Abstract
BACKGROUND
Type 2 diabetes mellitus (T2DM) is a risk factor for nonalcoholic fatty liver disease (NAFLD).
AIM
To determine the prevalence and clinical correlates of NAFLD in a large cohort of patients with T2DM.
METHODS
Four hundred thirty-seven participants with T2DM who consulted at Meijo Hospital from April 2019 to September 2020 and underwent computed tomography (CT) were assessed. The mean age was 74 ± 13 years, and 269 were men. Hepatic attenuation minus splenic attenuation (CTL−S) less than 1 Hounsfield unit was considered fatty liver. NAFLD was defined as fatty liver in the absence of significant alcohol consumption and hepatitis virus infection. A multiple logistic regression was used to assess the independent factors associated with NAFLD.
RESULTS
NAFLD was identified in 25.2% of the participants. Young age (odds ratio [OR] = −0.945; 95% confidence interval [CI]: 0.922–0.969), higher hemoglobin levels (OR = 1.501, 95%CI: 1.278–1.764), lower high-density lipoprotein (HDL) cholesterol levels (OR = 0.971, 95%CI: 0.953–0.989), and the absence of dialysis (OR = 0.109, 95%CI: 0.014–0.856) were independent predictors of NAFLD.
CONCLUSION
NAFLD was detected with CT in 25.2% of the participants. NAFLD was associated with younger age, higher hemoglobin levels, lower HDL cholesterol levels, and an absence of dialysis.
Keywords: Age, Computed tomography, Dialysis, Hemoglobin, Nonalcoholic fatty liver disease, Type 2 diabetes mellitus
Core Tip: Type 2 diabetes mellitus (T2DM) is a risk factor for nonalcoholic fatty liver disease (NAFLD). The prevalence of NAFLD by computed tomography (CT) has been reported in a few studies. The clinical correlates of NAFLD are often ambiguous. We determined the prevalence and clinical correlates of NAFLD determined by CT in a large cohort of patients with T2DM. The prevalence of NAFLD by CT was 25.2%. NAFLD was associated with younger age, higher hemoglobin levels, lower HDL cholesterol levels, and an absence of dialysis.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) frequently coexists with type 2 diabetes mellitus (T2DM). Both synergistically increase adverse outcomes[1,2]. NAFLD, T2DM, and obesity are epidemiologically correlated, but their causal interrelationships remain incompletely understood. Liu et al[3] proposed the hypothesis of disease subphenotyping in which genetically-driven NAFLD promotes T2DM and central obesity but protects against overall obesity. In contrast, genetically-driven T2DM and obesity increase the NAFLD risk.
A meta-analysis showed that the prevalence of NAFLD in patients with T2DM was 56% with ultrasonography (US) and proton magnetic resonance spectroscopy (MRI)[4]. The prevalence of NAFLD in patients with T2DM was highest in Europe (68%), but varies widely depending on the population. Three studies from Japan using US reported that the prevalence of NAFLD was 31%, 69%, and 61%[5-7]. The sensitivity of diagnosing NAFLD varies with the method. The prevalence of NAFLD in patients with T2DM detected by computed tomography (CT) was 10% in the United States[8], 22% in Turkey[9], and 27% in Japan[10].
In patients with T2DM, NAFLD is associated with an increased risk of overall death[11] but not with liver-related deaths[8]. Meanwhile, in NAFLD patients, T2DM is associated with advanced liver fibrosis[12] and increased mortality related to liver-related deaths[13]. NAFLD in patients with T2DM is associated with an increased risk of cardiovascular disease[7,9,10,14]. However, some reports have denied this association[8,15-17]. A positive association between NAFLD and nephropathy in patients with T2DM has been reported in some studies[18,19], while others did not find an association[20-22]. The association of NAFLD with cardiovascular risk and chronic kidney disease in the general population was reported to start in childhood[23,24].
We studied the prevalence of NAFLD in patients with T2DM in our hospital using CT and determined the factors associated with NAFLD in patients with T2DM.
MATERIALS AND METHODS
Study population
Data of patients with T2DM were retrieved from the hospital database. There were 724 Japanese diabetic patients who consulted at the Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital from April 2019 to September 2020. We excluded participants who had chronic hepatitis B (n = 6) and C (n = 28) infections, no assessment of hepatitis B surface antigen and hepatitis C virus antibody (n = 105), and an alcohol intake ≥ 20 g/d (n = 33). We also excluded 115 participants who did not have an abdominal CT examination; thus, 437 participants (269 men and 168 women) were included in the analysis (Figure 1). The mean age was 74 ± 13 years. There were 322 patients treated with oral hypoglycemic agents, 32 with insulin, one with a glucagon-like peptide-1 receptor agonist, and 82 with diet and exercise. All patients had more than a year with T2DM.
Figure 1.
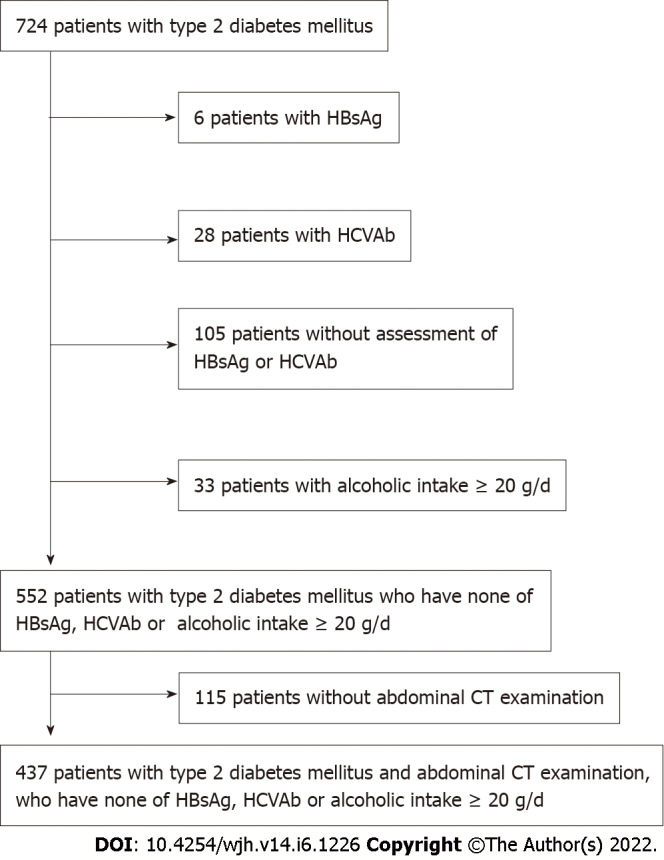
The participants in the present study consisted of 724 Japanese type 2 diabetic patients who consulted at Meijo Hospital from April 2019 to September 2020. We excluded participants who had chronic hepatitis B (n = 6) and C (n = 28) infection, no assessment of hepatitis B surface antigen (HBsAg) and hepatitis C virus antibody (n = 105), and alcohol intake ≥ 20 g/d (n = 33). We also excluded 115 participants with no abdominal computed tomography examinations. The final analysis included 437 participants (269 men and 168 women). HCVAb: Hepatitis C virus antibody; HBsAg: Hepatitis B surface antigen; CT: Computed tomography.
The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital (Approval No. 166). Written informed consent was waived because the data were analyzed anonymously based on information stored in the hospital database.
Abdominal CT examinations
A non-enhanced CT was performed using either a 16-section multidetector scanner (Aquilion 16; Canon Medical Systems, Tochigi, Japan), a 64-section multidetector scanner (Aquilion 64; Canon Medical Systems, Tochigi, Japan), or an 80-section multidetector scanner (Aquilion Prime SP/iEdition; Canon Medical Systems, Tochigi, Japan). The CT indications were to screen for diseases in the chest and abdomen in 430 patients. The CT was performed to investigate liver diseases in seven patients. Nine and three regions of interest were positioned at the liver or spleen, respectively, to avoid macroscopic vessels. Median hepatic or splenic attenuation values were obtained. Hepatic attenuation minus splenic attenuation (CTL−S) less than 1 Hounsfield unit was considered fatty liver[25].
Statistical analysis
Student's t-test was used to analyze differences in continuous variables between two groups. Categorical variables were compared with the Chi-squared or Fisher's exact test. Linear regression was performed to assess the relationship between two variables. Multiple logistic regression was performed to determine the independent factors associated with the presence of NAFLD. P values < 0.05 were considered significant. All analyses were performed with StatFlex version 6.0 for Windows (StatFlex, Osaka, Japan).
RESULTS
Fatty liver was detected in 110 of 437 patients (25.2%). It was significantly associated with male gender (P = 0.005), younger age (P < 0.001), greater height (P < 0.001) and weight (P < 0.001), higher body mass index (BMI) (P < 0.001), blood parameters [higher white blood cell counts (P = 0.021) and higher hemoglobin (P < 0.001)], altered liver function tests [higher albumin (P < 0.001), higher total bilirubin (P = 0.015), aspartate aminotransferase (P = 0.014), alanine aminotransferase (P < 0.001), and gamma glutamyl transpeptidase (P < 0.001)], kidney function [lower creatinine (P = 0.012) and higher estimated glomerular filtration rate (eGFR) (P = 0.004)], metabolic status [higher total cholesterol (P = 0.012), lower high density lipoprotein (HDL) cholesterol (P = 0.039), higher triglycerides (P < 0.001), and higher low density lipoprotein (LDL) cholesterol (P = 0.001)], a lower fibrosis-4 (FIB-4) index (P = 0.005), and non-hypertensive (P = 0.013) and non-dialysis patients (P = 0.003) (Table 1). Fatty liver also tended to be associated with higher HbA1c (P = 0.066).
Table 1.
Demographics of subjects
|
Variables
|
ALL (n = 437)
|
Fatty liver (n = 110)
|
Non-fatty liver (n = 327)
|
P
value
|
| Gender (Male/Female) | 269/168 | 80/30 | 189/138 | 0.005 |
| Age (yr) | 74.0 ± 13.0 | 64.7 ± 13.0 | 77.1 ± 11.0 | < 0.001 |
| Height (cm) | 159.4 ± 10.2 | 163.2 ± 10.0 | 158.2 ± 9.9 | < 0.001 |
| Body weight (kg) | 61.3 ± 14.5 | 70.0 ± 16.6 | 58.5 ± 12.5 | < 0.001 |
| BMI (kg/m2) | 24.0 ± 4.9 | 26.0 ± 4.7 | 23.3 ± 4.7 | < 0.001 |
| HbA1c (%) | 7.0 ± 1.2 | 7.2 ± 1.2 | 6.9 ± 1.2 | 0.066 |
| Blood glucose (mg/dl) | 164 ± 80 | 170 ± 116 | 161 ± 63 | 0.297 |
| White blood cells (/μL) | 6618 ± 2299 | 7056 ± 1649 | 6470 ± 2463 | 0.021 |
| Hemoglobin (g/dL) | 12.6 ± 2.4 | 14.2 ± 2.1 | 12.0 ± 2.2 | < 0.001 |
| Platelets (104/μL) | 20.7 ± 7.0 | 20.4 ± 5.5 | 20.8 ± 7.4 | 0.615 |
| Prothrombin time (%) | 89.6 ± 22.3 | 89.2 ± 23.6 | 89.7 ± 22.1 | 0.895 |
| Albumin (g/dL) | 3.7 ± 0.7 | 3.9 ± 0.6 | 3.6 ± 0.6 | < 0.001 |
| Total bilirubin (mg/dL) | 0.76 ± 0.50 | 0.86 ± 0.49 | 0.72 ± 0.49 | 0.015 |
| ALP (U/L) | 266 ± 156 | 247 ± 80 | 272 ± 176 | 0.182 |
| AST (U/L) | 25.3 ± 22.8 | 29.0 ± 20.2 | 23.0 ± 23.4 | 0.014 |
| ALT (U/L) | 23.5 ± 20.8 | 35.0 ± 27.4 | 19.0 ± 16.1 | < 0.001 |
| GGT (U/L) | 46.2 ± 63.1 | 68.0 ± 74.4 | 38.0 ± 56.6 | < 0.001 |
| Creatinine (mg/dL) | 1.71 ± 2.10 | 1.27 ± 1.58 | 1.85 ± 2.22 | 0.012 |
| eGFR (mL/min) | 52.0 ± 29.2 | 58.9 ± 21.9 | 49.6 ± 30.9 | 0.004 |
| Total cholesterol (mg/dL) | 181 ± 50 | 193 ± 64 | 177 ± 43 | 0.012 |
| HDL cholesterol (mg/dL) | 53 ± 17 | 50 ± 15 | 54 ± 17 | 0.039 |
| Triglyceride (mg/dL) | 160 ± 179 | 228 ± 299 | 134 ± 87 | < 0.001 |
| LDL cholesterol (mg/dL) | 101 ± 36 | 111 ± 40 | 96 ± 33 | 0.001 |
| FIB-4 index | 2.20 ± 1.56 | 1.84 ± 1.08 | 2.32 ± 1.67 | 0.005 |
| Cirrhosis (%) | 8 (1.8) | 1 (0.9) | 7 (2.1) | 0.405 |
| Hepatocellular carcinoma (%) | 7 (1.6) | 1 (0.9) | 6 (1.8) | 0.504 |
| Cerebrovascular accident (%) | 45 (10) | 9 (8.2) | 36 (11) | 0.399 |
| Cardiovasculart disease (%) | 137 (31) | 29 (26) | 108 (33) | 0.193 |
| Dyslipidemia (%) | 205 (47) | 53 (48) | 152 (46) | 0.758 |
| Hypertension (%) | 270 (62) | 57 (51) | 213 (65) | 0.013 |
| Dialysis (%) | 45 (10) | 3 (2.7) | 42 (12) | 0.003 |
Values are expessed as mean ± SD. Statistical analysis are conducted using the chi-squared test, Fisher’s exact test, or Student’s t test. ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanin aminotransferase; BMI: Body mass index; GGT: Gamma glutamyl transpeptidase; eGFR: Estimated glomelular filtration rate; HDL cholesterol: High density lipoprotein cholesterol; LDL cholesterol: Low density lipoprotein cholesterol; FIB-4 index: Fibrosis-4 index.
Multivariate logistic regression was used to elucidate the independent factors associated with fatty liver. Gender, age, BMI, HbA1c, white blood cell count, hemoglobin, albumin, eGFR, total cholesterol, HDL cholesterol, triglyceride, LDL cholesterol, hypertension, and dialysis therapy were analyzed. Age (P < 0.001), hemoglobin level (P < 0.001), HDL cholesterol level (P = 0.002), and absence of dialysis (P = 0.035) were independent factors associated with fatty liver (Table 2).
Table 2.
Multivariate analysis for factors associated with fatty liver
|
Variables
|
Odds ratio
|
95% confidence interval
|
P
value
|
| Age | 0.945 | 0.922-0.969 | < 0.001 |
| Hemoglobin | 1.501 | 1.278-1.764 | < 0.001 |
| HDL cholesterol | 0.971 | 0.953-0.989 | 0.002 |
| Dialysis | 0.109 | 0.014-0.856 | 0.035 |
HDL cholesterol: High density lipoprotein cholesterol.
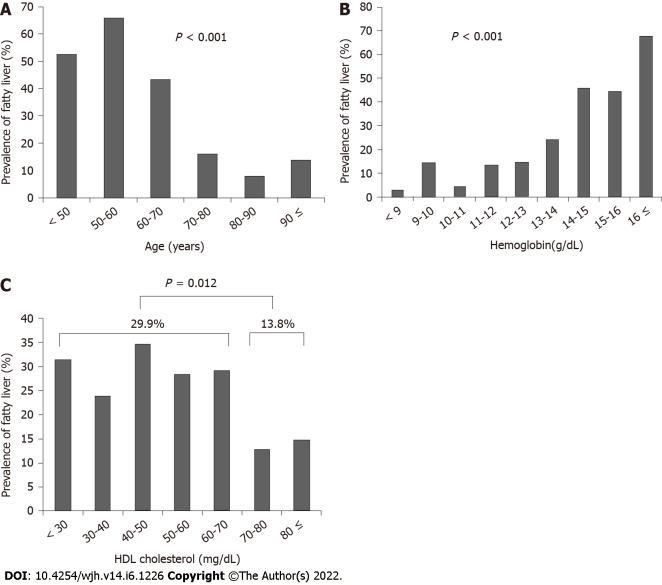
The patients were stratified according to age, hemoglobin level, and HDL cholesterol level, and the association with fatty liver was assessed. The prevalence of fatty liver significantly decreased with increasing age (P < 0.001) (Figure 2A), significantly increased as hemoglobin levels increased (P < 0.001) (Figure 2B), and was significantly higher in patients with HDL cholesterol < 70 mg/dL (29.9%) than patients with HDL cholesterol ≥ 70 mg/dL (13.8%) (P = 0.012) (Figure 2C).
Figure 2.
The patients were stratified according to age, hemoglobin level, and high-density lipoprotein cholesterol level, and the association with fatty liver was assessed. A: The prevalence of fatty liver significantly decreased with age (P < 0.001); B: The prevalence of fatty liver significantly increased as hemoglobin increased (P < 0.001); C: The prevalence of fatty liver was significantly higher in patients with high-density lipoprotein cholesterol of < 70 mg/dL (29.9%) than those with HDL cholesterol ≥ 70 mg/dL (13.8%) (P = 0.012). HDL: High-density lipoprotein
DISCUSSION
The prevalence of NAFLD in patients with T2DM detected by CT was 25.2%. NAFLD was associated with younger age, higher hemoglobin levels, lower HDL cholesterol levels, and the absence of dialysis.
A previous meta-analysis found that the prevalence of NAFLD in patients with T2DM was 56% with US or proton magnetic resonance spectroscopy[4]. The prevalence of NAFLD in patients with T2DM detected by CT was 10% in the United States[8], 22% in Turkey[9], and 27% in Japan[10]. Lee et al[26] reported that the sensitivity of US (92%) for detecting more than 30% hepatic steatosis was higher than CT (64%). The prevalence of NAFLD in this study is comparable to the reported prevalence in patients with T2DM diagnosed by CT in Japan. In a preliminary study of 179 subjects who attended health screening in our hospital (120 males and 59 females; 53.7 ± 10.8 years), CT detected fatty liver in 40 (22%). Thus, the prevalence of NAFLD in T2DM is higher than in the general population considering older age.
The prevalence of NAFLD significantly decreased with increasing age in this study. In contrast, Targher et al[14] reported that the prevalence of NAFLD detected by US in patients with T2DM increased with age; 65% among patients aged 40 to 59 and 75% among those aged 60 and older. The difference in this study compared to Targher's regarding the association of NAFLD and age may be attributed to the difference in the mean age of the participants (74 vs 64 years, respectively). By combining Targher's results and this study, it is suggested that the prevalence of NAFLD increases with age until about 60 years (as in Targher's study) and then decreases with age, as shown in this study. An "inverted U curve," in which the prevalence of NAFLD reaches a peak in late adulthood and decreases afterward has been reported in the general population[27-29]. However, a meta-analysis observed a consistent increase in the NAFLD prevalence across all age groups[30].
Poor nutritional status is more common in older people[31]. Age-related changes in appetite, health problems, and social problems predispose older adults to less food intake. Poor nutritional status in older people is also common in patients with T2DM[32]. The decreasing prevalence of NAFLD with age shown in the present study may be attributed to poor nutritional status. Age significantly negatively correlated with BMI in this study (r = −0.32, P < 0.001, data not shown). A high BMI is associated with NAFLD[28] and a low BMI is associated with poor nutritional status[33]. BMI was associated with NAFLD in this study by univariate analysis but not by multivariate analysis.
In this study, lower hemoglobin values were associated with a lower prevalence of NAFLD. Anemia is common in the elderly and an indicator of poor nutritional status[34]. Therefore, the lower prevalence of NAFLD in patients with lower hemoglobin values may be attributed to the poor nutritional status of these patients. Hemoglobin values also significantly positively correlated with BMI (r = 0.21, P < 0.001, data not shown).
Higher HDL cholesterol values were associated with a lower prevalence of NAFLD. This association between HDL cholesterol and NAFLD has previously been reported[28].
Dialysis treatment was associated with a lower prevalence of NAFLD. Diabetic nephropathy affects approximately 25% of patients with T2DM and is the leading cause of renal failure. Two studies reported that NAFLD is inversely associated with nephropathy in patients with T2DM, similar to this study[20,21]; however, some studies have reported a positive or no association[18,19,22]. The difference in the studied populations may cause this discrepancy. When the study population was not limited to patients with T2DM, a meta-analysis showed a positive association between NAFLD and chronic kidney disease[35]. However, in patients with T2DM, this association is still ambiguous.
This study did not find an association between NAFLD and cardiovascular disease. This association has been previously reported[7,9,10,14]; however, other studies deny it[8,15-17]. Higher HDL cholesterol values associated with a lower prevalence of NAFLD in this study reduce the risk of cardiovascular disease[36]. Thus a follow-up study may reveal an association between NAFLD and cardiovascular disease.
In this study, the number of patients with cirrhosis or hepatocellular carcinoma (HCC) was small, and cirrhosis and HCC were not associated with NAFLD. In a follow-up study of patients with T2DM (mean, 10.9 years), Adams et al[11] reported that 5 of 116 patients with NAFLD and none of 221 patients without NAFLD died from liver-related causes. Dunn et al[8] reported that NAFLD was not associated with liver-related outcomes (transplant, HCC, or encephalopathy) in patients with T2DM in a five-year retrospective cohort study. Further studies are needed to assess these associations.
There are three limitations to this study. First, it is a cross-sectional study. There may be a question of whether NAFLD decreases with age. Poor nutritional status in older people may be one reason why NAFLD decreases with age. It is also possible that we assessed a certain subpopulation of T2DM with a low risk of NAFLD and survival until older age, while the patients with a high risk of NAFLD dropped out until older age because of complications. Thus, the temporal association of NAFLD with the factors assessed in this study has to be clarified by prospective cohort studies. Second, fatty liver was diagnosed by CT in this study. The sensitivity of MRI, US, and CT for detecting a fatty liver of 5% or higher is 77%-80%, 53%-62%, and 50% compared with liver biopsy[37]. However, a liver biopsy is invasive and has a risk of severe complications. Thus noninvasive modalities, such as US, CT, and MRI, have been commonly used to detect fatty liver. MRI is expensive and scarce. The disadvantage of US is its subjective nature. The high liver iron content increases CT Hounsfield units and may obliterate the diagnosis of fatty liver. However, CT is widely available in Japan, and the diagnosis is objective. Thus, CT is a promising modality for diagnosing fatty liver. Third, the present study was performed in a single hospital. The prevalence found has to be reevaluated in multicenter studies.
CONCLUSION
The prevalence of NAFLD in patients with T2DM detected by CT was 25.2%. NAFLD was associated with age, hemoglobin level, HDL cholesterol level, and the absence of dialysis treatment.
ARTICLE HIGHLIGHTS
Research background
Type 2 diabetes mellitus (T2DM) is an established risk factor for the development of nonalcoholic fatty liver disease (NAFLD). Both synergistically increase adverse outcomes.
Research motivation
The prevalence of NAFLD assessed by computed tomography (CT) was reported only in a few studies. The clinical correlates of NAFLD are often ambiguous.
Research objectives
To determine the prevalence and clinical correlates of NAFLD assessed by CT in a large cohort of T2DM patients.
Research methods
Four hundred thirty-seven participants with T2DM who consulted at Meijo Hospital from April 2019 to September 2020 and underwent CT were assessed.
Research results
The prevalence of NAFLD as detected by CT was 25.2% in T2DM patients, and NAFLD was associated with a younger age, higher hemoglobin levels, lower high density lipoprotein cholesterol levels, and absence of dialysis treatment.
Research conclusions
The prevalence of NAFLD in T2DM is higher than in the general population considering older age and decreases with age.
Research perspectives
The association of NAFLD with age has to be clarified by prospective cohort studies.
Footnotes
Institutional review board statement: The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital (Approval No. 166).
Informed consent statement: Written informed consent was waived because the data were analyzed anonymously based on information in the hospital database.
Conflict-of-interest statement: Kentaro Yoshioka is a consultant to Sanwa Kagaku Kenkyusho Co., Ltd. and received a research grant from Sumitomo Dainippon Pharma Co., Ltd. and scholarship grants from Otsuka Pharmaceutical Co., Ltd., and AbbVie GK. The remaining authors declare no conflict of interest.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 10, 2022
First decision: March 8, 2022
Article in press: May 14, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Di Sessa A, Italy; Ulasoglu C, Turkey; Zhang LL, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Ryosuke Yamane, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Kentaro Yoshioka, Center for Liver Diseases, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan. kyoshiok@fujita-hu.ac.jp.
Kazuhiko Hayashi, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Yuko Shimizu, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Yuki Ito, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Komei Matsushita, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Michiyo Yoshizaki, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Go Kajikawa, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Taro Mizutani, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Atsuko Watarai, Department of Diabetes and Endocrinology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Kosuke Tachi, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Hidemi Goto, Department of Gastroenterology and Hepatology, Federation of National Public Service Personnel Mutual Aid Associations Meijo Hospital, Nagoya 460-0001, Aichi, Japan.
Data sharing statement
No additional data are available.
References
- 1.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shima T, Uto H, Ueki K, Takamura T, Kohgo Y, Kawata S, Yasui K, Park H, Nakamura N, Nakatou T, Tanaka N, Umemura A, Mizuno M, Tanaka J, Okanoue T. Clinicopathological features of liver injury in patients with type 2 diabetes mellitus and comparative study of histologically proven nonalcoholic fatty liver diseases with or without type 2 diabetes mellitus. J Gastroenterol. 2013;48:515–525. doi: 10.1007/s00535-012-0653-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, Pique-Regi R, Dong XC, Chen YE, Willer C, Liu W. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73:263–276. doi: 10.1016/j.jhep.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda K, Seki Y, Ichihi M, Okada T, Hirata A, Kogita S, Sawai Y, Igura T, Tsugawa M, Imai Y. Usefulness of ultrasonographic estimation of preperitoneal and subcutaneous fat thickness in the diagnosis of nonalcoholic fatty liver disease in diabetic patients. J Med Ultrason (2001) 2015;42:357–363. doi: 10.1007/s10396-015-0615-7. [DOI] [PubMed] [Google Scholar]
- 6.Miyasato M, Murase-Mishiba Y, Bessho M, Miyawaki M, Imbe H, Tsutsumi C, Tanimoto K, Imagawa A, Terasaki J, Hanafusa T. The cytokeratin-18 fragment level as a biomarker of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Clin Chim Acta. 2014;433:184–189. doi: 10.1016/j.cca.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi Y, Ito H, Komatsu Y, Oshikiri K, Antoku S, Abe M, Mifune M, Togane M. Non-alcoholic fatty liver disease is an independent predictor for macroangiopathy in Japanese type 2 diabetic patients: a cross-sectional study. Intern Med. 2012;51:1667–1675. doi: 10.2169/internalmedicine.51.7307. [DOI] [PubMed] [Google Scholar]
- 8.Dunn MA, Behari J, Rogal SS, O'Connell MR, Furlan A, Aghayev A, Gumus S, Saul MI, Bae KT. Hepatic steatosis in diabetic patients does not predict adverse liver-related or cardiovascular outcomes. Liver Int. 2013;33:1575–1582. doi: 10.1111/liv.12285. [DOI] [PubMed] [Google Scholar]
- 9.Idilman IS, Akata D, Hazirolan T, Doganay Erdogan B, Aytemir K, Karcaaltincaba M. Nonalcoholic fatty liver disease is associated with significant coronary artery disease in type 2 diabetic patients: a computed tomography angiography study 2: J Diabetes. 2015;7:279–286. doi: 10.1111/1753-0407.12172. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa K, Miyoshi T, Osawa K, Miki T, Toda H, Ejiri K, Yoshida M, Nanba Y, Nakamura K, Morita H, Ito H. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: a prospective cohort study. Cardiovasc Diabetol. 2021;20:8. doi: 10.1186/s12933-020-01192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–1229, 1229.e1. doi: 10.1016/j.cgh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 15.Cakır E, Ozbek M, Colak N, Cakal E, Delıbaşi T. Is NAFLD an independent risk factor for increased IMT in T2DM? Minerva Endocrinol. 2012;37:187–193. [PubMed] [Google Scholar]
- 16.Coracina A, Gaiani S, Cosma A, Pellizzari P, Pizzi C, de Kreutzenberg S, Cecchet D, Sacerdoti D, Tessari P. No association between the degree of liver steatosis and early signs of vasculopathy in T2DM. Nutr Metab Cardiovasc Dis. 2012;22:e11–e12. doi: 10.1016/j.numecd.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Petit JM, Guiu B, Terriat B, Loffroy R, Robin I, Petit V, Bouillet B, Brindisi MC, Duvillard L, Hillon P, Cercueil JP, Verges B. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:4103–4106. doi: 10.1210/jc.2009-0541. [DOI] [PubMed] [Google Scholar]
- 18.Casoinic F, Sâmpelean D, Bădău C, Prună L. Nonalcoholic fatty liver disease--a risk factor for microalbuminuria in type 2 diabetic patients. Rom J Intern Med. 2009;47:55–59. [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444–450. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim BY, Jung CH, Mok JO, Kang SK, Kim CH. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2014;5:170–175. doi: 10.1111/jdi.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv WS, Sun RX, Gao YY, Wen JP, Pan RF, Li L, Wang J, Xian YX, Cao CX, Zheng M. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol. 2013;19:3134–3142. doi: 10.3748/wjg.v19.i20.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan YT, Zhang C, Li L, Bi CS, Song X, Zhang ST. Non-alcoholic fatty liver disease is not related to the incidence of diabetic nephropathy in Type 2 Diabetes. Int J Mol Sci. 2012;13:14698–14706. doi: 10.3390/ijms131114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Sessa A, Umano GR, Miraglia Del Giudice E. The Association between Non-Alcoholic Fatty Liver Disease and Cardiovascular Risk in Children. Children (Basel) 2017;4 doi: 10.3390/children4070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Sessa A, Guarino S, Melone R, De Simone RF, Marzuillo P, Miraglia Del Giudice E. Relationship between nonalcoholic fatty liver disease and chronic kidney disease could start in childhood. World J Gastroenterol. 2021;27:5793–5795. doi: 10.3748/wjg.v27.i34.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, Lee SG, Yu ES. Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology. 2011;258:760–766. doi: 10.1148/radiol.10101233. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Alqahtani SA, Schattenberg JM. NAFLD in the Elderly. Clin Interv Aging. 2021;16:1633–1649. doi: 10.2147/CIA.S295524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 29.Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. doi: 10.1186/s12876-019-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 31.Volkert D. Malnutrition in older adults - urgent need for action: a plea for improving the nutritional situation of older adults. Gerontology. 2013;59:328–333. doi: 10.1159/000346142. [DOI] [PubMed] [Google Scholar]
- 32.Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients. 2020;12 doi: 10.3390/nu12113367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda K, Ishida Y, Nonogaki T, Mori N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin Nutr. 2020;39:180–184. doi: 10.1016/j.clnu.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Mitrache C, Passweg JR, Libura J, Petrikkos L, Seiler WO, Gratwohl A, Stähelin HB, Tichelli A. Anemia: an indicator for malnutrition in the elderly. Ann Hematol. 2001;80:295–298. doi: 10.1007/s002770100287. [DOI] [PubMed] [Google Scholar]
- 35.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arca M, Montali A, Valiante S, Campagna F, Pigna G, Paoletti V, Antonini R, Barillà F, Tanzilli G, Vestri A, Gaudio C. Usefulness of atherogenic dyslipidemia for predicting cardiovascular risk in patients with angiographically defined coronary artery disease. Am J Cardiol. 2007;100:1511–1516. doi: 10.1016/j.amjcard.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, Suh DJ, Kim KM, Bae MH, Lee JY, Lee SG, Yu ES. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.