa), trypsin inhibitor (20.1 kDa), and lysozyme from egg white (14.4 kDa). The protein band of the PLA depolymerase in an SDS-polyacrylamide gel was blotted onto a polyvinylidene difluoride sheet (16), and the N-terminal amino acid sequence of the protein was analyzed by using an Applied Biosystems model 491 protein sequencer.
RESULTS AND DISCUSSION
Isolation of PLA-degrading Amycolatopsis sp. strains and degradation of emulsified PLA by the strains.
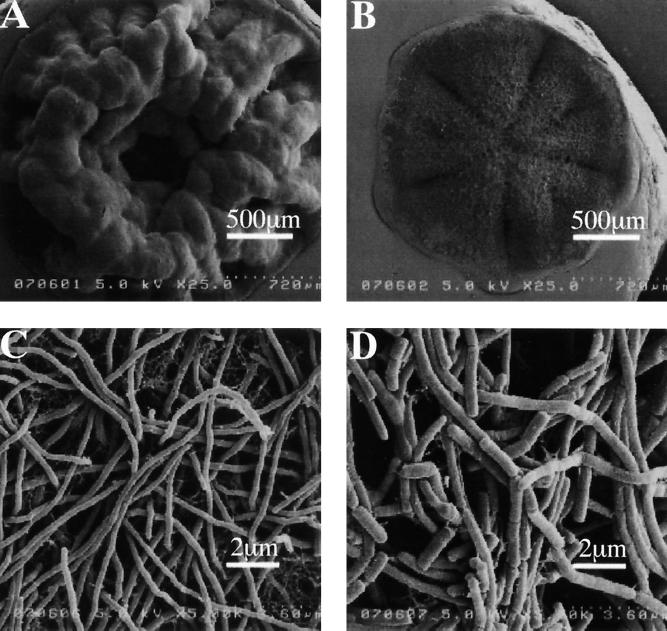
Soil samples were collected at 300 different places such as university campuses, house gardens, rice fields, weed fields, roadsides, riversides, seasides, and dumping grounds in Sendai City, Japan. Small portions of the suspensions of the soil samples were plated onto the PLA-emulsified minimal agar medium, and the plates were incubated at 37°C for 30 to 40 days. To prevent the plates from drying out, the plates were put into a plastic box, which was then placed in an incubator at 37°C. Two clear-zone-forming colonies were isolated by the screening of 300 soil samples on >1,000 plates of the PLA-emulsified agar medium, where 102 or 103 colonies appeared on each plate. The clear-zone-forming colonies were purified by repeated transfer onto PLA-emulsified mineral agar plates and ISP medium 1 plates. The isolates, designated strains K104-1 and K104-2, were studied for their morphologic, chemical, and biochemical properties (Fig. 1 and Table 1). (i) Both K104-1 and K104-2 formed actinomycete-like colonies with aerial and vegetative mycelia on or in the ISP medium 1 agar plate. Scanning electron microscopy for the colonies showed that K104-1 formed nonfragmented aerial mycelia, while K104-2 formed fragmented ones (Fig. 1). (ii) Analyses for diaminopimelic acid isomer, whole cell sugar pattern, major menaquinones, phospholipid type, and the occurrence of mycolic acid (Table 1) indicated that both K104-1 and K104-2 belong to the genus Amycolatopsis (7, 9). (iii) Biochemical properties such as production of soluble pigment, acid production from various sugars, decarboxylation of benzoate and citrate, decomposition of hypoxanthine and xanthine, production of amylase and nitrate reductase, and growth in 5% NaCl (Table 1) showed that both of the isolates were different from any species of Amycolatopsis described previously (9, 14). (iv) The nucleotide sequences of the 16S rRNA gene of the strains revealed 96 to 97% identity with those of Amycolatopsis alba, Amycolatopsis azurea, and Amycolatopsis coloradensis. Thus, we isolated two Amycolatopsis sp. strains, K104-1 and K104-2, which formed clear zones on the PLA-emulsified mineral agar plates.
FIG. 1.
Morphology of the PLA-degrading Amycolatopsis sp. strains K104-1 and K104-2. Strains K104-1 (A and C) and K104-2 (B and D) were grown on an ISP medium 1 agar plate, and their colonies were viewed by scanning electron microscopy at a ×25 magnification (A and B) and at a ×5,000 magnification (C and D), as described in Materials and Methods.
TABLE 1.
Chemical and biochemical properties of Amycolatopsis sp. strains K104-1 and K104-2
| Characteristic | K104-1 | K104-2 |
|---|---|---|
| Diaminopimelic acid isomer | Meso | Meso |
| Whole-cell sugar patterna | A | A |
| Major menaquinones | MK-9 (H4,H6)b | MK-9 (H4,H6)b |
| Phospholipid typec | PII | PII |
| Mycolic acid | Absent | Absent |
| Color of aerial mycelium | Brown | White |
| Production of soluble pigment | + | + |
| Color of soluble pigment | Brown | Dark brown |
| Acid production from: | ||
| Adonitol | − | − |
| l-Arabinose | + | + |
| Cellobiose | + | + |
| Erythritol | − | − |
| Galactose | + | + |
| Inositol | + | + |
| Lactose | + | + |
| Mannitol | + | + |
| Raffinose | − | − |
| Decarboxylation of: | ||
| Benzoate | − | − |
| Citrate | + | + |
| Decomposition of: | ||
| Hypoxanthine | + | + |
| Xanthine | − | − |
| Growth in 5% NaCl | ± | ± |
| Production of: | ||
| Amylase | − | − |
| Nitrate reductase | − | − |
Sugar types are designated according to the work of Lechevalier and Lechevalier (15). Type A, arabinose and galactose.
MK-9 (H4,H6), 2-methyl-II,III-tetrahydromultiprenyl-1,4-naphthoquinone and 2-methyl-II,III,VIII-hexahydromultiprenyl-1,4-naphthoquinone.
Phospholipid types are designated according to the work of Lechevalier and Lechevalier (15). Type PII, phosphatidylethanolamine phosphatidylglycerol, OH-phosphatidylethanolamine, phosphatidylinositol, phosphatidylinositol mannosides, and diphosphatidylglycerol.
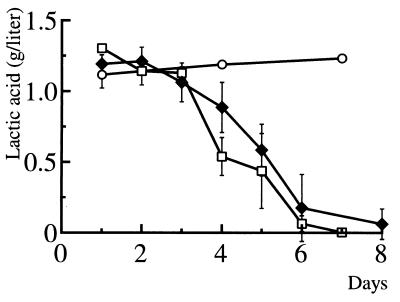
When the Amycolatopsis sp. strains (wet weight, approximately 1 g) were inoculated in 150 ml of the 0.1% (wt/vol) PLA-emulsified medium and cultivated at 37°C with vigorous shaking, both strains cleared the PLA emulsion within a week. To estimate degradation of the emulsified PLA by the strains, residual PLA was quantified as the concentration of lactic acid after alkaline hydrolysis of the culture fluid as described in Materials and Methods. As shown in Fig. 2, the concentration of lactic acid significantly decreased after the cultivation for 2 to 3 days, and >90% of PLA was consumed by the bacteria within 8 days. The concentration of lactic acid in the medium did not change unless the bacteria were inoculated (Fig. 2). These results indicated that strains K104-1 and K104-2 degraded and assimilated the emulsified high-molecular-weight PLA.
FIG. 2.
Degradation and assimilation of the emulsified PLA by the Amycolatopsis sp. strains. Strains K104-1 (□) and K104-2 (♦) were grown in PLA-emulsified mineral liquid medium at 37°C for different times. Residual PLA was hydrolyzed in 1 M NaOH at 100°C for 1 h, and the concentrations of lactic acid were quantified in triplicate for each time point as described in Materials and Methods. The concentration of PLA was also monitored for the noninoculated PLA-emulsified medium (○). The concentrations of lactic acid (mean values ± standard deviations) were calculated from three independent experiments.
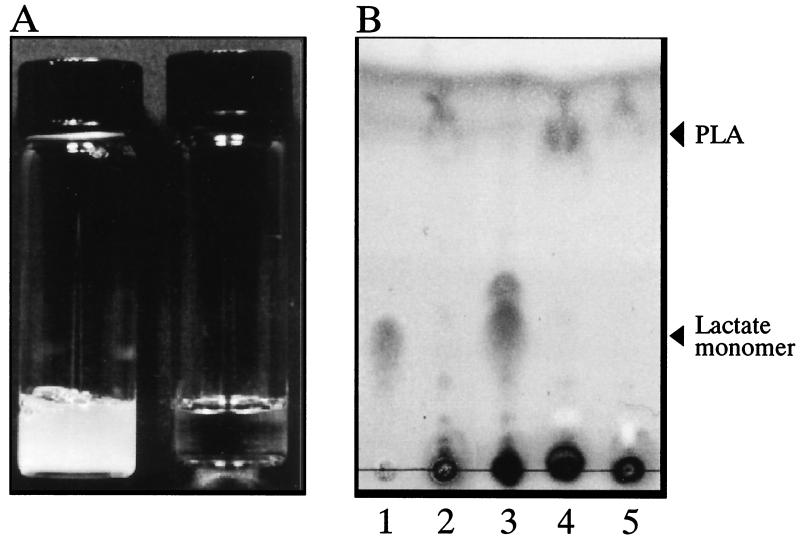
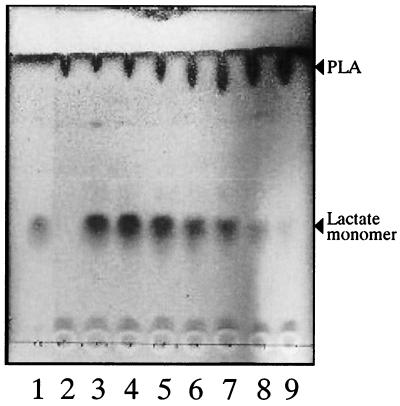
Since K104-1 degraded PLA more rapidly than K104-2 (Fig. 2), the culture supernatant of the strain was concentrated 100-fold and tested for PLA-degrading activity. When 0.1% (wt/vol) PLA emulsion was incubated with the concentrated culture supernatant at 37°C for 24 h, it was clarified (Fig. 3A) and acidified from pH 7.1 to 5.4. In contrast, no decrease in turbidity of the PLA emulsion was observed without the culture supernatant (Fig. 3A) or with the culture supernatant heated at 100°C for 5 min or treated with 1 mM phenylmethylsulfonyl fluoride (PMSF) (results not shown). Furthermore, no significant PLA-degrading activity was detected for the cell homogenate of K104-1, which was obtained with a French pressure cell at 1,200 kg/cm2 (results not shown). Degradation products from PLA were analyzed using thin-layer chromatography. When emulsified PLA was incubated with the concentrated supernatant of K104-1, the spots corresponding to monomeric and oligomeric lactate were visible and the spot corresponding to PLA disappeared (Fig. 3B, lane 3). The spot of oligomeric lactate in Fig. 3B (lane 3) may correspond to dimeric lactate, on the basis of its mobility on the thin-layer chromatography, and the spots corresponding to the lactate trimer and the lactate dimer were occasionally visible under the same conditions (results not shown). In contrast, the spots corresponding to the lactate monomer and oligomer were not observed when the PLA emulsion was incubated without the culture supernatant (Fig. 3B, lane 2) or with the culture supernatant preheated at 100°C for 5 min (Fig. 3B, lane 4) or pretreated with 1 mM PMSF (Fig. 3B, lane 5). These results indicated that an extracellular serine enzyme(s) from K104-1 degraded the emulsified high-molecular-weight PLA, forming monomeric and oligomeric lactate.
FIG. 3.
Degradation of the emulsified PLA by the culture supernatant of K104-1 (A) and thin-layer chromatography for the degradation products (B). (A) An emulsion of 0.1% PLA was incubated without (left tube) or with (right tube) the 100-fold-concentrated culture supernatant of K104-1 at 37°C for 24 h. (B) PLA emulsion was incubated without (lane 2) or with (lane 3) the 100-fold-concentrated culture supernatant preheated at 100°C for 5 min (lane 4) or pretreated with 1 mM PMSF (lane 5). A portion (0.1 ml) of the mixtures was lyophilized, solubilized in 1 M HCl, and applied to thin-layer chromatography plates. Lithium lactate was spotted in lanes 1 and 3.
Purification of an extracellular PLA depolymerase from K104-1 and molecular weight and N-terminal amino acid sequence of the purified protein.
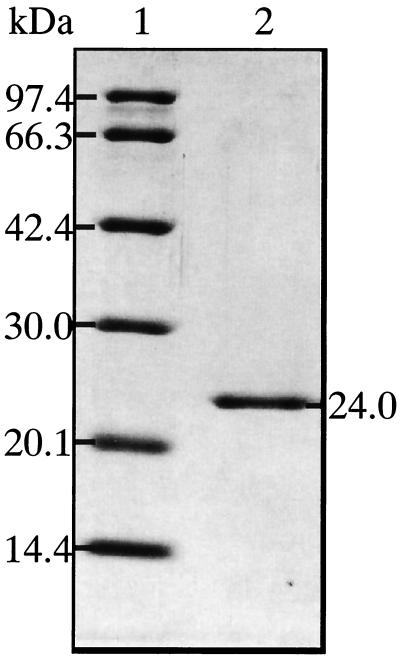
PLA-degrading activity was assayed as the ability to decrease turbidity of PLA emulsion: enzyme fractions were incubated with 0.1% (wt/vol) PLA emulsions in 10 mM potassium phosphate buffer (pH 7.1) at 37°C for 0 to 30 min, and turbidity of the PLA emulsion was monitored spectrophotometrically at 630 nm. A single PLA depolymerase was purified to homogeneity from the culture supernatants of K104-1 by successive column chromatography using CM-Toyopearl 650M, TSKgel CM-5PW, and TSKgel phenyl-5PW, as described in Materials and Methods. The purification of the enzyme is summarized in Table 2: yield and purification of the PLA depolymerase were 18% and 23-fold, respectively, and 93 μg of purified PLA depolymerase was obtained from 5 liters of the culture supernatant. The molecular mass of the purified PLA depolymerase was 24 kDa as determined by SDS-PAGE (Fig. 4). The isoelectric point (pI) of the enzyme was >10, as estimated by using a 5% polyacrylamide gel containing Ampholine at pH 7 to 11 (results not shown). The N-terminal amino acid sequence of the protein was determined for the initial 17 residues (Table 3). A similarity search of the DDBJ nucleic acid sequence database indicated that the N-terminal amino acid sequence of the purified PLA depolymerase was different from that of any other protein so far registered. However, the seven N-terminal amino acid residues of the purified PLA depolymerase were 100% identical to those of the fibrinolytic serine proteases (F-I-1 and F-I-2) from the earthworm Lumbricus rubellus (19) (Table 3). The purified PLA depolymerase also revealed 80% identity in the N-terminal 10 amino acid residues with the collagenolytic serine proteases from the hepatopancreas of the Kamchatka crab, Paralithodes camtshatica (12) (Table 3).
TABLE 2.
Purification of a PLA-degrading enzyme from the culture supernatant of Amycolatopsis sp. strain K104-1
| Step | Total protein (mg) | Total activity (U)a | Sp act (U/mg of protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Culture supernatant | 12.1 | 13.3 | 1.10 | 100 | 1 |
| CM-Toyopearl 650M | 0.881 | 6.69 | 7.58 | 50.1 | 6.9 |
| CM-5PW | 0.188 | 4.07 | 21.6 | 30.5 | 19.7 |
| Phenyl-5PW | 0.093 | 2.39 | 25.7 | 17.9 | 23.3 |
One unit of the PLA-degrading activity was defined as a 1-U decrease in absorbance at 630 nm per min under standard conditions.
FIG. 4.
SDS-PAGE for the purified PLA depolymerase from the culture supernatant of Amycolatopsis sp. K104-1. The purified enzyme preparation (1 μg of protein) was electrophoresed in an SDS-polyacrylamide gel (12.5%, wt/vol), and protein bands were stained with Coomassie brilliant blue R-250.
TABLE 3.
N-terminal amino acid residues of PLA depolymerase from Amycolatopsis strain K104-1, fibrinolytic proteases from an earthworm, and collagenolytic proteases from the Kamchatka crab
Degradation of high-molecular-weight PLA in emulsion and solid film by the purified PLA depolymerase.
Treatment of PLA emulsion with the purified enzyme at 37°C for 30 min decreased turbidity of the emulsion at a rate depending on the enzyme concentration (1 to 65 μg/ml) (results not shown). Thin-layer chromatography for the PLA emulsions showed that the spot corresponding to lactic acid appeared upon treatment of emulsified PLA with the enzyme, and the amount of lactic acid formed was dependent on the enzyme concentration (Fig. 5). Thus, the purified PLA depolymerase of K104-1 degraded the emulsified high-molecular-weight PLA to lactic acid.
FIG. 5.
Depolymerization of emulsified PLA by the purified PLA-degrading enzyme. PLA emulsion was incubated without (lane 2) or with various concentrations of the purified PLA depolymerase (65, 32.5, 16, 8, 4, 2, and 1 μg/ml; lanes 3 through 9) at 37°C for 30 min. The mixtures were lyophilized, solubilized in 1 M HCl, and analyzed by thin-layer chromatography. Lithium lactate was spotted in lane 1.
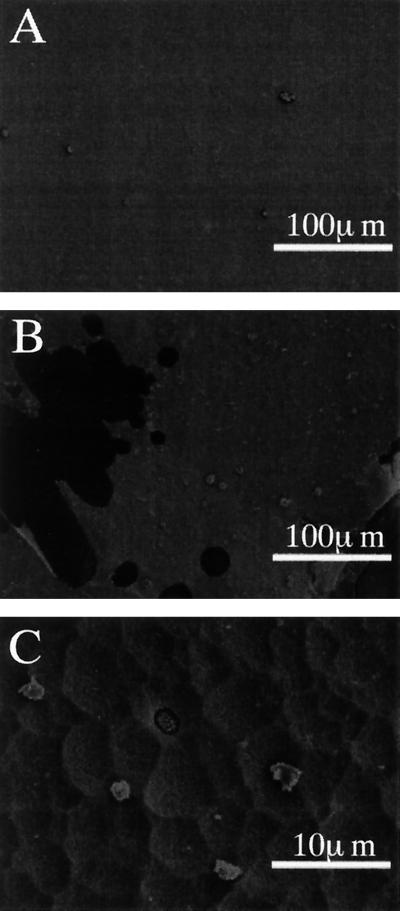
The PLA depolymerase was also tested for the ability to degrade a solid film of PLA (thickness, approximately 5 μm) under constant pH conditions. To avoid pH drop, the reaction mixture was put into a dialysis tube and dialyzed against 10 mM Tris HCl buffer (pH 8.6) during the enzyme treatment. When PLA films (5.0 to 5.5 mg) were treated with the PLA depolymerase (140 μg/ml) at 37°C for 48 h, the PLA films were disintegrated into small pieces and digested by the enzyme. The total weight of the residual film was 0.3 to 0.5 mg, indicating that >90% of the PLA film was digested by the enzyme treatment. Scanning electron microscopy of the resultant small pieces of the PLA film indicated that the PLA depolymerase scraped off the film surface (Fig. 6B) and made holes (Fig. 6C), leading to disintegration of the film. The film surface was smooth unless the PLA depolymerase was added (Fig. 6A). These results indicated that the PLA depolymerase gained access to the hydrophobic surface of the solid film, degrading the high-molecular-weight PLA.
FIG. 6.
Degradation of PLA film by the purified PLA depolymerase. PLA films (thickness, 5 μm) were treated with the purified PLA depolymerase (140 μg/ml) in a dialysis tube at 37°C for 48 h or left untreated, as described in Materials and Methods. Scanning electron micrographs of an untreated film at a ×1,800 magnification (A) and an enzyme-treated film at magnifications of ×1,800 (B) and ×180 (C) are shown.
Effect of pH and temperature on the PLA-degrading activity of the purified enzyme.
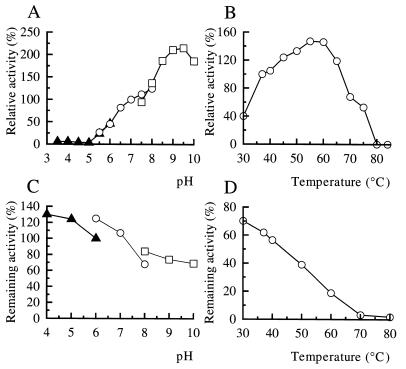
Degradation of the emulsified PLA by the purified enzyme was assayed under standard conditions except for pH (i.e., pH 3.5 to 10). In the pH range of >5, the PLA-degrading activity of the enzyme increased with increasing pH, reaching the maximal value at pH 9.5 (Fig. 7A). In contrast, no enzyme activity was detected under acidic conditions (pH < 5) (Fig. 7A). The PLA-degrading activity of the enzyme was assayed at temperatures of 30 to 100°C. The maximal activity of the enzyme was observed at 55 to 60°C, but no activity was detected at >80°C (Fig. 7B).
FIG. 7.
Effects of pH and temperature on the PLA-degrading activity of the purified PLA depolymerase. (A and B) Optimal pH and optimal temperature. PLA-degrading activity of the purified enzyme was assayed under standard conditions, except for pH (A) and temperature (B), as described in Materials and Methods. (C) Stability of the PLA depolymerase at different pHs. The purified enzyme preparation was kept at different pHs at 4°C for 24 h, and residual PLA-degrading activity was assayed under standard conditions. (D) Stability of the PLA-degrading activity. The purified enzyme was kept at different temperatures for 1 h, and residual activity was assayed under standard conditions. The PLA-degrading activity obtained under the standard conditions was defined as 100%. Buffers used in the assays for optimum pH (A) and pH stability (C) of the enzyme activity were 10 mM sodium acetate buffer (▴) for pH 3.5 to 6.0 (A) and for pH 4.0 to 6.0 (C), 10 mM potassium phosphate buffer (○) for pH 5.5 to 8.0 (A) and for pH 6.0 to 8.0 (C), and 10 mM Tris HCl buffer (□) for pH 7.5 to 10.0 (A) and for pH 8.0 to 10.0 (C).
Stability of the PLA depolymerase at various pHs and temperatures.
The enzyme was incubated at pHs of 4 to 10 at 37°C for 24 h, followed by an assay for residual activity under the standard conditions. As shown in Fig. 7C, the PLA depolymerase was quite stable in the whole pH range. PLA depolymerase was incubated at various temperatures between 30 and 100°C for 1 h, and residual activity was assayed. The PLA-degrading activity of the enzyme was decreased upon exposure to increasing temperature from 30 to 70°C and was abolished at temperatures of >80°C (Fig. 7D).
Substrate specificity of the PLA depolymerase and effect of various inhibitors on the purified enzyme.
The purified PLA depolymerase was tested for its hydrolytic activities for several representative substrates for protease, lipase, esterase, and poly(hydroxyalkanoate) depolymerase. As shown in Table 4, the PLA depolymerase degraded casein and fibrin, and specific activities of the caseinolysis and the fibrinolysis by the enzyme were 645 ± 28 U/mg of protein (n = 3) and approximately 2 U/mg of protein, respectively. However, the enzyme showed no significant hydrolytic activity for collagen type I, triolein, tributyrin, poly(β-hydroxybutyrate), and poly(ε-caprolactone) (Table 4). Thus, the PLA depolymerase exhibited caseinolytic and fibrinolytic activities, but it had neither lipase activity nor esterase activity for the other poly(hydroxyalkanoates) tested.
TABLE 4.
Substrate specificity of the purified PLA depolymerase
| Substrate (concna) | Hydrolysis |
|---|---|
| Casein (0.1) | + |
| Fibrin (0.3) | + |
| Collagen type I (0.1) | − |
| Triolein (0.1) | − |
| Tributyrin (0.1) | − |
| Poly(β-hydroxy)butyrate (0.1) | − |
| Poly(ε-caprolactone) (0.1) | − |
Concentrations are given as percentages (wt/vol).
The PLA-degrading and the caseinolytic activities of the purified enzyme were inhibited by diisopropyl fluorophosphate and PMSF (Table 5). However, neither of the enzyme activities was significantly affected by soybean trypsin inhibitor, N-tosyl-l-lysyl chloromethyl ketone, N-tosyl-l-phenylalanyl chloromethyl ketone, or Streptomyces subtilisin inhibitor (Table 5). These results indicated that the PLA depolymerase is a serine enzyme which was not inhibited by the specific inhibitors for trypsin-type, chymotrypsin-type, and subtilisin-type proteases. Furthermore, EDTA and EGTA did not inhibit the caseinolytic activity of the enzyme (Table 5), indicating that divalent cations, including Ca2+, may not be involved in the enzymatic activity.
TABLE 5.
Effects of various enzyme inhibitors on the PLA- and casein-degrading activities of the purified PLA depolymerasea
| Inhibitorb | Concn | Residual activity (%)
|
|
|---|---|---|---|
| PLA degrading | Caseinolytic | ||
| DFP | 1 mM | 3.7 | 10.5 |
| 10 mM | NDc | 3.0 | |
| PMSF | 1 mM | 33.4 | 69.9 |
| 10 mM | NDc | 14.6 | |
| STI | 25 μg/ml | 102 | 101 |
| TLCK | 1 mM | 107 | 81.3 |
| 10 mM | NDd | 83.9 | |
| TPCK | 1 mM | 101 | 95.1 |
| 10 mM | NDd | 88.8 | |
| SSI | 113 μg/mle | 99.0 | 106 |
| EDTA | 5 mM | NDd | 103 |
| EGTA | 5 mM | NDd | 107 |
The purified enzyme (3.4 × 10−3 U, 0.13 μg of protein) was incubated with 0.1% (wt/vol) PLA emulsion or with 1% (wt/vol) casein in the reaction mixture (50 μl) containing inhibitor, as described in Materials and Methods. The activity obtained without inhibitor was taken as 100%.
DFP, diisopropyl fluorophosphate; STI, soybean trypsin inhibitor; TLCK, N-tosyl-l-lysyl chloromethyl ketone; TPCK, N-tosyl-l-phenylalanyl chloromethyl ketone; SSI, Streptomyces subtilisin inhibitor.
ND, not determined because of aggregation of the inhibitors, which hindered the assay for decrease in turbidity of PLA emulsion.
ND, not determined because of aggregation of emulsified PLA in the presence of the inhibitors.
SSI inhibited >96% of proteinase K activity at this concentration.
It has been shown that proteinase K from T. album, the lipase from R. delemer, and the polyester polyurethane-degrading enzyme from C. acidovorans strain TB-35 have PLA-degrading activity (1, 5, 22, 28). The PLA depolymerase of K104-1 resembles proteinase K with respect to their capability of hydrolyzing high-molecular-weight PLA (Mn, 200,000) in solid film and their properties as alkaline serine proteases. However, the PLA-degrading and the caseinolytic activities of the PLA depolymerase were not susceptible to Streptomyces subtilisin inhibitor (Table 5), which inhibits the subtilisin family enzymes (CLAN SB-S8), including proteinase K. As shown in Table 4, the PLA depolymerase hydrolyzed neither triolein (a representative substrate for lipase) nor tributyrin (a substrate for esterase), which was the different characteristic of the lipase from R. delemer (28) and the polyester polyurethane-degrading enzyme from C. acidovorans strain TB-35 (1). Furthermore, the lipase from R. delemer and the polyester polyurethane-degrading enzyme from C. acidovorans strain TB-35 did not hydrolyze high-molecular-weight PLA (1, 28). Thus, the PLA depolymerase from K104-1 is a novel PLA-degrading serine enzyme.
In this study, PLA-degrading Amycolatopsis sp. strains K104-1 and K104-2 were isolated by screening soil samples on the PLA-emulsified mineral agar medium, and a unique extracellular PLA depolymerase was purified and characterized. It should be noted that K104-1 and K104-2 consumed 0.1% PLA within 8 days to propagate in the mineral medium (Fig. 2), indicating that the strains efficiently assimilated degradation products of PLA. The PLA-degrading Amycolatopsis sp. strain 3118, isolated by Ikura and Kudo, also degraded PLA film to grow in a minimal medium (10). In contrast, as reported by Pranamuda et al. (21), Amycolatopsis sp. strain HT32 did not grow in a PLA-emulsified mineral medium, although it efficiently degraded PLA. Recently, Pranamuda and Tokiwa tested various strains of Amycolatopsis for clear-zone-forming ability on a PLA medium and found that 15 out of 25 strains degraded emulsified PLA in the medium, while only a few of the PLA-degrading strains assimilated degradation products of PLA (22). Taken together, the capability of assimilating PLA would be limited to certain strains in the genus Amycolatopsis, although PLA-degrading activity may be widely distributed in the genus. A single PLA depolymerase was purified from the culture supernatant of K104-1 based on the assay for the ability to decrease turbidity of PLA emulsion. The most prominent characteristic of the purified enzyme was the ability to degrade high-molecular-weight PLA to lactic acid. The PLA depolymerase produced only lactic acid from PLA (Fig. 5), suggesting an exo-type mode of action for the enzyme. However, we cannot rule out the involvement of another enzyme(s) in the degradation of PLA by K104-1, because the culture supernatant of K104-1 produced lactate oligomers as well as a lactate monomer from PLA (Fig. 3). Therefore, it would be interesting to explore other PLA-degrading enzyme(s) in the culture supernatant of K104-1 using various enzymatic substrates, such as synthetic substrates for protease, esterase, and lipase. Further characterization of the PLA depolymerase and molecular cloning of the gene encoding the enzyme are in progress.
ACKNOWLEDGMENTS
This work was done as a part of the Research and Development Project for Recycling of Food Containers and Packaging supported by The Japanese Society for Food Science and Technology.
We are very grateful to Yoko Takahashi of Kitasato Institute (Tokyo, Japan) for useful suggestions on the taxonomy of the soil isolates. We thank Tsuruji Sato for his cooperation in scanning electron microscopy.
REFERENCES
- 1.Akutsu H, Nakajima-Kambe T, Nomura N, Nakahara T. Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl Environ Microbiol. 1998;64:62–67. doi: 10.1128/aem.64.1.62-67.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrup T, Mullertz S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952;40:346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- 3.Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. pp. 460–462. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Edwards U, Rogall T, Bloecker H, Emde M, Boetter E. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuzaki H, Yoshida M, Asano M, Kumakura M. Synthesis of copoly(d,l-lactic acid) with relatively low molecular weight and in vitro degradation. Eur Polym J. 1989;25:1019–1026. [Google Scholar]
- 7.Gordon R E, Barnett D A, Handerhan J E, Pang C H. Nocardia coeliaca, Nocardia autotrophica, and the nocardin strain. Int J Syst Bacteriol. 1974;24:54–63. [Google Scholar]
- 8.Hagihara B, Matsubara H, Nakai M, Okunuki K. Crystalline bacterial proteinase. I. Preparation of crystalline proteinase of Bacillus subtilis. J Biochem. 1958;45:185–194. [Google Scholar]
- 9.Henssen A, Kothe H W, Kroppenstedt R M. Transfer of Pseudonocardia azurea and “Pseudonocardia fastidiosa” to the genus Amycolatopsis, with emended species description. Int J Syst Bacteriol. 1987;37:292–295. [Google Scholar]
- 10.Ikura Y, Kudo T. Isolation of a microorganism capable of degrading poly-(l-lactide) J Gen Appl Microbiol. 1999;45:247–251. doi: 10.2323/jgam.45.247. [DOI] [PubMed] [Google Scholar]
- 11.Itaevaara M, Vikman M, Venelampi O. Window composting of biodegradable packaging materials. Compost Sci Util. 1997;5:84–92. [Google Scholar]
- 12.Klimova O V, Borukhov S I, Solovyeva N I, Balaevskaya T O, Strongin A Y. The isolation and properties of collagenolytic proteases from crab hepatopancreas. Biochem Biophys Res Commun. 1990;166:1411–1420. doi: 10.1016/0006-291x(90)91024-m. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lechevalier H A. Nocardioforms. In: Holt J G, et al., editors. Bergey's manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1458–1579. [Google Scholar]
- 15.Lechevalier P M, Lechevalier H A. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol. 1970;20:435–444. [Google Scholar]
- 16.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinyl difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 17.Minnikin D E, Alshamaony L, Goodfellow M. Differentiation of Mycobacterium, Nocardia, and related taxa by thin-layer chromatographic analysis of whole-organism methanolysates. J Gen Microbiol. 1975;88:200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- 18.Minnikin D E, O'Donnell A G, Goodfellow M, Alderson G, Athalye M, Schaal A, Parlett J H. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods. 1984;2:233–241. [Google Scholar]
- 19.Nakajima N, Mihara H, Sumi H. Characterization of potent fibrinolytic enzymes in earthworm, Lumbricus rubellus. Biosci Biotechnol Biochem. 1993;57:1726–1730. doi: 10.1271/bbb.57.1726. [DOI] [PubMed] [Google Scholar]
- 20.Nishida H, Tokiwa Y. Distribution of poly(β-hydroxybutyrate) and poly(ε-caprolactone) aerobic degrading microorganisms in different environments. J Environ Polym Degrad. 1993;1:227–233. [Google Scholar]
- 21.Pranamuda H, Tokiwa Y, Tanaka H. Polylactide degradation by an Amycolatopsis sp. Appl Environ Microbiol. 1997;63:1637–1640. doi: 10.1128/aem.63.4.1637-1640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranamuda H, Tokiwa Y. Degradation of poly(l-lactide) by strains belonging to genus Amycolatopsis. Biotechnol Lett. 1999;21:901–905. [Google Scholar]
- 23.Reeve S M, McCarthy S P, Downey M J, Gross R A. Polylactide stereochemistry: effect on enzymatic degradability. Macromolecules. 1994;27:825–831. [Google Scholar]
- 24.Sasagawa Y, Kamio Y, Matsubara Y, Matsubara Y, Suzuki K, Kojima H, Izaki K. Purification and properties of collagenase from Cytophaga sp. L43–1 strain. Biosci Biotechnol Biochem. 1993;57:1894–1898. doi: 10.1271/bbb.57.1894. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Murao S. Complex formation of microbial alkaline protease inhibitor (S-SI) with subtilisin BPN′ and its properties. Agric Biol Chem. 1974;38:2227–2233. [Google Scholar]
- 26.Staneck J L, Roberts G D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol. 1974;28:226–231. doi: 10.1128/am.28.2.226-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomita K, Kuroki Y, Nagai K. Isolation of thermophiles degrading poly(l-lactic acid) J Biosci Bioeng. 1999;87:752–755. doi: 10.1016/s1389-1723(99)80148-0. [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Li S M, Roussos S, Vert M. Screening of microorganisms for biodegradation of poly(lactic acid) and lactic acid-containing polymers. Appl Environ Microbiol. 1996;62:2392–2397. doi: 10.1128/aem.62.7.2393-2397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams D F. Enzymic hydrolysis of polylactic acid. Eng Med. 1981;10:5–7. [Google Scholar]