Abstract
Objectives
Anti-carbamylated protein antibodies (anti-CarPAs) are present in RA sera and have been associated with erosive disease. The exact targets of anti-CarPAs in vivo are currently not well known; we used a proteomic approach on serum and SF of RA patients to assess the human carbamylome and to identify carbamylated autoantigens as potential biomarkers in early RA.
Methods
Mass spectrometry was performed on SF and serum from RA patients. Carbamylated proteins present in both sample types were selected as candidate autoantigens for the establishment of ELISAs. A cohort of early RA patients was tested for positivity for specific anti-CarPAs.
Results
Eleven novel carbamylated proteins were identified, and five were selected as potential autoantigens for detection of anti-CarPAs. Among them, antibodies against carbamylated hemopexin (anti-CaHPX) and alpha-2-macroglobulin (anti-CaA2M) showed comparable diagnostic value to the established carbamylated foetal calf serum–based ELISA. A cohort of 189 early RA patients was studied. The combination of these new biomarkers with anti-citrullinated protein antibodies and RF identified 89% of early RA patients in our cohort. There was little correlation between the tested biomarkers, and each one of the tested antigens could identify a different subset of seronegative RA patients. Anti-CaA2M positivity showed clinical potential, being associated with higher disease disability.
Conclusion
We highlight the detection of novel carbamylated autoantigens in vivo using a combined proteomics approach in the SF and serum of RA patients. Anti-CaHPX and anti-CaA2M are promising clinical biomarkers, especially in seronegative RA.
Keywords: RA, carbamylated proteins, anti-CarP antibodies, carbamylome
Rheumatology key messages.
Eleven novel carbamylated proteins were detected via proteomic evaluation of the RA carbamylome.
Carbamylated hemopexin and carbamylated alpha-2-macroglobulin are two novel, clinically relevant, anti-CarPA targets in RA.
Anti-CarPAs against specific antigens allow the diagnosis of 60% of ACPA–/RF– patients in the CAP48 early arthritis (ERA) cohort.
Introduction
RA is a chronic inflammatory disease characterized by joint inflammation that leads to cartilage destruction, pain and disability. Protein posttranslational modifications have been a point of interest in RA ever since the description of ACPAs: the evolution of our knowledge culminated in the inclusion of ACPA positivity in the 2010 RA classification criteria [1], and the recent demonstration of their pathogenic role in disease [2].
However, approximately one-third of RA patients are seronegative for ACPAs, underlining the need for additional biomarkers [3]. Another posttranslational modification detected in RA is carbamylation, consisting of the non-enzymatic conversion of a lysine residue to homocitrulline. Carbamylation happens under the influence of cyanate in vitro and in vivo. The conditions promoting this procedure are mainly uraemia in chronic renal disease and inflammatory states [4].
Anti-carbamylated protein antibodies (Anti-CarPAs) have been the subject of numerous studies since their original description in 2011 (4). However, their cumulative effect in diagnosis of RA has been modest, even though their predictive value of erosive disease, especially in the absence of ACPA, has been repeatedly shown [4–8].
Unlike ACPA, for which several target antigens have been described and their role in disease partly elucidated, little is known about the specific targets of anti-CarPA in RA patients. As a result, an ELISA-based assay with carbamylated foetal calf serum (FCS) used as the antigen is the established method for anti-CarPA detection.
A few specific carbamylated antigens of human origin have been used so far to determine anti-CarPA specificities. However, the selection of proteins has often been based on analogy with ACPA—thus potentially increasing cross reactivity—or on informed guesses based on disease physiopathology. As such, antibodies against carbamylated vimentin, α-1-anti-trypsin and albumin have been described, although there are still insufficient lines of evidence underlying their involvement in the pathophysiology of RA [9–12].
In the present study, we used a Liquid Chromatography—Mass Spectrometry (LC-MS)–driven approach to discover novel carbamylated antigens, based on carbamylome research performed on serum and SF of RA patients. The use of the established carbamylated FCS (CaFCS)-based method might have several potential limitations, ranging from reproducibility due to cross-batch differences in FCS composition, to poor diagnostic performance due to the less optimized nature of the assay, as it is not yet commercially available. We aimed to determine the proteins that are preferentially carbamylated in vivo in serum and SF of RA patients and to explore the presence and clinical significance of anti-CarPA targeting those specific carbamylated antigens in two cohorts of RA patients.
We report the discovery of 11 original in vivo carbamylated proteins and of two novel antigenic targets of anti-CarPA. We also examine the potential of an anti-CarPA autoantibody panel for future diagnostic use.
Methods
Patients and study design
In this study we included early RA patients (n = 189) from the CAP48 Belgian cohort, a prospective cohort of early arthritis patients aged 50 years or less. All patients fulfilled the ACR/EULAR 2010 RA classification criteria [3]. Sera were obtained at the 12-month follow-up time point.
Sera of self-reported healthy controls [HCs, n = 44, mean age 41.8 (±14.7), 86% female] with no musculoskeletal symptoms and no previous personal or family history of rheumatic disease were also provided by the Rheumatology department of Erasme Hospital (Brussels, Belgium).
A cohort of established RA patients [n = 60, age 56 (±12), 73% female, ACPA+ 73%, RF+ 73%, disease duration 11 years (±10)] followed in the Rheumatology department (Erasme Hospital, Brussels, Belgium) was initially tested for anti-CaFCS antibodies. Eight highly positive sera, along with eight anti-CaFCS–negative sera were then selected for carbamylated protein detection via LC-MS. We also performed LC-MS on RA patient SF (n = 10) originating from inflamed knee joints, and 10 SF samples obtained from osteoarthritic knees were used as controls.
It was hypothesized that carbamylated proteins present in both serum and SF had a higher probability of being self-antigens. The candidate proteins identified in that way were subsequently carbamylated in vitro and used to create specific in-house ELISAs; initial testing and standardization were performed on the established RA and HC cohorts. The most promising antigens were then selected for application on the CAP48 early RA cohort,
This study was approved by the local ethics committee of Erasme—ULB (ref: P2018/601; Brussels, Belgium). All of the participants gave written informed consent.
Serum and SF were collected, centrifuged, aliquoted and stored at −80°C.
Baseline clinical characteristics
The baseline clinical characteristics of the early RA patients included in this study are summarized in Table 1.
Table 1.
Clinical characteristics of the patients included in the study
| Early RA (n = 189) | |
|---|---|
| Age, years (mean) | 36.1 (9.15) |
| Sex (F) | 151/185a (81.6%) |
| Disease duration, days, mean (s.d.) | 49 (79) |
| ACPA+ | 129/188 (68.6%)b |
| RF+ | 122/188 (64.9%)b |
| ACPA–/RF– | 47/188 (25%)b |
| ANA+ | 63/162 (38.9%)c |
| CRP, mg/dl [mean (s.d.)] | 1.67 (2.99) |
| Erosive disease | 46/174 (26.4%)d |
| Smokers (ever) | 41/164 (25%)e |
| DAS28 (mean) | 4.40 (1.33) |
| SJC (mean [s.d.]) | 6.4 (5.7) |
| TJC (mean [s.d.]) | 10.1 (8.2) |
| SDAI (mean [s.d.]) | 23.7 (13.9) |
| CDAI (mean [s.d.]) | 22.1 (13.4) |
| HAQ (mean [s.d.]) | 1.11 (0.73) |
| CS therapy | 35/185 (18.9%)a |
| csDMARDS | 47/186 (25.3%)f |
| bDMARDS | N/A |
Four missing values; bone missing value; c27 missing values; d15 missing values; e25 missing values; fthree missing values. DAS28: DAS calculated on 28 joints; SJC: swollen joint count; TJC: tender joint count; SDAI: Simplified Disease Activity Index; CDAI: Clinical Disease Activity Index; csDMARDs: conventional synthetic DMARDs; bDMARDs: biologic DMARDs; N/A: not applicable.
Clinical assessment
Baseline and follow-up clinical and biological data (described in Supplementary Data Section S1, available at Rheumatology online) were obtained for all RA patients from the CAP48 and Rheumatology biobank registry. The presence of bone erosions was assessed in a dichotomic fashion on plain radiographs of the hands.
ELISAs for antibody detection
Anti-CaFCS ELISA
Replicating the methods of Shi et al. [5], we created an in-house ELISA using carbamylated FCS, described in detail in Supplementary Data Section S1 (available at Rheumatology online).
Posttranslational modification of proteins
Alpha-1-antitrypsin (A1AT), alpha-2-macroglobulin (A2M), hemopexin (HPX), haptoglobin (HP), serum transferrin (TF) and albumin (ALB) (all commercially available proteins purified from human plasma—Sigma-Aldrich) were diluted in PBS to 100 μg/ml and were carbamylated by incubation with cyanate 1M for 24 h, followed by extensive dialysis against PBS using a Snakeskin Dialysis membrane, for a total of six 4-h dialysis cycles.
Carbamylation was confirmed using LC-MS analysis, and endogenous carbamylation was excluded by analysing the non-carbamylated form of each protein.
Anti-CarPA ELISAs using specific carbamylated antigens
Carbamylated antigens (A1AT, A2M, HPX, HP, ALB, TF) and the corresponding non-modified proteins were coated on 96-well plates (Nunc-Maxisorp 96-well plates, C-shaped) at a 10 ng/ml total protein concentration and incubated overnight at 4°C. The plates were then washed three times with PBS/0.05% Tween and blocked on ice for 6 h using a PBS/1% BSA blocking buffer. After washing, 50 μl of serum samples diluted 50 times in a PBS/1% BSA/0.05% Tween buffer was added to each well, in duplicates. After overnight incubation on ice, plates were washed and an anti-human IgG rabbit polyclonal antibody linked to horseradish peroxidase (DAKO) was applied at a 1:1000 dilution for 3.5 h, at 4°C. After a final wash, 50 μl of 3,3′,5,5′-tetramethylbenzidine substrate was added to the wells for 20 min, and the reaction was stopped using 50 μl of sulphuric acid at a 1M concentration.
Absorbance was subsequently read at 450 nm.
A standard serum was created using a (1:1:1) pool of three highly positive sera, while cut-off and antibody titres were calculated as described for the anti-CaFCS assay.
Statistical analysis
For statistical analysis, version 7.04 of the GraphPad Prism software was used. Testing for normality was done using the D’Agostino and Pearson normality test. Non-parametric variables were assessed using the Mann–Whitney U test. For normally distributed variables, Student’s t test was used, and ANOVA was performed for comparisons between >2 groups. Categorical datasets were assessed using χ2d.
Results
Determination of the human ‘carbamylome‘
We performed LC-MS on serum samples from RA patients with different anti-CarPA and ACPA status. A total of 20 serum samples were tested, including serum from four HCs (Supplementary Table S1, available at Rheumatology online).
The previously described carbamylated autoantigens A1AT and ALB were detected and, in accordance with previous studies, we also detected these proteins in HCs and in anti-CaFCS–negative patients. The sites of their in vivo carbamylation were determined (Supplementary Table S2, available at Rheumatology online).
Several novel carbamylated proteins were identified in the sera studied. Five proteins were carbamylated in samples from both HCs and patients with RA: A2M, apolipoprotein alpha-1 (ApoA1), HPX, ceruloplasmin and HP. Additionally, five other carbamylated proteins were only present in RA sera: apolipoprotein alpha-4 (ApoA4), haemoglobin beta (HbB), retinol-binding protein 4 (RBP4), TF and C3 complement (C3). Interestingly, the presence of these potentially RA-specific proteins was not related to the presence of anti-CaFCS antibodies. Lastly, we did not observe a relationship between ACPA/anti-CaFCS autoantibody status and the presence of carbamylated peptides in the serum.
We then performed LC-MS on RA patient SF originating from inflamed knee joints of established RA patients. A total of 10 samples were analysed, as well as 10 samples obtained from patients with OA used as controls. Carbamylated ALB was present in samples from both OA and RA patients. Other carbamylated proteins were only identified in RA SF: A1AT, C3, HP, A2M, HPX, fibrinogen gamma chain, beta-actin (ACTB), and gamma-actin (ACTG1).
When pooling the results from the two proteomic approaches, a total of 15 carbamylated proteins were detected, out of which 11 have never been previously reported (Supplementary Table S2, available at Rheumatology online). Of note, when considering a defined protein, the position of carbamylated amino acid residues was different from patient to patient, and the carbamylation sites also differed between serum and SF. Exceptions to this were A1AT (same position of carbamylation throughout all the tested sera) and HPX (same two positions carbamylated both in serum and SF). The carbamylated sequences were manually compared, but no sequence repetition that could point to a specific immunogenic amino acid sequence was detected.
Identification of new antibodies recognizing specific carbamylated antigens and investigation of their use as RA biomarkers
Using the results from our proteomic biomarker discovery study, we cross-referenced the SF and serum results and selected proteins that were found to be carbamylated in both samples as candidate autoantigens for anti-CarPA. Five candidate proteins were chosen: A1AT, ALB, A2M, HP and HPX, as well as TF, which was exclusively found in its carbamylated form in RA sera.
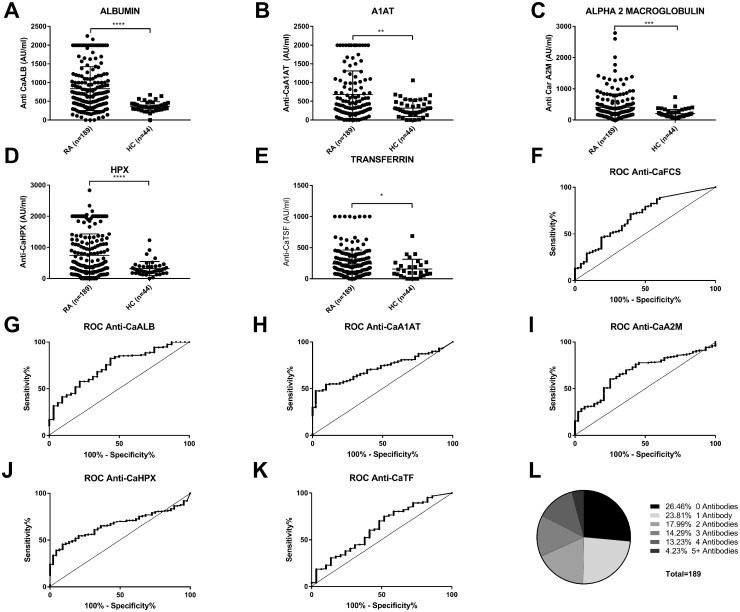
We carbamylated the candidate proteins in vitro and used them to create in-house ELISAs to detect anti-CarPA against those specific antigens. These assays were standardized by first testing a cohort of established RA patients (n = 60), and HCs (n = 44). We then validated each assay in the CAP48 early arthritis (ERA) cohort (n = 189). RA sera presented a significantly higher reactivity towards each of the carbamylated antigens when compared with that of HCs, with the exception of HP (Fig. 1A–E).
Fig. 1.
Anti-CarPA reactivity against specific antigens levels, receiver-operator curves, and specificity overlap
(A–E) Anti-CarPA reactivity against specific antigens levels in ERA patients (n=189) and HCs (n=44). (F–K) Receiver operator curves (ROCs) for the different anti-CarPA antibodies. The diagonal reference line indicates no discrimination between RA patients and HCs. (L) Anti-CarPA specificity overlap in ERA patients. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Table 2 summarizes the results of ELISA testing using specific carbamylated antigens, and their relationship with ACPA, RF and ANA positivity. ACPA were significantly more prevalent in anti-CaFCS+, anti-CaA1AT+, anti-CaTF+ and anti-CaHPX+ patients compared with their seronegative counterparts. Similarly, RF was more prevalent in anti-CarPA+ individuals except for anti-CaHPX. Finally, anti-CaA2M, anti-CaHPX and anti-CaA1AT were associated with ANA positivity. The clinical potential of each anti-CarPA was then explored by examining baseline and 12-month follow-up clinical data for all patients (Table 2).
Table 2.
Specific Anti-CarPA, their relationship with ACPA, RF and ANA, as well as with clinical characteristics in the CAP48 early RA cohort
| CAP48 cohort (n = 189) | Anti-CaFCS (n = 62) | Anti-CaALB (n = 56) | Anti-CaA1AT (n = 79) | Anti-CaA2M (n = 47) | Anti-CaHPX (n = 73) | Anti-CaTF (n = 19) | |
|---|---|---|---|---|---|---|---|
| Age [years, mean (s.d.)] | 36.1 (9.15) | 37.3 (9.65) | 35.7 (9.07) | 36.1 (9.16) | 35.6 (9.55) | 35.8 (9.36) | 38.6 (8.91) |
| Sex (F) | 151/185a (81.6%) | 50/61a (82%) | 44/54a (81.5%) | 64/75a (85.3%) | 36/46a (78.3%) | 60/70a (85.7%) | 15/18a (83.3%) |
| ACPA+ | 129/188a (68.6%) | 51/62 (82.3%) ** | 42/56 (75%) | 65/78 a (83.3%) *** | 38/47 (80.9%) | 58/73 (79.5%) * | 18/19 (94.7%) ** |
| RF+ | 122/188a (64.9%) | 49/58 a (84.5%) ** | 45/52 a (86.5%) *** | 62/75 a (82.7%) *** | 34/42 a (81%) * | 52/68a (76.5%) | 19/19 (100%) *** |
| ACPA–/RF– | 47/188a (25%) | 6/58a (10.3%) | 7/52a (13.5%) | 8/75a (10.6%) | 7/42a (16.6%) | 10/68a (14.7%) | 0/19 |
| ANA+ | 63/162a (38.9%) | 25/61a (40.9%) | 24/45a (53.3%) | 37/70 a (52.9%) ** | 25/42 a (59.5%) ** | 33/64 a (51.6%) ** | 5/18a (27.8%) |
| CRP [mg/dl; mean (s.d.)] | 1.67 (2.99) | 1.23 (1.78) | 1.25 (1.80) | 1.61 (2.56) | 0.92 (1.25) | 1.49 (2.18) | 1.03 (1.76) |
| Erosive disease (BL) | 46/174a (26.4%) | 14/61a (23%) | 9/52a (17.3%) | 18/74a (24.3%) | 10/46a (24.4%) | 17/69a (24.6%) | 6/19 (31.6%) |
| Smokers (ever) | 41/164a (25%) | 15/54a (27.8%) | 9/48a (18.8%) | 15/67a (22.4%) | 17/44 a (38.6%) * | 11/63a (17.5%) | 6/16a (37.5%) |
| DAS28 BL [mean (s.d.)] | 4.40 (1.33) | 4.34 (1.47) | 4.54 (1.31) | 4.40 (1.29) | 4.43 (1.20) | 4.28 (1.38) | 3.7 (1.03)* |
| SDAI BL [mean (s.d.)] | 23.7 (13.9) | 23.8 (16) | 25.8 (15.1) | 23.7 (14.2) | 23.8 (12.9) | 22.8 (15) | 17 (10)* |
| CDAI BL [mean (s.d.)] | 22.1 (13.4) | 23.4 (15.8) | 24.6 (14.5) | 22.6 (13.4) | 23.8 (12.9) | 21.4 (14.2) | 16 (9.8)* |
| HAQ BL [mean (s.d.)] | 1.11 (0.73) | 1.17 (0.74) | 1.18 (0.80) | 1.12 (0.78) | 1.26 (0.77) | 1.07 (0.81) | 0.74 (0.68)* |
| DAS28 12 mo [mean (s.d.)] | 2.71 (1.20) | 2.67 (1.23) | 2.78 (1.29) | 2.73 (1.15) | 2.76 (1.33) | 2.7 (1.19) | 2.3 (0.78) |
| SDAI 12 mo [mean (s.d.)] | 9.46 (9.99) | 8.73 (9.57) | 10.47 (11.42) | 9.49 (8.82) | 10.57 (11.81) | 9.14 (8.93) | 5.91 (4.74) |
| CDAI 12 mo [mean (s.d.)] | 8.88 (9.76) | 8.27 (9.58) | 9.76 (10.98) | 8.95 (8.39) | 9.77 (11.32) | 8.50 (8.41) | 5.32 (4.6) |
| HAQ 12 mo [mean (s.d.)] | 0.61 (0.66) | 0.65 (0.71) | 0.64 (0.69) | 0.63 (0.64) | 0.80 (0.77)* | 0.57 (0.69) | 0.53 (0.63) |
| ΔDAS28 [mean (s.d.)] | −1.77 (1.59) | −1.77 (1.60) | −1.78 (1.48) | −1.68 (1.34) | −1.71 (1.38) | −1.58 (1.49) | −1.57 (1.31) |
| ΔSDAI [mean (s.d.)] | −14.6 (14.6) | −15.2 (15.3) | −15.34 (14.4) | −14.1 (13.4) | −13.18 (12.2) | −13.69 (14.16) | −11.39 (11.21) |
| ΔCDAI [mean (s.d.)] | −13.5 (13.88) | −14.38 (14.48) | −14.81 (13.65) | −13.17 (13.12) | −13.07 (12) | −13.92 (14.15) | −10.91 (10.86) |
| ΔHAQ [mean (s.d.)] | −0.52 (0.85) | −0.54 (0.88) | −0.51 (0.82) | −0.49 (0.79) | −0.46 (0.95) | −0.48 (0.89) | −0.24 (0.68) |
missing values. BL: baseline; DAS28: DAS calculated on 28 joints; SDAI: Simplified Disease Activity Index; CDAI: Clinical Disease Activity Index; 12 mo: 12-month follow-up; Δ: delta (12-month value minus baseline value). *P < 0.05; **P < 0.01; ***P < 0.001; bold values denote statistical significance at the P < 0.05 level.
Anti-CaALB, anti-CaA1AT and anti-CaHPX did not show any meaningful clinical associations in our cohort. Anti-CaA2M antibodies were significantly more prevalent in patients with past or present smoking habit (P = 0.024). The presence of anti-CaA2M was also associated with a higher HAQ score after 12 months of treatment (P = 0.0294).
Conversely, anti-CaTF positivity was observed exclusively in seropositive (ACPA+/RF+) patients and was associated with a lower disease activity at baseline, as measured by the DAS calculated on 28 joints using CRP (DAS28-CRP), Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) scores. Out of the components of the composite scores, anti-CaTF positivity was associated with lower tender joint count compared with anti-CaTF–negative patients. Additionally, anti-CaTF–positive patients had significantly lower HAQ scores at baseline.
Relationship between antibodies against specific carbamylated antigens
We assessed the correlation between the different anti-CarPA levels in the ERA cohort using the Spearman correlation test (Supplementary Table S3, available at Rheumatology online). A χ2 test was also performed to compare antibodies pairwise, based on seropositivity (Supplementary Table S4, available at Rheumatology online). While almost all antibodies showed a positive association in terms of antibody positivity, and a degree of correlation with one another, the correlation coefficients indicate a poor correlation of the antibody levels in most cases (Supplementary Tables S3 and S4, available at Rheumatology online). Notable exceptions were moderate correlations between anti-CaALB and anti-CaA2M (ρ = 0.3316) as well as between anti-CaHPX and anti-CaA1AT (ρ = 0.4024) (Supplementary Table S3, available at Rheumatology online).
The presence of multiple specific antibodies in the serum of a patient did not predict a higher anti-CarPA value for a specific carbamylated antigen.
Detection of anti-CarPA in the Cap48 ERA cohort
When examining each antibody independently, we observed that the diagnostic performance of the anti-CaFCS assay [area under the curve (AUC) 0.686] was inferior to that of anti-CaALB (AUC 0.736) and anti-CaA1AT (AUC 0.720). It was however comparable with the diagnostic performance of anti-CaA2M (AUC 0.686) and anti-CaHPX (0.666) (Table 3, Fig. 1F–K).
Table 3.
Diagnostic value of specific-antigen anti-CarPA
| Specificity | Sensitivity | AUC | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|
| Anti-CaFCS | 92.8% | 35% | 0.686 | 88.4% | 44% | 4.86 | 0.71 |
| Anti-CaALB | 96.8% | 29.6% | 0.736 | 98.2% | 18.8% | 9.48 | 0.73 |
| Anti-CaA2M | 97.7% | 24.9% | 0.686 | 97.9% | 23.2% | 10.94 | 0.77 |
| Anti-CaHPX | 95.5% | 36.7% | 0.666 | 97.3% | 25% | 8.07 | 0.66 |
| Anti-CaTF | 96.6% | 10% | 0.625 | 95% | 14.1% | 2.92 | 0.93 |
| Anti-CaA1AT | 96.7% | 41.8% | 0.720 | 98.75% | 27.6% | 17.97 | 0.60 |
AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio.
When examining anti-CarPA overlap, we observed that a subset of patients (49.7% of all patient sera analysed) had reactivity for multiple carbamylated antigens. However, 23.8% of all patient sera analysed had reactivity towards a single carbamylated antigen (Fig. 1L).
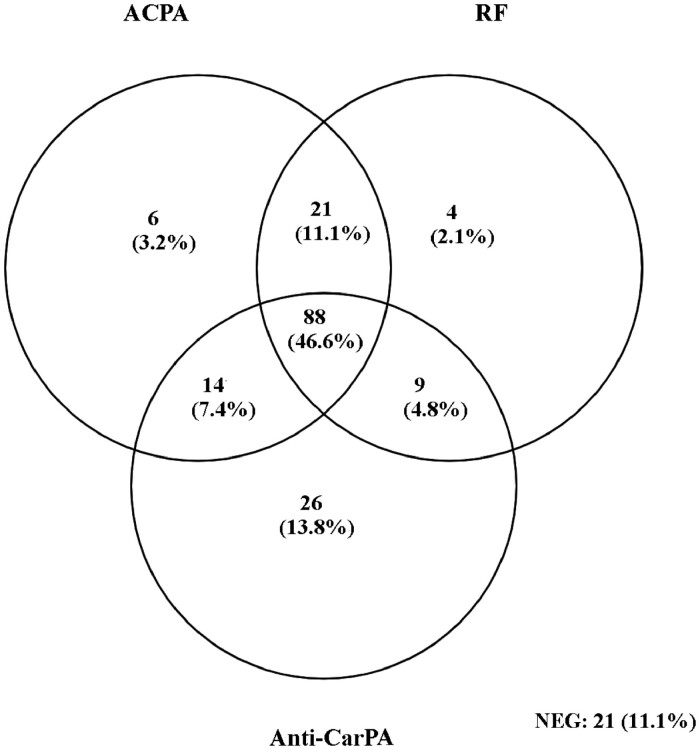
Overall, 73.5% of ERA patient sera were positive for at least one of the anti-CarPA (Fig. 2). In comparison, only 13.6% of HC sera were positive for at least one of the anti-CarPA, and only one serum was positive for two anti-CarPA; HC sera did not show positivity for three or more antibodies (data not shown).
Fig. 2.
Venn diagram of autoantibody positivity for ACPA, RF and the combined anti-CarPA (anti-CaA2M, anti-CaALB, anti-CaHPX, anti-CaA1AT) in the ERA cohort
NEG
: negative for all three antibodies.
When combined in parallel testing, the anti-CarPA assays managed to identify a further 13.8% of ERA ACPA–/RF– seronegative patients, lowering the percentage of seronegative patients from 24.9% (ACPA–/RF–) to 11.1% (ACPA–/RF–/Anti-CarPA–) (Fig. 2).
Anti-CarPA in seronegative (ACPA–/RF–) ERA patients
Based on our previous finding that anti-CaFCS antibodies were of prognostic value in seronegative (ACPA–/RF–) ERA patients [13], we performed a subgroup analysis of the seronegative portion of the ERA cohort. A total of 47 patients were included in the analysis for each of the candidate biomarkers.
Supplementary Table S5 (available at Rheumatology online) summarizes the results from the comparison of these patients based on their positivity for anti-CaALB. Reactivity against CaALB was associated with higher CDAI and SDAI scores at baseline, but not at 12 months of follow-up.
Anti-CaA2M–positive patients in the same subgroup exhibited higher baseline HAQ scores, as well as a better HAQ response to treatment as observed by the Delta HAQ (Supplementary Table S6, available at Rheumatology online).
The same analysis for anti-CaA1AT and anti-CaHPX did not show any prognostic role for these two autoantibodies in seronegative (ACPA–/RF–) ERA patients.
Discussion
In recent years, the dichotomy of seronegative and seropositive RA has been challenged, since numerous antibodies and antibody specificities can help close the serological gap [14]. The clinical value of anti-CarPA has been debated: from a diagnostic standpoint, anti-CaFCS only detects 12–16% of ACPA/RF-negative RA patients, making their additive value to the current clinical practice debatable [15]. Use of the three biomarkers in serial testing increases the specificity and detects individuals at high risk of developing RA in the asymptomatic phase of the disease, at the cost of lower sensitivity [16]. We hypothesized that the use of specific antigens—rather than the mixture of proteins contained in FCS—that are carbamylated in vivo in RA patients, would allow the detection of more seronegative patients. Indeed, we demonstrate that a combination of four carbamylated proteins detected close to 60% of all seronegative patients. Interestingly, each of the specific antigens had comparable or better diagnostic performance to that of anti-CaFCS, suggesting the need for new tests based on widely available purified proteins, as opposed to a protein mix that can vary from lot to lot.
The methodology for selecting target antigens other than the traditional carbamylated FCS varies between previous studies: some used either Western Blot or LC-MS analysis of in vitro carbamylated sera [9, 11] in order to select candidate biomarkers. Others relied on educated guesses based on prior knowledge of RA pathophysiology, and in particular ACPA antigenic targets [17, 18]. In some cases, mass spectrometry on patient tissue or SF was used to confirm the in vivo presence of the selected antigen [17]. Vimentin, ALB, A1AT, binding immunoglobulin protein, γ-fibrinogen, α-enolase, and prothrombin were identified as antigenic targets of anti-CarPA in RA [9–12, 17, 19–21]. Our study used an innovative approach to biomarker selection, using LC-MS analysis both on RA sera and on SF to assess the carbamylome in disease, and to select potential autoantigens that are pertinent to RA.
CaALB was previously evaluated as an antigen for anti-CarPA detection in a cohort of Japanese RA patients [9]. In that study, approximately one-third of patients exhibited positivity for anti-CaALB, with a fair correlation with anti-CaFCS. In our study, we found anti-CaALB antibodies to be equally prevalent in the ERA cohort, but no correlation was observed with the presence of anti-CaFCS antibodies. This discrepancy can be potentially explained by differences in the cohorts examined: we examined early RA patients with a higher proportion of seronegative patients, as opposed to patients with several years of disease duration who were predominantly ACPA positive, as in the study of Nakabo et al.
CaA1AT was recently reported as a self-antigen in established RA, with RA sera exhibiting reactivity towards it, and anti-CaA1AT showing diagnostic properties that were similar to our present findings [11]. Additionally, in contrast with our observations, Verheul et al. reported a strong correlation between anti-CaA1AT and anti-CaFCS antibodies; this was however based on comparing optical densities towards the carbamylated antigens directly, and not by comparing antibody levels, as in our study.
At the time of writing, only one previous study has addressed the human carbamylome [20]. It used a combined LC-MS approach performed on cartilage, synovium and SF, but did not examine patient sera in parallel. A total of 45 carbamylated peptides were reported, including ALB, vimentin, fibrinogen, fibronectin and the newly identified CaTF. The last three proteins were then tested as potential autoantigens in a small RA cohort: CaTF was more prevalent in ACPA+ RA, but in contrast to our results, it was also detected in a small subset of ACPA– RA. This difference could be attributed to the fact that we examined an early RA cohort with less seronegative patients as opposed to the 50% of seronegative patients examined by Verheul et al.
Our study is the first one to combine a parallel analysis of serum and SF, so as to achieve an understanding of the potential antigenic targets of anti-CarPA both on a systemic and on an articular level. Some of the proteins that we identified were previously described as carbamylation targets, (e.g. ALB, A1AT, and very recently TF). We now describe 11 novel in vivo carbamylated targets, mainly proteins implicated in inflammation. Moreover, our study is the first one to examine the association of targeted antibodies with clinical data in an early RA cohort. This has allowed us to also evaluate the clinical importance of antibodies previously described in the literature, such as A1AT and ALB.
Anti-CarPA, like ACPA, have been shown to react to multiple carbamylated epitopes. The degree of correlation between reactivity against the various antigens varies across the studies, although most of them report at most a moderate correlation [9, 11]. In our study, this relationship was poor for most of the proteins identified, a fact that may be attributed to the different affinities for epitopes of one anti-CarPA, or that may be due to the presence of two or more anti-CarPA originating from different B cell clones. As our study examined early RA patients, it is plausible that epitope spreading occurs with disease progression, as documented for ACPA.
It is possible that patients considered to be seronegative may simply be patients undetected by current diagnostic methods that are not sensitive enough. In the future, synthetic peptides based on the carbamylated sequences of our candidate antigens could thus be used for detection of anti-CarPA in the same way that CCP is currently used for detection of ACPA. The selection of a candidate amino acid sequence should be based on extensive testing, but our LC-MS data might provide interesting leads (Supplementary Table S2, available at Rheumatology online): matching carbamylated sequences in serum and SF (e.g. DLATGTMKERSWPAVG in HPX or ELPGEYSMKVTGEGCVY in A2M), or unique carbamylated sequences for a single protein (e.g. LSSWVLLMKYLGNATAI in serum A1AT) would be interesting candidates. A degree of caution would be required with such an approach, as the sites of carbamylation might vary from patient to patient, and the sample size in the published reports, including our own, is not large enough to account for that variability. As an example, Verheul et al. reported a candidate lysin residue at position 359 of A1AT that our LC-MS analysis did not detect [11].
The prognostic value of anti-CaFCS has been underlined in many studies: anti-CaFCS+ patients are at a higher risk of joint erosions, especially the ACPA-negative subset of patients [4]. They also experience more severe bone loss and osteoporosis, and they exhibit higher disease activity and disease burden [22–24]. Our previous study had confirmed these observations, showing that the ACPA-negative/anti-CaFCS–positive group of patients had higher disease activity than the double-negative group [13]. In the present study, we show for the first time that reactivity towards specific carbamylated antigens can help define interesting patient subsets within the RA spectrum: anti-CaA2M detected a subgroup of patients at greater risk of functional loss despite appropriate DMARD treatment, as observed by the higher disease disability scores after 12 months of follow-up compared with the anti-CaA2M– patients. On the other hand, anti-CaTF helped the detection of a subgroup within the ACPA-positive patients who have a less severe disease phenotype at presentation.
When applied to ACPA-/RF- patients, both anti-CaALB and anti-CaA2M were present in the subset of seronegative patients that had a more severe disease at presentation. Given the diagnostic challenge that seronegative RA poses, using these antibodies could promote the earlier detection of patients with a more severe disease phenotype, with the caveat that our results were obtained in a small seronegative patient cohort.
To summarize, our study provides new insights into the human RA carbamylome, as well as the targets of anti-CarPA in RA. The identified proteins provide new tools for early diagnosis as well as disease prognosis. We show that the use of specific human proteins to detect anti-CarPA increases the diagnostic accuracy of the testing. Future studies could expand on the clinical applications of anti-CarPA against specific antigens by examining populations of different ethnicity, larger cohorts and cohorts with a longer follow-up duration: this might reveal new and interesting clinical associations for each of the novel antigens. The construction of synthetic carbamylated peptides akin to the CCP, using the proteins we studied and the carbamylated sequences that our proteomic approach provided, may help progress towards a standardized method of anti-CarPA testing.
Funding: This study was supported by the ‘Fonds Erasme pour la Recherche Médicale’, Belgium and the King Baudouin Foundation, Belgium (P.S.). D.C. is a Senior Research Associate from the Fonds de la recherche scientifique-FNRS (FRS-FNRS). This project is supported by the charity fund CAP48 from Radio-télévision belge de la Communauté française (RTBF) (Project ‘Polyarthrite de l’enfant et du jeune adulte CAP48’).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data are available upon reasonable request by contacting the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Contributor Information
Paschalis Sidiras, Laboratory of Bone and Metabolic Biochemistry; Rheumatology Department, Hôpital Erasme.
Jessica Lechanteur, Laboratory of Bone and Metabolic Biochemistry.
Virginie Imbault, IRIBHM, Université libre de Bruxelles, Brussels, Belgium.
Tatiana Sokolova, Rheumatology, Institute de Recherche Expérimentale et Clinique (IREC), Cliniques Universitaires Saint-Luc—Université catholique de Louvain (UCL), Brussels, Belgium.
Patrick Durez, Rheumatology, Institute de Recherche Expérimentale et Clinique (IREC), Cliniques Universitaires Saint-Luc—Université catholique de Louvain (UCL), Brussels, Belgium.
Valérie Gangji, Laboratory of Bone and Metabolic Biochemistry.
David Communi, IRIBHM, Université libre de Bruxelles, Brussels, Belgium.
Joanne Rasschaert, Laboratory of Bone and Metabolic Biochemistry.
References
- 1. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 2. Krishnamurthy A, Joshua V, Haj Hensvold A. et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis–associated autoantibody-mediated bone loss. Ann Rheum Dis 2016;75:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratt AG, Isaacs JD.. Seronegative rheumatoid arthritis: pathogenetic and therapeutic aspects. Best Pract Res Clin Rheumatol 2014;28:651–9. [DOI] [PubMed] [Google Scholar]
- 4. Shi J, Knevel R, Suwannalai P. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA 2011;108:17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verheul MK, Shiozawa K, Levarht EW. et al. Anti-carbamylated protein antibodies in rheumatoid arthritis patients of Asian descent. Rheumatology (Oxford, England) 2015;54:1930–2. [DOI] [PubMed] [Google Scholar]
- 6. Yee A, Webb T, Seaman A. et al. Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol Res 2015;61:24–30. [DOI] [PubMed] [Google Scholar]
- 7. Pecani A, Alessandri C, Spinelli FR. et al. Prevalence, sensitivity and specificity of antibodies against carbamylated proteins in a monocentric cohort of patients with rheumatoid arthritis and other autoimmune rheumatic diseases. Arthritis Res Ther 2016;18:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ajeganova S, van Steenbergen HW, Verheul MK. et al. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor. Ann Rheum Dis 2017;76:112–8. [DOI] [PubMed] [Google Scholar]
- 9. Nakabo S, Hashimoto M, Ito S. et al. Carbamylated albumin is one of the target antigens of anti-carbamylated protein antibodies. Rheumatology (Oxford, England) 2017;56:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scinocca M, Bell DA, Racape M. et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol 2014;41:270–9. [DOI] [PubMed] [Google Scholar]
- 11. Verheul MK, Yee A, Seaman A. et al. Identification of carbamylated alpha 1 anti-trypsin (A1AT) as an antigenic target of anti-CarP antibodies in patients with rheumatoid arthritis. J Autoimmun 2017;80:77–84. [DOI] [PubMed] [Google Scholar]
- 12. Challener GJ, Jones JD, Pelzek AJ. et al. Anti-carbamylated protein antibody levels correlate with anti-Sa (citrullinated vimentin) antibody levels in rheumatoid arthritis. J Rheumatol 2016;43:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sidiras P, Spruyt D, Gangji V. et al. Antibodies against carbamylated proteins: prevalence and associated disease characteristics in Belgian patients with rheumatoid arthritis or other rheumatic diseases. Scand J Rheumatol 2021;50:118–6. [DOI] [PubMed] [Google Scholar]
- 14. Reed E, Hedström AK, Hansson M. et al. Presence of autoantibodies in “seronegative” rheumatoid arthritis associates with classical risk factors and high disease activity. Arthritis Res Ther 2020;22:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li L, Deng C, Chen S. et al. Meta-analysis: diagnostic accuracy of anti-carbamylated protein antibody for rheumatoid arthritis. PLoS One 2016;11:e0159000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verheul MK, Bohringer S, van Delft MAM. et al. Triple positivity for anti-citrullinated protein autoantibodies, rheumatoid factor, and anti-carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol (Hoboken, NJ) 2018;70:1721–31. [DOI] [PubMed] [Google Scholar]
- 17. Dekkers JS, Verheul MK, Stoop JN. et al. Breach of autoreactive B cell tolerance by post-translationally modified proteins. Ann Rheum Dis 2017;76:1449–57. [DOI] [PubMed] [Google Scholar]
- 18. Reed E, Jiang X, Kharlamova N. et al. Antibodies to carbamylated alpha-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res Ther 2016;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu HC, Lai PH, Lai NS. et al. Increased serum levels of anti-carbamylated 78-kDa glucose-regulated protein antibody in patients with rheumatoid arthritis. Int J Mol Sci 2016;17:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verheul MK, Janssen GMC, de Ru A. et al. Mass-spectrometric identification of carbamylated proteins present in the joints of rheumatoid arthritis patients and controls. Clin Exp Rheumatol 2021;39:570–7. [PubMed] [Google Scholar]
- 21. Alunno A, Bistoni O, Pratesi F. et al. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford, England) 2018;57:850–5. [DOI] [PubMed] [Google Scholar]
- 22. Humphreys J, Verheul M, Barton A. et al. Association of anti-carbamylated protein antibodies with long-term disability and increased disease activity in patients with early inflammatory arthritis: results from the Norfolk Arthritis Register. Lancet (London, England) 2015;385:S44. [DOI] [PubMed] [Google Scholar]
- 23. Regueiro C, Ortiz AM, Boveda MD. et al. Association of high titers of anti-carbamylated protein antibodies with decreased bone mineral density in early arthritis patients. PLoS One 2018;13:e0202583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Truchetet ME, Dublanc S, Barnetche T. et al. ; Fédération Hospitalo-Universitaire ACRONIM. Association of the presence of anti-carbamylated protein antibodies in early arthritis with a poorer clinical and radiologic outcome: data from the French ESPOIR cohort. Arthritis Rheumatol (Hoboken, NJ) 2017;69:2292–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by contacting the corresponding author.