ABSTRACT
Background
The associations of red and processed meat with chronic disease risk remain to be clarified, in part because of measurement error in self-reported diet.
Objectives
We sought to develop metabolomics-based biomarkers for red and processed meat, and to evaluate associations of biomarker-calibrated meat intake with chronic disease risk among postmenopausal women.
Methods
Study participants were women who were members of the Women's Health Initiative (WHI) study cohorts. These participants were postmenopausal women aged 50–79 y when enrolled during 1993–1998 at 40 US clinical centers with embedded human feeding and nutrition biomarker studies. Literature reports of metabolomics correlates of meat consumption were used to develop meat intake biomarkers from serum and 24-h urine metabolites in a 153-participant feeding study (2010–2014). Resulting biomarkers were used in a 450-participant biomarker study (2007–2009) to develop linear regression calibration equations that adjust FFQ intakes for random and systematic measurement error. Biomarker-calibrated meat intakes were associated with cardiovascular disease, cancer, and diabetes incidence among 81,954 WHI participants (1993–2020).
Results
Biomarkers and calibration equations meeting prespecified criteria were developed for consumption of red meat and red plus processed meat combined, but not for processed meat consumption. Following control for nondietary confounding factors, hazard ratios were calculated for a 40% increment above the red meat median intake for coronary artery disease (HR: 1.10; 95% CI: 1.07, 1.14), heart failure (HR: 1.26; 95% CI: 1.20, 1.33), breast cancer (HR: 1.10; 95% CI: 1.07, 1.13) for, total invasive cancer (HR: 1.07; 95% CI: 1.05, 1.09), and diabetes (HR: 1.37; 95% CI: 1.34, 1.39). HRs for red plus processed meat intake were similar. HRs were close to the null, and mostly nonsignificant following additional control for dietary potential confounding factors, including calibrated total energy consumption.
Conclusions
A relatively high-meat dietary pattern is associated with somewhat higher chronic disease risks. These elevations appear to be largely attributable to the dietary pattern, rather than to consumption of red or processed meat per se.
Keywords: cancer, cardiovascular disease, diabetes, metabolomics, red and processed meat
Introduction
Dietary intake biomarkers have had an increasingly important place in the nutritional epidemiology research agenda, following decades of principal reliance on self-reported dietary data. For example, our own group has used the established doubly labeled water (DLW) biomarker of total energy consumption (1), and the urinary nitrogen (UN) biomarker of total protein intake (2) to adjust self-reported data for measurement error in these dietary variables. Also, we have associated the resulting “biomarker-calibrated intakes” with subsequent chronic disease incidence in Women's Health Initiative (WHI) cohorts. These studies revealed (3, 4) strong systematic biases in self-reported energy and protein intake, whether based on FFQ, 4-d food record (4DFR), or three 24-h dietary recalls (24HRs). Such biases, if overlooked, lead to attenuated and distorted association estimates for these dietary variables in relation to chronic disease risk in WHI cohorts (5–8).
A major issue in nutritional epidemiology is potential for systematic bias in dietary assessments. For total energy, measurement error in self-reported diet depends systematically on such participant characteristics as BMI (in kg/m2), age, and ethnicity and also includes substantial random error, leading to distorted chronic disease association estimates. Measurement error may also affect the assessment of variables needed for confounding control or for mediation analysis in observational studies.
Our WHI nutrition studies, recently summarized in (9), have also included biomarker development for certain macronutrient and micronutrient dietary exposures, and these again revealed various nutrient and chronic disease associations in WHI cohorts that were mostly not evident or were severely attenuated in the absence of biomarker-based measurement error adjustment.
Here we consider a similar developmental process for red meat, processed meat, and red and processed (R + P) meat combined, both for absolute intakes and for their ratios to total energy intake. Conceptually, the study of food groups does not differ in a major way from the study of nutrients, except that with foods it may be more likely that there is a subset reporting no consumption, precluding analysis using the usual log- intake transformation of dietary variables in HR modeling. Here, to retain zero consumers, we employ a log (1 + intake/median intake) transformation for meat intake variables in HR analyses.
The study of meat intake in relation to chronic disease is motivated in part by a systematic review (10) in which the authors concluded that evidence of chronic disease benefits for a reduction in red or processed meat is of low certainty, and that any benefits are probably small. In comparison, Dietary Guidelines for Americans (11) advise limiting or avoiding intake of red and processed meat, as do the World Cancer Research Fund/American Institute for Cancer Research (12) and American Cancer Society (13) reports, based on a substantial history of observational epidemiologic research.
Methods
Study cohorts and data
During 1993–1998, a total of 48,835 participants were randomly assigned to the WHI Dietary Modification (DM) trial, with 29,294 randomly assigned to the usual dietary modification comparison group (DM-C), and an additional 93,676 participants enrolled in the companion prospective WHI Observational Study (OS) (14). All participants were postmenopausal and aged 50–79 y when enrolled at 40 US clinical centers. The WHI FFQ was used to assess frequency and portion size of 122 foods and food combinations over the preceding 3-mo period (15) and was administered at enrollment and year 1 in the DM trial and at enrollment in the OS, among other times. Completion of a 4-d food record (4DFR) was a DM trial eligibility requirement, and 24-h dietary recalls (24HRs) were obtained periodically on cohort subsamples. Nutrient content estimates were derived using the University of Minnesota's Nutrition Data System for Research (NDSR® version 2005). All WHI participants provided core questionnaires at enrollment, including medical history, reproductive history, family history, personal habits, medications, and dietary supplements, and provided a fasting blood sample (14).
Nutrition and Physical Activity Assessment Study
The Nutrition and Physical Activity Assessment Study (NPAAS) biomarker study (4), conducted among 450 OS participants during 2007–2009, examined measurement properties of dietary self-reports for nutritional variables having an established intake biomarker, and used these biomarker data to adjust dietary self-reports for measurement error in disease association analyses. Participants were recruited at 9 clinical centers, with an overrepresentation of racial/ethnic minority women and of women having relatively high BMI. There were 2 clinic visits separated by 2 wk. The first included measured height and weight; DLW dosing and spot urine collections for total energy assessment; completion of FFQ, dietary supplement, and other questionnaires, and fasting blood specimen collection. Participants brought 4DFRs and 24-h urine specimens to the second visit, provided a fasting blood specimen and additional spot urine specimens. Three 24HRs were obtained over the subsequent 2–3 mo. NPAAS baseline characteristics have been reported (4).
NPAAS Feeding Study
The NPAAS Feeding Study (NPAAS-FS) was conducted in the Seattle area during 2010–2014 among 153 women who were WHI particiants (16). Fourteen of the NPAAS-FS participants were previously enrolled in NPAAS. Participants were provided food and beverages over a 2-wk feeding period, with individualized diets that were intended to approximate their usual diets, so that blood and urine concentrations would stabilize quickly and intake variations in the study cohort would be retained. Meat-related biomarkers were derived primarily from serum and 24-h urine metabolomics profiles from specimens obtained at the end of the feeding period. Baseline demographic and lifestyle characteristics for participants in the NPAAS-FS have been reported (16).
Biomarkers, calibration equations, and disease associations for meat-related variables
As detailed in (17), fasting serum samples were analyzed using LC/MS, with 303 metabolites targeted. Lipid metabolites were measured using lipidizer/differential mobility spectrometry, targeting 1070 lipids. Urine samples (24-h), analyzed by NMR spectroscopy, yielded 57 metabolite concentrations. Urine samples, analyzed using untargeted GC-MS, resulted in 275 metabolites. To reduce the dimensionality of the metabolomics data for biomarker development, we searched the literature for metabolites reported to be correlated with the intake of meat or related dietary variables. We began with metabolomic correlates of meat and seafood intake provided in an extensive 2019 literature review by Cuparencu et al. (18). Then we searched PubMed for biomarkers of meat using the search terms “metabolomics OR metabolites AND meat” and “biomarkers OR markers AND meat,” using primary research studies and review papers restricted to humans and dated 2019 and later, including the highly relevant publication by Wedekind et al. (19). We further included 2 heme metabolites (glycine and biliverdin) to acknowledge the source of “red” in red meat. From this list of correlates, we identified those that were reliably measured on our metabolomics platforms and restricted the set of metabolites considered for biomarker development to the resulting reliably measured set. As detailed in (17), metabolite data quality was assessed based on CVs for blinded quality control samples that were analyzed along with the study samples. Available measures in NPAAS-FS for biomarker development also include DLW for total energy intake (1) and UN for total protein intake (2).
We considered biomarkers for daily grams of red meat (beef, lamb, pork, veal, game, organ), processed meat (sausage, bacon, cold cuts), R + P meat, energy from red meat/total energy intake, energy from processed meat/total energy intake, and energy from R + P meat/total energy intake, using linear regression of feeding study intake on metabolite concentrations and other variables. Meat-related variables for which biomarker equations have cross-validated percentage of variation explained (CV-R2) of ≥36% in NPAAS-FS were considered for calibration equation development, as in our previous development work (16, 20). This 36% criterion is motivated by benchmark R2 values of ∼50% for DLW energy intake and ∼40% for UN protein intake.
Calibration equations for meat-related variables having a suitable biomarker were developed from linear regression of biomarker values on corresponding self-reported intake values and personal characteristics in NPAAS. Calibration equations meeting criteria for meat-related variables, including adjusted R2 values (20) of ≥36%, were used to generate biomarker-calibrated intakes in larger WHI cohorts for use in disease association analyses.
Outcome ascertainment, follow-up, and disease categories
Clinical outcomes were reported biannually in the DM trial and annually in the OS, by self-administered questionnaire (21) from enrollment in 1993–1998 to the end of the intervention period (31 March 2005), and annually thereafter in both cohorts. An initial report of cardiovascular disease (CVD) during cohort follow-up was confirmed by review of medical records by physician–adjudicators. Additionally, coronary heart disease (CHD), defined as nonfatal myocardial infarction (MI) plus CHD death, stroke (ischemic plus hemorrhagic), heart failure, and all deaths were centrally reviewed by expert physician investigator committees. Invasive cancers, except nonmelanoma skin cancer, were centrally coded using the National Cancer Institute Surveillance, Epidemiology, and End Results Program procedures. Prevalent type 2 diabetes (T2D) at baseline was self-reported during eligibility screening. Incident T2D during follow-up was documented by self-report at each annual contact. These reports have been shown to be consistent with medication inventories of oral agents or insulin (22).
Following the intervention period, WHI participants had the opportunity to enroll in additional follow-up through 30 September 2010 and subsequently for open-ended follow-up, with >80% of women doing so on each occasion. Cancer, diabetes, and all-cause mortality (including National Death Index matching) outcomes through 28 February 2020 are included here. Follow-up for CVD incidence is included only through 30 September 2010, since self-reports for most WHI participants were not adjudicated after that date. Heart failure adjudication in WHI cohorts stopped after 31 March 2005. The median follow-up duration was 11.3 y for CVD incidence, 7.8 y for heart failure, and ∼20 y for cancer, diabetes, and mortality. Disease outcome categories were those used in our previously reported study (20).
Statistical methods
To allow for the possibility of zero intakes for the meat-related variables, the NPAAS-FS–provided intakes were used as response variables in linear regression for biomarker development, without the logarithmic transformation used in our previous biomarker development research (20). These variables were each regressed linearly on serum and 24-h urine metabolite concentration measurements, with the potential addition of linear terms in DLW energy and UN protein biomarkers, as well as personal characteristics, including race/ethnicity, education, age, BMI, self-reported leisure activity, and baseline FFQ intake. The inclusion of baseline FFQ intakes aims to avoid a potential bias in subsequent disease association analyses, resulting from the fact thatthe association analyses are conditioned on baseline dietary intake data. Baseline here is defined as the time of enrollment in the OS and 1 y following enrollment in the DM-C, the latter choice to avoid intake bias related to the use of the FFQ energy from fat of ≥32% of total energy as a DM trial eligibility criterion. A P < 0.1 threshold was used to select and retain variables in a stepwise variable selection procedure for potential biomarker development.
The biomarker equations for meat-related variables having CV-R2 of ≥36% from the metabolite selection approach described above were used to calculate biomarker-based intakes for the 436 NPAAS participants who were not NPAAS-FS participants. These NPAAS-based biomarker intakes were regressed linearly on concurrent NPAAS FFQ meat intake assessments and personal characteristics to develop calibration equations. Calibration equations were accepted for meat-related variables if the linear regression adjusted R2 was ≥36%. The R2 adjustment was obtained by dividing the regression R2 by the correlation between NPAAS and NPAAS-FS biomarker-based intakes for the meat variables, as further explained in (4), for the 14 participants in both substudies (specimens separated by ∼4 y) as in our previous analyses (20). Disease-specific individual characteristics used in calibration equation development are listed in Supplemental Table 1. Briefly, covariates considered for CVD equations were age (linear); family income; education; cigarette smoking history; alcohol consumption; leisure physical activity; any dietary supplement use; prior menopausal hormone use; hypertension; personal history of cancer; family history of MI, stroke, or diabetes; use of medications to lower blood pressure, blood lipids, or blood glucose; and season in which the FFQ was completed. Invasive cancer equations included these same variables, exclusive of personal history of CVD and of family history of MI, stroke, or diabetes, and inclusive of Gail model 5-y breast cancer risk score, family history of colorectal cancer, and personal history of colon polyp removal. T2D analyses included the same variables as the CVD analyses except for family history of MI or stroke. These same procedures were used to develop calibration equations with 4DFRs or three 24HRs, rather than FFQs, as the principal explanatory variables.
Biomarker-calibrated intakes using FFQs were calculated for the 81,954 participants having all data needed for confounding control, 16,639 from the DM-C, and 65,015 from the OS for meat intake variables having a suitable calibration equation. These, as well as FFQ meat intakes without biomarker calibration, were right skewed. These variables were transformed to log (1 + meat intake/cohort median intake), yielding approximate normality, prior to their entry into Cox regression models (23) along with disease-specific potential confounding factors. Baseline hazard rates in the Cox model analyses were stratified on baseline age (i.e., year 1 for participants in DM-C, enrollment for participants in OS) in 5-y categories, on cohort (DM-C or OS), and in the DM-C, also on participation in the WHI hormone therapy trials (estrogen, estrogen placebo, estrogen plus progestin, estrogen plus progestin placebo, not randomized). Estimated HRs for a 40% increment above the median in meat intake are presented for display purposes. This increment is well within the self-reported variation in meat intake. With this model, the HR for a 40% increment above the median intake equals (1.2)b, where b is the transformed meat variable coefficient estimate from Cox regression. Corresponding HRs for 20% and −40% meat intake increments relative to the median meat intake, for example, are (1.1)b and (0.8)b, respectively. HRs and 95% CIs for these or other fractional changes in meat intake relative to the median intake are readily obtained using log (1. + meat intake/median meat intake) transformation and the so-called delta method. Note, for example, that significance of the departure of the HR from one is not affected by the 40% increment choice compared with other possible increment choices. Both nondietary and dietary potential confounding variables were entertained in the HR analyses. Dietary potential confounding factors that are not biomarker calibrated were log-transformed prior to inclusion in the Cox regression model. The same set of characteristics described above (Supplemental Table 1), exclusive of BMI in some analyses, was considered for inclusion in HR models as nondietary potential confounding factors, with P < 0.1 used for inclusion and for retention in a stepwise HR modeling procedure. Missing data rates were generally low for specific covariates, but ≥20% participants had missing data on one or more modeled covariates in some of our analyses. Participants were excluded from outcome-specific analyses if any modeled covariate was missing. Based on sensitivity analyses that dropped covariates having relatively high missingness rates, thereby including additional participants, this exclusion is not expected to materially affect disease association estimates. Participants having CVD, invasive cancer, or treated T2D prior to WHI enrollment were excluded from respective CVD, cancer, or diabetes analyses. Also, to allow for possible confounding by other dietary variables, log-transformed baseline FFQ dietary variables were considered for inclusion in the log-HR model in some analyses. The additional dietary intakes considered for inclusion, beyond calibrated meat intake and calibrated total energy intake (see below) were log–saturated fat and log-sodium, the major correlates of meat intake in WHI cohorts.
Disease occurrence time for a case’ was defined as days from baseline’ (year 1 in the DM-C and enrollment in the OS) to diagnosis. Censoring time for noncases’ was d from baseline to the earliest date of death without the outcome under study, last contact, or 31 March 2005 for heart failure, 30 September 2010 for other CVD incidence outcomes, or 28 February 2020 for cancer, diabetes, and mortality outcomes. Because of uncertainty in the coefficients in the calibrated intake estimating equations, a sandwich-type’ estimator was used to estimate the variance of log-HR parameter estimates, and to estimate corresponding CIs (24–26).
Analyses were repeated with a quadratic term in transformed meat intake added to the log-HR model. Quadratic coefficients estimates were mostly nonsignificant, and about equally divided between positive and negative values across outcomes, suggesting substantially linear log-HRs in relation to transformed meat intake.
HR analyses were carried out also with DLW-calibrated log-total energy intake, along with the transformed meat intake variables, included in the HR model. The meat variables and total energy were jointly calibrated using multivariate multiple linear regression. Because BMI may be an important mediator of the relation between meat variables and chronic disease risk, initial analyses exclude BMI from the disease risk model. Analyses were also conducted with BMI added to the disease risk model, along with the previously mentioned other FFQ dietary confounding factors.
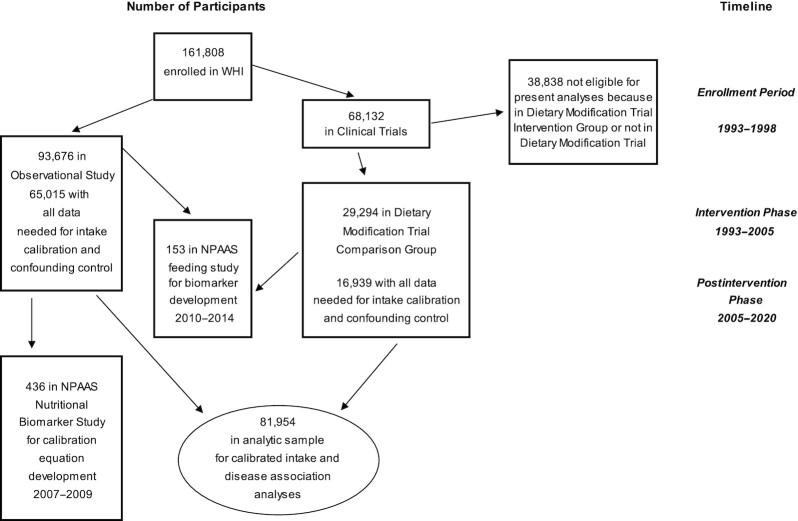
Figure 1 shows cohorts and participant flow in the WHI DM-C and the OS, and in the NPAAS and NPAAS-FS subsets, over the intervention and postintervention study phases.
FIGURE 1.
Study samples and flow in the Women's Health Initiative cohorts of postmenopausal women aged 50–79 y at enrollment during 1993–1998 at 40 US clinical centers and in NPAAS and NPAAS–Feeding Study subcohorts. NPAAS, Nutrition and Physical Activity Assessment Study; WHI, Women's Health Initiative.
Ethics
The WHI is funded primarily by the National Heart, Lung, and Blood Institute. Participants provided written informed consent for their overall WHI, NPAAS, and NPAAS-FS activities. Related protocols were approved by the Institutional Review Boards at the Fred Hutchinson Cancer Research Center and at each participating clinical center.
Results
Biomarker development
Supplemental Table 2 shows the set of targeted metabolites considered for meat intake biomarker development. Also, DLW energy, UN protein, BMI, as well as corresponding baseline FFQ intakes, were considered as variables that may increase the linear regression CV-R2 for meat variables among the 153 participants in NPAAS-FS. Using a P < 0.1 threshold used to select and to retain variables in a stepwise variable selection procedure, one obtains CV-R2 values of 38.8% for absolute intake of red meat and 45.0% for absolute intake of R + P meat. CV-R2 values for energy from red meat/total energy intake (21.4%), energy from R + P meat/total energy intake (26.0%), absolute processed meat (8.0%), and energy from processed meat/total energy intake (15.1%) did not satisfy biomarker development criteria, and these meat variables were not considered further. There were 19, 33, and 6 participants who had zero intake of red, processed, and R + P meat, respectively, during the 2-wk feeding period. The use of linear regression without log-transformation allowed these participants to be retained in the biomarker development, and this led to noticeably larger CV-R2 values than would otherwise have been the case. Table 1 provides details of the linear regression biomarker equations for red and R + P meat.
TABLE 1.
Linear regression biomarker equations for absolute intake (g/d) of red meat and red plus processed meat on serum and urine metabolites and other variables1
| Variable | Coefficient | R 2 | CV-R2 |
|---|---|---|---|
| Red meat | |||
| (Intercept) | −24.5 | ||
| Creatine (urine) | 11.5 | 11.3% | 9.2% |
| Trimethylamine (urine) | 22.8 | 0.6% | 0.5% |
| Guanidinoacetate (urine) | 40.2 | 8.6% | 7.0% |
| Hydroxyproline (serum) | 18.4 | 5.4% | 4.4% |
| Biliverdin (serum) | –4.2 | 0.9% | 0.8% |
| Lysophosphatidylcholine (LPC 22:5)2 (serum) | 13.8 | 4.4% | 3.6% |
| Phosphatidylcholine (PC 38:0)3 (serum) | –7.4 | 2.9% | 2.4% |
| BMI | 0.9 | 1.7% | 1.4% |
| Urinary nitrogen | 16.3 | 1.9% | 1.5% |
| Baseline FFQ red meat, g/d | 0.2 | 9.7% | 7.9% |
| Total | 47.4% | 38.8% | |
| Red + processed meat | |||
| (Intercept) | –224.7 | ||
| Creatine (urine) | 13.5 | 9.2% | 7.6% |
| Trimethylamine (urine) | 25.4 | 0.3% | 0.2% |
| Trimethylamine.N.oxide (urine) | –10.4 | 6.9% | 5.7% |
| Guanidinoacetate (urine) | –47.5 | 5.2% | 4.3% |
| Acetylcarnitine (serum) | 13.9 | 3.2% | 2.6% |
| Hydroxyproline (serum) | 24.2 | 5.8% | 4.8% |
| Biliverdin (serum) | -5.1 | 1.6% | 1.3% |
| Lysophosphatidylcholine (LPC 22:5)3 (serum) | 12.6 | 2.5% | 2.0% |
| Phosphatidylcholine (PC 38:0)3 (serum) | –8.8 | 3.0% | 2.5% |
| Phosphatidylcholine (PC 38:4)3 (serum) | 14.4 | 0.6% | 0.5% |
| BMI | 1.5 | 3.3% | 2.7% |
| Urinary nitrogen | 33.1 | 3.8% | 3.1% |
| Baseline FFQ Total meat, g/d | 0.2 | 9.5% | 7.8% |
| Total | 54.9% | 45.0% | |
Metabolites measured using NMR, LCMS, lipidomics, and GC/MS. Other variables considered include doubly labeled water-assessed total energy, urine nitrogen-assessed total protein, BMI, and baseline FFQ meat intake.
In LPC 22:5, 22 indicates number of carbons and 5 indicates number of double bonds in the fatty acid chain.
In PC 38:0/38:4, 38 indicates the number of carbon atoms and 0 and 4 indicate the number of double bonds in the 2 fatty acid chains.
Calibration equation development
Linear regression among NPAAS participants not in NPAAS-FS (n = 436) yields R2 values for biomarker-assessed red meat on corresponding FFQ red meat and personal characteristics taking values 27.1%, 29.5%, and 27.4%, respectively, according to whether CVD, cancer, or T2D covariates were used in calibration equation development. These increase to 39.5%, 43.0%, and 39.9% following adjustment for temporal variation in the red meat biomarker. Similarly, R2 values for R + P meat were 33.4%, 36.2%, and 33.4%, respectively, using CVD, cancer, and diabetes covariates, and these increase to 42.2%, 45.8%, and 42.3% following adjustment for temporal biomarker variation. These adjusted R2 values met our 36% calibration equation criterion for both red and R + P meat variables. Supplemental Table 3 shows details for these calibration equations separately for CVD, cancer, and diabetes covariates. Adjusted R2 values were not meaningfully increased by using 4DFRs or three 24HRs rather than FFQs in calibration equation development (data not shown).
Calibrated meat consumption and chronic disease risk
Supplemental Table 4, from (20), shows distributional details for various participant characteristics for the larger DM-C (n = 16,939) and OS (n = 65,015) cohorts used for disease association analyses. Table 2 displays certain participant characteristics and dietary intakes frequencies across quintiles of calibrated red meat intake for this large combined cohort (n = 81,954). Participants having a relatively high calibrated red meat intake also tended to have high BMI, high total, saturated, monounsaturated fat, and polyunsaturated fat, and high sodium intake, as assessed by FFQ, as well as high calibrated-total energy intake. The left side of Table 3 shows HRs (95% CIs) for a 40% increment beyond the median in calibrated FFQ meat variable intakes in relation to CVD outcomes, for the 2 meat-related variables having a suitable calibration equation. These analyses include nondietary potential confounding factors (Supplemental Table 1), but not BMI or dietary potential confounding factors. Positive associations were estimated for several CVD outcomes, for both red and R + P meat intake following biomarker calibration. For red meat, estimated HRs (95% CIs) were 1.09 (1.05, 1.14) for CHD, 1.04 (1.00, 1.08) for stroke, 1.08 (1.06, 1.11) for total CVD, and 1.26 (1.20, 1.33) for heart failure. Corresponding CVD HRs for R + P meat were similar to those for red meat alone. Corresponding HRs for a 40% increment in self-reported FFQ red or R + P meat intake (Supplemental Table 5) without biomarker calibration, were much closer to the null and were mostly not significantly different from one.
TABLE 2.
Baseline characteristics and estimated daily dietary intakes by quintiles of calibrated red meat intake (g/d) in a cohort of 81,954 Women's Health Initiative participants enrolled during 1993–1998 at 40 US clinical centers1
| Q1 (<15.1) (n = 15,639) | Q2 (15.1 to <20.4) (n = 15,640) | Q3 (20.4 to <25.5) (n = 15,640) | Q4 (25.5 to <32.4) (n = 15,638) | Q5 (≥32.4) (n = 15,640) | |
|---|---|---|---|---|---|
| Age at baseline, y | |||||
| 50–54 | 2695 (17.2) | 2110 (13.5) | 1978 (12.7) | 1769 (11.3) | 1921 (12.3) |
| 55–59 | 3473 (22.2) | 3250 (20.8) | 2960 (18.9) | 2961 (18.9) | 3183 (20.4) |
| 60–64 | 3484 (22.3) | 3520 (22.5) | 3532 (22.6) | 3567 (22.8) | 3874 (24.8) |
| 65–69 | 3063 (19.6) | 3407 (21.8) | 3453 (22.1) | 3671 (23.5) | 3494 (22.3) |
| 70–74 | 2075 (13.3) | 2308 (14.8) | 2615 (16.7) | 2557 (16.4) | 2295 (14.7) |
| 75–79 | 849 (5.4) | 1045 (6.7) | 1102 (7.1) | 1113 (7.1) | 873 (5.6) |
| BMI | |||||
| <25 | 12,417 (79.4) | 8785 (56.2) | 5591 (35.8) | 2796 (17.9) | 955 (6.1) |
| 25 to <30 | 3006 (19.2) | 5833 (37.3) | 7443 (47.6) | 7037 (45.0) | 3545 (22.7) |
| ≥30 | 216 (1.4) | 1022 (6.5) | 2606 (16.7) | 5805 (37.1) | 11,140 (71.2) |
| Calibrated total energy, 1000 kcal | 2.1 [1.9, 2.4] | 2.2 [1.9, 2.4] | 2.2 [2.0, 2.5] | 2.3 [2.0, 2.6] | 2.5 [2.1, 3.0] |
| FFQ red meat, g | 12.8 [1.7, 47.5] | 19.6 [3.1, 63.1] | 27.0 [5.6, 77.3] | 38.1 [9.7, 97.0] | 64.3 [17.3,166.0] |
| FFQ red plus processed meat, g | 18.1 [2.6, 63.4] | 27.0 [4.9, 79.4] | 36.2 [8.7, 95.5] | 49.2 [14.1,118.0] | 80.1 [24.1,196.1] |
| FFQ total energy, 1000 kcal | 1.2 [0.7, 2.2] | 1.4 [0.8, 2.3] | 1.4 [0.8, 2.5] | 1.6 [0.8, 2.6] | 1.8 [1.0, 3.3] |
| FFQ total fat, g | 37.0 [16.6, 78.3] | 42.2 [19.6, 86.7] | 47.6 [22.1, 97.3] | 54.4 [25.3,109.8] | 71.1 [32.4,149.8] |
| FFQ saturated fat, g | 11.8 [4.9, 27.1] | 13.7 [5.9, 30.4] | 15.6 [6.9, 33.7] | 18.1 [8.0, 38.3] | 23.9 [10.5, 52.3] |
| FFQ polyunsaturated fat, g | 8.1 [3.6, 17.8] | 9.0 [4.1, 19.1] | 9.9 [4.5, 21.1] | 11.0 [4.9, 23.5] | 13.8 [6.0, 30.6] |
| FFQ monounsaturated fat, g | 13.7 [5.7, 30.1] | 15.7 [7.0, 33.1] | 17.8 [8.0, 37.1] | 20.5 [9.2, 42.1] | 27.0 [12.0, 57.6] |
| FFQ sodium, g | 2.1 [1.1, 3.8] | 2.2 [1.2, 4.0] | 2.4 [1.3, 4.3] | 2.6 [1.4, 4.7] | 3.1 [1.6, 5.8] |
| FFQ fiber, g | 15.7 [7.7, 30.1] | 15.7 [7.8, 29.2] | 15.9 [8.1, 29.3] | 16.0 [8.1, 29.6] | 16.9 [8.6, 31.1] |
| FFQ fruit and vegetable, serving | 3.9 [1.5, 8.5] | 3.8 [1.4, 8.2] | 3.8 [1.4, 8.1] | 3.7 [1.4, 8.0] | 3.6 [1.3, 8.0] |
Tabular entries are n (%), or geometric mean [5th, 95th percentile]. Q, quartile.
Calibrated using cardiovascular disease set of covariates.
TABLE 3.
Cardiovascular disease risk HRs and 95% CIs for a 40% increment above the median for red meat and for red plus processed meat in a cohort of 81,954 Women's Health Initiative participants of postmenopausal US women enrolled during 1993–1998 at 40 US clinical centers and followed through February 20201
| No adjustment for BMI or dietary correlates | With adjustment for BMI and dietary correlates | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome (n = participants with events) | Red meat intake, g/d | P value | Red + processed Meat Intake, g/d | P value | Red meat intake, g/d | P value | Red + processed meat intake, g/d | P value |
| Nonfatal MI (2102) | 1.11 (1.06, 1.16) | <0.001 | 1.10 (1.06, 1.14) | <0.001 | 1.06 (0.97, 1.15) | 0.19 | 1.07 (0.98, 1.17) | 0.14 |
| Coronary death (3254) | 1.10 (1.07, 1.14) | <0.001 | 1.10 (1.07, 1.14) | <0.001 | 0.99 (0.92, 1.06) | 0.72 | 1.01 (0.94, 1.09) | 0.80 |
| Total CHD (2869) | 1.09 (1.05, 1.14) | <0.001 | 1.09 (1.05, 1.13) | <0.001 | 1.02 (0.95, 1.10) | 0.56 | 1.04 (0.97, 1.12) | 0.29 |
| Ischemic stroke (1776) | 1.07 (1.02, 1.12) | 0.003 | 1.06 (1.02, 1.11) | 0.005 | 1.03 (0.95, 1.13) | 0.46 | 1.02 (0.93, 1.12) | 0.68 |
| Hemorrhagic stroke (395) | 0.87 (0.78, 0.96) | 0.01 | 0.86 (0.79, 0.95) | 0.002 | 0.99 (0.82, 1.20) | 0.91 | 0.97 (0.79, 1.19) | 0.77 |
| Total stroke (2425) | 1.04 (1.00, 1.08) | 0.05 | 1.03 (1.00, 1.07) | 0.09 | 1.02 (0.94, 1.10) | 0.66 | 1.01 (0.93, 1.10) | 0.78 |
| CHD + stroke (5023) | 1.07 (1.05, 1.11) | <0.001 | 1.07 (1.04, 1.09) | <0.001 | 1.02 (0.97, 1.08) | 0.38 | 1.03 (0.98, 1.09) | 0.26 |
| CABG + PCI (3119) | 1.11 (1.07, 1.14) | <0.001 | 1.11 (1.07, 1.14) | <0.001 | 1.03 (0.96, 1.10) | 0.42 | 1.04 (0.97, 1.12) | 0.30 |
| Total CVD2 (6964) | 1.08 (1.06, 1.11) | <0.001 | 1.08 (1.05, 1.10) | <0.001 | 1.02 (0.98, 1.07) | 0.33 | 1.03 (0.99, 1.09) | 0.18 |
| Heart failure (1381) | 1.26 (1.20, 1.33) | <0.001 | 1.24 (1.18, 1.30) | <0.001 | 1.09 (0.98, 1.20) | 0.11 | 1.10 (0.99, 1.23) | 0.09 |
Values are HRs (95% CIs). HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, or ≥75 y), and with adjustment for a disease-specific set of potential confounding factors. Median intakes of red meat were 24.7 g/d with biomarker calibration and 31.8 g/d without biomarker calibration and of red plus processed meat were 34.8 g/d with biomarker calibration and 42.2 g/d without biomarker calibration. CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; DM-C, dietary modification comparison group; MI, myocardial infarction.; OS, observational study.
Total CVD comprised CHD + CABG + PCI + stroke.
The right side of Table 3 shows corresponding calibrated meat intake HRs in analyses that include jointly calibrated total energy, as well as BMI, FFQ saturated fat, and FFQ sodium. The meat intake HRs were all nonsignificant following these further adjustments. Corresponding CVD HRs without biomarker calibration (Supplemental Table 5, right side) are again close to the null and mostly nonsignificant.
Table 4 provides corresponding HRs for (invasive) cancer incidence. In the absence of adjustment for dietary correlates (Table 4, left side), calibrated red meat had HR (95% CI) of 1.10 (1.07, 1.13) for breast cancer, 1.12 (1.06, 1.18) for colon cancer, 1.25 (1.18, 1.33) for endometrial cancer, 1.15 (1.04, 1.27) for kidney cancer, 1.12 (1.09, 1.14) for obesity-related cancer, and 1.07 (1.05, 1.09) for total invasive cancer. There was also an estimated inverse association, with HR (95% CI) of 0.89 (0.81, 0.97), for ovarian cancer. Once again, HRs for R + P meat were close to those for red meat alone. These associations, however, were mostly nonsignificant following control for calibrated total energy, BMI, and FFQ dietary potential confounding factors (Table 4, right side), with a possible residual positive association with colon cancer, and possible inverse associations with endometrium, ovarian, and kidney cancer. Corresponding HRs without biomarker calibration (Supplemental Table 6) were again much attenuated toward the null.
TABLE 4.
Cancer incidence HRs and 95% CIs for a 40% increment above the median for red meat and for red plus processed meat in a Women's Health Initiative cohort of 81,954 postmenopausal US women enrolled during 1993–1998 at 40 US clinical centers and followed through February 20201
| No adjustment for BMI or dietary correlates | With adjustment for BMI and dietary correlates | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer site (n = participants with events) | Red meat intake, g/d | P value | Red + processed meat intake, g/d | P value | Red meat intake, g/d | P value | Red + processed meat intake, g/d | P value |
| Breast (5139) | 1.10 (1.07, 1.13) | <0.001 | 1.09 (1.07, 1.12) | <0.001 | 1.03 (0.98, 1.09) | 0.24 | 1.01 (0.96, 1.06) | 0.68 |
| Colon (1060) | 1.12 (1.06, 1.18) | <0.001 | 1.11 (1.05, 1.16) | <0.001 | 1.12 (1.00, 1.26) | 0.06 | 1.11 (0.98, 1.24) | 0.08 |
| Rectum (158) | 1.01 (0.86, 1.17) | 0.94 | 1.02 (0.89, 1.17) | 0.78 | 0.98 (0.72, 1.33) | 0.89 | 1.04 (0.79, 1.37) | 0.79 |
| Endometrium (881) | 1.25 (1.18, 1.33) | <0.001 | 1.24 (1.18, 1.31) | <0.001 | 0.88 (0.77, 1.01) | 0.08 | 0.86 (0.75, 0.98) | 0.03 |
| Ovary (471) | 0.89 (0.81, 0.97) | 0.01 | 0.91 (0.84, 0.98) | 0.02 | 0.75 (0.61, 0.91) | 0.003 | 0.79 (0.67, 0.94) | 0.008 |
| Leukemia (439) | 1.01 (0.92, 1.11) | 0.82 | 1.03 (0.95, 1.11) | 0.50 | 0.89 (0.73, 1.08) | 0.24 | 0.99 (0.83, 1.18) | 0.89 |
| Lung (1426) | 0.98 (0.93, 1.03) | 0.34 | 0.98 (0.94, 1.02) | 0.36 | 0.95 (0.86, 1.05) | 0.33 | 0.96 (0.87, 1.06) | 0.40 |
| Lymphoma (804) | 1.05 (0.98, 1.12) | 0.20 | 1.04 (0.97, 1.10) | 0.27 | 1.08 (0.94, 1.24) | 0.26 | 1.07 (0.94, 1.21) | 0.34 |
| Bladder (166) | 0.93 (0.81, 1.06) | 0.28 | 0.93 (0.83, 1.05) | 0.24 | 0.78 (0.58, 1.06) | 0.12 | 0.71 (0.53, 0.96) | 0.02 |
| Kidney (309) | 1.15 (1.04, 1.27) | 0.006 | 1.19 (1.09, 1.30) | <0.001 | 0.74 (0.58, 0.93) | 0.01 | 0.85 (0.68, 1.06) | 0.15 |
| Pancreas (416) | 1.04 (0.95, 1.15) | 0.36 | 1.04 (0.96, 1.13) | 0.31 | 0.99 (0.82, 1.20) | 0.95 | 1.00 (0.83, 1.20) | 0.97 |
| Obesity related2 (7313) | 1.12 (1.09, 1.14) | <0.001 | 1.11 (1.09, 1.13) | <0.001 | 1.01 (0.96, 1.05) | 0.79 | 1.00 (0.95, 1.04) | 0.83 |
| Total invasive (12,804) | 1.07 (1.05, 1.09) | <0.001 | 1.07 (1.05, 1.08) | <0.001 | 0.98 (0.95, 1.01) | 0.23 | 0.98 (0.95, 1.02) | 0.33 |
Values are HRs (95% CIs). HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, or ≥75 y), and with adjustment for a disease-specific set of potential confounding factors. Red meat median intakes were 24.7 g/d with biomarker calibration and 31.8 g/d without biomarker calibration, and red plus processed meat were 34.8 g/d with biomarker calibration and 42.2 g/d without biomarker calibration.
Obesity-related cancer defined here as breast, colon, rectum, endometrium, or kidney cancer.
Table 5 (left side) shows very strong associations of calibrated red and R + P meat intake with T2D incidence, with respective 40% increment HRs (95% CIs) of 1.37 (1.34, 1.39) and 1.35 (1.33, 1.37). These associations were considerably reduced but still highly significant following additional control for calibrated total energy, BMI, and FFQ dietary potential confounders (Table 5, right side), with HRs (95% CIs) of 1.08 (1.04, 1.12) and 1.09 (1.05, 1.13) for red and R + P meat, respectively. Corresponding diabetes associations were also evident without biomarker calibration, but these again are substantially attenuated toward the null (Supplemental Table 7).
TABLE 5.
T2D incidence HRs, and 95% CIs for a 40% increment above the median intake for red meat and for red plus processed meat in a Women's Health Initiative cohorts of 81,954 postmenopausal US women enrolled during 1993–1998 at 40 US clinical centers and followed through February 20201
| No adjustment for BMI or dietary correlates | With adjustment for BMI and dietary correlates | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome (n = participants with outcome) | Red meat intake, g/d | P value | Red + processed meat intake, g/d | P value | Red meat intake, g/d | P value | Red + processed meat intake, g/d | P value |
| T2D (12,145) | 1.37 (1.34, 1.39) | <0.001 | 1.35 (1.33, 1.37) | <0.001 | 1.08 (1.04, 1.12) | <0.001 | 1.09 (1.05, 1.13) | <0.001 |
Values are HRs (95% CIs). Red meat median intakes were 24.7 g/d with biomarker calibration and 31.8 g/d without biomarker calibration, and red plus processed meat median intakes were 34.8 g/d with biomarker calibration and 42.2 g/d without biomarker calibration. DM-C, dietary modification comparison group; OS, observational study; T2D, type 2 diabetes.
HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, or ≥75 y), and with adjustment for a disease-specific set of potential confounding factors.
Additional analyses were carried out that further added log-FFQ fiber and log-FFQ fruit and vegetable servings (see Table 2) to the analyses on the right side of Tables 3–5. These inclusions had very little further influence on HRs for any of the clinical outcomes (results not shown).
Discussion
Our analyses follow the approach used in previous reports of using values of our newly identified biomarkers in a WHI nutrition biomarker study to develop calibration equations that estimate intake based on concurrent self-reported dietary data and participant characteristics. Resulting calibration equations meeting criteria were developed for both red meat and R + P meat. Metabolites contributing to biomarker measures for red and R + P meat were biologically plausible and included amino acids and their derivatives, fatty acids and fatty acid transporters, degradation products of heme, and metabolites of muscle and other protein-rich foods. Transferability of these biomarkers to other cohorts is a topic of current research in WHI. The potential for transferability may be enhanced by allowance for participant characteristics in biomarker specification.
A possible reason we were unable to develop a metabolomics-based biomarker for processed meat was the rather small amount of processed meat intake in this cohort (Table 2). Also, in our experience, one may not be able to develop adequate biomarkers for a fraction of energy dietary variable simply by dividing biomarkers meeting criteria for the numerator and denominator of the ratio, motivating our (unsuccessful) consideration of separate biomarker developments for meat density measures.
To include the small fraction of participants without any reported red or R + P meat intake, both biomarker equations and calibration equations were based on linear regression without the log-transformation of intakes used in our previous reports, (e.g., 20). Here, the resulting calibrated meat variables were transformed to log (1. + meat intake/median cohort meat intake) to enhance normal distribution approximations and the applicability of measurement error modeling assumptions for Cox regression analyses. There was little evidence for major departures from the HR model used for the transformed meat variables considered in our analyses, based on further analyses that added a quadratic term in the transformed meat variables to the log HR model.
The inclusion of both baseline FFQ dietary data in biomarker equation development and contemporary FFQ data in calibration equation development merits some comment: The 2 sets of equations derive from data on distinct participants and are taken at quite different points in time: baseline 1993–1998 for biomarker equation development compared with 2007–2009 for calibration equation development. Dependency in the FFQ measurement errors for the 2 sets of dietary assessments therefore seems unlikely, and this potential is further reduced by consideration of variables such as BMI that could relate to the systematic bias component of FFQ measurement error in calibration equation development.
Calibration equations for red and R + P meat allow intake for these variables to be estimated using baseline data available for participants in larger WHI cohorts. This is also the case for calibration equations for these meat intakes and total energy jointly, the latter using DLW measures as biomarkers for total energy consumption.
These methods and resources allow us to contribute to the important but controversial topic of meat intake and chronic disease risk, here with biomarker calibration of meat intake, in the setting of postmenopausal US women. There is considerable epidemiologic literature reporting positive associations between red meat, and especially processed meat, intake with CVD, cancer, and diabetes. For example, a 2015 International Agency for Research on Cancer working group determined following a comprehensive literature review (27) that red meat is probably carcinogenic to humans and judged that evidence was sufficient to classify processed meat as carcinogenic. A recent systematic review of meat intake and heart disease demonstrated that there was substantial evidence that red meat and processed meat consumption were associated with higher rates of ischemic heart disease (28). Also, red meat and processed meat have consistently related positively to diabetes risk in systematic reviews of the observational epidemiologic literature (e.g., 29, 30).
Our analyses in WHI cohorts without control for potential dietary confounders (left sides of Tables 3–5), generally agree with this literature. For example, for red meat, the HR estimates for a 40% increment above the median intake were 1.11 for nonfatal MI; 1.10 for coronary death; 1.26 for heart failure; 1.10, 1.12, and 1.25 for cancers of the breast, colon, and endometrium, respectively; and 1.37 for T2D. Note that some of these associations (e.g., for coronary death, colon cancer, endometrial cancer) were significant only following biomarker calibration of the FFQ meat intake, a circumstance that would not be expected unless the measurement error adjustment included correction for systematic bias in self-reported FFQ meat intake.
The HRs in these analyses (Tables 3–5, left side) can be regarded as estimating associations for a relatively high meat dietary pattern, with its attendant high fat, high energy, and high sodium intakes (Table 2). Following adjustment for these dietary correlates (and BMI) the HRs were much reduced, and mostly nonsignificant, though the T2D HR remained significantly elevated. These latter analyses may entail some overadjustment since the high fat, high energy content of the meat itself contributes to the HR elevations just listed. For example, the change in direction of the HR for endometrial cancer following control for dietary correlates may reflect the sensitivity of these cancers to energy consumption. Further analyses showed that most, but not all, of the HR reductions in Tables 3–5 following control for dietary correlates can be attributed to control for biomarker-calibrated total energy.
Strengths of this study include its prospective cohort design with careful outcome ascertainment and long-term follow-up, and the development and use of novel biomarkers for meat intake. These biomarkers are primarily based on metabolomics profiles in blood and urine.
Limitations of the study include the observational study design, which does not ensure an absence of confounding, as high-meat consumers differ in many respects from low- or nonmeat consumers (e.g., Table 2). Also, processed meat intake was low in this population, precluding separate biomarker identification for these meats. Red meat intake with median FFQ intake of 31.8 g/d is also rather modest in amount compared with a value of ∼52.8 g/d after allowing for a negative trend of intake with age for US females based on 24-h data (31). As a final limitation, the cohort studied here includes only postmenopausal US women, and results may differ in other populations.
In summary, biomarker-based calibrated meat intake analyses provide a novel look at the association between meat consumption and the risk for major chronic diseases. Positive associations with a relatively high meat dietary pattern that are large enough to be of public health importance emerged for each of CVD, cancer, and diabetes. However, with the exception of diabetes, these associations appear to be almost entirely attributable to the high-fat, high-energy, and high-sodium intake associated with a high-meat dietary pattern, rather than to the meat per se in this population of postmenopausal US women.
Supplementary Material
Acknowledgments
We acknowledge the following investigators in the WHI Program: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland): Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet Anderson, Ross L. Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA): JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC): Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA): Marcia L. Stefanick; (The Ohio State University, Columbus, OH): Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ): Cynthia A. Thomson; (University at Buffalo, Buffalo, NY): Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL): Marian Limacher; (University of Iowa, Iowa City/Davenport, IA): Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA): Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC): Sally Shumaker; (University of Nevada, Reno, NV): Robert Brunner; Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC): Mark Espeland. For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. The author's responsibilities were as follows—RLP, LFT, MLN, MP, RBW, LS: helped design the WHI program; MLN, LFT, JWL, MP, RLP: carried out the WHI biomarker and feeding studies; JWL, DR, GANG, CZ, DOB, SLN, MLN, LFT, RLP: contributed to metabolite biomarker development activities; CZ, MP, RLP: carried out data analyses for the current manuscript; all authors: participated in critical review of manuscript drafts; and all authors: read and approved the final manuscript.
Notes
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services (contracts HHSN268201600046C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN271201600004C); and National Cancer Institute grants R01 CA119171 and P30 CA15704, and NIH instrumentation grant S10 OD021562.
Author disclosures: Dr. Marian Neuhouser is an Associate Editor on the Journal of Nutrition and played no role in the Journal's evaluation of the manuscript. All authors report no conflicts of interest.
Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, and the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives. The contents of the paper are solely the responsibility of the authors. The WHI is funded primarily by the National Heart, Lung, and Blood Institute. Participants provided written informed consent for their overall WHI, NPAAS, and NPAAS-FS activities. Related protocols were approved by the Institutional Review Boards at the Fred Hutchinson Cancer Research Center, and at each participating clinical center.
Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CHD, coronary heart disease; CR, confidence region; CT, clinical trial; CV, cross validation; CVD, cardiovascular disease; DLW, doubly labeled water; DM, dietary modification; DM-C, dietary modification comparison group; MI, myocardial infarction; NPAAS, Nutrition and Physical Activity Assessment Study; NPAAS-FS, NPAAS feeding study; OS, observational study; QC, quality control; R + P, red plus processed; T2D, Type 2 diabetes; UN, urinary nitrogen; WHI, Women's Health Initiative; 4DFR, 4-day food record; 24HRs, 24-h dietary recall.
Contributor Information
Cheng Zheng, Department of Biostatistics, University of Nebraska Medical Center, Omaha, NE, USA.
Mary Pettinger, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
G A Nagana Gowda, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Johanna W Lampe, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Daniel Raftery, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Lesley F Tinker, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Ying Huang, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Sandi L Navarro, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Diane M O'Brien, Institute for Arctic Biology, University of Alaska, Fairbanks, AK, USA.
Linda Snetselaar, College of Public Health, University of Iowa, Iowa City, IA, USA.
Simin Liu, Department of Epidemiology, School of Public Health, Brown University, Providence, RI, USA.
Robert B Wallace, College of Public Health, University of Iowa, Iowa City, IA, USA.
Marian L Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Ross L Prentice, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; School of Public Health, University of Washington, Seattle, WA, USA.
Data Availability
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode as described on the Women's Health Initiative website (www.whi.org).
References
- 1. Schoeller DA, Hnilicka JM. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J Nutr. 1996;126:348S–54S. [PubMed] [Google Scholar]
- 2. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(3):921S–4S. [DOI] [PubMed] [Google Scholar]
- 3. Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Van Horn Let al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol. 2008;167(10):1247–59. [DOI] [PubMed] [Google Scholar]
- 4. Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan Bet al. Evaluation and comparison of food records, recalls and frequencies for energy and protein assessment using recovery biomarkers. Am J Epidemiol. 2011;174(5):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prentice RL, Huang Y, Kuller LH, Tinker LF, Van Horn L, Stefanick ML, et al. Biomarker-calibrated energy and protein consumption and cardiovascular disease risk among postmenopausal women. Epidemiology. 2011;22(2):170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prentice RL, Shaw PA, Bingham SA, Beresford SA, Caan B, Neuhouser MLet al. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am J Epidemiol. 2009;169(8):977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tinker LF, Sarto GE, Howard BV, Huang Y, Neuhouser ML, Mossavar-Rahmani Yet al. Biomarker-calibrated dietary energy and protein intake association with diabetes risk among postmenopausal women from the Women's Health Initiative. Am J Clin Nutr. 2011;94(6):1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng C, Beresford SAA, Van Horn L, Tinker LF, Thomson CA, Neuhouser MLet al. Simultaneous association of total energy consumption and activity-related energy expenditure with cardiovascular disease, cancer, and diabetes risk among postmenopausal women. Am J Epidemiol. 2014;180(5):526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prentice RL, Howard BV, Van Horn L, Neuhouser ML, Anderson GL, Tinker LFet al. Nutritional epidemiology and the Women's Health Initiative: a review. Am J Clin Nutr. 2021;113(5):1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnston BC, Zeraatkar D, Han MA, Vernooij RWM, Valli C, El Dib Ret al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann Intern Med. 2019;171(10):756–64. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Department of Agriculture, U.S. Department of Health and Human Services . 2015–2020 Dietary guidelines for Americans. 8th ed.Accessed at [Internet], https://health.gov/dietaryguidelines/2015/guidelines on 17 December 2019. [Google Scholar]
- 12. World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) . Continuous Update Project Expert Report 2018. Meat, fish and dairy products and the risk of cancer. Available from[Internet]: dietandcancerreport.com. [Google Scholar]
- 13. Rock CL, Thomson C, Gansler T, Gapstur SM, McCulloch ML, Patel AVet al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70:245–71. [DOI] [PubMed] [Google Scholar]
- 14. Women's Health Initiative Study Group . Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 15. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. [DOI] [PubMed] [Google Scholar]
- 16. Lampe JW, Huang Y, Neuhouser ML, Tinker LF, Song X, Schoeller DAet al. Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am J Clin Nutr. 2017;105(2):466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng C, Gowda GAN, Raftery D, Neuhouser ML, Tinker LF, Prentice RLet al. Evaluation of potential metabolomic-based biomarkers of protein, carbohydrate and fat intakes using a controlled feeding study. Eur J Nutr. 2021;60(8):4207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuparencu C, Praticó G, Hemeryck LY, Sri Harsha PSC, Noerman S, Rombouts Cet al. Biomarkers of meat and seafood intake: an extensive literature review. Genes Nutr. 2019;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wedekind R, Kiss A, Keski-Rahkonen P, Viallon V, Rothwell JA, Cross AJet al. A metabolic study of red and processed meat intake and acylcarnetine concentration in human urine and blood. Am J Clin Nutr. 2020;112(2):381–8. [DOI] [PubMed] [Google Scholar]
- 20. Prentice L, Pettinger M, Neuhouser ML, Raftery D, Zheng C, Nagana Gowda GAet al. Biomarker-calibrated macronutrient intake and chronic disease risk among postmenopausal women. J Nutr. 2021;151(8):2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt Met al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9):S122–8. [DOI] [PubMed] [Google Scholar]
- 22. Margolis KL, Qi L, Brzyski R, Bonds DE, Howard BV, Kempainen Set al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5(3):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cox DR. Regression analysis and life tables (with discussion). J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 24. Prentice RL. Covariate measurement errors and parameter estimation in a failure time regression model. Biometrika. 1982;69(2):331–42. [Google Scholar]
- 25. Wang CY, Hsu L, Feng ZD, Prentice RL. Regression calibration in failure time regression. Biometrics. 1997;53(1):131–45. [PubMed] [Google Scholar]
- 26. Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement error in nonlinear models, a modern perspective. 2nd ed.Boca Raton, FL: Chapman and Hall/CRC; 2006. [Google Scholar]
- 27. IARC monographs on the evaluation of carcinogenic risks to humans; Volume 114: Red Meat and Processed Meat, 2018: International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- 28. Papier K, Knuppel A, Syam N, Jebb SA, Key TJ. Meat consumption and risk of ischemic heart disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021 Jul 2;1–12. doi: 10.1080/10408398.2021.1949575. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 29. Aune D, Ursin G, Veierod MD. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52(11):2277–87. [DOI] [PubMed] [Google Scholar]
- 30. Neuenschisander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the United States. Public Health Nutr. 2011;14(4):575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode as described on the Women's Health Initiative website (www.whi.org).