Abstract
Objective
Tocilizumab plus prednisone induces sustained glucocorticoid-free remission in patients with GCA. However, its long-term benefits in new-onset vs relapsing disease are uncertain, and the value of weekly vs every-other-week dosing has not been evaluated.
Methods
In Giant-Cell Arteritis Actemra (GiACTA) part 1, patients with new-onset or relapsing GCA received blinded tocilizumab weekly (TCZ QW), tocilizumab every-other-week (TCZ Q2W) or placebo for 52 weeks, with a prednisone taper. In part 2 (open-label), patients were treated at investigator discretion for 104 weeks. In this analysis, patients were evaluated according to their original treatment assignments, and outcomes beyond 52 weeks were assessed. Outcomes of interest included time to first flare and cumulative glucocorticoid exposure over 3 years according to baseline disease status.
Results
Part 1 enrolled 250 patients; 215 entered part 2. At baseline, 48% had new-onset disease and 52% had relapsing disease. In patients with new-onset and relapsing disease, the median time to first flare in the TCZ QW group was 577 and 575 days, respectively, vs 479 and 428 days with TCZ Q2W and 179 and 224 days with placebo; the median cumulative glucocorticoid dose was 3068 mg and 2191 mg with TCZ QW, 4080 mg and 2353 mg with TCZ Q2W, and 4639 mg and 6178 mg with placebo.
Conclusion
TCZ QW delayed the time to flare and reduced the cumulative glucocorticoid dose in patients with relapsing GCA and new-onset GCA. These data support initiating TCZ QW as part of first-line therapy in all patients with active GCA.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT01791153.
Keywords: biologic therapies, cardiovascular, clinical trials and methods, giant cell arteritis, inflammation
Rheumatology key messages.
Tocilizumab delayed time to first flare and reduced glucocorticoid exposure in new-onset and relapsing GCA.
Tocilizumab dosed weekly delayed time to first flare to a greater degree than every-other-week dosing.
Introduction
GCA is an inflammatory disorder associated with vascular and inflammatory symptoms, including headache, jaw claudication, fever, weight loss and visual disturbances that can lead to irreversible blindness [1, 2]. To minimize these consequences, patients with GCA are commonly treated with high-dose glucocorticoids, which are initially effective for symptom management but may not be sufficient to prevent disease flares at lower doses. Up to 80% of patients experience disease flares over the course of 1 year if glucocorticoids are tapered completely, and the flare rate is between 35% and 75% even if patients continue on some dose of prednisone while in remission [3–9]. Many patients require additional prednisone to treat flares or refractory symptoms; however, prolonged glucocorticoid use is associated with significant toxicity [2]. Given that GCA occurs almost exclusively in older adults, with a peak incidence occurring between 71 and 80 years [10], the potential for glucocorticoid toxicity is especially concerning [11, 12].
The IL-6 receptor alpha inhibitor tocilizumab was approved for the treatment of patients with GCA, based on positive results from the Giant-Cell Arteritis Actemra (GiACTA) trial [9]. GiACTA was a two-part, randomized, placebo-controlled, multicentre phase 3 study of patients with either new-onset or relapsing GCA [9, 13]. Part 1 consisted of a 52-week double-blind period; this was followed by a 104-week open-label phase (part 2). In part 1, tocilizumab administered weekly or every other week in combination with prednisone taper was superior to placebo plus prednisone taper at inducing sustained remission at week 52, while also significantly reducing the cumulative glucocorticoid dose [9]. In part 2, patients assigned to receive tocilizumab in part 1 experienced fewer flares and, over the 3-year study period, maintained a lower cumulative glucocorticoid exposure than those assigned to receive placebo in part 1 [14].
Subgroup analysis of GiACTA part 1 suggested potential differences in tocilizumab efficacy between new-onset and relapsing disease. Specifically, data from part 1 suggested that tocilizumab dosed weekly (TCZ QW) or every other week (TCZ Q2W) was similarly effective at preventing flares and reducing glucocorticoid exposure at the week 52 time point in patients with new-onset disease. In contrast, TCZ QW was more effective than TCZ Q2W for these outcomes in patients with relapsing disease at baseline. One consequence of these findings has been a reluctance on the part of some clinicians to reserve the use of tocilizumab for patients with relapsing disease and to treat patients with newly diagnosed GCA with prednisone monotherapy. Therefore, in the current analysis, we investigated the maintenance of clinical remission, time to first flare, and cumulative glucocorticoid exposure separately for patients with new-onset and relapsing GCA at baseline over the 3-year period of the GiACTA clinical trial. Additionally, data are limited regarding the effectiveness of different tocilizumab doses after 1 year of treatment in patients with new-onset and relapsing disease; therefore, we also examined the effectiveness of TCZ QW compared with TCZ Q2W in both patient groups.
Methods
Study design and assessments
Details of the GiACTA (ClinicalTrials.gov, NCT01791153) trial design have been published [9, 13, 14]. In brief, GiACTA was a two-part study consisting of a 52-week double-blind period (part 1) followed by a 104-week open-label phase (part 2). Patients entering part 1 were randomly assigned (2:1:1:1 ratio) to receive 162 mg s.c. TCZ QW with a 26-week prednisone taper, 162 mg s.c. tocilizumab every-other-week with a 26-week prednisone taper, s.c. placebo weekly with a 26-week prednisone taper or s.c. placebo weekly with a 52-week prednisone taper. Randomization was stratified by baseline prednisone dose (>30 mg/day and ≤30 mg/day prednisone). Patients completing part 1 were eligible to enter part 2, in which open-label treatment was left to the investigator’s discretion; however, investigators remained blinded to the original treatment assignments from part 1. Permitted treatments in part 2 were any combination of weekly tocilizumab 162 mg, prednisone, and/or MTX, or no treatment.
The primary efficacy end point in part 1 was the proportion of patients achieving sustained glucocorticoid-free remission, defined as remission from week 12 through week 52 and adherence to the prednisone taper. In part 1, remission was defined as the absence of flare and the normalization of CRP concentration to <1 mg/dl. Disease flare during the entire trial was determined by the investigator and was defined as the recurrence of signs or symptoms of GCA, or ESR ≥30 mm/h attributable to GCA, that required treatment intensification or both. In accordance with the protocol, patients entering part 2 in clinical remission stopped blinded tocilizumab injections. In part 2, the primary efficacy end point was maintenance of clinical remission, defined as the absence of flare throughout part 2 after the achievement of clinical remission at the end of part 1, regardless of CRP level.
The GiACTA protocol was approved by the ethics committee or institutional review board at each site (Supplementary Appendix, available at Rheumatology online), and the study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
Patients
Adults with active new-onset (diagnosis ≤6 weeks before baseline) or relapsing (diagnosis >6 weeks before baseline and previous treatment with ≥40 mg/day prednisone or equivalent for ≥2 weeks at any time) GCA were included. Diagnosis of GCA required patients to be ≥50 years of age and have a history of characteristic clinical manifestations (e.g. cranial or PMR symptoms) and inflammatory marker elevation (ESR ≥50 mm/h or CRP ≥2.45 mg/dl). Diagnostic confirmation via histology (i.e. temporal artery biopsy) or vascular imaging was also required. Additional details of the patient population have been published [9, 13–15].
Statistical analysis
Statistical considerations for GiACTA have been reported [9, 13, 14]. Efficacy analyses, including maintenance of clinical remission from week 52 through week 156, are reported using descriptive statistics. Time to flare was calculated using a Cox proportional hazards model and adjusted for starting prednisone dose (≤30 mg/day or >30 mg/day), with censoring on study withdrawal. Hazard ratios along with 95% CIs are presented. Cumulative glucocorticoid exposure was compared between groups using the van Elteren test stratified by starting prednisone dose (≤30 mg/day or >30 mg/day).
All study arm designations in this analysis (e.g. TCZ QW) refer to treatment assignment in part 1. The two placebo groups were combined for this analysis. Patients who were never in remission were censored at day 1, and patients who withdrew prematurely were censored from the time of withdrawal.
Results
Patients
A total of 251 patients were randomly assigned in part 1; of those, 250 received study treatment and were included in the intention-to-treat and safety populations. Of the 250 patients, 215 (86%) completed part 1 and transitioned to part 2 at week 52. Of those, 184 patients (86%) were in clinical remission [TCZ QW, 81 (95%); TCW Q2W, 36 (90%); PBO, 67 (74%)] and stopped receiving blinded injections when they entered part 2. During part 2, 7 patients (3.3%) withdrew from the study for safety reasons, and 11 patients (5.1%) withdrew for non-safety reasons. One hundred and ninety-seven patients completed the study through week 156 [14].
Baseline characteristics were generally balanced across treatment groups (Table 1). Most patients were women (75%), and their mean age was 69 years. In all groups, approximately half the patients had new-onset disease at baseline (TCZ QW, 47%; TCZ Q2W, 53%; PBO, 46%) and the remainder had relapsing disease. Patients with new-onset disease had a mean disease duration ranging from 29 to 30 days across the treatment groups, and patients with relapsing disease had a mean disease duration ranging from 520 to 553 days (Table 1).
Table 1.
Demographic and baseline characteristics (ITT population)
| TCZ QW |
TCZ Q2W |
Pooled PBO |
||||
|---|---|---|---|---|---|---|
| New-onset | Relapsing | New-onset | Relapsing | New-onset | Relapsing | |
| (n = 47) | (n = 53) | (n = 26) | (n = 23) | (n = 46) | (n = 55) | |
| Age, years | 70 (8.7) | 70 (8.4) | 68 (7.9) | 71 (8.7) | 69 (8.6) | 68 (7.3) |
| Female sex, n (%) | 37 (79) | 41 (77) | 18 (69) | 16 (70) | 34 (74) | 41 (75) |
| Weight, kg | 68 (13.5) | 72 (13.9) | 71 (14.5) | 71 (17.5) | 67 (11.7) | 76 (17.2) |
| Duration of GCA, days | 29 (10.2) | 553 (687.8) | 30 (8.7) | 520 (651.7) | 29 (10.1) | 544 (593.8) |
| Prednisone dose, n (%) | ||||||
| ≤30 mg/day | 15 (32) | 37 (70) | 9 (35) | 15 (65) | 22 (48) | 31 (56) |
| >30 mg/day | 32 (68) | 16 (30) | 17 (65) | 8 (35) | 24 (52) | 24 (44) |
All data are mean (s.d.) unless otherwise stated. ITT: intention-to-treat; PBO: placebo; QW: once weekly; Q2W: every 2 weeks; TCZ: tocilizumab.
Time to first flare
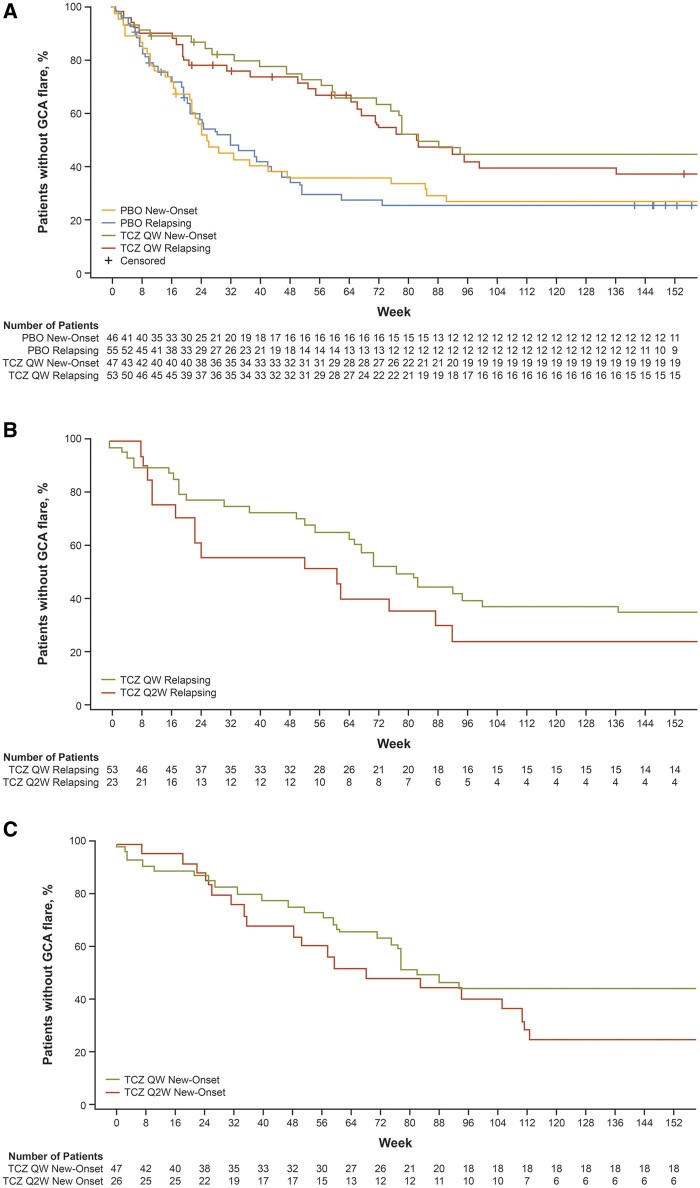
A higher proportion of patients in the TCZ QW group, compared with the TCZ Q2W and PBO groups, did not experience flares over the 3-year study period (48% vs 31% and 30%, respectively; Table 2). Among the patients with new-onset disease, 49% in the TCZ QW group remained flare-free compared with 27% in the TCZ Q2W group and 28% in the PBO group. Among those with relapsing disease at baseline, 47% in the TCZ QW group remained flare-free compared with 35% in the TCZ Q2W group and 31% in the PBO group. The hazard ratios for flare over 3 years for patients with relapsing disease in the TCZ QW group compared with the PBO group was 0.55 (95% CI: 0.34, 0.90). Patients with relapsing disease in the TCZ Q2W group also had a lower risk for flare than those in the PBO group, but this comparison was not statistically significant [hazard ratios 0.80 (95% CI: 0.44, 1.46)].
Table 2.
Time to first flare after clinical remission by disease onset at baseline
| TCZ QW |
TCZ Q2W |
Pooled PBO |
||||
|---|---|---|---|---|---|---|
| New-onset | Relapsing | New-onset | Relapsing | New-onset | Relapsing | |
| (n = 47) | (n = 53) | (n = 26) | (n = 23) | (n = 46) | (n = 55) | |
| Patients with flare, n (%) | 24 (51) | 28 (53) | 19 (73) | 15 (65) | 33 (71) | 38 (69) |
| Patients without flare, n (%) | 23 (49) | 25 (47) | 7 (27) | 8 (35) | 13 (28) | 17 (31) |
| Time to flare, days | ||||||
| Median | 577 | 575 | 479 | 428 | 179 | 224 |
| 95% CI | 499, NE | 463, NE | 341, 778 | 162, 645 | 149, 331 | 148, 322 |
| Range | 1–1267 | 1–1194 | 50–1156 | 1–1173 | 5–1171 | 1–1158 |
| Risk for flare throughout 3 yearsa | ||||||
| Hazard ratio | 0.51 | 0.55 | 0.76 | 0.80 | NA | NA |
| 95% CI | 0.30, 0.87 | 0.34, 0.90 | 0.43, 1.35 | 0.44, 1.46 | NA | NA |
TCZ vs PBO; includes relapsing patients only. Patients who were never in remission were censored at day 1. Patients who withdrew were censored at the time of withdrawal. NA: not applicable; NE: not evaluable: PBO: placebo; QW: once weekly; Q2W: every 2 weeks; TCZ: tocilizumab.
The median time to first flare following clinical remission was longer for patients originally assigned to tocilizumab in either dosing regimen, compared with the placebo group, and this was true for patients with new-onset GCA and those with relapsing disease. The TCZ QW group demonstrated the longest time to first flare of all groups studied (Table 2). Among the patients with new-onset disease at baseline, the median time to first flare was 577 days (95% CI: 499, not evaluable) in the TCZ QW group, 479 days (95% CI: 341, 778) in the TCZ Q2W group, and 179 days (95% CI: 149, 331) in the PBO group. For patients with relapsing disease at baseline, the median times to first flare in these three treatment groups were 575 days (95% CI: 463, not evaluable), 428 days (95% CI: 162, 645), and 224 days (95% CI: 148, 322), respectively.
A clear separation was observed between patients receiving tocilizumab and those receiving placebo throughout the study, regardless of whether patients had new-onset or relapsing disease at baseline (Fig. 1).
Fig. 1.
Time to first flare following clinical remission
Time to first flare following clinical remission by disease status at baseline for (A) placebo versus weekly tocilizumab, relapsing and new-onset; (B) weekly versus every-other-week tocilizumab, relapsing; (C) weekly versus every-other-week tocilizumab, new-onset. Treatment groups refer to originally assigned treatment in part 1. Patients who were never in remission were censored at day 1, and patients who withdrew were censored at time of withdrawal. PBO: placebo; QW: once weekly; Q2W: every 2 weeks; TCZ: tocilizumab.
Glucocorticoid exposure
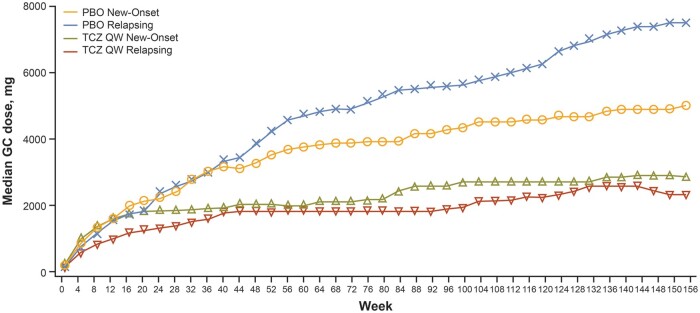
The median cumulative glucocorticoid dose over the 3-year study was generally lower for those in the TCZ QW group than in the PBO group, regardless of whether patients had new-onset or relapsing disease at baseline (Table 3, Fig. 2). For patients with new-onset disease, the median glucocorticoid exposure was 3068 mg [interquartile range (IQR): 1862–6283] in the TCZ QW group (P = 0.0331 vs PBO group), 4080 mg (IQR: 2604–6931) in the TCZ Q2W group (P = 0.3233 vs PBO group), and 4639 mg (IQR: 3147–6768) in the PBO group. For patients with relapsing disease at baseline, the median glucocorticoid exposures were 2191 mg (IQR: 1354–4690) in the TCZ QW group (P < 0.0001 vs PBO group), 2352 mg (IQR: 1517–5419) in the TCZ Q2W group (P = 0.0088 vs PBO group) and 6178 mg (IQR: 2918–10 919) in the PBO group.
Table 3.
Cumulative glucocorticoid exposure by disease status at baseline
| TCZ QW |
TCZ Q2W |
Pooled PBO |
||||
|---|---|---|---|---|---|---|
| New-onset | Relapsing | New-onset | Relapsing | New-onset | Relapsing | |
| (n = 47) | (n = 53) | (n = 26) | (n = 23) | (n = 46) | (n = 55) | |
| Cumulative glucocorticoid dose, mg | ||||||
| Median | 3068 | 2191 | 4080 | 2352 | 4639 | 6178 |
| IQR | 1862–6283 | 1354–4690 | 2604–6931 | 1517–5419 | 3147–6768 | 2918–10 919 |
| P-value (vs pooled PBO) | P = 0.0331 | P < 0.0001 | P = 0.3233 | P = 0.0088 | – | – |
Van Elteren’s test was used to calculate P values for the differences in cumulative glucocorticoid exposure between each of the TCZ groups and the pooled PBO group. The analysis was stratified by starting prednisone dose (≤30 mg/day or >30 mg/day). IQR: interquartile range; PBO: placebo; QW: once weekly; Q2W: every 2 weeks; TCZ: tocilizumab.
Fig. 2.
Cumulative glucocorticoid dose
Cumulative GC dose by disease status at baseline for placebo versus weekly tocilizumab. Treatment groups refer to originally assigned treatment in part 1. GC: glucocorticoid; PBO: placebo; QW: once weekly; TCZ: tocilizumab.
Discussion
In clinical trials of patients with vasculitis, patients with new-onset disease at the time of trial enrolment often fare better than those whose disease is relapsing at trial enrolment, at least over the short term. The reasons for this are not clear, but this pattern has been observed in both of the most carefully studied forms of systemic vasculitis, GCA, and ANCA-associated vasculitis [9, 16]. In part 1 of the GiACTA trial, the outcomes of patients with new-onset disease at baseline who were randomly assigned to TCZ Q2W did not clearly differ from the outcomes of patients who received TCZ QW. In contrast, TCZ QW was more effective than TCZ Q2W for these outcomes in patients with relapsing disease at baseline. We undertook the current study to understand whether these differences between patients with new-onset and relapsing GCA persisted over time, or whether the potential importance of higher dosing of IL-6-receptor blockade through TCZ QW doses might become more apparent over time.
Our study results clearly demonstrate the long-term benefits of TCZ QW over TCZ Q2W dosing, not only for patients with relapsing disease but also for those with new-onset disease. In this analysis, 52 weeks of treatment with tocilizumab delayed the time to first flare with an obvious dose–response pattern. Patients treated with TCZ QW had longer median times to first disease flare compared with those treated with TCZ Q2W, who in turn had longer median times to first flare compared with patients randomized initially to placebo. These findings were consistent across both the new-onset and relapsing disease subsets of patients. These findings are important because they confirm the benefits of early treatment with TCZ QW extend beyond the time of the primary end point in the GiACTA trial—i.e. 52 weeks. GCA remains a long-term, chronic disease for many patients, and extending disease control for the longest possible period of time without needing to resume glucocorticoid therapy has important implications for preventing complications of both the disease and its treatment.
Furthermore, the randomization of patients to TCZ QW at baseline (as opposed to TCZ Q2W or placebo) had profound implications for their cumulative glucocorticoid exposure over the next 3 years in both patients with new-onset GCA and those with relapsing GCA at baseline. This finding was true even though patients were treated according to investigator discretion after the 52-week time point, illustrating that superior disease control early in GCA has an important impact well beyond the time points chosen in most clinical trials for measurement of the primary end point. This also has substantial implications for clinical practice. Overall, the results of our study underscore the importance of optimal disease control early in the course of GCA therapy, and the extent to which this affects long-term disease control and the potential for treatment-induced morbidity from glucocorticoids.
This study has both strengths and weaknesses. Among its strengths are that the data are derived from the largest randomized, double-blind, placebo-controlled trial in GCA to date and that the original treatment assignments remained blinded to investigators and patients throughout the 3-year study. One potential limitation is the heterogeneous nature of treatment in part 2 of GiACTA. Treatment beyond the initial 52-week double-blind period (part 1) was left to the discretion of the investigator based on patient disease activity. Although that could have affected the interpretation of certain results and precluded the assessment of long-term safety, the design of the study is reflective of real-world practice, where individual treatment decisions are not constrained by study design. This also serves to highlight the long-term efficacy of tocilizumab in patients with GCA, as over 40% of patients did not experience relapse despite having received only 52 weeks of tocilizumab treatment [14].
In conclusion, the results presented here suggest that treatment with TCZ QW delays time to flare and reduces glucocorticoid exposure in patients with both new-onset and relapsing GCA. These results also suggest that TCZ QW should be initiated as soon as possible in patients with GCA, regardless of whether they already have a history of disease at the time of presentation for treatment. A patient with new-onset GCA may later develop relapsing disease, and both patient types deserve optimal therapy to be initiated from the earliest time point after the recognition of active disease.
Supplementary Material
Acknowledgements
The authors thank the patients, investigators and study site personnel for their valuable contributions to the GiACTA study. Writing and editorial assistance was provided by Stacie Dilks, PhD, of ApotheCom (San Diego, CA) and was funded by F. Hoffmann-La Roche Ltd.
Funding: This work was supported by F. Hoffmann-La Roche Ltd. The sponsor (Roche) participated in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication.
Disclosure statement: J.H.S. has received research grants and consulting fees from Roche/Genentech and was the global principal investigator in the GiACTA trial.
H.S. is an employee and a shareholder of Roche.
S.H.U. has received research support from Genentech and consulting fees from Kiniksa, Janssen and Sanofi.
M.A. has served on advisory boards for Roche.
D.B. has nothing to disclose.
E.B. has received consulting and speaker fees from Roche, paid to her institution.
M.C.C. has received a research grant from Kiniksa and consulting/advisory, attendance at a scientific meeting, or lecturing fees from GlaxoSmithKline, Janssen, AbbVie, Roche, Kiniksa and Vifor.
B.D. has received research grants and consultant fees from Roche, Chugai, Sanofi and AbbVie.
J.R. has received advisory board and speaker fees from Roche and Chugai.
C.S. has nothing to disclose.
R.S. has received research grants from Roche, Genentech, GlaxoSmithKline and Corbus and consultant fees from GlaxoSmithKline.
M.B. is an employee of Roche/Genentech.
Data availability statement
Qualified researchers may request access to individual-patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). Further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
John H Stone, Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA, USA.
Helen Spotswood, Roche Products Ltd, Welwyn Garden City, UK.
Sebastian H Unizony, Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA, USA.
Martin Aringer, University Medical Center and Faculty of Medicine, TU Dresden, Dresden, Germany.
Daniel Blockmans, Department of General Internal Medicine, University Hospitals Gasthuisberg, Leuven, Belgium.
Elisabeth Brouwer, Department of Rheumatology and Clinical Immunology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Maria C Cid, Department of Autoimmune Diseases, Hospital Clínic, University of Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain.
Bhaskar Dasgupta, Mid and South Essex NHS Foundation Trust, Southend University Hospital, Westcliff-on-Sea, UK.
Juergen Rech, Department of Internal Medicine 3—Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg, Universitätsklinikum Erlangen, Erlangen, Germany.
Carlo Salvarani, Division of Rheumatology, AUSL IRCCS Reggio Emilia and University of Modena and Reggio Emilia, Reggio Emilia, Italy.
Robert Spiera, Department of Medicine, Hospital for Special Surgery, New York, NY.
Min Bao, Genentech, South San Francisco, CA, USA.
References
- 1. Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B.. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology 2017;56:506–15. [DOI] [PubMed] [Google Scholar]
- 2. Serling-Boyd N, Stone JH.. Recent advances in the diagnosis and management of giant cell arteritis. Curr Opin Rheumatol 2020;32:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Restuccia G, Boiardi L, Cavazza A. et al. Flares in biopsy-proven giant cell arteritis in northern Italy: characteristics and predictors in a long-term follow-up study. Medicine 2016;95:e3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labarca C, Koster MJ, Crowson CS. et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology 2016;55:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alba MA, Garcia-Martinez A, Prieto-Gonzalez S. et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine 2014;93:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muratore F, Boiardi L, Restuccia G. et al. Relapses and long-term remission in large vessel giant cell arteritis in northern Italy: characteristics and predictors in a long-term follow-up study. Semin Arthritis Rheum 2020;50:549–58. [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Lado L, Calviño-Díaz C, Piñeiro A. et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine 2011;90:186–93. [DOI] [PubMed] [Google Scholar]
- 8. Kermani TA, Warrington KJ, Cuthbertson D. et al. ; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015;42:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone JH, Tuckwell K, Dimonaco S. et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. [DOI] [PubMed] [Google Scholar]
- 10. Sharma A, Mohammad A, Turesson C.. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: a systematic literature review. Semin Arthritis Rheum 2020;50:1040–8. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JC, Sarsour K, Collinson N. et al. Incidence of outcomes potentially associated with corticosteroid therapy in patients with giant cell arteritis. Semin Arthritis Rheum 2017;46:650–6. [DOI] [PubMed] [Google Scholar]
- 12. Wilson JC, Sarsour K, Collinson N. et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): a nested case–control analysis. Semin Arthritis Rheum 2017;46:819–27. [DOI] [PubMed] [Google Scholar]
- 13. Unizony SH, Dasgupta B, Fisheleva E. et al. Design of the tocilizumab in giant cell arteritis trial. Int J Rheumatol 2013;2013:912562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone JH, Han J, Aringer M. et al. Long-term impact of tocilizumab treatment on giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatol 2021;3:e328–36. [DOI] [PubMed] [Google Scholar]
- 15. Tuckwell K, Collinson N, Dimonaco S. et al. ; GiACTA Investigators. Newly diagnosed vs. relapsing giant cell arteritis: baseline data from the GiACTA trial. Semin Arthritis Rheum 2017;46:657–64. [DOI] [PubMed] [Google Scholar]
- 16.WGET Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. New Engl J Med 2005;352:351–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual-patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). Further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).