Abstract
BACKGROUND
Transjugular intrahepatic portosystemic shunt (TIPS) is used to treat complications of portal hypertension, such as ascites and variceal bleeding (VB). While liver doppler ultrasound (DUS) is used to assess TIPS patency, trans-shunt venography (TSV) is the gold standard.
AIM
To determine the accuracy of DUS to assess TIPS dysfunction and for need for revision.
METHODS
Retrospective review of patients referred for TIPS revision from 2008-2021. Demographics, DUS parameters at baseline and at the DUS preceding TIPS revision, TSV data were collected. Receiver operating characteristics curves, sensitivity, specificity, performance for doppler to predict need for revision were performed. Univariate and multivariate analyses were used to predict clinical factors associated with need for TIPS revision.
RESULTS
The cohort consisted of 89 patients with cirrhosis (64% men, 76% white, 31% alcohol as etiology); median age 59 years. Indication for initial TIPS were VB (41%), refractory ascites (51%), and other (8%). TIPS was revised in 44%. On univariate analysis, factors associated with need for TIPS revision were male (P = 0.03), initial indication for TIPS (P = 0.05) and indication for revision (P = 0.01). Revision of TIPS was associated with lower mortality (26% vs 46%) and significantly lower rates of transplant (13% vs 24%; P = 0.1). In predicting need for TIPS revision, DUS has a 40% sensitivity, 45% specificity, PPV 78%, and NPV 14%. The most accurate location for shunt velocity measure was distal velocity (Area under the curve: 0.79; P = 0.0007).
CONCLUSION
DUS has poor overall sensitivity and specificity in predicting need for TIPS revision. Non-invasive methods of predicting TIPS dysfunction are needed since those needing TIPS revision had better survival.
Keywords: Transjugular intrahepatic portosystemic shunt, Doppler ultrasound, Portal hypertension
Core Tip: Transjugular intrahepatic portosystemic shunt (TIPS) is used to treat complications of portal hypertension, however methods to assess TIPS patency are highly variable. Herein, we present a retrospective review of patients referred for TIPS revision from 2008-2021, and demonstrate that doppler ultrasound has poor overall sensitivity and specificity in predicting need for TIPS revision. Non-invasive methods of predicting TIPS dysfunction are needed since those needing TIPS revision had better survival.
INTRODUCTION
Complications of cirrhosis can include ascites and variceal bleeding due to portal hypertension. When ascites and variceal bleeding are refractory to diuretics and endoscopic therapy, respectively, transjugular intrahepatic portosystemic shunt (TIPS) can be considered. Since 1989, TIPS has been used for complications of portal hypertension with high clinical and technical success rates[1]. Formerly, bare metal stents were used and propone to dysfunction from narrowing[2]. However, patency rates have increased over the past 20 years due to the advent of available expandable polytetraflouroethylene (ePTFE) covered stents[3]. With ePTFE stents, TIPS patency rates have improved significantly, with studies showing 93% and 75.9% patency at 1 and 3 years, respectively[4]. However, TIPS dysfunction, including occlusion, stenosis, and encephalopathy still occur and are potentially deleterious complications.
Though there are no guidelines to suggest optimal timing of TIPS surveillance or thresholds for shunt dysfunction, clinical symptoms such as recurrence of ascites or variceal bleeding should prompt investigation. TIPS dysfunction due to stenosis is defined as greater than 50% reduction in lumen diameter on angiography or porto-systemic gradient (PSG), above 12 mmHg[5]. Currently, the gold standard is trans-shunt venography (TSV); however, this test is costly and invasive. Though isotope studies, computed tomography (CT), magnetic resonance imaging have been used as non-invasive methods of evaluating TIPS, doppler ultrasound (DUS) is the most widely accepted method[1]. The direction of blood flow can be cephalic (toward the heart) vs caudal (away from the heart) with the side of the TIPS closest to the heart termed the distal, cephalic, or hepatic vein end, whereas the proximal side has been deemed caudal, or portal vein end.
Though easily accessible, the utility of DUS to assess need to TIPS revision is poorly defined. The variability of results could be explained by the absence of a consensus definition of shunt dysfunction, differences in doppler measurements and the small number of patients reported in these case series. To our knowledge, there are limited prospective studies assessing the accuracy of DUS for assessing TIPS dysfunction. Although several studies have attempted to identify the optimal cut-off for TIPS dysfunction, there remains a significant amount of variability in terms of accuracy. Some argue that a lower limit of normal shunt velocity should be used. On the contrary, assuming that focal stenosis could lead to higher velocities at the stenotic level (a.k.a. Bernoulli’s principle), one could seek an upper limit of normal as well. Additionally, others have used the main portal vein velocity, or the difference between the maximum and minimum peak intra-stent velocities as indicators of malfunction[6-9].
To address this gap in knowledge, our aim was to determine the accuracy of DUS in assessing the need for TIPS revision using clinical and predictive factors.
MATERIALS AND METHODS
A retrospective study at a tertiary academic medical center that performs liver transplantation was performed under IRB approval. Adult patients from January 2008 to January 2021 who underwent TIPS revision were identified and reviewed. The patient’s electronic medical records were reviewed for demographic, clinical, and radiologic information at the time of TIPS revision. Of 100 patients identified, 11 were excluded; 9 for TIPS revision for worsening hepatic encephalopathy not based on DUS, and 2 for incomplete data. Therefore, 89 subjects were included in the final analysis.
Information on demographics (age, race), indication for initial TIPS (recurrent ascites or variceal bleeding, abnormal DUS), Model for End-Stage Liver Disease (MELD) at time of TIPS placement and at time of revision, DUS parameters (proximal, mid, distal velocities), TSV specificities (mm dilation, PSG before and after TIPS revision), presence or absence of stenosis, need for intervention, and clinical outcomes (death, transplant) were included.
Baseline TIPS patency at our institution was assessed by performing DUS 2-4 wk after TIPS placement, 6 and 12 mo after TIPS placement, and thereafter, assessed at routine HCC screening surveillance intervals. Additionally, assessment of TIPS patency was pursued if there are clinical signs of portal hypertension (i.e., recurrent ascites or variceal bleeding). The abnormal flow rates during TSV that led to a venography study are reported as “at revision,” whereas the baseline flow rates from the penultimate doppler preceding the venography are reported as “pre-TIPS baseline.” The normal range of flow is 90-190 cm/second; any gradient change of greater than 50 cm/second across the stent is considered abnormal and concerning for stenosis. TIPS venographic abnormalities included shunt occlusion, shunt stenosis, and/or elevation of the portosystemic gradient above 12mmHg. If present, the shunt was revised.
Descriptive statistics were used to describe the cohort. Mean (standard deviation, SD) and medians (interquartile range, IQR) were used for normalized and non-normalized data, respectively and compared by student’s t-test or Wilcoxon rank sum. Proportions were compared by chi-square or Fisher’s exact test as appropriate. Receiver operating characteristics curves, sensitivity, specificity, and performance of DUS to predict need for TIPS revision were performed. Univariate and multivariate analyses were used to predict clinical factors associated with need for TIPS revision.
RESULTS
The cohort consisted of 89 patients with cirrhosis (64% men, 76% white), with age range from 51-62 (median age of 59 years). The etiology of liver disease was alcohol (31%), hepatitis C virus (16%), non-alcoholic steatohepatitis (20%), other (30%). Indication for initial TIPS were refractory variceal bleeding (41%), refractory ascites (51%), and other causes (8%) (Table 1).
Table 1.
Characteristics of the cohort
| Characteristics | Mean (SD) | Median (IQR) |
| Age (n = 89) | 56.5 (9.8) | 59 (51-62) |
| % male | 64 | |
| % White/Black/other | 76/18/3 | |
| Etiology of Liver disease % | ||
| EtOH | 31 | |
| HCV | 16 | |
| NASH | 20 | |
| Other | 30 | |
| Indication for TIPS % | ||
| VB | 41 | |
| Refractory ascites | 51 | |
| other | 8 | |
| MELD at initial TIPS | 16.6 (6.1) | |
| PSG before TIPS mmHg | 15.5 (4.5) | 15 (12.5-18) |
| PSG after TIPS mmHg | 6.17 (2.54) | 6 (4-8) |
| TIPS dilation mm | 8.41 (0.91) | |
| TIPS revision (d) | 514 (670) | 311 (54-661) |
| Indication for revision (% doppler) | 74 | |
| MELD at revision | 17.3 (6.8) | |
| Doppler abnormal % | 82 | |
| High vel/low vel/clinical % | 36/31/30 | |
| Doppler flow at revision Doppler prox cm/s | 122 (58) | 127 (77-169) |
| Doppler flow at revision doppler mid | 135 (73) | 140 (77-185) |
| Doppler flow at revision Doppler distal | 141 (103) | 122 (57-205) |
| TIPS occluded % | 10 | |
| Doppler flow pre-TIPS baseline prox cm/s | 125 (43) | 122 (100-146) |
| Doppler flow pre-TIPs baseline mid | 133 (42) | 140 (109-161) |
| Doppler flow pre-TIPS baseline distal | 128 (52) | 128 (89-155) |
| Change prox | 45 (36) | 36 (12-80) |
| % change prox | -0.01 (.47) | -0.03 (-0.253-0.312) |
| Change mid | 55 (50) | 45.5 (16.9-74.2) |
| % change mid | 0.11 (0.66) | 1 (-20-33) |
| Change distal | 69 (76) | 48.7 (20-92) |
| % change distal | 0.1 (0.89) | -12% (-45-38) |
| PSG pre TIPS revision mmHg | 14 (12) | 12 (9-15) |
| PSG after TIPS revision mmHg | 8.32 (3.7) | 8 (6-10) |
| Outcome (Alive/LT/Dead) % | 49/13/37 | |
| TIPS stenosis (Y) % | 43% | |
| TIPS revised | 44% |
SD: Standard deviation; IQR: Interquartile range; HCV: Hepatitis C virus; NASH: Non-alcoholic steatohepatitis; TIPS: Transjugular intrahepatic portosystemic shunt; VB: Variceal bleeding; MELD: Model for End-Stage Liver Disease; PSG: Porto-systemic gradient.
The mean MELD at initial TIPS was 16.6 (SD: 6.1), PSG 15.5 mmHg (millimeters of mercury) (SD: 4.5) pre-TIPS and 6.17 mmHg (SD: 2.54), post-TIPS. The mean TIPS diameter was 8.41 (SD: 0.91) mm. The median of days to TIPS revision was 311 (54-661).
TIPS revision was prompted by either 1) clinical factors such as recurrent ascites (23%), or 2) an abnormal flow noted on the doppler ultrasound which was performed as part of our patency assessment protocol in clinic. Therefore, referral to interventional radiology for TIPS assessment were due to high doppler velocity (indicative of early TIPS dysfunction) in 23%, low velocity suggestive of late dysfunction in 51%, or clinical factors such as recurrent ascites in 23% (Table 2). Overall, 82% of the dopplers had abnormal flow. Fourty-four percent had true stenosis that required revision of TIPS; however, during venography, 56% of patients who were referred for revision, had widely patent TIPS.
Table 2.
Comparison of those who underwent transjugular intrahepatic portosystemic shunt revision
|
Characteristic
|
Yes revision (n = 39)
|
No revision (n = 50)
|
P
value
|
| Age | 56.7 (12) | 57.3 (7.6) | |
| % male/female | 51/49 | 74/26 | 0.0266 |
| % White/Black/other | 72/23/5 | 80/18/2 | |
| Etiology of liver disease % | 0.08 | ||
| EtOH | 26 | 36 | |
| HCV | 11 | 20 | |
| NASH | 21 | 20 | |
| Indication for TIPS % | 0.05 | ||
| VB | 53 | 50 | |
| Refractory ascites | 34 | 46 | |
| MELD at initial TIPS | 15.8 (6.5) | 17.2 (5.8) | |
| PSG before TIPS mmHg | 15.9 (5.1) | 15.1 (4.0) | |
| PSG after TIPS mmHg | 5.89 (3.1) | 6.3 (2.1) | |
| TIPS dilation mm | 8.6 (1.04) | 8.2 (0.76) | |
| Days to TIPS revision | 572 (740) | 466 (612) | |
| Indication for revision (% doppler) | 84 | 59 | 0.006 |
| MELD at revision | 15.5 (6.8) | 18.7 (6.5) | 0.03 |
| Revision indication | 84 [flow issue] | 66 [clinical] | .04 |
| Doppler abnormal % | 78 [> 5% change proximal flow] | 86 [< 5% change] | 0.39 |
| High vel/low vel/clinical % | 23/51/23 | 46/16/36 | 0.0028 |
| DF at revision Doppler prox cm/s | 103 (64) | 134 (21) | 0.0356 |
| DF at revision Doppler mid | 109 (92) | 151 (52) | 0.017 |
| DF at revision Doppler distal | 86.6 (72) | 174 (105) | 0.0003 |
| TIPS occluded % | 23 | 2 | 0.0019 |
| DF pre-TIPS baseline prox cm/s | 112 (50) | 133 (36) | 0.044 |
| DF pre-TIPs baseline mid | 125 (52) | 140 (32) | 0.16 |
| DF pre-TIPS baseline distal | 117 (58) | 136 (45) | 0.13 |
| Change prox | 42 (37) | 47 (36) | |
| % change prox | -0.11 (0.42) | 0.10 (0.49) | |
| Change mid | 69 (69) | 46 (30) | 0.1 |
| % change mid | -0.057 (0.69) | 0.16 (0.51) | 0.17/.05 (Wilcoxon) |
| Change distal | 62.9 (51) | 73 (89) | |
| % change distal | -0.20 (0.76) | 0.32 (0.92) | 0.021/0.0014 (Wilcoxan) |
| PSG pre TIPS revision mmHg | 15.5 (6.1) | 13.1(14.8) | |
| PSG after TIPS revision mmHg | 8.11 (4.3) | 8.46 (3.3) | |
| Outcome (Alive/LT/Dead) % | 61/13/26 | 40/14/46 | 0.1 |
| TIPS stenosis (Y) % | 100 | 46 | < 0.0001 |
DF: Doppler flow; HCV: Hepatitis C virus; NASH: Non-alcoholic steatohepatitis; TIPS: Transjugular intrahepatic portosystemic shunt; VB: Variceal bleeding; MELD: Model for End-Stage Liver Disease; PSG: Porto-systemic gradient.
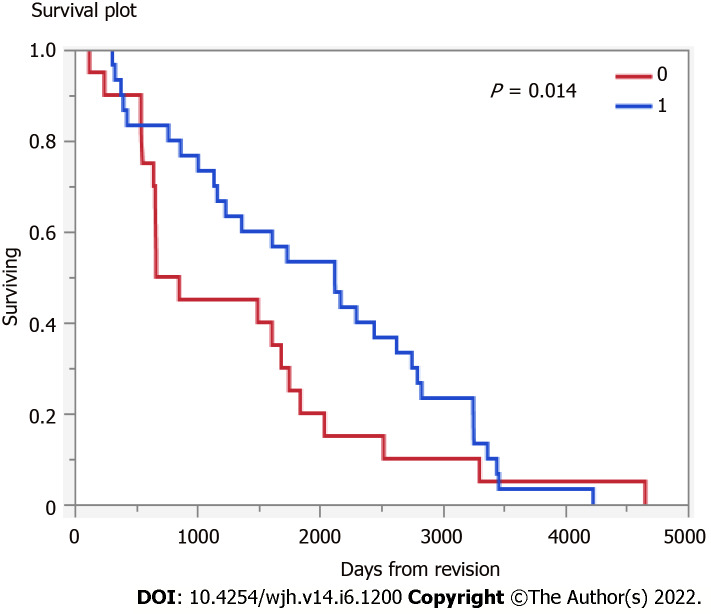
MELD at TIPS revision was 15.5 (SD: 6.8). Among those undergoing TIPS revision (n = 39) followed a median 1503 (IQR 663-2491) days, 13% underwent subsequent liver transplant and 26% died, therefore, the transplant free survival was 61%. Those that underwent TIPS revision had higher transplant free survival (Figure 1) (P = 0.14).
Figure 1.
Transplant free survival (blue: Transjugular intrahepatic portosystemic shunt revision; red: Did not undergo transjugular intrahepatic portosystemic shunt revision).
On univariate analysis, factors associated with need for TIPS revision were male gender (P = 0.026), initial indication for TIPS (P = 0.05) and indication for TIPS revision (P = 0.006). On multivariate analysis, only gender was associated with TIPS revision (P = 0.023). While TIPS flow in the proximal TIPS at the baseline doppler was lower in the revision group than in the non-revision group (P = 0.04), TIPS flow was lower at the time of revision at all parts of the stent (all P < 0.01).
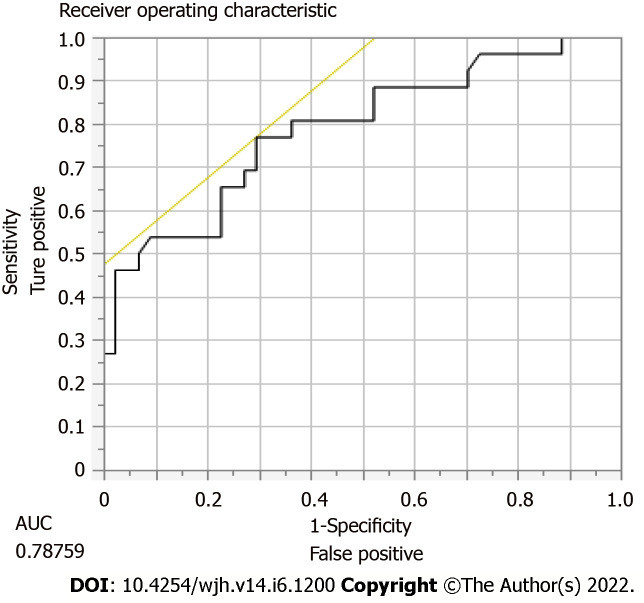
DUS has a 40% sensitivity, 45% specificity, PPV 78%, and NPV 14% of predicting TIPS stenosis or occlusion requiring intervention. In order to calculate these statistical values, we compared whether or not the DUS was abnormal vs if TIPS revision was performed by radiology. The most accurate location for shunt velocity measure was distal velocity [Area under the curve (AUC): 0.79; P = 0.0007] (Figure 2), compared to proximal (AUC 0.65) and mid (AUC 0.71) velocities (Tables 3 and 4). A distal flow value of 114 cm/s or less had 77% sensitivity, 70% specificity, PPV 60%, NPV 84% for predicting need for revision.
Figure 2.
Sensitivity and specific of distal velocity in need for transjugular intrahepatic portosystemic shunt revision.
Table 3.
Area under the curve based on intra-transjugular intrahepatic portosystemic shunt velocity
|
TIPS velocity
|
Area under the curve
|
| Proximal flow velocity | 0.65 |
| Mid flow velocity | 0.71 |
| Distal flow velocity | 0.79 (P = 0.0007) |
TIPS: Transjugular intrahepatic portosystemic shunt.
Table 4.
Performance of doppler ultrasound in predicting need for transjugular intrahepatic portosystemic shunt revision
|
Performance characteristic
|
|
| Sensitivity | 40% |
| Specificity | 45% |
| Negative predictive value | 14% |
| Positive predictive value | 78% |
DISCUSSION
This study of patients referred for TIPS revision over a 13-year period found that DUS overall has poor sensitivity and specificity for predicting TIPS dysfunction. However, distal velocity seemed to be the most accurate location for determining shunt velocity in this study. Those that underwent TIPS revision were found to have higher transplant free survival.
We observed that significantly more men required TIPS revision than women. We did not observe a statistically significant difference in regards to MELD score or PSG. This is in contrast to prior studies by Brants et al[3] who define TIPS dysfunction as an occluded shunt, increase in PSG > 12 mmHg, or stenosis of at least 50% of the shunt diameter[3]. In addition, at the time of revision, patients who needed a TIPS TSV had lower MELD scores, perhaps suggesting that our revision group had fewer decompensations than the non-revision group which could have influenced the survival outcomes of this study. As such, we found that revision of TIPS was associated with lower mortality (26% vs 46%) and significantly lower rates of transplant (13% vs 24%; P = 0.1).
While DUS is accessible and non-invasive to detect TIPS dysfunction, studies have shown that DUS is inaccurate and variable in detecting TIPS dysfunction[10]. Much of the established literature has examined bare metal stents alone; however, less is known about the accuracy of DUS in the evaluation of covered stents. In vitro model such as DUS has its limitations in accuracy compared to an in vivo model because gradient measurements are multifactorial including resistance through the TIPS, resistance through hepatic parenchyma, and presence of collateral vessels[9].
Many factors can influence interpretation of doppler ultrasound. Because the stent is a three-dimensional structure that may not be located within a given plane, an area of focal stenosis could be incorrectly assessed[10]. Inherent to its technique, ultrasound is affected by operator experience. For instance, if only the intravascular portions are assessed, rather than the intraparenchymal segments, a TIPS may be mislabeled as patent. Finally, clinically factors such as obesity, ascites, breathing patterns could impact the ultrasound examination.
To date, there is a lack of well-designed multi-center trials that prospectively explore the accuracy of DUS and clinical factors in predicting TIPS dysfunction. The currently available results are inconsistent and variable due to the absence of a consensus definition of shunt dysfunction, differences in doppler measurements, and the small number of patients included in these series. Because stenosis can lead to a decreased velocity and slower flow, some studies have identified a lower limit of normal for peak shunt velocity, whereas, others have explored an upper limit of normal assuming that focal stenosis would lead to elevated velocities at the stenosis level.
A study of 43 patients using a mean portal vein velocity of < 30 cm/sec and a distal shunt velocity of < 90 cm/sec and > 220 cm/sec, Kanterman et al[5], reported a 94% sensitivity and 72% specificity if either parameter was abnormal. This study is in keeping with our results where a distal flow of < 114 cm/sec predicted need for TIPS revision with a 70% specificity. However, Chong et al[6] used a lower threshold, 50 cm/sec, which was 100% sensitive and 93% specific for predicting TIPS stenosis. This was based only on a series of 28 patients[1,6]. In our study, at the time of TIPS revision, the velocities at all portions of the stent were significantly decreased, however, the distal shunt velocity outperformed the proximal and mid shunt velocities. Though, a study by Benito et al[1] of 105 patients found that a middle shunt velocity threshold of 98cm/sec had the highest receiver operating characteristic with a 46% sensitivity and 79% specificity.
TIPS patency rates have increased over the past 20 years since the introduction of covered ePTFE stents, as compared to bare metal stents. Our study only included patients with ePTFE stents. This is in contrast to a study by Engstrom et al[11] where peak shunt velocities from covered and bare metal TIPS showed comparable sensitivities when using either depressed or elevated velocity criteria. However, they reported that a depressed velocity was more specific in covered TIPS, whereas, an elevated velocity was more specific in bare metal TIPS.
Our study is limited due to its retrospective design and lack of predefined DUS criteria to define TIPS dysfunction. In our analysis, we considered the normal range of velocity flow to be 90-190 cm/second, with an abnormal flow to be greater than 50 cm/second increase from previous ultrasound. Furthermore, our small sample size limits the generalizability of our findings. Although previous studies have included the main portal vein velocities, we chose to only focus on clinical parameters and TIPS velocities.
Although the gold standard for assessment of TIPS function is venography with portosystemic pressure gradient measurements, this procedure remains invasive and can be cost-prohibitive. Recently, color-doppler ultrasound, spleen and liver stiffness measurements via point shear wave elastography have shown promise in potentially serving as non-invasive methods to assess for dysfunction[12-16]. Helical CT angiography may also play a role, although future studies are needed to validate these findings[17]. However, these newer methods are not widely available and have not been used to assess TIPS dysfunction.
In summary, if TIPS is placed in the carefully selected patient, it could be life-saving. However, an important consideration is TIPS stenosis that could lead to recurrence of hepatic decompensation. Therefore, an inexpensive, non-invasive, and accurate screening method for early detection of TIPS stenosis is needed. In this study, distal velocity may be able to predict TIPS stenosis with acceptable accuracy while improving transplant free survival rates. However, multi-center prospective studies with a larger cohort are needed to confirm these findings.
CONCLUSION
DUS has poor overall sensitivity and specificity in predicting need for TIPS revision. Non-invasive methods of predicting TIPS dysfunction are needed since those needing TIPS revision had better survival.
ARTICLE HIGHLIGHTS
Research background
Portal hypertension as a result of cirrhosis can lead to complications such as variceal bleeding and ascites. Refractory variceal bleeding or ascites can be treated with Transjugular intrahepatic portosystemic shunt (TIPS), an expandable polytetrafluoroethylene covered stent used to decrease portal pressures. However, a complication of this procedure is stent stenosis.
Research motivation
There are currently no guidelines to assist providers in ensuring TIPS patency. Our study aims to assess the accuracy of doppler ultrasound in predicting need for TIPS revision, compared to trans-shunt venography (TSV) as the gold standard.
Research objectives
To determine the accuracy of doppler ultrasound to assess TIPS dysfunction and for need for revision.
Research methods
Retrospective chart review of patients referred for TIPS revision from 2008-2021 at a tertiary medical center. We collected demographical data, doppler ultrasound (DUS) parameters at baseline and at the DUS preceding TIPS revision, TSV data were collected. Receiver operating characteristics curves, sensitivity, specificity, performance for doppler to predict need for revision were performed.
Research results
The cohort consisted of 89 patients with cirrhosis (64% men, 76% white, 31% alcohol as etiology); median age 59 years. TIPS was revised in 44%. On univariate analysis, factors associated with need for TIPS revision were male (P = 0.03), initial indication for TIPS (P = 0.05) and indication for revision (P = 0.01). Revision of TIPS was associated with lower mortality (26% vs 46%) and significantly lower rates of transplant (13% vs 24%; P = 0.1). In predicting need for TIPS revision, DUS has a 40% sensitivity, 45% specificity, PPV 78%, and NPV 14%. The most accurate location for shunt velocity measure was distal velocity (AUC 0.79; P = 0.0007).
Research conclusions
DUS has poor overall sensitivity and specificity in predicting need for TIPS revision.
Research perspectives
Future research should include multi-center prospective trials using our proposed cut-off of a distal shunt velocity of less than 114 cm/second, to determine if this is the optimal cut-off to predict need for TIPS revision.
Footnotes
Institutional review board statement: Study was approved by IRB ( IRB HM20022488).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: No conflicts of interest for all authors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 30, 2022
First decision: March 25, 2022
Article in press: May 22, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Goral V, Turkey; Rabago LR, Spain A-Editor: Zhu JQ S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
Contributor Information
Nikki Duong, Division of Gastroenterology, Hepatology, and Nutrition, Virginia Commonwealth University Medical Center, Richmond, VA 23219, United States. nduong91@gmail.com.
Marcus Healey, Department of Internal Medicine, Virginia Commonwealth University Medical Center, Richmond, VA 23219, United States.
Kunal Patel, Division of Interventional Radiology, Virginia Commonwealth University Medical Center, Richmond, VA 23219, United States.
Brian J Strife, Division of Interventional Radiology, Virginia Commonwealth University Medical Center, Richmond, VA 23219, United States.
Richard K Sterling, Division of Gastroenterology, Hepatology, and Nutrition, Virginia Commonwealth University Medical Center, Richmond, VA 23219, United States.
Data sharing statement
No additional data available.
References
- 1.Benito A. Doppler ultrasound for TIPS: does it work? Abdominal Imaging . 2004:45–52. doi: 10.1007/s00261-003-0088-9. [DOI] [PubMed] [Google Scholar]
- 2.Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol. 2014;20:16996–17010. doi: 10.3748/wjg.v20.i45.16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brants L, Leiderman M, Veitsman E, Ofer A, Beck-Razi N. Role of Doppler Ultrasound Combined With Clinical Features in the Diagnosis of Transjugular Intrahepatic Portosystemic Shunt Dysfunction in the Era of Covered Stents. J Ultrasound Med. 2020;39:2373–2377. doi: 10.1002/jum.15346. [DOI] [PubMed] [Google Scholar]
- 4.Luo X, Zhao M, Wang X, Jiang M, Yu J, Li X, Yang L. Long-term patency and clinical outcome of the transjugular intrahepatic portosystemic shunt using the expanded polytetrafluoroethylene stent-graft. PLoS One. 2019;14:e0212658. doi: 10.1371/journal.pone.0212658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168:467–472. doi: 10.2214/ajr.168.2.9016228. [DOI] [PubMed] [Google Scholar]
- 6.Chong WK, Malisch TA, Mazer MJ, Lind CD, Wor- rell JA, Richards WO. Transjugular intrahepatic per- tosystemic shunt: US assessment with maximum flow velocity. Radiol . 1993;189:789–793. doi: 10.1148/radiology.189.3.8234705. [DOI] [PubMed] [Google Scholar]
- 7.Manatsathit W, Samant H, Panjawatanan P, Braseth A, Suh J, Esmadi M, Wiedel N, Ingviya T. Performance of ultrasound for detection of transjugular intrahepatic portosystemic shunt dysfunction: a meta-analysis. Abdom Radiol (NY) 2019;44:2392–2402. doi: 10.1007/s00261-019-01981-w. [DOI] [PubMed] [Google Scholar]
- 8.Foshager MC, Ferral H, Nazarian GK, Castañeda-Zúñiga WR, Letourneau JG. Duplex sonography after transjugular intrahepatic portosystemic shunts (TIPS): normal hemodynamic findings and efficacy in predicting shunt patency and stenosis. AJR Am J Roentgenol. 1995;165:1–7. doi: 10.2214/ajr.165.1.7785564. [DOI] [PubMed] [Google Scholar]
- 9.Dodd GD 3rd, Zajko AB, Orons PD, Martin MS, Eichner LS, Santaguida LA. Detection of transjugular intrahepatic portosystemic shunt dysfunction: value of duplex Doppler sonography. AJR Am J Roentgenol. 1995;164:1119–1124. doi: 10.2214/ajr.164.5.7717217. [DOI] [PubMed] [Google Scholar]
- 10.Wachsberg RH. Doppler ultrasound evaluation of transjugular intrahepatic portosystemic shunt function: pitfalls and artifacts. Ultrasound Q. 2003;19:139–148. doi: 10.1097/00013644-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom BI, Horvath JJ, Suhocki PV, Smith AD, Hertzberg BS, Smith TP, Kim CY. Covered transjugular intrahepatic portosystemic shunts: accuracy of ultrasound in detecting shunt malfunction. AJR Am J Roentgenol. 2013;200:904–908. doi: 10.2214/AJR.12.8761. [DOI] [PubMed] [Google Scholar]
- 12.Bureau C, Pagan JCG, Layrargues GP. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int . 2007;27:742–747. doi: 10.1111/j.1478-3231.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy , Timothy P. Long-term follow-up after TIPS: use of Doppler velocity criteria for detecting elevation of the portosystemic gradient. J vascular intervent radiol . 1998:275–281. doi: 10.1016/s1051-0443(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 14.Foucher , Juliette Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. European J gastroenterol hepatol . 2006:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Giunta M, La Mura V, Conti CB, Casazza G, Tosetti G, Gridavilla D, Segato S, Nicolini A, Primignani M, Lampertico P, Fraquelli M. The Role of Spleen and Liver Elastography and Color-Doppler Ultrasound in the Assessment of Transjugular Intrahepatic Portosystemic Shunt Function. Ultrasound Med Biol. 2020;46:1641–1650. doi: 10.1016/j.ultrasmedbio.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Han H, Yang J, Jin WK, Li X, Zhang F, Zhuge YZ, Wu M, Yang B. Diagnostic value of conventional ultrasound and shear wave elastography in detecting transjugular intrahepatic portosystemic shunt dysfunction. Acta Radiol. 2021;62:1575–1582. doi: 10.1177/0284185120975183. [DOI] [PubMed] [Google Scholar]
- 17.Chopra S, Dodd GD 3rd, Chintapalli KN, Rhim H, Encarnacion CE, Palmaz JC, Esola CC, Ghiatas AA. Transjugular intrahepatic portosystemic shunt: accuracy of helical CT angiography in the detection of shunt abnormalities. Radiology. 2000;215:115–122. doi: 10.1148/radiology.215.1.r00ap51115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data available.