Abstract
Background:
Diflunisal is a non-steroidal anti-inflammatory drug that stabilizes transthyretin (TTR) and reduces neurologic deterioration in patients with polyneuropathy caused by hereditary transthyretin amyloidosis (ATTRv).
Methods:
We conducted a retrospective cohort study of patients with wild-type transthyretin cardiac amyloidosis (ATTRwt-CM) treated with diflunisal for at least one year between 2009 and 2016 at the Boston University Amyloidosis Center. Baseline and one year follow up characteristics were measured, including plasma chemistries and echocardiography. Cox proportional hazards analysis assessed the primary outcome of all-cause mortality.
Results:
A total of 104 ATTRwt-CM patients were evaluated with 35 patients receiving diflunisal. Patients in the diflunisal group were younger (73.8 years vs 76.8 years, p = 0.034), with lower B-type natriuretic peptide (BNP, 335 +/− 367 vs 520 +/− 296 pg/mL, p = 0.006), similar troponin I (0.1 +/− 0.1 vs 0.2 +/− 0.3 ng/mL, p = 0.09), and better renal function (eGFR 67 +/− 17 vs 53 +/− 18 mL/min/1.73m2, p = 0.0002) at baseline. Over a median follow-up of 3.2 years, 52 deaths occurred. Diflunisal administration was associated with improved survival in unadjusted analysis (HR 0.13, 95% CI 0.05 – 0.36, p < 0.001) that persisted after adjustment for age, baseline BNP, eGFR, troponin I, interventricular septal thickness, and left ventricular ejection fraction (HR 0.18, 95% CI 0.06 – 0.51, p = 0.0006). Over the observation period, no significant changes in BNP, troponin I, interventricular septal thickness or left ventricular ejection fraction were observed with diflunisal treatment. A total of 14 patients (40%) discontinued diflunisal in this study, but only 3 within the first year. Mean eGFR in treated patients was 59 ml/min/1.73m2 at 1 year (change from baseline p=0.03).
Conclusion:
Diflunisal administration in ATTRwt-CM was associated with improved survival and overall stability in clinical and echocardiographic markers of disease with decrement renal function.
Keywords: cardiac amyloidosis, transthyretin, diflunisal, heart failure, echocardiography
Introduction
Transthyretin cardiac amyloidosis (ATTR-CM) is a restrictive cardiomyopathy resulting from amyloid deposits composed of misfolded transthyretin (TTR) protein. ATTR-CM can result from either genetically normal (ATTRwt) or genetically abnormal variant (ATTRv) protein. Amyloidogenic TTR tetramers dissociate into monomers, misfold, and aggregate as amyloid fibrils that deposit primarily in the heart, soft tissue, and the peripheral and autonomic nervous system. ATTRwt amyloidosis is an under recognized cause of heart failure in older individuals (over age 60 years) that may account for 5% to around 15% of heart failure with preserved ejection fraction (HFpEF),with a described male predominance [1–6]. ATTRwt amyloidosis is characterized by progressive heart failure, atrial and ventricular arrhythmias, advanced conduction disease, and eventually death with median survival of approximately 3.6 years if untreated [7].
Previously treatable only by organ transplantations, contemporary strategies for ATTRv polyneuropathy involve either TTR gene silencing or TTR tetramer stabilization [8]. Diflunisal is a non-steroidal anti-inflammatory drug (NSAID) that stabilizes TTR tetramer in vitro [9,10]. Clinically, diflunisal inhibited the rate of neurologic deterioration in patients with ATTRv polyneuropathy in a placebo-controlled randomized trial [11]. As diflunisal can be safely administered to selected patients with ATTR-CM [12], we recently reported that diflunisal stabilizes echocardiographic parameters of left ventricular function [13]. Furthermore, diflunisal confers enhanced stability to TTR tetramers as assessed by serum TTR levels that associate with attenuation of cardiac biomarker and left ventricular ejection fraction decline [14]. Retrospective cohort data suggested that a TTR stabilization strategy with either diflunisal or the recently approved therapy tafamidis (Vyndaqel/Vyndamax, Pfizer, Inc.) reduced disease progression and increased survival in patients with ATTR-CM[15]. Most recently, tafamidis was shown to improve survival and hospitalization rates for heart failure among patients with ATTR-CM in a prospective, randomized, placebo-controlled clinical trial [16]. Tafamidis, however, costs $225,000 per year rendering it the most expensive cardiovascular therapeutic yet marketed. We hypothesized that generic diflunisal may favorably impact survival, providing an inexpensive alternative to tafamidis treatment for selected patients with ATTRwt-CM.
Methods
Study population
We conducted a retrospective cohort study of 104 patients with ATTRwt-CM referred to the Boston University Amyloidosis Center between 2009 and 2016. Diagnosis of ATTRwt-CM was confirmed by cardiac biopsy with Congo red staining and immunohistochemistry/mass spectrometry confirming ATTR in 89 patients, by non-cardiac biopsy in addition to pyrophosphate scan in 9 patients, and pyrophosphate scan alone (with absence of a plasma cell dyscrasia) in 6 patients. Bidirectional TTR gene sequencing was performed in all patients confirming the absence of mutation. The decision to treat with diflunisal per standard clinical practice at our Center ensured eGFR > 45 mL/min/1.73 m2 and stable New York Heart Association (NYHA) class I-III heart failure. The administered dose of diflunisal was 250 mg twice daily. Reassessment of renal function was recommended within 1 to 2 weeks of treatment initiation, as per the treatment protocol at our center. Review of clinical records affirmed initiation of diflunisal treatment. Patients for whom diflunisal was administered for any duration were included in the diflunisal group. The control group included patients for whom diflunisal was not recommended, as well as those patients for whom there was missing data regarding initiation of diflunisal therapy even if recommended. Patients without baseline testing at our center, those enrolled in clinical trials and those for whom treatment recommendations could not be ascertained were not included in this study. Medical history, clinical characteristics, laboratory studies, and echocardiography parameters were collected from the electronic health record and assessed at baseline and at one year follow up, when available. Date of death was obtained from medical records or publicly available online obituaries. Participants provided written informed consent to participate in the study, which was approved by the Boston University Medical Campus Institutional Review Board and conformed with the principles of the Declaration of Helsinki.
Echocardiography
Echocardiograms were obtained at baseline and at one-year follow-up, or one-year post diflunisal initiation, as applicable. All echocardiograms were obtained on Philips iE33 (Philips Healthcare, Amsterdam, Netherlands) machines and images were reviewed on a Philips Xcelera viewer by a single echocardiographer (OS). Echocardiograms were assessed for end-diastolic interventricular septal thickness (IVS), inferolateral or posterior wall thickness (PWT), and left ventricular ejection fraction (LVEF), according to chamber quantification guidelines from the American Society of Echocardiography [17].
Statistical analysis
All statistical analyses were conducted using SAS (version 9.3). Univariable regression was employed to compare baseline demographic and clinical characteristics between patients who were treated with diflunisal and untreated controls. Log transformation was performed for BNP to account for non-normality. Differences in baseline characteristics between those treated with diflunisal vs. controls were determined by t-testing for continuous and chi-squared tests for categorical variables. Differences between baseline and 1-year variables was determined by pairwise t-testing. Kaplan-Meier estimates were used to estimate survival distributions in the two risk groups. Due to the small number of events that occurred after year six of follow-up, we censored the survival data at year six. Cox proportional hazards multivariable regression models were developed to assess differences between the two groups in all-cause mortality while adjusting for baseline covariates including age, BNP, eGFR, troponin I, interventricular septal thickness, and LVEF. To minimize the risk of overfitting, additional models with fewer variables that included NYHA functional class, diuretic use, posterior wall thickness, and angiotensin converting enzyme inhibitor/receptor blocker administration were developed. Linear regression was performed to assess for the association of survival with covariates. In order to address residual confounding, we compared survival in selected patients with eGFR > 45 mL/min/1.73 m2 and NYHA functional class I or II at first visit in each arm. To address missing data regarding diflunisal administration in a small number of patients (n=9), a sensitivity analysis was performed excluding subjects without follow-up information and reassessing adjusted differences in mortality between the diflunisal and control cohorts.
Results
Patient characteristics
The study population (n=104) was composed of 101 male patients (97%), and all patients were evaluated at least once at the Boston University Amyloidosis Center between 2009 and 2016. We followed patients for clinical outcomes until April 2019. The overall average age was 75.8 ± 6.8 years at baseline clinic visit (Table 1). There were a total of 84 White (82%) patients, with a nonsignificant trend towards more White patients in the diflunisal group (91% vs 77%, p = 0.20). Patients in the control group were significantly older (mean age 76.8 years in the control group vs 73.8 years in the diflunisal group, p = 0.03). There were no significant differences between the patients who received diflunisal and those who did not with respect to sex and NYHA heart failure class. Cardiovascular comorbidities were relatively equally distributed between the two groups, with the exception of hypertension, which was more prevalent in the diflunisal cohort (37% in the diflunisal cohort vs 17% in the control group, P = 0.02) Baseline renal function was better in patients who received diflunisal (eGFR 67 ± 17 mL/min/1.73 m2 vs 53 ± 18 mL/min/1.73 m2, CKD-Epi, p = 0.0002). Of those who were treated with diflunisal, fewer were also taking diuretics (69% vs 91%, p = 0.003) and angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) (29% vs 48%, p = 0.06). The use of beta blockers was similar among the two groups.
Table 1:
Baseline characteristics
| Diflunisal (n=35) | Control (n=69) | p value | |
|---|---|---|---|
| Age, years | 73.8 ± 7.0 | 76.8 ± 6.5 | 0.03 |
| Male | 34 (97.1%) | 67 (97.1%) | 0.99 |
| Race: white | 31 (91.2%) | 53 (76.8%) | 0.20 |
| Time from diagnosis, years | 0.41 ± 0.50 | 0.47 ± 0.9 | 0.72 |
| Hypertension | 13 (37%) | 12 (17%) | 0.02 |
| Diabetes | 5 (14%) | 10 (14%) | 1.00 |
| Coronary Artery Disease | 8 (23%) | 21 (30%) | 0.45 |
| Atrial fibrillation | 21 (60%) | 47 (68%) | 0.42 |
| Creatinine, mg/dL | 1.14 ± 0.33 | 1.41 ± 0.43 | < 0.01 |
| eGFR mL/min/1.73m2 | 67.3 ± 17.4 | 53.2 ± 17.6 | < 0.001 |
| BNP, pg/mL | 335.0 ± 365.6 | 5 ± 296.1 | < 0.01 |
| logBNP | 5.48 ± 0.8 | 6.07 ± 0.7 | < 0.001 |
| Troponin I, ng/ | 0.1 ± 0.1 | 0.2 ± 0.34 | 0.09 |
| NYHA class | |||
| I | 13 (37.1%) | 17 (24.6%) | |
| II | 16 (45.7%) | 23 (33.3%) | |
| III | 5 (14.3%) | 26 (37.7%) | |
| Diuretic use | 24 (68.6%) | 63 (91.3%) | < 0.01 |
| Beta blocker use | 18 (51.4%) | 46 (66.7%) | 0.13 |
| ACEi or ARB use | 10 (28.6%) | 33 (47.8%) | 0.06 |
| Left ventricular ejection fraction, % | 53.1 ± 12.0 | 45.0 ± 11.5 | < 0.01 |
| Interventricular septal thickness, mm | 16.6 ± 2.7 | 15.8 ± 2.3 | 0.11 |
| Posterior wall thickness, mm | 16.5 ± 2.2 | 15.8 ± 2.4 | 0.01 |
Continuous variables expressed as mean +/− standard deviation, and categorical variables as number (%). eGFR was calculated using the CKD-Epi equation.
The median follow-up time was 3.2 years in the control arm, and 4 years in the diflunisal arm. Fifty-eight patients (56%) had in-person one year follow-up at our amyloidosis center, such that labs and echo data were obtained at follow-up. For the remaining patients, follow-up consisted of telephone or email communication. Diflunisal therapy was recommended in 44 patients (42%), however, initiation of diflunisal therapy was confirmed in 35 patients (34%) and these patients formed the diflunisal cohort. The remaining 9 patients were not started on diflunisal therapy due to the preference of their local physicians, or due to missing data prohibiting us from confirming diflunisal use. These patients were included in the control group. Diflunisal administration was on average for 4.0 ± 2.3 years. Three patients (9%) discontinued diflunisal within 1 year. Eleven patients (31%) discontinued diflunisal after 1 year, and all fourteen patients remained included in the diflunisal group (Table 2). The most common reason for diflunisal discontinuation was for worsening renal function in 8 patients (57%). In addition, 2 patients discontinued for volume overload, 1 patient discontinued owing to gastrointestinal bleeding, 1 patient discontinued due to fatigue, 2 patients were discontinued at the discretion of their physician to reduce the risk of bleeding with initiation of systemic anticoagulation, and 2 patients discontinued after greater than 5 years on diflunisal for enrollment in a clinical trial. The remaining 21 patients in the diflunisal group remained on the medication through the end of the observation period.
Table 2:
Reason for diflunisal discontinuation
| Subject ID | Diflunisal duration of treatment (years) | Reason for discontinuation |
|---|---|---|
| 01 | 5.8 | Reduction in creatinine clearance |
| 02 | 0.4 | Fatigue |
| 03 | 1.0 | Reduction in creatinine clearance |
| 04 | 8.4 | Enrollment in another clinical trial |
| 05 | 4.8 | Reduction in creatinine clearance |
| 06 | 2.8 | Gastrointestinal bleeding |
| 07 | 5.0 | Enrollment in another clinical trial |
| 08 | 2.5 | Initiation of systemic anticoagulation* |
| 09 | 1.6 | Reduction in creatinine clearance |
| 10 | 0.8 | Reduction in creatinine clearance |
| 11 | 3.0 | Reduction in creatinine clearance |
| 12 | 3.0 | Reduction in creatinine clearance |
| 13 | 0.1 | Volume overload |
| 14 | 2.9 | Initiation of systemic anticoagulation* |
Diflunisal was discontinued at the discretion of the treating physician to reduce the risk of bleeding with initiation of systemic anticoagulation.
Survival
After a median follow-up of 3.4 years, there were a total of 52 deaths, with 4 (11.4%) in the diflunisal group and 48 (69.6%) in the control group (Figure 1). Among baseline characteristics, age, creatinine, troponin, BNP, and LVEF were associated with mortality in univariable analysis (Table 3). The unadjusted median survival was greater in patients treated with diflunisal than in the control group (median survival 3.3 years in control vs not reached in the diflunisal group, HR for death 0.13, 95% CI 0.05 – 0.36, p < 0.0001). This effect persisted after adjustment for age, baseline BNP, creatinine, troponin I, intraventricular septal thickness, and LVEF (HR 0.18, 95% CI 0.061 – 0.51, p = 0.0013) (Table 4). Furthermore, diflunisal continued to be significantly associated with improved mortality after adjusting for baseline NYHA class and baseline diuretic use, in addition to the variables above (HR 0.19, 95% CI 0.064 – 0.54, p = 0.0019) (Supplementary Table 1). In separate multivariable models, we included posterior wall thickness and baseline ace-inhibitor/angiotensin receptor blocker use as covariates (Supplementary Table 2), and reduced the number of covariates to five (age, baseline creatinine, baseline troponin I, septal thickness and LVEF) in order to ensure that we were not overfitting our previous multivariable models given the relatively small number of outcomes (Supplementary Table 3). Diflunisal continued to be significantly associated with improved mortality in these additional multivariable regression models. The associations for these supplementary models were similar to our original multivariable model, and there was no indication of a lack of convergence in the original multivariable model, thus suggesting a low chance of overfitting.
Figure 1:
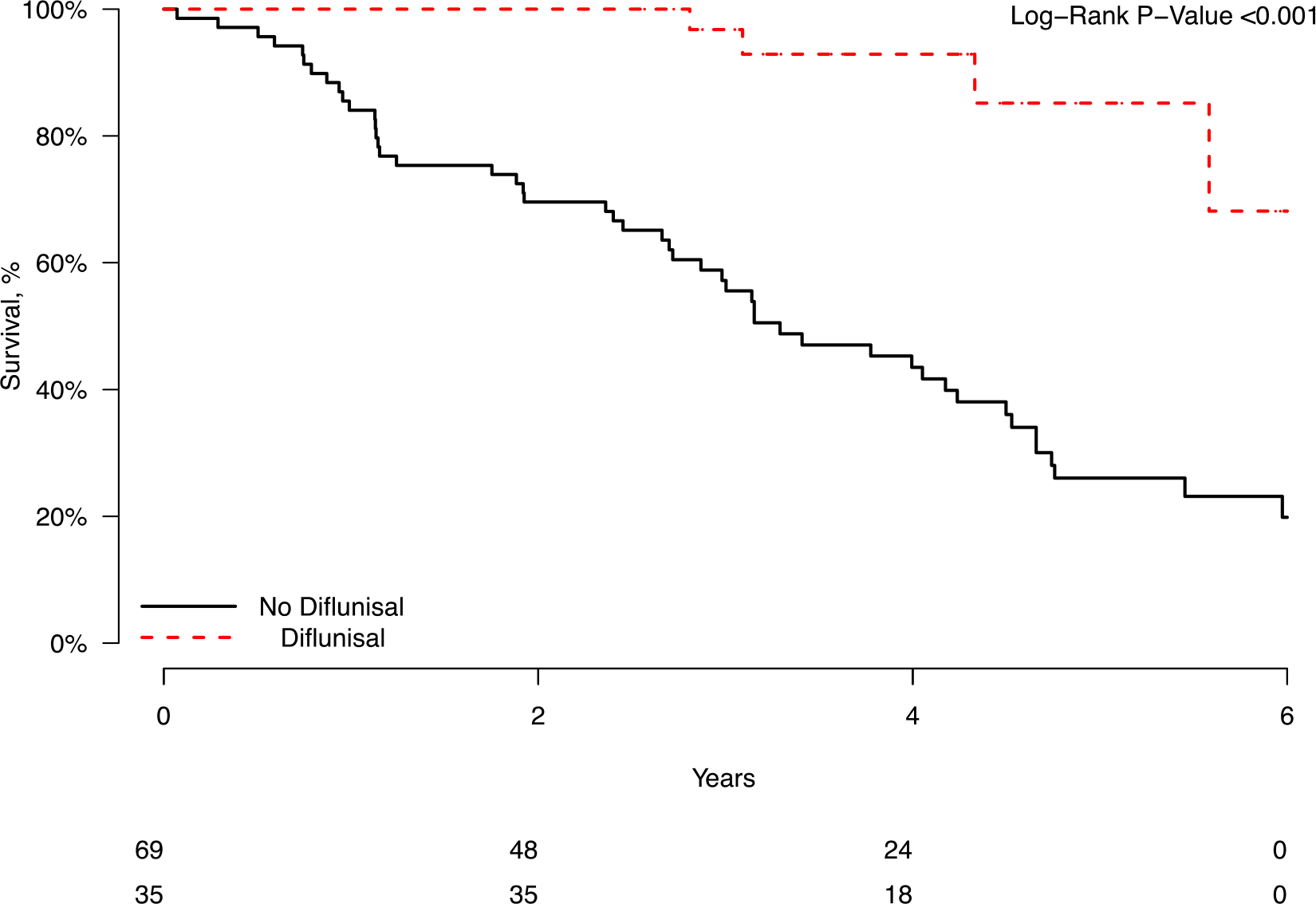
Kaplan-Meier curve showing unadjusted survival in patients treated with diflunisal for at least 1 year.
Table 3:
Association of baseline characteristics with mortality in univariate analysis
| Variable | Hazard Ratio | 95% Confidence Interval for Hazard Ratio | P value |
|---|---|---|---|
| Age | 1.041 | 0.996 –1.088 | 0.07 |
| BNP | 1.001 | 1.001 – 1.002 | < 0.001 |
| Log BNP | 2.061 | 1.365 – 3.112 | < 0.001 |
| Creatinine | 2.098 | 1.177 – 3.739 | 0.01 |
| eGFR | 0.980 | 0.966 – 0.994 | 0.01 |
| Interventricular septal thickness | 0.998 | 0.898 – 1.109 | 0.97 |
| Posterior wall thickness | 1.012 | 0.905 – 1.132 | 0.83 |
| LVEF | 0.959 | 0.937 – 0.982 | < 0.001 |
| NYHA | 1.154 | 0.850 – 1.566 | 0.36 |
| Troponin | 7.170 | 3.303 – 15.564 | < 0.001 |
Table 4:
Multivariable assessment of the association of diflunisal use with mortality: Cox proportional hazards model 1.
| Variable | Hazard Ratio | 95% Confidence Interval for Hazard Ratio | P value |
|---|---|---|---|
| Diflunisal | 0.159 | 0.061–0.51 | 0.46 |
| Age | 1.011 | 0.97–1.05 | 0.47 |
| logBNP | 1.001 | 1.000–1.002 | 0.18 |
| Creatinine | 1.138 | 0.52–2.30 | 0.81 |
| Troponin | 5.693 | 2.58–13.14 | <0.001 |
| Interventricular septal thickness | 1.001 | 0.89–1.13 | 0.97 |
| LVEF | 0.980 | 0.96–1.01 | 0.15 |
We performed a supplementary analysis of patients with baseline serum BNP greater than the overall median BNP in our population (400 pg/mL). In the diflunisal group, 10 patients (28.6%) had a baseline serum BNP greater than the median, while this was true for 42 patients (60.9%) in the control group (Supplementary Figure 1). In this cohort of patients with baseline serum BNP greater than the median BNP of the overall cohort, there were 3 deaths in the diflunisal group (30%), while 32 patients died in the control group (76%). At 2 years from initial visit, there was 1 death in the diflunisal group (10%) and 13 deaths in the control group (31%).
In an additional supplementary analysis, we sought to further address residual confounding in our cohort by comparing the primary outcome in patients in the control group who would have been eligible for diflunisal by our center’s criteria (eGFR > 45 mL/min/1.73 m2 and NYHA functional class I or II at first visit). These criteria yielded 28 patients in the control group and 23 patients in the diflunisal group. By univariate analysis, diflunisal continued to be associated with reduced mortality (HR 0.14, 95% CI 0.04 – 0.49). The small sample sizes of the cohorts in this analysis precluded meaningful multivariable adjustments.
A sensitivity analysis, performed after excluding the 9 patients for whom diflunisal was recommended but not initiated, showed similarly reduced mortality associated with diflunisal after adjustment for the baseline covariates above (HR 0.16, 95% CI 0.05 – 0.45).
Secondary end-points
At baseline, patients who were treated with diflunisal had lower BNP than those in the control group (335 ± 366 pg/mL vs 520 ± 296 pg/mL, p = 0.006,Table 1), with similar troponin I levels (0.1 ± 0.1 ng/mL vs 0.2 ± 0.34 ng/mL, p = 0.093). At one year follow up, there were nonsignificant improvements in BNP (logBNP −0.7 ± 0.6 pg/mL vs 0.2 ± 0.8, p = 0.19) and troponin I (−0.01 ± 0.08 ng/mL vs 0.13 ± 0.55 ± ng/mL, p = 0.15) in the diflunisal group (Table 5). Patients in the diflunisal group had worsening renal function at follow up (eGFR −9.0 ± 11.7 vs −2.4 ± 8.2, p = 0.03). Worsening renal function prompted discontinuation of diflunisal in 57% of patients, but most of these discontinuations occurred after one year of treatment.
Table 5:
Differences between treated and untreated patients at 1 year follow up.
| Untreated n = 69 | Diflunisal n = 35 | p value | |||
|---|---|---|---|---|---|
| Baseline | 1 year follow up | Baseline | 1 year follow up | ||
| NYHA class | 0.46 | ||||
| I | 17 (24.6) | 3 (4.3) | 13 (37.1) | 9 (25.7) | |
| II | 23 (33.3) | 2 (2.9) | 16 (45.7) | 18 (51.4) | |
| III | 26 (37.7) | 19 (27.5) | 5 (14.3) | 6 (17.1) | |
| eGFR, mL/min/1.73m2 | 53.2 ± 17.6 | 51.2 ± 20.1 | 67.3 ± 17.4 | 59.1 ± 16.7 | 0.03 |
| BNP, pg/mL | 520.1 ± 296.1 | 595.8 ± 452.7 | 335.0 ± 365.6 | 300.5 ± 247.4 | 0.11 |
| logBNP | 6.1 ± 0.7 | 6.1 ± 0.7 | 5.5 ± 0.8 | 5.4 ± 0.8 | 0.19 |
| Troponin I, ng/mL | 0.2 ± 0.34 | 0.25 ± 0.6 | 0.1 ± 0.1 | 0.09 ± 0.07 | 0.12 |
| LVEF, % | 45.0 ± 11.5 | 43.7 ± 12.9 | 53.1 ± 12.0 | 54.1 ± 10.2 | 0.27 |
| Interventricular septal thickness, mm | 15.8 ± 2.3 | 16.6 ± 2.0 | 16.6 ± 2.7 | 17.2 ± 2.33 | 0.15 |
| Posterior wall thickness, mm | 15.8 ± 2.4 | 16.3 ± 1.9 | 16.5 ± 2.2 | 16.7 ± 2.1 | 0.04 |
Continuous variables expressed as mean +/− standard deviation and categorical variables as number (%). p values reflect change differences between follow-up and baseline for treated versus untreated patients. eGFR was calculated using the CKD-Epi equation.
Imaging findings
Baseline and 1-year echocardiographic parameters are summarized in Tables 1 and 5. Patients treated with diflunisal had lower LVEF at baseline, with similar interventricular septal and posterior wall thickness. At one year follow up, diflunisal treatment was associated with stability in LV systolic function, posterior wall thickness and interventricular septal thickness. In contrast, there was a small but significant increase in posterior wall thickness in the control group at the 1-year follow-up assessment (0.69 +/− 1.65 mm, p for difference between groups = 0.04).
Discussion
In this retrospective study, diflunisal use was associated with a marked improvement in survival in selected patients with ATTRwt-CM. To our knowledge, these data represent the largest report of diflunisal treatment in predominately (86%) biopsy-proven ATTRwt-CM. Our data demonstrate that the survival curves diverge within the first year, with an 84% reduction in mortality after adjustment for age, baseline cardiac biomarkers, renal function, LV ejection fraction and interventricular septal thickening after nearly 4 years treatment. Further adjustments for baseline NYHA functional class and baseline diuretic or ace-inhibitor/ARB use continued to show a significant mortality reduction in the diflunisal group. Recognizing the role of selection bias in treatment group allocation, we compared outcomes for patients in either cohort who would have met criteria for diflunisal use at our center. While this analysis was affected by relatively small sample sizes, diflunisal use continued to be associated with a significant improvement in mortality. These results are similar to those of Rosenblum and colleagues who reported a relative mortality reduction of 63% after adjusting for covariates, in their retrospective cohort study of ATTRwt and ATTRv amyloidosis patients treated with diflunisal or tafamidis [15]. In contrast to the Rosenblum data, our study involved only patients with ATTRwt-CM (the most common type) treated with or without diflunisal. Patients who received tafamidis were excluded from our study. Thus, as an important distinction from the Rosenblum study, our study allows us to assess the effects of a single TTR stabilizer, diflunisal, in ATTRwt-CM, therefore avoiding interactions with the effect of tafamidis or the type of ATTR on mortality in this cohort.
Furthermore, while diflunisal and tafamidis are mechanistically similar, the cost of diflunisal is on the order of $500–600 per year versus $225,000 per year for tafamidis. While co-pay assistance programs exist, the drug remains expensive to many patients. A recently reported description of “real world” cost impact of tafamidis showed that median and mean out-of-pocket monthly costs of tafamidis were $1909 and $3082, before financial assistance, even as insurers paid 89% of the cost, on average. For those who were able to afford the initial out-of-pocket copay without the need for financial assistance, the median and mean out-of-pocket costs of tafamidis were $250 and $1683[18]. Recent cost-effective analysis suggests that a 92.6% reduction in list price would be required to render tafamidis cost-effective at accepted quality-adjust life-year thresholds [19]. As such a reduction appears unlikely, it is important to determine the efficacy of inexpensive alternatives. Our data suggest that diflunisal could indeed represent a low-cost alternative to tafamidis in carefully selected patients for the treatment of ATTRwt cardiac amyloidosis. However, given diflunisal’s side effect profile and lack of randomized clinical trial data in a cardiomyopathy cohort, tafamidis will remain the first TTR stabilizer of choice.
In light of the ATTR-ACT Study findings, our data suggest that TTR stabilization, irrespective of agent, is associated with improved survival in patients with ATTRwt-CM and class I-III NYHA heart failure [16]. It is important to note that diflunisal was administered to patients based on baseline eGFR > 45 ml/min/1.73m2 and stable NYHA heart failure class III or better. Thus, the drug is not appropriate for all patients with ATTRwt-CM, and must be carefully monitored. While patients treated with diflunisal were likely in an early stage of ATTRwt-CM disease, the benefit continued to be seen after adjustment for baseline differences in disease stage including age, biomarkers, systolic function, diuretic use, ace-inhibitor/ARB use, functional class, and renal function. This survival benefit in the diflunisal cohort was also seen when diflunisal patients were compared to control patients with similar renal function and NYHA class, who would have met criteria for diflunisal use at our center. Indeed, the survival observed in our untreated cohort (median of 3.3 years) reflects our prior experience and that of others [20], [21]. It is also important to note that the subjects included in this study were diagnosed and treated prior to the approval of tafamidis, providing the ethical equipoise that permitted diflunisal administration.
In our current study, diflunisal treatment also associated with stabilization of LV wall thickness and cardiac biomarkers, with a mild decline in renal function due to the NSAID effect of diflunisal. These findings complement our earlier study which demonstrated stability in LV wall thickness and LV ejection fraction and an improvement in LV global longitudinal strain (GLS) and LA volume in patients treated with diflunisal for ATTR-CM [13].
The use of NSAIDs carries potential for renal and gastrointestinal side effects. In a study of 23 patients with ATTR cardiac amyloidosis, diflunisal was found to be safe from a renal standpoint, with only one patient demonstrating an increase in serum creatinine after a median treatment time of 15 months [22]. In another study assessing the safety and tolerability of diflunisal in treatment of cardiac amyloidosis, a 6% decline in eGFR was noted among 13 patients over a median study period of about 1 year [12]. In our study, 40% of patients in the diflunisal cohort discontinued treatment with diflunisal due to side effects, which is a significant limitation of this drug. Of the total discontinuations, 57% were due to renal dysfunction and 7% were due to GI intolerance. There were 3 patients in our study who discontinued diflunisal prior to 1 year follow up. These patients were included in the diflunisal group for analysis, given that they would more likely bias toward the null hypothesis. These observations outline a significant potential challenge with the use of diflunisal and underscore the need for continued monitoring, especially if diflunisal is used beyond one year. Overall, patients in this cohort who were able to tolerate diflunisal could do so for a median of three years. Nonetheless, discontinuations of diflunisal due to renal dysfunction are a significant concern, especially in light of the favorable side effect profile of tafamidis. In the ATTR-ACT trial, drug discontinuation due to adverse effects was more common in the placebo arm [16]. This highlights the superior tolerability of tafamidis as compared to diflunisal.
Limitations
This is an observational retrospective study inherently limited by selection bias, as treatment allocations were made by treating physicians based on clinical characteristics. There were significant differences in baseline characteristics of patients who were treated with diflunisal as compared to the control group. The control group was likely disproportionately affected by comorbidities resulting in a higher mortality rate than would be observed in early-stage cardiac amyloidosis. Thus, one valid critique of our study is that we simply selected earlier stage and healthier patients for diflunisal therapy who by natural history would be expected to fare better [20].We acknowledge this inherent bias and attempted to adjust for differences in baseline characteristics including severity of cardiac dysfunction (as determined by biomarkers, baseline NYHA functional class, baseline diuretic and ace-inhibitor/ARB use and echocardiographic features) and renal function. While one could rightly argue that diflunisal treated patients had a less advanced disease stage, the magnitude of hazard ratio reduction with diflunisal therapy strongly suggests a contribution of drug treatment effect upon survival. We adjusted for these baseline differences which reflect disease severity, but additional confounders were likely present. Since disease staging relies on baseline NT-proBNP and baseline eGFR values, we attempted to analyze our cohort further using biomarkers. Renal function is one of the criteria used for determining eligibility for diflunisal use at our center, hence this metric would, of necessity be different between the two groups at baseline. Since our center routinely used serum BNP and not NT-proBNP at the time of this study, we assessed the proportion of patients in each cohort who had a baseline serum BNP value above the median for the entire cohort, in a supplementary analysis (Supplementary Figure 1). In this group of patients with baseline serum BNP above the median value for the entire cohort, at two years from initial visit, 10% of patients in the diflunisal group died as compared to 31% of patients in the control group. We performed another supplementary analysis comparing outcomes of patients in either group who met criteria for diflunisal use at our center. Diflunisal remained associated with improved survival in this analysis. Thus, we posit that despite significant baseline differences in disease severity between the two groups, diflunisal use still appears to be associated with improved survival in cohorts that are relatively better matched. Our small sample size prevents us from including more variables in our Cox proportional hazards models or using other methods for baseline covariate adjustments.
As this is a study of patients seen in consultation at a referral center, it is also limited by missing data, as there may have been additional data obtained by the patient’s local physician, and adherence to diflunisal is unknown. A supplementary analysis excluding 9 patients for whom diflunisal was recommended without documentation of drug initiation continued to show a significant reduction in all-cause mortality in the diflunisal cohort. There were 2 patients who were initially treated with diflunisal and later enrolled in a clinical trial, however we included these patients given that they were enrolled after greater than 5 years on diflunisal therapy. Another limitation of our study is our inability to ascertain cause of death for many of our patients as their deaths were ascertained by searching publicly available databases. These limitations stated, our study, in addition to other similar observational studies, provides impetus for a randomized clinical trial comparing diflunisal to tafamidis.
In summary, our findings suggest a role for diflunisal in patients with ATTRwt cardiomyopathy. Given the retrospective nature of our study and the potential for residual confounding despite the adjustments outlined above, our findings should be viewed as hypothesis-generating and suggest the need for external validation with larger cohorts perhaps including prospective, randomized clinical trials. In the meantime, tafamidis, as the only current FDA-approved medication for this disease with efficacy and safety demonstrated in a rigorous clinical trial, must remain a first line therapy option. Tafamidis, however, is limited by significant financial constraints. As such, diflunisal may be a viable and more cost-effective alternative for selected patients with preserved renal function who are unable to afford tafamidis and for whom clinical trial enrollment is not an option.
Supplementary Material
Funding
This study was supported, in part, by R01 HL139671to FLR.
Abbreviations:
- ATTR
amyloidogenic transthyretin
- ATTRv
hereditary transthyretin or variant transthyretin
- ATTRwt
wild-type transthyretin amyloidosis
- IVS
interventricular septal thickness
- LVEF
left ventricular ejection fraction
- PWT
posterior wall thickness
- TTR
transthyretin
Footnotes
Disclosure statement
FLR has received research funding from Pfizer, Eidos Therapeutics, Alnylam Pharmaceuticals, and Akcea Therapeutics. JLB reports consulting fees from Alnylam Pharmaceuticals and Ionis Pharmaceuticals, and has served on the scientific advisory boards of Intellia Therapeutics and Corino Therapeutics, and on an advisory committee for Ionis Pharmaceuticals.
The remaining authors have nothing to disclose.
References
- 1.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594. [DOI] [PubMed] [Google Scholar]
- 3.Bennani Smires Y, Victor G, Ribes D, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32(9):1403–1413. [DOI] [PubMed] [Google Scholar]
- 4.Lo Presti S, Horvath SA, Mihos CG, et al. Transthyretin Cardiac amyloidosis as diagnosed by 99mTc-PYP scanning in patients with acute heart failure and preserved ejection fraction. Crit Pathw Cardiol. 2019;18(4):195–199. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Lopez E, Gagliardi C, Dominguez F, et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38(24):1895–1904. [DOI] [PubMed] [Google Scholar]
- 6.Ruberg FL, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(22):2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grogan M, Scott CG, Kyle RA, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020. [DOI] [PubMed] [Google Scholar]
- 8.Castano A, Drachman BM, Judge D, et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green NS, Palaninathan SK, Sacchettini JC, et al. Synthesis and characterization of potent bivalent amyloidosis inhibitors that bind prior to transthyretin tetramerization. J Am Chem Soc. 2003;125(44):13404–13414. [DOI] [PubMed] [Google Scholar]
- 10.Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest. 2004;84(5):545–552. [DOI] [PubMed] [Google Scholar]
- 11.Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castano A, Helmke S, Alvarez J, et al. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18(6):315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohrmann G, Pipilas A, Mussinelli R, et al. Stabilization of cardiac function with diflunisal in transthyretin (ATTR) cardiac amyloidosis. J Card Fail. 2019;26(9):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson JLS, Arvanitis M, Koch CM, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail. 2018;11(2):e004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblum H, Castano A, Alvarez J, et al. TTR (transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail. 2018;11(4):e004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18.Masri A, Chen H, Wong C, et al. Initial experience prescribing commercial tafamidis, the most expensive cardiac medication in history. JAMA Cardiol. 2020;5(9):1066–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazi DS, Bellows BK, Baron SJ, et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141(15):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connors LH, Sam F, Skinner M, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806. [DOI] [PubMed] [Google Scholar]
- 22.Ikram A, Donnelly JP, Sperry BW, et al. Diflunisal tolerability in transthyretin cardiac amyloidosis: a single center’s experience. Amyloid. 2018;25(3):197–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


