Abstract
Background
Since introducing COVID-19 vaccines, many neurological complications such as acute transverse myelitis have been reported in the literature. This study aims to identify the clinical characteristics, radiological findings, and prognostic factors in patients with COVID-19 vaccine-associated transverse myelitis (TM).
Methods
We systematically reviewed Scopus, Pubmed, Cochrane library, Google Scholar, and preprint databases using appropriate keywords from inception till 8th April 2022. Besides, we manually searched the reference lists of the included studies and relevant previous reviews.
Results
We included 28 studies identifying 31 post-COVID-19 vaccination myelitis patients (17 female and 14 male). The mean age of the included patients was 52±19 years. ChAdOx1 nCoV-19 vaccine (Oxford-AstraZeneca) was the most common type of vaccine in association with myelitis (12 out of 31), followed by Pfizer (8 out of 31), Moderna (7 out of 31), Sinopharm (3 out of 31), and Janssen vaccine (1 out of 31). The myelitis occurred in 24 and 7 patients after administering the first and second dose of the vaccine, respectively. 21 and 10 patients had good recovery (Modified Rankin Score (MRS) <3 at the follow-up) and poor recovery (MRS≥3 at the follow-up) from myelitis, respectively. Age (OR 1.09, 95%CI 1.01–1.18, pvalue 0.02), and MRS at admission (OR 17.67, 95%CI 1.46–213.76, pvalue 0.024) were two independent risk factors for poor recovery from myelitis.
Conclusion
The patients with higher age and MRS at admission had a worse prognosis and needed timely and more aggressive therapeutic strategies.
Keywords: COVID-19, Vaccine, Vaccination, Transverse myelitis, Prognosis
1. Introduction
Transverse myelitis (TM) is an inflammatory disorder of the spinal cord that occurs due to different etiologies, such as infections, autoimmune, and demyelinating diseases (Frohman and Wingerchuk, 2010). Acute transverse myelitis (ATM) results in motor, sensory, and autonomic dysfunction, which remains with a relatively severe disability in one-third of patients (Transverse Myelitis Consortium Working Group 2002). The rapid spread of a novel coronavirus (Severe Acute Respiratory Syndrome-Coronavirus-2: SARS-CoV-2) worldwide and a high mortality and morbidity rate of coronavirus 2019 (COVID-19) resulted in a tremendous global attempt for vaccine development. Since the COVID-19 pandemic, several hundred vaccines have reached the preclinical stage, and over 60 vaccines have entered clinical trials (Mellet and Pepper, 2021). Many studies were investigated to evaluate the efficacy and safety profile of the COVID-19 vaccines. The Pfizer BNT162b2, Moderna mRNA-1273, and Oxford-AstraZeneca vaccines have been shown 95%, 94.1%, and 70.4% efficacy after the second dose, respectively (Voysey et al., 2021). Although most of the complications post COVID-19 vaccination are mild and spontaneously resolved, there are increasing reports of the serious neurological adverse events, including Guillain-Barre syndrome (GBS), Bell's palsy, seizures, encephalitis, ATM, acute disseminated encephalomyelitis (ADEM), stroke, and cerebral venous sinus thrombosis in the literature (Garg and Paliwal, 2021; Goss et al., 2021). The clinical trials of the safety profile of the approved vaccines reported three cases of ATM in association with the Oxford-AstraZeneca vaccine (Ling et al., 2021; Mahase, 2020). Besides, the Centers for Disease Control (CDC)’s Vaccine Adverse Event Reporting System (VAERS) announced the occurrence of 9 cases of ATM with Pfizer, Moderna, and Johnson & Johnson's COVID-19 vaccines (Goss et al., 2021).
The precise pathophysiologic mechanisms and the causality role of the COVID-19 vaccine in developing these neurological complications are still unclear, even though cross-reactivity and overactivity of the immune system and direct neurotoxicity have been suggested as an etiopathogenic mechanisms (Piyasirisilp and Hemachudha, 2002).
In this study, we aimed to review the clinical characteristics and radiological features of patients with COVID-19 vaccine-associated myelitis and determine the prognostic factors in these individuals to improve the outcome of such patients by implementing appropriate treatment strategies.
2. Materials and methods
We performed our systematic review using Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline (Moher et al., 2010). This study was registered in the international prospective register of systematic reviews (PROSPERO) (registration number: CRD42021288656). Moreover, the ethics committee and institutional review board of Shiraz University of Medical Sciences reviewed and approved this study protocol (Approval No# IR.sums.med.rec.1401.129).
2.1. Literature search strategy
The search was performed on Pubmed, Scopus, Google Scholar, Cochrane library, and preprint (bioRxiv, medRxiv) databases by two independent investigators (NK and MR) from inception till 8th April 2022. The literature search was conducted using the following keywords: “COVID-19″ OR “SARS-CoV-2″ OR “2019 novel coronavirus” OR “coronavirus disease 2019″ OR “2019 nCOV” AND “Vaccine” OR “Vaccination” OR “Immunization” AND “Myelitis” OR “Myelopathy” OR “Spinal Cord” OR “Neurological” OR “Neurologic” OR “Neurology” OR “Nervous System”. The reference lists of the included studies and previous reviews were also reviewed, and relevant reports were selected.
2.2. Study selection
2.2.1. Inclusion criteria
-
1-
Observational (cohort, case-control, cross-sectional, case series, and case report) studies with at least one reported case of TM after any COVID-19 vaccine.
-
2-
The time interval between vaccination and development of TM should be within 42 days (according to the World Health Organization Global Advisory Committee on Vaccine safety- WHO GACVS).
-
3-
No language limitation.
2.2.2. Exclusion criteria
-
1-
Systematic reviews, narrative reviews, editorials, and commentaries, unless reporting an original case of TM following COVID-19 vaccine administration.
-
2-
Patients with a diagnosis of multiple sclerosis (MS), neuromyelitis optica spectrum disorder
-
3-
(NMOSD), myelin oligodendrocyte glycoprotein antibody disease (MOGAD), or ADEM.
-
4-
Positive anti-aquaporin-4 antibody, or myelin oligodendrocyte glycoprotein.
-
5-
Markedly positive vasculitis panel.
-
6-
Not reporting clinical characteristics, diagnostic work-ups, or outcomes.
2.3. Data extraction
Two independent reviewers (MS and HM) assessed the searched articles and checked them regarding duplication and fulfilling the inclusion and exclusion criteria. Any discrepancies between the reviewers’ searches were discussed with a senior reviewer (VRO) until the attainment of an agreement. We extracted the following data from the included reports: study characteristics; demographic data, including age and sex; past medical history (PMH); COVID-19 real-time polymerase chain reaction (RT-PCR); vaccine type and dose; the interval between COVID-19 vaccination and development of symptoms/signs related to TM; neurological symptoms and signs, radiological, cerebrospinal fluid (CSF) analysis, and laboratory findings related to TM; treatment regimen of transverse myelitis; and outcome (We categorized patients with modified Rankin scale (MRS) < 3 and ≥3 at the follow-up as good and partial recovery, respectively (Annunziata et al., 2019)). We grouped patients according to the clinical symptoms and signs into the motor; sensory; sphincter; motor-sensory; motor-sphincter; sensory-sphincter; and motor-sensory-sphincter symptoms/signs. Moreover, we categorized patients with spinal cord lesions extending over three or more vertebral segments as longitudinally extensive transverse myelitis (LETM). Besides, considering the affected spinal cord's width, we classified patients as complete (the entire width of the spinal cord is affected) or partial TM. Diagnosis of ATM was made according to the Transverse Myelitis Consortium Working Group (TMCWG) (Transverse Myelitis Consortium Working Group 2002).
2.4. Quality assessment of study and risk of bias
The quality and risk of bias of case reports, case series, and cross-sectional studies were evaluated using the Joanna Briggs Institute (JBI) critical appraisal checklists (Moola et al., 2020; Munn et al., 2020). We attempted to stay away from selection bias by contacting the corresponding authors for each manuscript in which the details of clinical characteristics, diagnostic work-ups, and outcomes were not indicated.
2.5. Outcome measures
The primary outcome measure was the prognostic factors of COVID-19 vaccine-associated myelitis. The secondary outcome of the present study was the determination of the demographic, clinical characteristics, and radiological features of patients with COVID-19 vaccine-associated myelitis.
2.6. Statistical analysis
Statistical analysis was conducted using SPSS software version 26, and a pvalue less than 0.05 was considered statistically significant. Qualitative values were described using number and percent; in contrast, quantitative data were reported using mean and standard deviation. The comparison between clinical characteristics, radiological features, and laboratory findings of groups (good recovery versus partial recovery) was assessed using the Pearson chi‐square test (for categorical variables) and independent t-test, and the Mann-Whitney test (for continuous variables). Besides, multivariate forward stepwise logistic regression analysis was performed to identify the independent risk factors of poor recovery from vaccine-associated myelitis.
3. Results
3.1. Study selection
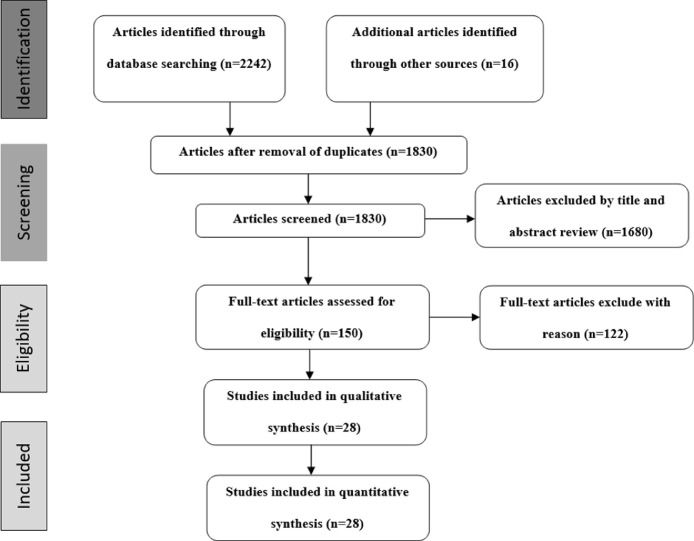
The PRISMA flowchart (Fig. 1 ) showed a search strategy for our review. The present study consisted of 28 studies, including 26 case reports, one case series, and one cross-sectional, and the pooled data of 31 patients with COVID-19 vaccine-associated ATM is extracted (Sriwastava et al., 2021; Sepahvand et al., 2022; Tan et al., 2021; Corrêa et al., 2021; Vegezzi et al., 2021; Hsiao et al., 2021; Pagenkopf and Südmeyer, 2021; Notghi et al., 2021; Gao et al., 2021; Malhotra et al., 2021; E Khan et al., 2022; Erdem et al., 2021; Hirose et al., 2021; Tahir et al., 2021; Miyaue et al., 2021; Fujikawa et al., 2021; Kaulen et al., 2022; McLean and Trefts, 2021; Alabkal et al., 2021; Fitzsimmons and Nance, 2021; Nakano et al., 2022; Spataro et al., 2022; Cabral et al., 2022; Z Khan et al., 2022; Maroufi et al., 2022; Eom et al., 2022; Netravathi et al., 2022; Esechie et al., 2022). All of the selected studies were written in the English language.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow charts show the studies’ selection processes.
3.2. Quality assessment of study
The quality assessment results of the included articles were shown in Supplementary Table 1, Supplementary Table 2, and Supplementary Table 3. Overall, all of the selected studies had a reasonable risk of bias.
3.3. Patients’ demographics and clinical characteristics
The demographics and clinical characteristics of the previously reported cases with COVID-19 vaccine-associated transverse myelitis are shown in Table 1 .
Table 1.
Demographic, clinical, radiological, and laboratory findings of the reported patients with COVID-19 vaccine-associated myelitis.
| First Author/Country | Age/Sex | Comorbidities | Name of vaccine | Type of vaccine | Dose of vaccine | The interval between the vaccine and neurological symptoms onset(days) | The interval between the start of neurological symptoms to maximum disability (days) | TM clinical symptoms/neurological examinations | TM MRI features | Complete or incomplete TM (width* of the lesion)/longitudinal extension of the lesion⁎⁎ | Treatment | Results of paraclinics | Recovery at the last follow-up¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Sriwastava et al.10/ USA | 67/F | Negative | Moderna(m-RNA 1273) | mRNA-based vaccine | First | 1 | 4 | Lower extremities weakness with muscle strength of grade 4/5. Reflexes were brisk at the knee. | Longitudinally extensive lesion from C1 to C3 with patchy enhancement. | Incomplete/ LETM | IVIg and PLEX. | CSF showed normal protein, glucose, and no pleocytosis. NMO, MOG, and OCB were negative. | Partial recovery |
| 2.Sepahvand et al.11 /Iran | 71/M | DM, HTN, IHD | Sinopharm(BBIBP-CorV) | Inactivated vaccine | First | 5 | 4 | Left hemiparesis (muscle strength of grade 3/5), urinary retention, hypoesthesia of the right upper and lower limbs and right side of the trunk, as well as impaired position and vibration senses and positive Babinski sign in the left lower limb. | Longitudinally extensive lesion from the medulla to C3. | Incomplete/LETM | MTP 6 gs followed by oral prednisolone. | CSF showed normal protein, glucose, and no pleocytosis. NMO, MOG, OCB, and vasculitis were negative. | Good recovery |
| 3.Tan et al.12/Malaysia | 25/F | Negative | ChAdOx1 nCOV-19 vaccine | Viral vector | First | 5 | 11 | Lower limbs myalgia followed by lower extremities weakness (muscle strength of grade 3/5), exaggerated deep tendon reflexes at the knees and ankles, bilateral positive Babinski sign, numbness and allodynia below the T8 spinal level, and urinary retention. | Multi-segment T2W hyperintensities (T3-T5, T7-T8, and T11-L1), showed variable cord enhancement post-contrast at T7-T8 lesions. |
Incomplete/LETM | MTP 5 gs | CSF showed no pleocytosis and normal glucose with an elevated protein of 546 mg/L (normal range: 150 −400). OCB, NMO, MOG, and vasculitis were negative. | Good recovery |
| 4.Corrˆea et al.13/ Brazil |
65/M | Negative | ChAdOX1 nCoV-19 vaccine | Viral vector | First | 8 | NM | Tetraparesis | Hyperintense lesion extending from C4-C6 with no contrast enhancement. | Incomplete/LETM | MTP 5 gs followed by tapering oral prednisolone. | CSF showed no pleocytosis, normal CSF glucose with an elevated protein of 70 mg/dl, and a negative infectious myelopathy panel (toxo-plasmosis, influenza, HSV, VZV, CMV, and syphilis). OCB, NMO, MOG, and vasculitis were negative. | Good recovery |
| 5.Vegezzi et al.14/Italy | 44/F | Negative | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 4 | 3 | Paraparesthesia and numbness in lower extremities as well as reduced light touch and pinprick up to the ankles and brisk deep tendon reflexes in the lower limbs. | Hyperintensities in posterior paramedian at T7-T8 and left lateral cord at T10-T11 with mild patchy enhancement. | Incomplete/TM | MTP 5 gs followed by tapering oral prednisolone 1 mg/kg/d | CSF showed mild lymphocytic pleocytosis (6cells/µl), mildly elevated protein (76.7 mg/dl), and negative viral myelitis panel (HSV-1/2, VZV, Enterovirus, EBV, and CMV). NMO, OCB, MOG, and vasculitis were negative. | Good recovery |
| 6.Hsiao et al.15/Taiwan | 41/M | DM | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 21 | 21 | Left peripheral facial palsy that completely resolved and followed by paresthesia, and loss of sensation in lower limbs (loss of pinprick sensation below T4 bilaterally, loss of joint position, and vibration over both lower limbs), asymmetrical lower extremities weakness (LT>RT), as well as increased bilateral knee reflexes. | Hyperintense lesion with contrast enhancement over the spinal cord at the T1 to T6. | Incomplete/LETM | MTP 5 gs followed by tapering oral prednisolone 1 mg/kg/d. | CSF showed mild lymphocytic pleocytosis (WBC: 11 cells/ul), mildly elevated protein (44.3 mg/dl), and a negative infectious myelopathy panel. Vasculitis, and NMO were negative. | Good recovery |
| 7.Pagenkopf et al.16/Germany | 45/M | Atopic dermatitis | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 8 | 4 | Thoracic back pain with urinary retention followed by flaccid tetraparesis dominantly in lower limbs with sensory level at T9. | Longitudinally extensive lesion from C3 to T2 without gadolinium enhancement. | Complete/ LETM | MTP 5 gs followed by oral tapering prednisolone. | CSF showed PMN pleocytosis of 481 cells/μl (67% PMN), increased protein (140 mg/dl), increased lactate (3,98 mmol/l) and decreased glucose (CSF/serum ratio 0,43), and negative infectious myelopathy panel (cryptococcus, E-coli, isteria monocytogenes, neisseria meningitidis, streptococcus agalactiae, streptococcus pneumonia,haemophilus influenza, HSV1/2, VZV, HHV-6, CMV, EBV, tick- borne encephalitis, neuroborreliosis, enteroviridae, coxsackie, west nile virus, mycoplasma, tuberculosis, syphilis, HIV, hepatitis B and hepatitis C). OCB,, NMO, MOG, vasculitis, and autoimmune panel (Hu, Ri, ANNA-3, Yo, Tr, Myelin, Ma/Ta, GAD65, Amphiphysin, glutamate receptors of type NMDA and AMPA, GABA-a- receptor, GABA-b-receptor, LGI1, CASPR2, ZIC4, DPPX, Glycin- receptor, mGluR1, mGluR5, GluRD2, Rho GTPase, ITPR1, CARPVIII, Homer 3, Revoverin, Neurochondrin, Flotillin, and IgLON5) were negative. B12 serum level was normal. | Good recovery |
| 8.Notghi et al.17/UK | 58/M | DM, Pulmonary sarcoidosis | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 7 | 7 | Progressive numbness of lower limbs (severe allodynia up to neck level and absence of vibration sensation below the groin), and urinary retention followed by weakness of lower limbs and inability to walk. | First: Longitudinally extensive lesion from T2 to T10 with foci of gadolinium enhancement; Second with one-week interval without treatment: extension of the lesion from C1 to T10 with no gadolinium enhancement. |
Complete/ LETM | MTP 5 gs followed by 5 sessions of PLEX followed by tapering oral prednisolone | CSF showed elevated protein 168 mg/dL with lymphocytic pleocytosis (11 cells/ul) and negative infectious myelopathy panel (HIV, HTLV-1, borrelia, hepatitis B&C, syphilis, HHV-6, mycoplasma). OCB in CSF and serum was similar. Vasculitis, MOG, NMO, and autoimmune panel (antiHu-YO, Ri) were negative. ACE, B12, copper, and folate levels were normal. FDG-PET scan demonstrated no active sarcoidosis nodule. | Partial recovery |
| 9.Gao et al.18/Taiwan | 76/F | HTN, Right-sided impaired hearing | Moderna(m-RNA 1273) | mRNA-based vaccine | First | 2 | 1 | Right upper and lower limbs and sacral paresthesia (decreased proprioceptive sensation below the right T4 dermatome), as well as gait unsteadiness with preserved muscle strength. The deep tendon reflex of the right limbs were relatively brisk. The Babinski sign showed a right extensor plantar response. | Longitudinally extensive intramedullary hyperintensity in the cervical cord at the C2–C5 levels on T2W images with contrast ring enhancement at the C3 level. | Incomplete/LETM | MTP 5 gs followed by tapering oral prednisolone. | CSF showed mild pleocytosis (15cells/µL) with neutrophil predominance (73%) and increased protein levels (57.2 mg/dL; normal limit: 15–45 mg/dL). CSF RPR, treponema pallidum hemagglutination, HIV, infectious myelopathy panel, and cytology were all negative. Vasculitis, NMO, and OCB were negative. B12 was deficient (131, >211). | Good recovery |
| 10.Malhotra et al.19/India | 36/M | Negative | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 8 | NM | Abnormal sensation in lower limbs ascending to manubrium sterni, exaggerated deep tendon reflexes in lower limbs with extensor plantar responses. |
Hyperintense lesion at the dorsal aspect of cord at the level of C6-C7 with ring enhancement. | Incomplete/TM | MTP 5 gs | CSF showed mildly elevated protein (54 mg/dl) with no pleocytosis and a negative infectious myelopathy panel. NMO, MOG, and vasculitis were negative. | Good recovery |
| 11.Khan et al.20/USA | 67/F | CAD, CKD, Neuropathy, Colon rupture | Moderna(m-RNA 1273) | mRNA-based vaccine | First | 1 | NM | Bilateral upper and lower extremities weakness (RT>LT)(muscle strength of grade 3/5 and 4/5 in the right lower and upper extremities, respectively; tingling in the right lower extremity and bilateral loss of vibration up to the ankle; Exaggerated deep tendon reflexes in lower limbs with bilateral positive Babinski signs. | Hyperintense lesion in the upper cervical spine and cord edema extending from C1-C3 with patchy post-contrast enhancement. | Incomplete/LETM | MTP 3 gs followed by PLEX 5 sessions | CSF showed cell count 2, glucose 77 mg/dl, serum glucose 125 mg/dl, CSF protein 56 mg/dl, negative infectious myelopathy panel (SARS-COV-2 RT-PCR, gram stain, culture, Cryptococcus, HSV, and VDRL). OCB in CSF and serum was similar. Autoimmune and paraneoplastic panel, NMO, MOG, vasculitis, HTLVI/II, and HIV were negative. | Good recovery |
| 12.Erdem et al.21/Turkey | 78/F | HTN, DM, Breast cancer in complete remission | Sinopharm(BBIBP-CorV) | Inactivated vaccine | Second | 21 | 1 | Bilateral upper and lower extremities weakness(muscle strength of 0/5 at the right upper limb, 4/5 at the left proximal upper limb, 0/5 at the left distal upper limb, 4/5 at both lower limbs), difficulty in ambulating, paresthesias, and hypoesthesia of bilateral upper extremities, as well as decreased vibratory senses in bilateral lower extremities, urinary retention, and bilateral positive Babinski signs. |
Longitudinally extensive intramedullary T2W hyperintensity from the C1 to the T3. | Complete/ LETM | MTP 4 gs, PLEX 5 sessions. | CSF showed a normal cell count 2cells/µl with elevated protein level (56 mg/dL) with normal glucose. NMO, MOG, Vasculitis, OCB and tumor markers were negative; IgG index, B12, and ACE were normal. | Partial recovery |
| 13.Hirose et al.22/Japan | 70/M | HTN, Hyperuricemia, Alcoholic liver cirrhosis | Moderna(m-RNA 1273) | mRNA-based vaccine | First | 7 | NM | Bilateral lower extremities hypoesthesia (impaired bilateral perceptions to pinprick predominantly on the left side, and vibration predominantly on the right side below the level of the 8th thoracic dermatome), and mild paraparesis predominantly on the right side (muscle strength of grade 4/5); exaggerated deep tendon reflexes in lower limbs with bilateral positive Babinski signs. | Multiple high-intense areas on the T2W image located at the T1/2 and T 5/6 vertebral levels with weak gadolinium enhancement. |
Incomplete/TM | MTP 5 gs followed by tapering oral prednisolone. | CSF showed a normal white blood cell count (1 cell/μL), an increased level of proteins (52 mg/dL), and negative HSV, VZV, and autoimmune panel (Amphiphysin, Hu, Yo, CV2, Ri, Ma2/Ta, recoverin, Tr, GAD65, NMDAR, AMPAR, GABAbR, LGI1, Casper2, IgLON5, and DPPX); NMO, MOG, HIV, HTLV-1, and vasculitis were negative; OCB was positive. | Good recovery |
| 14.Tahir et al.23/USA | 44/F | Negative | Janssen(Johnson & Johnson)(Ad26.COV2.S) | Human adenovirus vector vaccine | First | 7 | 2 | Low back pain, numbness (decreased vibration in bilateral toes) and mild paresthesia in the neck and abdomen, exaggerated deep tendon reflexes in bilateral upper and lower extremities, positive Babinski signs, and urinary retention; Muscle strengths were preserved. | The longitudinally extensive hyperintense lesion from the C2–3 segment into the upper thoracic spine. | Incomplete/LETM | MTP 3 gs followed by PLEX 5 sessions. | CSF showed a white blood cell count of 100 cells/ul with 96% lymphocytes, 3% monocytes, and 1% eosinophils, glucose 71 mg/dL, protein 43 mg/dL; CSF bacterial, viral, and fungal cultures were negative; OCB, NMO, and vasculitis were negative. | Good recovery |
| 15.Miyaue et al.24/Japan | 75/M | HTN, HLP, Prostate cancer | Pfizer(BNT162b2) | mRNA-based COVID-19 vaccine | First | 3 | 11 | Ascending paresthesia followed by low back pain, reduced sensation during urination and defecation (total sensory loss below the level of the umbilicus), severe weakness in both legs(paraplegia), and loss of deep tendon reflexes in both legs. | The longitudinally extensive hyperin-ense lesion from the lower thoracic to the lumbar spine with no gadolinium enhancement. | Complete/ LETM | MTP 6 gs and PLEX 7 sessions followed by oral prednisolone 1 mg/kg/d. | CSF showed pleocytosis (33 cells/μL with 54% lymphocytes), an elevated protein level (155 mg/dL) with markedly elevated MBP (8580 pg/mL); NMO, MOG, and autoimmune panel (amphiphysin, CV2, PNMA2 (Ma2/Ta), Ri, Yo, u, recoverin, SOX1, titin, zic4, GAD65, and Tr) were negative. | Poor recovery |
| 16.Fujikawa et al.25/USA | 46/F | B12 deficiency | Moderna(m-RNA 1273) | mRNA-based vaccine | First | 2 | 4 | Constant, shooting, upper back pain in between her shoulder blades that radiated to her arms and lower chest, paresthesia distal to the T10 dermatome (decreased sensation to light touch and sharp materials), and bilateral lower extremities weakness (proximal and distal left lower-extremity strength ⅗ and right lower-extremity strength ⅘), followed by partial urinary retention. Also, there was hyporeflexia of the bilateral ankle, patella, and biceps deep tendon reflexes. | Hyperintense lesion involving the central gray matter at C6-T2 without enhancement. | Incomplete/LETM | MTP 5 gs followed by tapering oral prednisolone. | CSF showed no pleocytosis with normal protein level, negative VDRL, and Lyme IgM and IgG; NMO and vasculitis were negative; B12 level at 245 pg/mL (reference of range 254–1320 pg/mL). | Good recovery |
| 17.Kaulen et al.26/Germany | 55/F | Negative | Pfizer(BNT162b2) | mRNA-based COVID-19 vaccine | First | 3 | NM | Hypoesthesia in lower extremities. | Hyperintense T8 myelitis, mild homogeneous contrast uptake. | NM/TM | Wait and watch | CSF showed 8 lymphocytes, and positive OCB; Anti-SS‐A, SS‐B, anti gangliosidoses antibody panel, autoimmune panel (Hu, Ri, Yo PCA‐2 and Tr/DNER‐IgG, myelin, Ma/Ta, GAD65, amphiphysin, NMDA‐R, AMOA‐R, GABAA/B‐R, LGI2, CASPR2, ZIC3, ZIC4, DPPX, glycin‐R, mGluR1, mGluR5, Rho‐GTPase activating protein 26, ITPR1, homer 3, recoverin, neurochondrin, GluRD2, flotillin 1/2, IgLON5, PNMA2, SOX1, titin, Zic4, GAD65, VGKC, SOX‐1, PCA‐2 antibodies), myositis panel (ANA, Mi‐2 alpha, Mi‐2 beta, TIF1 gamma, MDA5, NXP2, SAE1, Ku, PM100, PM75, Jo‐1, SRP, PL‐7, PL‐12, EJ, OJ, Ro‐52, cN‐1A IgG), NMO, and MOG were negative. | Partial recovery |
| 18.Kaulen et al.26/Germany | 34/F | Negative | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 14 | NM | Ascending hypoesthesia up to T9, and weakness of the right leg. | Hyperintense lesion T 4–9 with contrast medium enhancement. | NM/LETM | MTP | CSF showed 5 lymphocytes, and positive OCB; Anti-SS‐A, SS‐B, anti gangliosidoses antibody panel, autoimmune panel (Hu, Ri, Yo PCA‐2 and Tr/DNER‐IgG, myelin, Ma/Ta, GAD65, amphiphysin, NMDA‐R, AMOA‐R, GABAA/B‐R, LGI2, CASPR2, ZIC3, ZIC4, DPPX, glycin‐R, mGluR1, mGluR5, Rho‐GTPase activating protein 26, ITPR1, homer 3, recoverin, neurochondrin, GluRD2, flotillin 1/2, IgLON5, PNMA2, SOX1, titin, Zic4, GAD65, VGKC, SOX‐1, PCA‐2 antibodies), myositis panel (ANA, Mi‐2 alpha, Mi‐2 beta, TIF1 gamma, MDA5, NXP2, SAE1, Ku, PM100, PM75, Jo‐1, SRP, PL‐7, PL‐12, EJ, OJ, Ro‐52, cN‐1A IgG), NMO, and MOG were negative. | Partial recovery |
| 19.Mclean et al.27/USA | 69/F | Cancer cervix, Hypothyroidism | Pfizer(BNT162b2) | mRNA-based vaccine | First | 2 | 1 | Bilateral lower extremities, handgrip and finger extension weakness, paresthesia extended from lower extremities to both hands, and urinary incontinence. Also, deep tendon reflexes were noted to be slightly exaggerated. |
Extensive T2W signal abnormalities were seen particularly in the anterior aspect, as well as the mid-cord extending from C3–4 down to T2–3. | Incomplete/LETM | MTP 5 gs. | CSF showed normal analysis, negative OCB, and infectious myelopathy panel, including (VDRL, HSV, and Lyme); NMO and MOG were negative; TSH, folate, SPEP, UPEP, ESR, ANA, HIV, rheumatoid factor, RPR, hepatitis screening, ade-novirus antibody, ACE, anti-dsDNA, ANCA, CMV, copper, EBV, zoster, West-Nile virus, enterovirus, HTLV-1, Sjogren's, vitamin E, and autoimmune panel (anti-Hu, anti CV2, anti amphiphysin) were normal. | Partial recovery |
| 20.Alabkal et al.28/Canada | 26/F | Pancreatitis | Pfizer(BNT162b2) | mRNA-based vaccine | First | 3 | 12 | Progressive saddle anesthesia and bilateral paresthesias, numbness, and intermittent allodynia ascending the plantar aspects of her feet up the posterior legs, extending to the perineum (Sensation to temperature, pinprick, and proprioception symmetrically decreased in S1-S4 distribution including the soles of the feet but with posterior leg sparing on objective testing) followed by lack of sensation with defecation, urination, wiping, and sexual intercourse. Also, deep tendon reflexes were brisk, and muscle strength of the right extensor hallucis longus was grading of 4+/5. |
Short segment T2W hyperintensity with diffuse enhancement at T5. | Incomplete/TM | MTP 5 gs. | CSF showed pleocytosis(19cells/ul, 98%lymphocyte), normal protein (0.34 g/l), IgG index (0.68), and negative infectious myelopathy panel (CSF bacterial and fungal cultures, acid-fast bacilli, cryptococcal antigen, HSV, VZV, enterovirus, Borrelia burgdorferi, and syphilis); NMO, MOG, rheumatologic panel (ANA, C3, C4, rheumatoid factor), serum protein electrophoresis, and HIV were negative. | Partial recovery |
| 21.Fitzsimmons et al.29/USA | 63/M | Negative | Moderna(m-RNA 1273) | mRNA-based vaccine | Second | 1 | 1.5 | Aching and slight numbness in the calves and ankles of both legs, more prominent in the left leg, episode of an involuntary erection followed by difficulty in ambulation, urinary retention, constipation, and progression of numbness to both buttocks and back of the thighs, and inability to stand. Also, patellar and Achilles reflexes were brisk. |
Increased T2W signal intensity in the distal thoracic spinal cord and conus with questionable associated enhancement. | NM/LETM | IVIg 0.5 g/kg/d for 2 days, MTP 5 gs followed by 1 mg/kg/day oral prednisolone. | CSF showed 3 nucleated cells, normal protein 37 mg/dl, and glucose 74 mg/dl; Autoimmune antibody panel NMO, MOG, vasculitis, and OCB were negative except for mild elevated anti-SSA antibody. | Partial recovery |
| 22.Nakano et al.30/Japan | 85/M | Negative | Pfizer(BNT162b2) | mRNA-based vaccine | Second | 3 | 12 | Progressive gait disturbance (proximal-dominant weakness (RT>LT), numbness in lower extremities (distal-dominant hypoesthesia in the lower extremities), and urinary retention. Also, hyporeflexia in both upper and lower limbs was noted. |
Longitudinally hyperintense lesion at the T3–5 vertebral levels on T2W imaging. | Incomplete/LETM | 2 courses of 0.5 gram MTP for 3 days followed by prednisolone 40 mg/day | CSF showed predominantly monomorphonuclear pleocytosis (11 cells/μL), with elevated protein levels (120 mg/dL), normal IgG index(0.67), normal MBP (58pg/ml), negative OCB and CSF cytology; NMO was negative. | No improvement |
| 23.Spataro et al.31/Italy | 20/F | Negative | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 3 | 12 | Muscle tenderness, weakness (muscle strength proximally (iliopsoas and quadriceps) and distally (ankle dorsiflexion and plantar flexion) 2–3/5), bilateral legs paresthesia (Tactile and pinprick sensation was decreased from T4 dermatome downward), and urinary retention. Also, mild spasticity, very brisk patellar and achilles tendon reflexes, and equivocal Babinski signs were noted bilaterally. | Normal brain and spinal MRI | Negative/ Negative | MTP 5 gs followed by betamethasone IM injection 4 mg for consecutive 15 days. | CSF showed increased protein, normal glucose, and 2 cells/µL (primarily lymphocytes). ANA and anti-dsDNA were negative. | Good recovery |
| 24.Cabral et al.32/Portugal | 33/M | Negative | Pfizer(BNT162b2) | mRNA-based vaccine | Second | 2 | 2 | Progressive weakness of the lower limbs (proximal muscle strengths of 4+ and 4 in the left and right legs, respectively), numbness in lower limbs (decreased thermic sensation below the T12), urinary retention, and nocturnal low back pain. Also, achilles deep tendon reflexes were brisk and plantar reflexes were equivocal. |
Normal brain and spinal MRI. | Negative/ Negative | Negative | CSF showed mononucleated pleocytosis (24 cells/ µL) and mild hyperproteinorrhachia (56 mg/dL); OCB was negative. | Good recovery |
| 25.Khan et al.33/Pakistan | 61/F | Asthma, HTN | Sinopharm (BBIBP-CorV) | Inactivated vaccine | Second | 4 | NM | Sudden onset of abnormal sensations (hypoesthesia in the upper and lower limbs) and weakness in bilateral upper and lower limbs. Neurological examination revealed upper motor neuron disease signs, including hyperreflexia, hypertonia, and spasticity, in both upper and lower extremities. |
A hyperintense lesion in C5–6 with contrast enhancement. | NM/TM | MTP 1 g/day | CSF showed normal analysis; NMO, ANA, and RF were negative. | Good recovery |
| 26.Maroufi et al.34/Iran | 31/F | Hyperthyroidism during pregnancy | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 21 | 14 | Progressive lower limbs paraparesis (strength +4/5 in the right lower limb and 3/5 in the left lower limb) associated with paresthesia and pain (decreased pinprick sensation in lower limbs, impaired proprioceptive sensation in bilateral toes, and saddle anesthesia) more severe on the left side as well as urinary retention and fecal incontinence. | Cord expansion and hyperintense lesion of the spinal cord from T10 to L1 segment with heterogeneous enhancement. | NM/LETM | MTP 1 gram for 7 days followed by tapering oral prednisolone 50 mg. | CSF showed pleocytosis(40 cells/ µL with 97% lymphocyte), elevated protein (62 mg/dl), normal sugar and lactate; ACE, OCB, NMO, MOG, paraneoplastic and rheumatologic panel were negative. | Good recovery |
| 27.Eom et al.35/Korea | 81/M | HTN, DM | Pfizer(BNT162b2) | mRNA-based vaccine | Second | 3 | 14 | Bilateral hand weakness (strength 2/5), numbness/paresthesia in both hands and fingers in association with exaggerated deep tendon reflexes. | High signal intensity and multifocal nodular enhancement from the C1 to C3. | Incomplete/TM | MTP 1 gram for 5 days followed by tapering oral prednisolone | CSF showed normal analysis; NMO, MOG, vasculitis, and paraneoplastic panel were negative. | Partial recovery |
| 28.Eom et al.35/Korea | 23/F | Negative | Pfizer(BNT162b2) | mRNA-based vaccine | First | 21 | 3 | Sudden onset tingling sensation (normal sensory exam) in both thighs followed by weakness of both legs (strength 1/5) and urinary retention. | High signal intensity without contrast enhancement at the anterior portion of the conus medullaris. | Incomplete/TM | MTP 1 gram for 5 days followed by tapering oral prednisolone. | CSF showed normal analysis; NMO, MOG, and vasculitis were negative. | Good recovery |
| 29.Netravathi et al.36/India | 50/F | NM | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 28 | 21 | Bilateral feet paresthesias (decreased distal vibration sense) in association with lower limbs weakness (finger extensor). | Focal cervical syrinx (C7-T1) and demyelination across C6. | NM/TM | MTP 1 gram for 5 days followed by tapering oral prednisolone. | CSF showed (2 cells/ µL with 100% lymphocyte), protein (28 mg/dl), and normal glucose. PCNA was mildly elevated. NMO, MOG, and ANCA were negative. | Good recovery |
| 30.Netravathi et al.36/India | 31/M | NM | ChAdOx1 nCoV-19 vaccine | Viral vector | First | 14 | 5 | Bladder disturbances followed by progressive numbness of lower limbs (decreased sensations below L1) and lower limbs weakness(strength1/5). | Long segment cervicothoracic hyperintensity with subtle enhancement. | NM/LETM | MTP 1 gram for 5 days and 7 sessions of PLEX followed by oral prednisolone and Rituximab 1 gram. | CSF showed PMN pleocytosis (370 cells/ µL), elevated protein (174 mg/dl), and normal glucose; NMO, MOG, and vasculitis panel were negative. | Good recovery |
| 31.Esechie et al.37/USA | 58/M | SCLC on atezolizumab and prophylactic whole brain radiation, HTN, BPH, back pain on the spinal stimulator | Moderna(m-RNA 1273) | mRNA-based vaccine | Second | 1 | 2 | Acute onset of lower extremities weakness (strength 0/5) and loss of sensation from his chest down to his lower extremities (diminished sensation to all modalities below the left T4 and the right T5 sensory dermatome). He also had urinary retention. | A large gadolinium-enhancing lesion in the cervicothoracic cord spanning from C7 to T7; MRI of the brain revealed small enhancing lesions in the left frontal lobe and both occipital lobes consistent with metastasis to the brain. | Complete/ LETM | MTP 1 gram for 5 days and 3 sessions of PLEX. | CSF showed WBC (25 cells/μl), protein 94 mg/dl, normal glucose, negative OCB, and normal IgG index; NMO, MOG, and infectious myelopathy panel were negative. | Partial recovery |
Complete and incomplete are defined as the entire and partial width of the spinal cord being affected, respectively.
LETM is defined as a spinal cord lesion extending over three or more vertebral segments.
We consider the modified Rankin scale (MRS) at the last follow-up of the reported patients as an indicator of he rate of recovery (MRS < 3 as a good recovery, and MRS ≥3 as a partial recovery).
TM: transverse myelitis; MRI: magnetic resonance imaging; F: female; C: cervical; LETM: longitudinally extensive transverse myelitis; IVIg: intravenous immunoglobulin; PLEX: plasma exchange; CSF: cerebrospinal fluid; NMO: neuromyelitis optica; MOG: myelin oligodendrocyte glycoprotein; OCB: oligoclonal bands; M: male; DM: diabetes mellitus; HTN: hypertension; IHD: ischemic heart disease; MTP: pulse methylprednisolone; T2W: T2-weighted; T: thoracic; L: lumbar; NM: not mentioned; HSV: herpes simplex virus; VZV: varicella-zoster virus; EBV: Epstein-Barr virus; CMV: cytomegalovirus; LT: left; RT: right; HHV-6: human herpesvirus 6; HIV: human immunodeficiency virus; HTLV-1: human T-lymphotropic virus type 1; FDG-PET scan: fluorodeoxyglucose-positron emission tomography scan; CAD: coronary artery disease; CKD: chronic kidney disease; SARS-CoV-2: severe acute respiratory syndrome-coronavirus-2; VDRL: venereal disease research laboratory test; ACE: angiotensin-converting enzyme; HLP: hyperlipidemia; TSH: thyroid stimulating hormone; SPEP: serum protein electrophoresis; UPEP: urine protein electrophoresis; ESR: erythrocyte sedimentation rate; RPR: Rapid plasma Reagin; ANA: anti-nuclear antibody; S: sacral; MBP: myelin basic protein; RF: rheumatoid factor; PCNA: proliferating cell nuclear antigen; SCLC: small cell lung cancer; BPH: benign prostatic hyperplasia.
A total of 31 cases were reported from 15 countries: 7 cases from the USA; 3 cases from Germany, India, and Japan; 2 cases from Italy, Iran, Korea, and Taiwan; and 1 case from each Brazil, Canada, Malaysia, Pakistan, Portugal, Turkey, and UK. 12 cases occurred after ChAdOx1 nCoV-19(Oxford-AstraZeneca) vaccine, 8 cases after Pfizer (BNT162b2) vaccine, 7 cases after Moderna (m-RNA 1273), 3 cases after Sinopharm (BBIBP-CorV), and 1 case after Janssen (Johnson & Johnson) vaccine. Two patients (patient 3 and patient 6) decided to receive a second dose of vaccine after the development of ATM following the first dose. Because these two patients experienced myelitis post-ChAdOx1 nCoV-19(Oxford-AstraZeneca) vaccination, they administered mRNA-based vaccine 5-week (patient 3) and 14-week (patient 6) after the first dose.
The mean age of the patients was 52±19 years. The frequency of females and males was 17/31(55%) and 14/31(45%), respectively. In 24/31(77%) and 7/31(23%) cases, neurological symptoms started following the first and second doses of the vaccine, respectively.
9 out of 31 patients (29%) had a previous history of autoimmune disease, including five diabetes mellitus (DM), one atopic dermatitis, one asthma, one sarcoidosis, one hyperthyroidism, and one hypothyroidism. Besides, four patients had a history of malignancy, including breast, prostate, cervix, and small cell lung cancer (patients 12, 15, 19, and 31). The other comorbidities were as follows: hypertension (7/31), coronary artery disease (2/31), hyperlipidemia (2/31), B12 deficiency (1/31), neuropathy (1/31), pancreatitis (1/31), chronic kidney disease (1/31), alcoholic liver cirrhosis (1/31), and hyperuricemia (1/31).
Considering clinical symptoms/signs spectrum, 2/31(6%); 4/31(13%); 7/31(23%); 1/31(3%); 1/31(3%) and 16/31(52%) patients had motor; sensory; motor-sensory; motor-sphincter; sensory-sphincter; and motor-sensory-sphincter symptoms/signs, respectively. The Modified Rankin Score (MRS) at admission was 3.7 ± 1.2. The median time interval between vaccination and development of neurological symptoms/signs was eight days (1–28 days) (data available for 31/31 cases), and the average time between starting neurological symptoms and reaching maximal disability was seven days (1–21 days) (data available for 24/31 patients).
Two cases (patients 6 and 14) had facial palsy in addition to the TM; patient 6 had facial palsy one week before the development of TM, and patient 14 developed facial palsy during the treatment course of TM.
In 9/31 (29%) of patients, there was a history of flu-like symptoms, such as fever, chills, body aching, fatigue, and cough following vaccination, and 22/31 (71%) reported cases had no history of constitutional symptoms after vaccine administration.
According to TMCWG diagnostic criteria, 23 patients had a “definite TM” diagnosis, 7 cases were “possible TM”, and one patient was “suspected TM”.
3.4. CSF and laboratory findings
As shown in Table 1 in detail, CSF analysis was performed in all patients, which showed pleocytosis in 15/31 patients (10 cases had mononuclear pleocytosis, 4 cases had polymorphonuclear pleocytosis, and an undetermined type of pleocytosis in one case (patient 31). In 8 out of 31 patients, there was elevated protein without pleocytosis in the CSF analysis. Besides, 8 out of 31 patients had a completely normal CSF study. CSF-specific oligoclonal bands (OCB) were assessed in 21/31 patients and showed positive results in 3 cases (patients 13, 17, and 18). Myelin basic protein (MBP) was measured in 2/31 patients, which was positive in one case (patient 15). Moreover, infectious etiologies for myelitis were investigated in 17 out of 31 patients and were negative in all cases.
Serological work-up for autoimmune disease was investigated in all patients. Anti-aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies were checked in 29 and 23 out of 31 patients, respectively, and were negative in all cases. Vasculitis and rheumatologic screening were evaluated in 26/31 patients, which were negative in all patients except for mildly elevated anti-SS-A and weakly positive proliferating cell nuclear antigen (PCNA), each in one patient (patients 21 and 29, respectively). Paraneoplastic and anti-neuronal antibodies panel were investigated in 10/31 patients, which showed negative results. B12 serum level was checked in 5/31 patients and showed mild deficiency in 2 cases (patients 9 and 16). Angiotensin-converting enzyme (ACE) was assessed in 4 patients, which returned negative results. Moreover, FDG-PET was performed on one patient with a previous history of pulmonary sarcoidosis (patient 8) and showed no active sarcoidosis nodule.
3.5. Radiological findings
In 29/31(93.5%) of patients, spinal MRI showed a hyperintense lesion on T2-weighted (T2W) sequence, and 2 out of 31 patients had negative spinal MRI (patients 23 and 24). The most frequently affected spinal subsegment was thoracic (19/29, 65.5%), followed by cervical (14/29, 48.3%). In 20/29 (69%) patients, myelitis was longitudinally extensive and extended over an average of 5.4 ± 3.4 vertebral body segments (range from 3 to 17 vertebral segments). 18 out of 29(62%) patients showed T2W hyperintense lesions with gadolinium enhancement. Besides, 5 out of 22 (22.7%) and 17 out of 22 (77.3%) patients had complete and partial myelitis, respectively. Brain MRI was unremarkable in all patients except for one case (patient 31), which was consistent with a diagnosis of brain metastasis. Details are provided in Table 1.
3.6. Treatment and outcome
29 out of 31 (93.5%) patients received one or more types of immunotherapy, and two cases (patients 17 and 24) were managed conservatively (“wait and watch”). 20 out of 29 cases (69%) only received pulse methylprednisolone (MTP). The pulse MTP followed by plasma exchange was administered on 6/29(21%). One patient (patient 30) received the combination of three immunomodulatory treatments (MTP + plasma exchange + rituximab) (Table 1). Furthermore, in two patients (patients 9 and 16) B12 was prescribed in addition to the immunotherapy (Table 1). Data on the follow-up of the patients were mostly limited to the hospital course or the first two weeks after discharge.21 out of 31 (67.7%) had a good recovery after treatment, and 10 out of 31 (32.3%) had poor or partial responses to treatment. During the hospitalization, two patients died due to pneumosepsis (patient 22) and cardiac arrest due to pulmonary thromboembolism and cardiac arrhythmia (patient 31).
3.7. Prognostic factors of COVID-19 vaccine-associated myelitis
As shown in Table 2 , higher age (Pearson correlation coefficient 0.47; pvalue=0.003), higher MRS at admission (Pearson correlation coefficient 0.60; pvalue=0.001), and second dose of vaccine (Pearson correlation coefficient 0.45; pvalue=0.02) presented a worst prognostic effects on the outcome of patients with COVID-19 vaccine-associated myelitis. On the contrary, the ChAdOx1 nCoV-19 vaccine (Pearson correlation coefficient −0.40; pvalue=0.04) was associated with a better outcome. After performing multivariate forward stepwise logistic regression analysis, age (OR 1.09, 95%CI 1.01–1.18, pvalue 0.02), and MRS at admission (OR 17.67, 95%CI 1.46–213.76, pvalue 0.024) were two independent risk factors for poor recovery from COVID-19 vaccine-associated myelitis.
Table 2.
Prognostic factors of COVID-19 vaccine-associated myelitis.
| Variables | Good recovery* N(%) or Mean±SD | Poor recovery* N(%) or Mean±SD | OR(95%CI) | Pvalue |
|---|---|---|---|---|
| Age | 46.3 ± 16.9 | 65.5 ± 17.7 | NA | 0.003 |
| Sex Male Female |
9/21(42.8) 12/21(57.2) |
5/10(50) 5/10(50) |
0.75(0.16–3.4) |
1.00 |
| Type of vaccine ChAdOx1 nCoV-19 Pfizer(BNT162b2) Moderna Sinopharm Janssen |
11/21(52.4) 3/21(14.3) 5/21(23.8) 1/21(4.75 1/21(4.75) |
1/10(10) 5/10(50) 2/10(20) 2/10(20) 0/10(0) |
0.1(0.01–0.95) 6(1.05–34.2) 0.8(0.13–5.1) 5(0.4–63.2) 0.95(0.86–1.05) |
0.04 0.07 1.00 1.00 1.00 |
| Dose of vaccine First Second |
19/21(90.5) 2/21(9.5) |
5/10(50) 5/10(50) |
9.5(1.4–64.4) |
0.02 |
| Interval between symptoms onset to nadir <7 days ≥7 days |
8/14(57.2) 6/14(42.8) |
5/10(50) 5/10(50) |
1.07(0.2–5.77) |
1.00 |
| Clinical symptoms/signs spectrum Motor Sensory Motor-Sensory Sensory-Sphincter Motor-Sphincter Motor-Sensory- Sphincter |
1/21(4.75) 4/21(19.1) 5/21(23.8) 1/21(4.75) 0/21(0) 10/21(47.6) |
1/10(10) 0/10(0) 2/10(20) 0/10(0) 1/10(10) 6/10(60) |
2.2(0.12–39.6) 0.81(0.65–0.99) 0.8(0.12–5.1) 0.95(0.86–1.05) 1.1(0.9–1.36) 1.65(0.36–7.6) |
1.00 0.27 1.00 1.00 0.32 0.70 |
| CSF analysis CSF pleocytosis (>5 cells/µl) CSF protein >45 mg/dl |
10/21(47.6) 13/21(61.9) |
4/10(40) 5/10(50) |
0.73(0.16–3.4) 0.61(0.13–2.8) |
1.00 0.70 |
| MRI GD enhancing lesion Yes No |
13/21(61.9) 8/21(38.1) |
5/10(50) 5/10(50) |
0.61(0.13–2.8) |
0.70 |
| LETM Yes No |
13/21(61.9) 8/21(38.1) |
7/10(70) 3/10(30) |
1.43(0.28–7.2) |
1.000 |
| Width of the spinal cord involvement Complete Partial |
1/13(7.7) 12/13(92.3) |
4/9(44.4) 5/9(55.6) |
9.6(0.84–108.7) |
0.11 |
| Treatment MTP MTP+PLEX MTP+IVIG PLEX+IVIG MTP+PLEX+RTX No treatment |
15/21(71.5) 2/21(9.5) 1/21(4.75) 0/21(0) 1/21(4.75) 2/21(9.5) |
5/10(50) 4/10(40) 0/10(0) 1/10(10) 0/10(0) 0/10(7.7) |
0.4(0.08–1.9) 6.3(0.92–43.6) 0.95(0.86–1.05) 1.1(0.9–1.4) 0.95(0.86–1.05) 0.9(0.78–1.04) |
0.42 0.06 1.00 0.32 1.00 1.00 |
| MRS at admission | 3.2 ± 1.1 | 4.7 ± 0.48 | NA | 0.001 |
Good recovery and poor recovery means MRS<3 and MRS≥3, respectively, at the follow-up period.
SD: standard deviation; OR: odds ratio; CI: confidence interval; NA: not applicable; COVID-19: coronavirus disease 2019; CSF: cerebrospinal fluid; MRI: magnetic resonance imaging; GD: gadolinium; LETM: longitudinally extensive transverse myelitis; MTP: pulse methylprednisolone; PLEX: plasma exchange; IVIG: intravenous immunoglobuline; RTX: rituximab; MRS: Modified Rankin Score.
4. Discussion
The vaccine-associated neurological complications were first reported in 1885 with Pasteur's rabies vaccine. In 1977, 21 cases of GBS and central nervous system (CNS) demyelination, including ADEM, chronic leukoencephalitis, and myelitis, were reported in association with the suckling mouse brain (SMB) rabies vaccine (Toro et al., 1977). Besides, CNS demyelination has been reported in association with other vaccines, including influenza, human papilloma virus, hepatitis A or B, measles, rubella, yellow fever, anthrax, meningococcus, and tetanus (Karussis and Petrou, 2014). The concern about the potential association between CNS inflammation and the COVID-19 vaccine increased in 2020 when the interim analysis of four randomized clinical trials showed 3 cases of ATM associated with the ChAdOx1 nCoV-19 vaccine. Two patients were considered previously unrecognized multiple sclerosis (unlikely to be related to the vaccine), while the other was deemed possibly related to the vaccine (Voysey et al., 2021).
This study is the first systematic review that comprehensively investigated the clinical and radiological characteristics as well as prognostic factors in individuals with post-COVID-19 vaccination idiopathic TM. To the best of our knowledge, thus far, no study focusing only on idiopathic TM following COVID-19 vaccination exists, and only one systematic review concerning CNS demyelination (MS, NMOSD, ADEM, and TM) in association with the COVID-19 vaccine has been reported in the literature (Ismail and Salama, 2022).
In our study, age rendered a significant role in the outcome of patients with myelitis, and older individuals had a poor prognosis. Similarly, age had a negative prognostic effect on the individuals with ATM in the pre-COVID-19 era (Annunziata et al., 2019). Our results revealed that although post-COVID-19 vaccination ATM was more common in females, sex did not have a significant prognostic effect on the outcome. These findings are in line with Annunziata et al. suggestions in patients with ATM in the pre-COVID-19 era (Annunziata et al., 2019). On the contrary, Schulte et al. suggested that males were more commonly affected in COVID-19 infection-associated ATM (Schulte et al., 2021). This discrepancy might be explained by the slight differences between the pathophysiologic mechanisms of post-vaccination and post-COVID-19 infection ATM. The major mechanism in the pathogenesis of ATM in the pre-COVID-19 era and post-vaccination is the aberrant activation of the autoimmune processes, which is more frequently seen in females (E Khan et al., 2022; Shoenfeld and Aron-Maor, 2000). However, COVID-19 infection can trigger myelitis through direct invasion of the spinal cord in addition to the activation of immune-mediated processes (Chu et al., 2020).
Our study showed that ATM was more frequently associated with Oxford-AstraZeneca (chimpanzee adenovirus vector-based) vaccine. The exact pathophysiologic mechanisms for vaccine-induced ATM remain to be elucidated; however, vaccine-related factors in alliance with the genetic susceptibility of the individuals have been suggested. The molecular mimicry between infectious antigens and self-antigens is one of the main proposed mechanisms for developing demyelination post-vaccination. Besides, adjuvants (substances that increase the antigen-specific immune responses) could aberrantly provoke innate and adaptive immune responses. Toll-like receptors TLR-7 and TLR-8 promote type I interferon production and polyclonal activation of B lymphocytes, resulting in the expansion of autoreactive T cells. The activation of autoreactive T cells accompanied by macrophages secreting cytokines cause further recruitment of T-helper cells and consequently induces local inflammation (Shoenfeld and Aron-Maor, 2000; Blank et al., 2007; Lehmann et al., 1992; Murali-Krishna et al., 1998; Aharon-Maor and Shoenfeld, 2000; Velikova and Georgiev, 2021). Moreover, mRNA vaccines may promote several pro-inflammatory cascades through elevation in the serum level of inflammatory cytokines (Talotta, 2021). Recent studies emphasized the pathogenic role of Interleukin-6(IL-6) and IL-17 in myelitis (Kerr and Ayetey, 2002; Graber et al., 2008). IL-17, through the regulation of cytokines, including tumor necrosis factor α (TNF- α), IL-1β, and IL-6, stimulates astrocytes to produce IL-6 (Vera-Lastra et al., 2013). The elevation of the level of IL-6 in the CSF of patients with myelitis predicts a poor prognosis (Graber et al., 2008). Our analysis demonstrated that the second dose of vaccine rendered a poor prognosis. One proposed suggestion for this finding is increasing the serum level of IL-6, particularly after administering the second dose of the vaccine (Bergamaschi et al., 2021). As mentioned earlier, the elevation of the IL-6 in the CSF of patients with myelitis is associated with poor recovery. Our study's median time interval between vaccination and initiation of neurological symptoms/signs is similar to the interval between the first clinical presentation of COVID-19 infection and myelitis signs/symptoms (Schulte et al., 2021). However, the interval between the first neurological presentations to the maximal disability is lower in cases of COVID-19 infection myelitis compared with vaccine-associated myelitis (2 days vs. 7 days) (Schulte et al., 2021). Comparing the severity of neurological symptoms in post-vaccination myelitis with COVID-19 infection-associated myelitis (Schulte et al., 2021; Hooshmandi et al., 2022) revealed that sensory symptoms/signs had a frequency of 13% and 5% in post-vaccination and post COVID-19 infection individuals, respectively. On the other hand, motor-sensory-sphincter symptoms/signs were more common in post-COVID-19 infection than post-vaccination myelitis (65% vs. 55%). Overall, it seems that neurological symptoms/signs are more severe and reach their maximal intensity in a shorter period in post-COVID-19 infection individuals than in those with post-vaccination myelitis. In this study, although the spectrum of clinical symptoms/signs had no significant prognostic effects on the outcome of patients with ATM, the severity of clinical symptoms/signs at admission assessed via the MRS scale presented as a risk factor for recovery from COVID-19 vaccine-associated myelitis. In contrast to our findings, Annunziata et al. (Annunziata et al., 2019) suggested that motor-sensory symptoms with or without sphincter dysfunction at admission were associated with a poor prognosis in the follow-up period. Besides, Cobo Calvo et al. (Cobo Calvo et al., 2013) proposed that the presence of urinary sphincter dysfunction predicted a worse outcome. The small sample size of this study might affect our findings; as a result, further studies with a larger sample size are essential to substantiate the exact effect of the clinical symptoms and signs spectrum on the prognosis of patients with COVID-19 vaccine-associated ATM. Our results showed that the gadolinium-enhancing lesion, the length, and the width of the lesion in the spinal cord did not affect the prognosis and degree of recovery, consistent with the previous findings (Annunziata et al., 2019). However, Cobo Calvo et al. (Cobo Calvo et al., 2013) suggested that patients with LETM had a poor prognosis and greater disability. This discrepancy might be due to the differences in the prognosis assessment, which is usually based on qualitative clinical parameters, MRS cut off point (we used MRS<3 as a marker of good recovery, similar to Annunziata et al., but Cobo Calvo et al. used MRS<2), and duration of follow-up. This study indicated that higher age and MRS at admission were associated with a poor outcome. Therefore, older individuals with severe clinical symptoms/signs at the onset of the disease had the worst prognosis. Identifying the risk factors for poor prognosis could help deliver more aggressive therapy promptly to individuals with COVID-19 vaccine-associated ATM who have poor prognostic factors to reduce morbidity and mortality at the follow-up.
Comparing the incidence of idiopathic TM post-COVID-19 vaccine with the incidence of 0.5 per million population per year of COVID-19 infection-associated TM (Román et al., 2021) suggests that the risk of TM related to COVID-19 vaccine are significantly lower than COVID-19 infection. Besides, vaccination is the most effective tool in preventing severe COVID-19 disease and the following complications (FDA authorizes Johnson and Johnson COVID-19 vaccine 2021). Moreover, our results showed that the intensity of myelitis attack post-vaccination is less severe than post-COVID-19 infection. Consequently, the beneficial effects of the COVID-19 vaccine in confronting the pandemic exceed the potential risk of spinal cord inflammation. However, close medical surveillance of the potential neurologic adverse effects is crucial to accurately determine these vaccines’ safety profile.
4.1. Limitation
One limitation of the present study is that most existing studies about COVID-19 vaccine-associated myelitis are case reports, case series, and cross-sectional studies with different duration of follow-up. Consequently, high-quality cohort and case-control studies with large sample sizes and relatively the same follow-up time are necessary to accurately identify the prognostic factors in COVID-19 vaccine-associated ATM. Another limitation is the short duration of follow-up (most of the available data about the follow-up of patients with COVID-19 vaccine-associated ATM is limited to the hospital course or the first two weeks after discharge). Considering that 13% of patients with idiopathic ATM may convert to MS in the long-term follow-up (Cobo Calvo et al., 2013) and the degree of the recovery from myelitis attack may be changed in the follow-up period, further studies with a longer duration of follow-up are warranted.
5. Conclusion
The present study is a systematic review concerning the clinical/radiological characteristics and prognostic factors of patients with post-COVID-19 vaccination myelitis. This study showed that higher age and MRS at admission are the worst prognostic factors associated with a higher degree of disability in the short-term follow-up. The COVID-19 vaccine-associated ATM patients who had one or both of these risk factors are at risk of poor recovery from myelitis attacks and require a timely and more aggressive therapeutic approach.
Author contributions
All authors contributed to the study conception and design. Material preparation, and data collection were performed by NK, MR, MS, and HM. Data analysis was performed by VRO. The first draft of the manuscript was written by VRO, NK, MR, MS, and HM. Review and critique of the manuscript was performed by VRO and MAS. All authors read and approved the final version of the manuscript.
Funding
None.
Ethical standard statement
Ethical board approval is not applicable in this case report. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.104032.
Appendix. Supplementary materials
References
- Frohman E.M., Wingerchuk D.M. Clinical practice. Transverse myelitis. N. Engl. J. Med. 2010;363(6):564–572. doi: 10.1056/NEJMcp1001112. [DOI] [PubMed] [Google Scholar]
- Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499–505. doi: 10.1212/wnl.59.4.499. [DOI] [PubMed] [Google Scholar]
- Mellet J., Pepper M.S. A COVID-19 vaccine: big strides come with big challenges. Vaccines (Basel) 2021;9(1):39. doi: 10.3390/vaccines9010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (Azd1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet Lond Engl. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R.K., Paliwal V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2021;31:1–38. doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID -19 vaccines. Ann. Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Zhong J., Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J. Med. Virol. 2021;93(12):6486–6495. doi: 10.1002/jmv.27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: Oxford researchers halt vaccine trial while adverse reaction is investigated. BMJ. 2020;9:m3525. doi: 10.1136/bmj.m3525. [DOI] [PubMed] [Google Scholar]
- Piyasirisilp S., Hemachudha T. Neurological adverse events associated with vaccination. Curr. Opin. Neurol. 2002;15(3):333–338. doi: 10.1097/00019052-200206000-00018. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Annunziata P., Masi G., Cioni C., Gastaldi M., Marchioni E., D'amico E., et al. Clinical, laboratory features, and prognostic factors in adult acute transverse myelitis: an Italian multicenter study. Neurol. Sci. 2019;40(7):1383–1391. doi: 10.1007/s10072-019-03830-6. [DOI] [PubMed] [Google Scholar]
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. Chapter 7: systematic reviews of etiology and risk. In: aromataris E, Munn Z (Editors) JBI Man. Evid. Synth. 2020 JBI. [Google Scholar]
- Munn Z., Barker T.H., Moola S., Tufanaru C., Stern C., McArthur A., et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- Sriwastava S., Shrestha A.K., Khalid S.H., Colantonio M.A., Nwafor D., Srivastava S. Spectrum of neuroimaging findings in post-covid-19 vaccination: a case series and review of literature. Neurol. Int. 2021;13(4):622–639. doi: 10.3390/neurolint13040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepahvand M., Yazdi N., Rohani M., Emamikhah M. Cervical longitudinally extensive myelitis after vaccination with inactivated virus-based COVID-19 vaccine. Radiol. Case Rep. 2022;17(2):303–305. doi: 10.1016/j.radcr.2021.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.Y., Yusof Khan A.H.K., Mohd Yaakob M.N., Abdul Rashid A.M., Loh W.C., Baharin J., et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol. 2021;21(1):395. doi: 10.1186/s12883-021-02427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa D.G., Cañete L.A.Q., dos Santos G.A.C., de Oliveira R.V., Brandão C.O., da Cruz L.C.H. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: cause or coincidence? Clin. Imaging. 2021;80:348–352. doi: 10.1016/j.clinimag.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegezzi E., Ravaglia S., Buongarzone G., Bini P., Diamanti L., Gastaldi M., et al. Acute myelitis and ChAdOx1 nCoV-19 vaccine: casual or causal association? J. Neuroimmunol. 2021;359 doi: 10.1016/j.jneuroim.2021.577686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.T., Tsai M.J., Chen Y.H., Hsu C.F. Acute transverse myelitis after covid-19 vaccination. Medicina (Lithuania) 2021;57(10):1010. doi: 10.3390/medicina57101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notghi A.A.A., Atley J., Silva M. Lessons of the month 1: longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin. Med. (Lond.) 2021;21(5):e535–e538. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.J., Tseng H.P., Lin C.L., Shiu J.S., Lee M.H., Liu C.H. Acute transverse myelitis following covid-19 vaccination. Vaccines (Basel) 2021;9(9):1008. doi: 10.3390/vaccines9091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra H.S., Gupta P., Prabhu V., Kumar Garg R., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021;114(8):591–593. doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J. Neurol. 2022;269(3):1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem N.Ş., Demirci S., Özel T., Mamadova K., Karaali K., Çelik H.T., et al. Acute transverse myelitis after inactivated covid-19 vaccine. Ideggyogy Sz. 2021;74(7–08):273–276. doi: 10.18071/isz.74.0273. [DOI] [PubMed] [Google Scholar]
- Hirose S., Hara M., Koda K., Natori N., Yokota Y., Ninomiya S., et al. Acute autoimmune transverse myelitis following COVID-19 vaccination: a case report. Med. (Baltimore). 2021;100(51):e28423. doi: 10.1097/MD.0000000000028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir N., Koorapati G., Prasad S., Jeelani H.M., Sherchan R., Shrestha J., et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021;13(7):e16624. doi: 10.7759/cureus.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaue N., Yoshida A., Yamanishi Y., Tada S., Ando R., Hosokawa Y., et al. A case of refractory longitudinally extensive transverse myelitis after severe acute respiratory syndrome coronavirus 2 vaccination in a japanese man. Intern. Med. 2021;61(5):739–742. doi: 10.2169/internalmedicine.8747-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa P., Shah F.A., Braford M., Patel K., Madey J. Neuromyelitis optica in a healthy female after severe acute respiratory syndrome coronavirus 2 mRNA-1273 vaccine. Cureus. 2021;13(9):e17961. doi: 10.7759/cureus.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen L.D., Doubrovinskaia S., Mooshage C., Jordan B., Purrucker J., Haubner C., et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur. J. Neurol. 2022;29(2):555–563. doi: 10.1111/ene.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P., Trefts L. Transverse myelitis 48 h after the administration of an mRNA COVID 19 vaccine. Neuroimmunol. Rep. 2021;1 [Google Scholar]
- Alabkal J., Rebchuk A.D., Lyndon D., Randhawa N. Incomplete subacute transverse myelitis following vaccination with Pfizer-BioNTech COVID-19 mRNA vaccine: a case report. Cureus. 2021;13(12):e20460. doi: 10.7759/cureus.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons W., Nance C.S. Sudden onset of myelitis after COVID-19 vaccination: an under-recognized severe rare adverse event. SSRN J. 2021;7 [Google Scholar]
- Nakano H., Yamaguchi K., Kawabata K., Asakawa M., Matsumoto Y. Acute transverse myelitis after BNT162b2 vaccination against COVID-19: report of a fatal case and review of the literature. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2021.120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro R., Fisco G., La Bella V. Reversible radiculomyelitis after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2022;15(2) doi: 10.1136/bcr-2021-247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G., Gonçalves C., Serrazina F., Sá F. MRI negative myelitis induced by Pfizer-BioNTech COVID-19 vaccine. J. Clin. Neurol. 2022;18(1):120–122. doi: 10.3988/jcn.2022.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Khattak A.A., Rafiq N., Amin A., Abdullah M. Interstitial lung disease and transverse myelitis: a possible complication of COVID-19 vaccine. Cureus. 2022;14(2):e21875. doi: 10.7759/cureus.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroufi S.F., Naderi Behdani F., Rezania F., Tanhapour Khotbehsara S., Mirzaasgari Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: case report and review of literature. Hum. Vaccin. Immunother. 2022:1–4. doi: 10.1080/21645515.2022.2040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom H., Kim S.W., Kim M., Kim Y.E., Kim J.H., Shin H.Y., et al. Case reports of acute transverse myelitis associated with mRNA vaccine for COVID-19. J. Korean Med. Sci. 2022;37(7):e52. doi: 10.3346/jkms.2022.37.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netravathi M., Dhamija K., Gupta M., Tamborska A., Nalini A., Holla V.V., et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult. Scler. Relat. Disord. 2022;60 doi: 10.1016/j.msard.2022.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esechie A., Fang X., Banerjee P., Rai P., Thottempudi N. A case report of longitudinal extensive transverse myelitis: immunotherapy related adverse effect vs COVID-19 related immunization complications. Int. J. Neurosci. 2022:1–4. doi: 10.1080/00207454.2022.2050907. [DOI] [PubMed] [Google Scholar]
- Toro G., Vergara I., Román G.C. Neuroparalytic accidents of antirabies vaccination with suckling mouse brain vaccine. Clinical and pathologic study of 21 cases. Arch. Neurol. 1977;34:694–700. doi: 10.1001/archneur.1977.00500230064011. [DOI] [PubMed] [Google Scholar]
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelination. Autoimmun. Rev. 2014;13:215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Ismail I.I., Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte E.C., Hauer L., Kunz A.B., Sellner J. Systematic review of cases of acute myelitis in individuals with COVID-19. Eur. J. Neurol. 2021;28(10):3230–3244. doi: 10.1111/ene.14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Aron-Maor A. Vaccination and autoimmunity—‘vaccinosisʻ: a dangerous liaison? J. Autoimmun. 2000;14(1):1–10. doi: 10.1006/jaut.1999.0346. [DOI] [PubMed] [Google Scholar]
- Chu H., Chan J.F., Yuen T.T., Shuai H., Yuan S., Wang Y., et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M., Barzilai O., Shoenfeld Y. Molecular mimicry and auto-immunity. Clin. Rev. Allergy Immunol. 2007;32(1):111–118. doi: 10.1007/BF02686087. [DOI] [PubMed] [Google Scholar]
- Lehmann P.V., Forsthuber T., Miller A., Sercarz E.E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Aharon-Maor A., Shoenfeld Y. The good, the bad and the ugly of vaccination. Isr. Med. Assoc. J. 2000;2(3):225–227. [PubMed] [Google Scholar]
- Velikova T., Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021;41(3):509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to "potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases". Clin. Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D.A., Ayetey H. Immunopathogenesis of acute transverse myelitis. Curr. Opin. Neurol. 2002;15:339–347. doi: 10.1097/00019052-200206000-00019. [DOI] [PubMed] [Google Scholar]
- Graber J.J., Allie S.R., Mullen K.M., Jones M.V., Wang T., Krishnan C., et al. Interleukin–17 in transverse myelitis and multiple sclerosis. J. Immunol. 2008;196:124–132. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Vera-Lastra O., Medina G., Cruz-Dominguez M.D.P., Jara L.J., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfelds'syndrome): clinical and immunological spectrum. Expert Rev. Immunol. 2013;9:361–373. doi: 10.1586/eci.13.2. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C., Terpos E., Rosati M., Angel M., Bear J., Stellas D., et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021;36(6) doi: 10.1016/j.celrep.2021.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshmandi E., Foroughi A.A., Poursadeghfard M., KianiAra F., Ostovan V.R., Nazeri M. Transverse myelitis as a rare neurological complication of Coronavirus Disease 2019: a case report and literature review. Iran J. Med. Sci. 2022 doi: 10.30476/ijms.2022.92813.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo Calvo A., Mañé Martínez M.A., Alentorn-Palau A., Bruna Escuer J., Romero Pinel L., Martínez-Yélamos S. Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol. 2013;13:135. doi: 10.1186/1471-2377-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute transverse myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA authorizes Johnson & Johnson COVID-19 vaccine Med. Lett. Drugs Ther. 2021;63(1620):41–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.