Abstract
Objectives
To investigate key factors that may contribute to the variability of rituximab-mediated peripheral and renal B cell depletion (BCD) in SLE.
Methods
We analysed: (i) CD19+ B cell counts in patients with SLE before and 1, 2, 3 and 6 months after treatment with rituximab, comparing them with RA patients; (ii) the presence of B cells in renal biopsies after rituximab therapy; (iii) whether the duration of BCD correlated with patient demographics and B cell expression of CD20 and FcγRIIb; and (iv) the effect of B cell activation factor (BAFF) on the efficiency of rituximab and obinutuzumab at inducing BCD in whole blood assays, in vitro.
Results
In SLE (n = 71), the duration of BCD was shorter compared with RA (n = 27). B cells were detectable in renal biopsy samples (n = 6) after treatment with rituximab in all patients with poor response while peripheral blood B cells remained low or undetectable in the same patients. There were no significant relationships between peripheral BCD and patient age, disease duration, serum C3 levels or the level of expression of B cell surface proteins CD20 and FcγRIIb. Obinutuzumab was more efficient than rituximab at inducing BCD in whole blood assays, regardless of excess BAFF.
Conclusions
BCD in SLE is less efficient than in RA. Renal B cell presence following rituximab treatment was associated with poor outcomes. No significant relationships between any measured B cell related, clinical or laboratory parameters and the efficiency of BCD by rituximab was found. Obinutuzumab was superior to rituximab at inducing BCD.
Keywords: B cell depletion, rituximab, SLE, LN, obinutuzumab
Rheumatology key messages.
B cell depletion in SLE is less efficient than RA regardless of clinical and laboratory parameters.
Disparity in peripheral and renal B-cell depletion is notable in rituximab refractory LN.
Obinutuzumab was superior to rituximab at inducing B cell depletion in vitro, regardless of B cell activation factor.
Introduction
In patients with SLE, incomplete peripheral blood B cell depletion (BCD) contributes to poor response to therapy using the anti-CD20 mAb, rituximab [1, 2]. The presence of renal interstitial B cells in patients with LN is associated with renal dysfunction and histologically active lesions [3]. Rituximab treatment for refractory LN results in variable peripheral BCD [4] and the lupus nephritis assessment with rituximab (LUNAR) study found that complete peripheral BCD (0 cells/µl) lasting >71 days was associated with complete response [4, 5]. In a study of seven patients with LN who responded well to rituximab, BCD in the kidneys was reported in all patients on repeat renal biopsy at 3–12 months after rituximab [6]. Therefore, improving BCD may enhance clinical response [7, 8]. However, we do not know whether peripheral blood BCD reflects BCD in the kidney and if incomplete BCD in the kidney fails to disrupt inflammatory lesions, contributing to poor response.
Anti-CD20 mAbs can employ multiple effector mechanisms including complement-dependent cell cytotoxicity (CDC), antibody-dependent cellular cytotoxicity, antibody-dependent cell phagocytosis and direct cell death [9]. There are two main types of anti-CD20 mAbs: Type I anti-CD20 mAbs cluster the target B cell antigen, CD20, and recruit the inhibitory FcγRIIb, CD32b, leading to their internalization to a significantly greater extent than Type II anti-CD20 mAbs such as obinutuzumab [10–12]. Clustering of anti-CD20 mAbs facilitates efficient CDC, whereas internalization impairs other Fc-mediated effector functions such as antibody-dependent cellular cytotoxicity [10] and antibody-dependent cell phagocytosis.
In SLE, both inherent and acquired defects in effector mechanisms compromise the efficiency of anti-CD20 mAbs. We have previously shown that B cell intrinsic mechanisms such as B cell internalization of rituximab [13] and B cell extrinsic factors such as rituximab pharmacokinetics [14] influence its efficiency. SLE-related defects in complement system [15], phagocytosis [16] and elevated serum B cell activation factor (BAFF) levels [17] may also limit the efficiency of rituximab in SLE [17–20]. BAFF antagonizes deletion of self-reactive B cells in transgenic mice [21], and promotes anti-apoptotic protein expression on B cells, which may compromise rituximab-induced apoptotic direct cell death [10]. In SS, BAFF was reported to modulate repopulation of B cells in patients treated with rituximab [22]. However, there are no current data comparing the duration of BCD in SLE with other autoimmune diseases without such disease-related defects in the complement system or phagocytosis such as RA, where the efficacy is more predictable [23]. Furthermore, we do not know whether peripheral blood BCD reflects BCD in the kidney and data about the relationship between renal BCD and clinical response to rituximab is limited. Therefore, it is important to understand whether host- and/or disease-related factors influence the efficiency of anti-CD20 mAb-mediated BCD in SLE.
In SLE, host-specific factors such as FcγRIIIa polymorphisms are reported to influence the efficiency of rituximab [19], but the effect of patient demographics is not known. Given SLE-related complement defects [18], it is also important to probe the relationship between serum C3 levels and the efficiency of BCD. Furthermore, the relationship between B cell intrinsic factors such as expression of the target antigen, CD20, and FcγRIIb (CD32b) that mediates internalization of rituximab, and extrinsic factors such as the effect of excess BAFF on the efficiency of anti-CD20 mAbs is not known.
Following on from our previous studies [10, 13, 14], here we compared the duration of BCD in patients with SLE and RA, analysed BCD in the kidney samples from patients with refractory LN with poor response to rituximab and explored the relationship between possible factors influencing the efficiency of rituximab, in vivo and in vitro. A recent phase II study reported good clinical efficacy of obinutuzumab in proliferative LN [24]. Therefore, we investigated whether obinutuzumab, given its superiority in inducing BCD in vitro [10], was capable of delivering superior BCD in the presence of excess BAFF.
Methods
Patients
This was a retrospective study. All patients participating in this study fulfilled the ACR (ACR/SLICC) classification criteria for SLE or (ACR/EULAR) diagnostic criteria for RA, respectively. All study participants provided written informed consent according to the declaration of Helsinki and in the remit of the London-Bentham Research Ethics Committee approval of the study. All patients were treated with rituximab (1 g × 2, given 2 weeks apart) at University College Hospital, London, UK. Patient demographics, and clinical and laboratory parameters of the cohort have been described previously [10, 13, 25]. Rituximab was a gift from the pharmacy of University College Hospital. Roche Innovation Center Zurich, Switzerland provided obinutuzumab [26, 27].
Flow cytometry
Fluorochrome-conjugated mAbs anti-CD3 [phycoerythrin (PE)-Cy7], anti-CD19 (Alexa Fluor 700), anti-CD45 (PE), anti-CD20 (FITC) and anti-CD32 (PE) were obtained from BD Biosciences (Oxford, UK) and Biolegend, London, UK. In addition to forward- and side-scatter characteristics, B cells were identified as CD19+ and T cells as CD3+, by flow cytometry using a Becton Dickinson LSR Fortessa cell analyser (supplementary Figs S1 and S2, available at Rheumatology online). Peripheral blood mononuclear cells were separated from whole blood by Ficoll-Hypaque density gradient and B cells were isolated using EasySep Human B Cell Enrichment Kit (Stemcell Technologies, Cambridge, UK).
Whole blood BCD assays
Whole blood BCD assays were performed as described previously [10]. Briefly, freshly drawn, heparinized whole blood (100 µl) was incubated with or without mAbs at 1 µg/ml for 24 h at 37°C and 5% CO2 and subsequently analysed by flow cytometry. Samples were incubated with BAFF at 100 ng/ml for some experiments. The cytotoxicity index (CTI) of mAbs was calculated from the proportion of B cells to T cells on flow cytometry, as described previously [13].
Immunohistochemistry on renal biopsies
Renal biopsy was performed in six patients with suspected active LN despite previous or ongoing treatment with immunosuppression and following treatment with rituximab (1 g × 2, given 2 weeks apart). All patients had a renal biopsy between 5 months and 11 years after rituximab treatment. Immunohistochemistry was performed on formalin-fixed paraffin-embedded sections using standard techniques to stain for B cells using 2 different antibodies: anti-PAX-5 (Becton Dickinson, UK) and anti-CD79 (Dako, Agilent Technologies LDA UK Limited) at a dilution of 1:200 and incubated for 1 h at room temperature. Antigen retrieval was performed using citrate buffer. Following the addition of a secondary antibody and a washing step, Avidin-Biotin Complex solution was added. After a further washing step, the slides were developed using 3,3-diaminobenzidine.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software (version 5). Mann–Whitney U test or paired t-tests were used to compare unpaired or paired samples, respectively. Spearman rank sum test (r2) was used to analyse relationships between variables and r2 > 0.45 and P < 0.05 were considered significant.
Results
Duration of BCD in RA and SLE
The duration of BCD was assessed in 27 patients with RA and 71 patients with SLE. Before treatment with rituximab, patients with RA had significantly higher number of CD19+ cells (median 0.2260 × 109/l, range 0.012–0.663) in peripheral circulation, compared with patients with SLE (median 0.097 × 109/l, range 0.01–1.274) (Fig. 1A) (Mann–Whitney U test). In contrast, at both 3 and 6 months after treatment with rituximab, the number of CD19+ cells was significantly lower in patients with RA compared with patients with SLE. No significant differences were noted at 1 month after rituximab, although some patients with SLE had CD19+ cell counts >0.5 × 109/l. These results showed that after rituximab treatment the duration of BCD was shorter in patients with SLE compared with patients with RA.
Fig. 1.
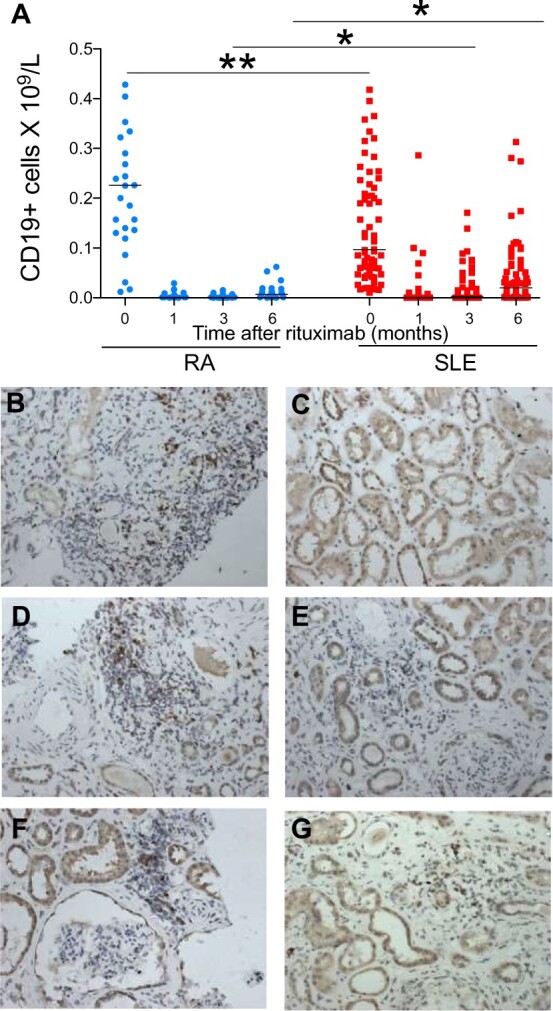
B cell depletion following rituximab
(A) Peripheral and renal B cell depletion with rituximab in patients with RA and SLE. CD19+ B cell counts in peripheral blood before and after treatment with rituximab in patients with RA (n = 27) and SLE (n = 71). (B–G) Immunohistochemistry photomicrographs ×200 of anti-CD79 staining in renal biopsies, which illustrates the degree of B cell infiltration in the biopsies from patients 1–6, respectively. *P < 0.05; **P < 0.001.
BCD in the kidney
A repeat renal biopsy was performed in patients with suspected active LN for the following indications: decline in renal function in four patients (with nephrotic-range proteinuria in three patients) and nephrotic syndrome with preserved renal function in the remaining two patients. Renal biopsy was performed at a median of 12 weeks (range 2–15 weeks’ time from second or only dose of rituximab).
At the time of the renal biopsy following rituximab, the median creatinine was 375 μmol/l (range 54–764), median urine protein creatinine ratio was 632 mg/mmol (range 85–969) and median albumin was 26 g/l (range 18–32). Table 1 demonstrates the individual results and class of LN on both renal biopsies as well as treatment details.
Table 1.
Renal biopsy data and parameters before and after rituximab
| Patient | Prior-renal biopsy class after RTX | Previous treatment | Duration between biopsies | Class of renal biopsy after RTX | Treatment at time of biopsy | Creatinine (μmol/l) | PCR (mg/mmol) |
|---|---|---|---|---|---|---|---|
| 1 | IV + V | CS, CYC, MMF, AZA, CSA | 7 years | V | CS, MMF | 66 | 371 |
| 2 | N/A | CS, AZA, MMF, CSA | N/A | V | CS, MMF | 54 | 637 |
| 3 | IV | CS, CYC | 11 years | IV-GA/C | CS | 764 | 969 |
| 4 | IV G/A | CS, CYC, MMF | 8 months | VI | CS, CYC | 394 | 883 |
| 5 | IV + V | CS, MMF | 5 months | IV | CS, MMF | 461 | 85 |
| 6 | IV-G + V | CS, CYC | 5 months | IV-GA/C, V | CS, ×1 dose CYC | 356 | 627 |
AZA: azathioprine; CS: corticosteroids; CYC: cyclophosphamide; MMF: mycophenolate mofetil; PCR: protein–creatinine ratio; N/A: not available; RTX: rituximab.
During this episode of active disease at the time of the biopsy, three patients had elevated anti-dsDNA antibody levels of 1766, 76 and 124 IU/ml, and three other patients had normal levels (10, 15 and 19 IU/ml). Of these patients with normal anti-dsDNA antibody levels, two had higher levels prior to treatment with rituximab. Five patients had low serum C3 levels (<0.9 g/l).
Patients 2 and 4 had minimal staining with anti-CD79. The remaining four patients had detectable B cells demonstrated by anti-CD79 staining (Fig. 1B–G). Immunohistochemistry with anti-Pax-5 showed similar staining patterns to that with anti-CD79 in four patients (Fig. 1C). Two patients without detectable renal B cells on both staining patterns had Class V and Class VI LN; therefore, staining was only present in proliferative glomerular lesions.
Peripheral B cells in patients with LN
Complete BCD was defined as ≤ 0.005 × 109/l. Patient 2 with a Class V lesion and normal renal function at follow-up had no detectable B cell infiltration, despite a CD19 count of 0.03 × 109/l at the time of renal biopsy. This patient had briefly reached a nadir CD19 count of 0.006 but reconstituted rapidly without detectable renal B cell infiltration. In this cohort of patients, patient 2 is the only patient with normal renal function at long-term follow-up. Patient 1 also with a Class V lesion and a CD19 count of 0.042 × 109/l demonstrated renal B cell infiltration. Patients 3, 5 and 6 had significantly suppressed CD19 count (≤0.002) with all three patients having detectable anti-CD79 staining in renal biopsy samples. Patients 1, 3, 5 and 6 either progressed rapidly to end-stage renal failure or advanced chronic kidney disease (CKD). Table 2 demonstrates the correlation between renal biopsy B cells and peripheral B cells and their clinical outcomes. Patients with lower levels of renal B cells, irrespective of the peripheral B cell count, had better clinical outcomes.
Table 2.
Peripheral CD19+ B cell counts and staining for B cells in renal biopsy samples and patient outcomes following rituximab treatment
| Patient | Class of LN | Renal biopsy B cell staining |
Peripheral B cells | Outcome at latest f/u | |
|---|---|---|---|---|---|
| Anti-CD79 | Anti-Pax-5 | ||||
| 1 | V | ↑ | ↑ | ↑ 0.042 × 109/l | Progressive CKD |
| 2 | V | → | → | ↑ 0.03 × 109/l | Remission (normal renal function) |
| 3 | IV-GA/C | ↑ | ↑ | ↓ (≤0.002) | Dialysis → ESRF |
| 4 | VI | → | → | ↓ (≤0.002) | Stable CKD (on PD) |
| 5 | IV | ↑ | ↑ | ↓ (≤0.002) | Stable CKD |
| 6 | IV-GA/C | ↑ | ↑ | ↓ (≤0.002) | Advanced CKD, dialysis dependent |
→ minimal staining; ↑detectable B cells; ↓ suppressed counts (≤0.005). CKD: chronic kidney disease; ESRF: end stage renal failure; f/u: follow-up.
B cell expression of CD20 and FcγRIIb in RA and SLE
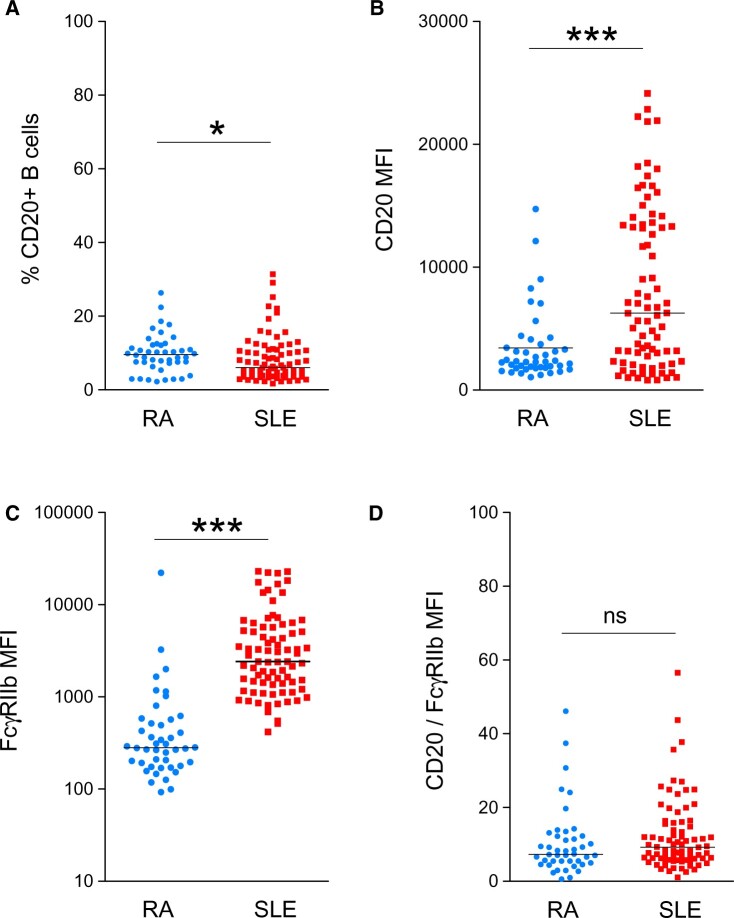
Expression of CD20 and the inhibitory FcγRIIb on malignant B cells influences clinical response to rituximab in lymphoma [12, 28, 29]. Therefore, their expression on B cells from patients with RA (n = 44) and SLE (n = 81) were compared.
The median [interquartile range (IQR)] frequency of CD20+ B cells was significantly greater in patients with RA compared with those with SLE, with 9.6% (7.3–12.1%) and 6% (3.9–10.8%), respectively (Fig. 2A). In contrast, B cell expression of CD20 [mean fluorescence intensity (MFI)] was significantly lower in patients with RA compared with those with SLE with median (IQR) 2292 (1787–3654) and 6261 (2334–13 446), respectively (Fig. 2B). However, the MFI of CD20 varied remarkably, particularly in patients with SLE.
Fig. 2.
The MFI of CD20 and FcγRIIb on B cells from patients with RA and SLE
(A) The frequency of CD20+ B cells in patients with RA and SLE; (B) the MFI of CD20 in patients with RA and SLE; (C) the MFI of FcγRIIb in patients with RA and SLE; and (D) the ratio of MFI of CD20 and MFI of FcγRIIb of CD20+ B cells from patients with RA and SLE. MFI, mean fluorescence intensity; *P < 0.05; ***P < 0.0001; ns: not significant.
B cell expression of FcγRIIb (CD32) (MFI) was also significantly lower in patients with RA compared with those with SLE, with median (IQR) 280 (181–555) and 2414 (1378–5707), respectively. The MFI of FcγRIIb varied remarkably between patients with RA and SLE (Fig. 2C). Further, there was no difference in the relative B cell expression of CD20 and FcγRIIb (ratio MFI of CD20: MFI of FcγRIIb) between patients with RA and SLE (Fig. 2D).
Thus, these results found no significant relationship between the relative expression of CD20 and FcγRIIb between patients with RA and SLE.
Relationships between the efficiency of type I anti-CD20 mAb and patient demographics
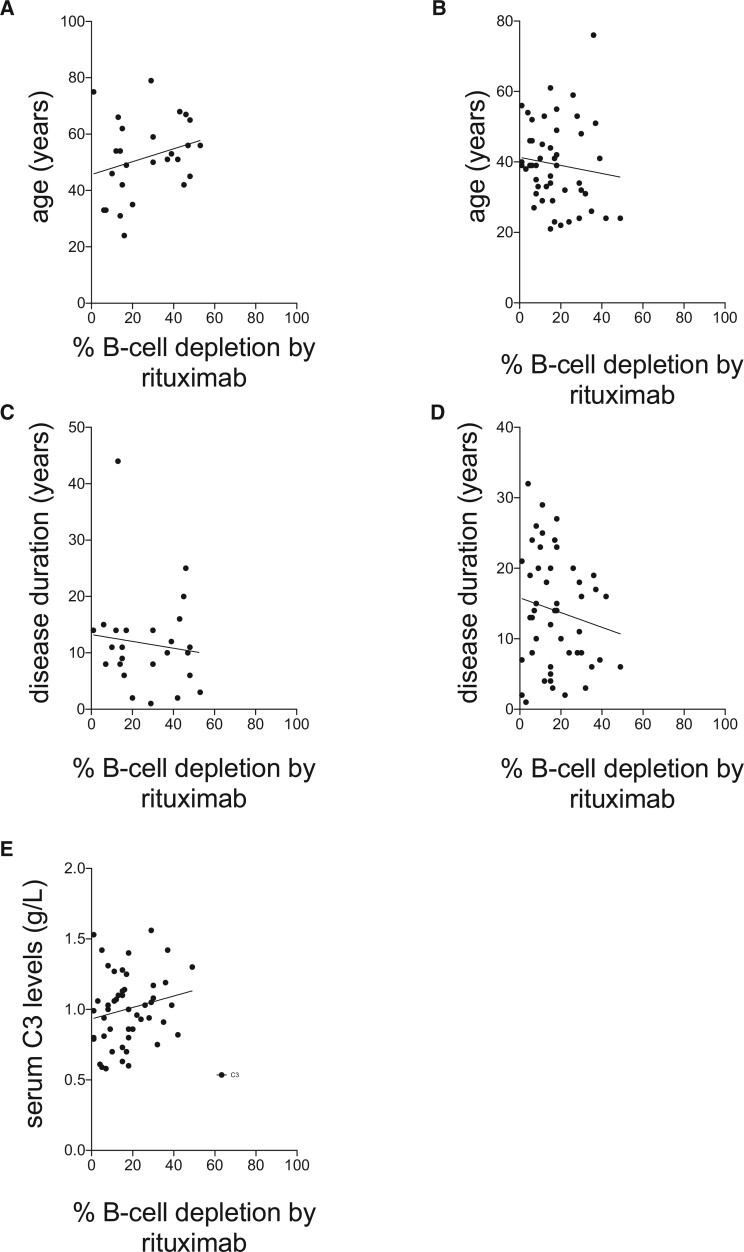
To assess the effects of patient characteristics on the efficiency of anti-CD20 mAbs, whole blood BCD assays were performed. There were no significant relationships between the percentages of BCD achieved by rituximab in the whole blood BCD assay in samples from patients with SLE and age (years) of patients with RA or SLE with r2, Spearman’s correlation coefficient, values of 0.07 (P > 0.1) and 0.01 (P > 0.4), respectively, or disease duration of patients with RA or SLE with r2, Spearman’s correlation coefficient, values of 0.01 (P > 0.5) and 0.02 (P > 0.2) or serum C3 levels with spearman r2 of –0.17 (P = 0.37), respectively (Fig. 3).
Fig. 3.
Relationships between patient demographics and BCD in vitro
Relationships between rituximab-mediated BCD and age of patients with (A) RA and (B) SLE, and disease duration of patients with (C) RA and (D) SLE, and (E) serum C3 levels in patients with SLE. BCD: B cell depletion.
BCD in vitro with type I and II anti-CD20 mAbs: relationship with B cell expression of CD20 and FcγRIIb
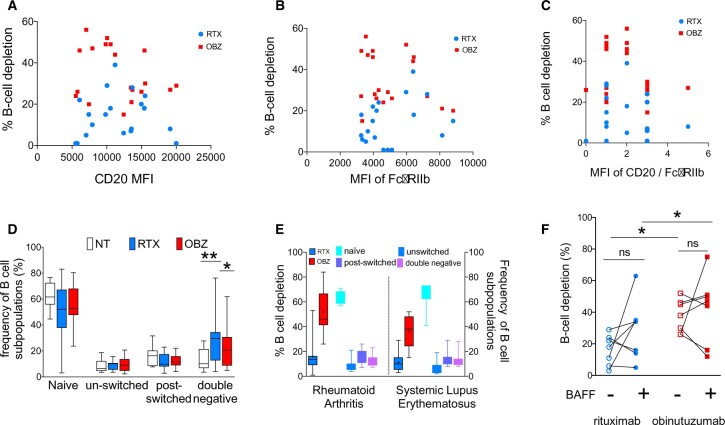
CD20 expression on B cells varied remarkably between patients with SLE with a mean (s.d.) of 11 555 (4354). However, there were no significant relationships between B cell expression of CD20 and the CTI of rituximab or obinutuzumab in the whole blood assay in samples from patients with SLE, with r2, Spearman’s correlation coefficient, values of –0.11 (P = 0.63) and 0.19 (P = 0.42), respectively (Fig. 4A).
Fig. 4.
BCD in vitro with rituximab and obinutuzumab: relationship with B cell expression of CD20 and FcγRIIb; B cell subpopulations and the effect of BAFF
Relationship between percentage BCD with rituximab and obinutuzumab in whole blood assays and (A) the MFI of CD20, (B) the MFI FcγRIIb and (C) the MFI of CD20/FcγRIIb on B cells from patients with SLE (n = 19). (D) Composition of B cell subpopulations in the whole blood BCD assay. The frequency of various B cell subpopulations in samples incubated with or without mAbs in samples from patients with RA (n = 9). (E) CTI of mAbs in whole blood BCD assay and the distribution of B cell subpopulations in RA (n = 10) and SLE (n = 9) in peripheral blood prior to incubation with mAbs. (F) The effect of excess BAFF on the efficiency of BCD. Each symbol represents an individual sample and the unfilled symbols represent samples incubated without excess BAFF and the filled symbols represent samples incubated with excess BAFF. Naïve, IgD+CD27–; unswitched memory B cells, IgD+CD27+; switched memory B cells, IgD–CD27+; double negative, IgD–CD27–. BAFF: B cell activation factor; BCD: B cell depletion; CTI: cytotoxicity index; MFI, mean fluorescence intensity; NT, not treated; RTX, rituximab; OBZ, obinutuzumab; *P < 0.05 and **P < 0.001; ns: not significant.
Expression of FcγRIIb was less variable than that of CD20 with a mean (s.d.) of 5008 (1687). No significant correlations were found between the expression of CD20, FcγRIIb or their relative expression (ratio of MFI of CD20/FcγRIIb) to the CTI of rituximab or obinutuzumab in patients with SLE with r2, Spearman’s correlation coefficient, values of –0.31 (P = 0.19) and 0.25 (P = 0.29), respectively (Fig. 4A–C).
BCD in vitro with type I and II anti-CD20 mAbs: relationship with the composition of B cell subpopulations
B cell subpopulations may differ in their susceptibility to deletion by mAbs [30]. Therefore, the distribution of B cell subpopulations in samples from nine patients with SLE incubated with anti-CD20 mAbs for 24 h in the whole blood BCD assay was analysed. There were no differences between B cell subpopulations in samples incubated with the two mAbs except for the frequency of double (CD19+IgD–CD27–) negative cells, which was higher in samples incubated with rituximab compared with samples incubated without mAbs and those incubated with obinutuzumab (Fig. 4D). The CTI of obinutuzumab was significantly greater than the CTI of rituximab in both RA (n = 10) and SLE (n = 9) (Fig. 4E). However, there were no significant differences in the frequencies of B cell subpopulations between the cohort of patients with RA and SLE (Fig. 4E). Collectively, these results suggest that obinutuzumab was more efficient at BCD than rituximab regardless of the composition of B cell subpopulations.
Efficiency of type I and II anti-CD20 mAbs and the effect of excess BAFF
Patients with SLE may have high BAFF levels detectable in the serum [31]. Therefore, the effect of BAFF on the efficiency of anti-CD20 mAbs at inducing BCD was assessed by comparing the CTI of anti-CD20 mAbs in samples incubated in the presence or absence of BAFF at 100 ng/ml in the whole blood assay using freshly drawn blood samples from patients with RA (n = 3) and SLE (n = 4).
There were no consistent effects of excess BAFF on the efficiency of mAbs in the whole blood BCD assay in samples from patients with SLE (n = 7, Fig. 4F). There were no significant differences between the CTI of rituximab or obinutuzumab in the absence or presence of excess BAFF in the whole blood assay (P = 0.1875 and P > 0.05, respectively). The median (range) CTI of rituximab in the absence of excess BAFF was 18 (1–29) and in the presence of excess BAFF the median (range) CTI was 34 (14–63). The median (range) CTI of obinutuzumab in the absence of excess BAFF was 45 (26–52) and in the presence of excess BAFF the median (range) CTI was 50 (12–75). However, even in this small number of samples the CTI of obinutuzumab was significantly greater than rituximab regardless of excess BAFF (P < 0.05).
Discussion
Here, we found that BCD in SLE was of shorter duration than in RA. We report a disparity between peripheral blood BCD and renal BCD in individual patients with LN. Although this study did not find any significant relationships between the possible contributing factors investigated and BCD, obinutuzumab was superior to rituximab at inducing BCD, in vitro, regardless of excess BAFF.
Despite treatment with the same dose of rituximab, the duration of BCD was remarkably variable between individual patients with RA and SLE, as reported previously [1–3, 32–34]. Before treatment with rituximab, patients with RA had higher CD19+ B cell counts compared with patients with SLE. However, the observation that CD19+ B cells were detectable in peripheral circulation at an earlier time point after treatment with rituximab in patients with SLE compared with RA suggests that BCD was less pronounced and/or that repopulation of distinct B cell subpopulations occurred sooner in patients with SLE compared with those with RA and/or that CD19+CD20– plasma blasts and plasma cells, which may, at least in part, explain the disparity in clinical response reported in clinical trials involving patients with RA and SLE.
Despite disappointing trial evidence [4], rituximab is used especially in those patients who are refractory to standard treatment [35]. In LN, recruitment of macrophages, T cells and B cells into the interstitium contributes to inflammation [36], and in Class IV LN both activity and chronicity correlate with B cell infiltration in the interstitium rather than in the glomerulus [37]. Our data indicate a clinically relevant discrepancy in BCD between peripheral blood and the kidney in patients with LN with the persistence of interstitial B cells (as demonstrated by CD79- and Pax-5-positive cells), which was associated with progressive CKD and end-stage renal failure in four patients, whereas the patient with preserved renal function had no detectable B cells in the kidney. The only patient without any detectable B cells by either marker (CD79 or Pax-5) had a Class V lesion and at long-term follow-up has normal kidney function and remains in remission. Despite undetectable peripheral CD19+ B cells, the persistence of B cells following rituximab was associated with risk of progressive CKD and end-stage renal failure. Taken together with the observations that rituximab treatment leads to resorption of immune deposits in responding patients with LN [38], our findings suggest that improving BCD in the kidney may enhance clinical response to BCD therapy in LN.
We have previously reported that BCD in SLE is less efficient regardless of serum rituximab levels [14], which suggested that alternative resistance mechanisms operate to reduce the efficiency of rituximab. Although the study was not primarily designed to evaluate the effect of each parameter, we found no significant correlation between the CTI of rituximab and/or obinutuzumab and patient’s age, duration of disease or serum C3 levels. In contrast to some B cell malignancies, B cell expression of FcγRIIb and/or CD20 did not correlate with the in vitro efficiency of mAbs in either RA or SLE, with respect to the target cell expression of FcγRIIb [28, 39] and CD20 [40, 41]. Thus, although B cell expression of CD20 and/or FcγRIIb was variable between individuals and also in B cell subpopulations, the findings described here suggest that the thresholds of B cell expression of CD20 and FcγRIIb and/or baseline composition of B cell subpopulations do not seem to influence the efficiency of anti-CD20 mAbs in inducing BCD, in vitro, in RA and SLE patient samples.
There were no remarkable differences in susceptibility of B cell subpopulations to anti-CD20 mAbs in whole blood BCD assays. Allowing for the limitation that alterations in one B cell compartment would alter the frequency of other B cell subpopulations, the results suggest that IgD-CD27-B cells might resist depletion with rituximab, but not obinutuzumab in in vitro whole blood BCD assays.
BAFF inhibits apoptosis in B cells by up-regulating the anti-apoptotic factors Bcl-2 and Bcl-XL [42]. Therefore, BAFF may antagonize rituximab-mediated apoptotic cell death whereas BAFF may have limited effect on obinutuzumab-induced lysosome-mediated direct cell death [10, 18]. While the results of trials investigating the efficacy of combination therapy with rituximab and belimumab are awaited, a recent mechanistic study of the combination therapy in 15 patients followed up for 2 years reported that poor BCD was more common in non-responders than in responders [43]. Furthermore, patients who do not respond to rituximab due to incomplete depletion may benefit from treatment with an alternative anti-CD20 agent rather than belimumab [8]. Here, we found no consistent effects of BAFF on the efficiency of mAb-mediated BCD in this small number of samples. However, the efficiency of mAbs was not reduced in the presence of excess BAFF, whereas obinutuzumab induced superior BCD to rituximab regardless of additional BAFF in whole blood assays.
The main limitations of this study include that experiments to investigate the mechanisms behind the variability in BCD as well as comparing the efficiency of Type I and II anti-CD20 mAbs were carried out in vitro. In contrast to randomized controlled trials of rituximab in LN [4, 44], which failed to demonstrate superiority over standard of care, a phase II study demonstrated efficient peripheral BCD and renal responses with obinutuzumab compared with placebo when added to MMF and steroids [24]. Our data correspond with this in that obinutuzumab was more efficient than rituximab at inducing BCD in whole blood assays.
Thus, the main conclusions are: (i) B cell depletion in SLE is less efficient than RA; (ii) renal B cell infiltration following rituximab treatment is associated with poor outcomes; (iii) no significant relationships between the measured B cell intrinsic and extrinsic factors and the efficiency of BCD by rituximab; and (iv) obinutuzumab was superior to rituximab at inducing BCD in vitro, regardless of excess BAFF.
Taken together, this study highlights the potential of obinutuzumab, a commercially available, mechanistically different Type II anti-CD20 mAb with an afucosylated Fc portion not disposed to internalization (unlike rituximab), as an alternative B cell depleting agent in RA and SLE.
Supplementary Material
Acknowledgements
V.R.R. was funded by an Arthritis Research UK Fellowship (20488) and an Medical Research Council Clinical Academic Research Partnership (MRC-CARP, MR/T024968/1) award, and supported by Doris Hillier Grant (2013 and 2017) and a research grant from Roche Glycart, Basel. V.R.R., L.K., D.A.I. and M.J.L. acknowledge the support of the Biomedical Research Centre (BRC) award to University College London and University College Hospital. The authors would like to thank Pauline Buck, Eve McLoughlin, Nichola Whitbread and Samantha Moore for their help with recruiting and obtaining samples from patients for this study. Obinutuzumab was provided by Hoffmann La Roche.
Funding: The study was part funded by Versus Arthritis grant (20488), Doris Hillier Grant 2017 and Roche Glycart. VRR’s work was supported by Medical Research Council grant (MR/T024968/1).
Disclosure statement: V.R.R. has received a research grant from Roche. C.K. is an employee of and shareholder of Roche. M.S.C. is a retained consultant for BioInvent International and has performed educational and advisory roles for Baxalta and Boehringer Ingleheim. He has received research funding from Roche, Gilead, iteos, Bioinvent International, Union Chimique Belge (UCB) and GlaxoSmithKline. M.J.L. acted as a consultant for Roche and Genentech and received funds less than $10 000 and support to attend educational conferences.
Contributor Information
Venkat R Reddy, Department of Rheumatology, University College London Hospitals NHS Foundation Trust; Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Ruth J Pepper, Department of Rheumatology, University College London Hospitals NHS Foundation Trust; Department of Renal Medicine (formerly Centre for Nephrology), Royal Free Hospital, London, UK.
Kavina Shah, Department of Rheumatology, University College London Hospitals NHS Foundation Trust; Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Geraldine Cambridge, Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Scott R Henderson, Department of Renal Medicine (formerly Centre for Nephrology), Royal Free Hospital, London, UK.
Christian Klein, Cancer Immunotherapy Discovery, Oncology Discovery & Translational AreaRoche Pharma Research & Early Development, Roche Innovation Center, Zurich, Switzerland.
Loren Kell, Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Samuel J Taylor, Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
David A Isenberg, Department of Rheumatology, University College London Hospitals NHS Foundation Trust; Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Mark S Cragg, Centre for Cancer Immunology, Faculty of Medicine, University of Southampton, Southampton, UK.
Maria J Leandro, Department of Rheumatology, University College London Hospitals NHS Foundation Trust; Centre for Rheumatology and Bloomsbury Rheumatology Unit, University College London.
Data availability statement
The data are available from the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Vital EM, Dass S, Buch MH. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011;63:3038–47. [DOI] [PubMed] [Google Scholar]
- 2. Albert D, Dunham J, Khan S. et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis 2008;67:1724–31. [DOI] [PubMed] [Google Scholar]
- 3. Looney RJ, Anolik JH, Campbell D. et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 2004;50:2580–9. [DOI] [PubMed] [Google Scholar]
- 4. Rovin BH, Furie R, Latinis K. et al. ; LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- 5. Gomez Mendez LM, Cascino MD, Garg J. et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol 2018;13:1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunnarsson I, Sundelin B, Jónsdóttir T. et al. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum 2007;56:1263–72. [DOI] [PubMed] [Google Scholar]
- 7. Md Yusof MY, Shaw D, El-Sherbiny YM. et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis 2017;76:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassan SU, Md Yusof MY, Emery P, Dass S, Vital EM.. Biologic sequencing in systemic lupus erythematosus: after secondary non-response to rituximab, switching to humanised anti-CD20 agent is more effective than belimumab. Front Med (Lausanne) 2020;7:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glennie MJ, French RR, Cragg MS, Taylor RP.. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol 2007;44:3823–37. [DOI] [PubMed] [Google Scholar]
- 10. Reddy V, Klein C, Isenberg DA. et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford) 2017;56:1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein C, Lammens A, Schäfer W. et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs 2013;5:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim SH, Beers SA, French RR. et al. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica 2010;95:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddy V, Cambridge G, Isenberg DA. et al. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy V, Croca S, Gerona D. et al. Serum rituximab levels and efficiency of B cell depletion: differences between patients with rheumatoid arthritis and systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:951–2. [DOI] [PubMed] [Google Scholar]
- 15. Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res 2002;4(Suppl 3):S279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahuja A, Teichmann LL, Wang H. et al. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in Lupus. J Immunol 2011;187:3888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter LM, Isenberg DA, Ehrenstein MR.. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum 2013;65:2672–9. [DOI] [PubMed] [Google Scholar]
- 18. Reddy V, Dahal LN, Cragg MS, Leandro M.. Optimising B-cell depletion in autoimmune disease: is obinutuzumab the answer? Drug Discov Today 2016;21:1330–8. [DOI] [PubMed] [Google Scholar]
- 19. Anolik JH, Campbell D, Felgar RE. et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 2003;48:455–9. [DOI] [PubMed] [Google Scholar]
- 20. Quartuccio L, Fabris M, Moretti M. et al. Resistance to rituximab therapy and local BAFF overexpression in Sjogren's syndrome-related myoepithelial sialadenitis and low-grade parotid B-cell lymphoma. Open Rheumatol J 2008;2:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thien M, Phan TG, Gardam S. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 2004;20:785–98. [DOI] [PubMed] [Google Scholar]
- 22. Pers JO, Devauchelle V, Daridon C. et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren’s syndrome. Arthritis Rheum 2007;56:1464–77. [DOI] [PubMed] [Google Scholar]
- 23. Edwards JCW, Szczepański L, Szechiński J. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81. [DOI] [PubMed] [Google Scholar]
- 24. Furie R, Cascino MD, Garg JP. et al. O35 B-cell depletion and response in a randomized, controlled trial of obinutuzumab for proliferative lupus nephritis. Lupus Sci Med 2020;7:A27–8. [Google Scholar]
- 25. Reddy V, Martinez L, Isenberg DA, Leandro MJ, Cambridge G.. Pragmatic treatment of patients with systemic lupus erythematosus with rituximab: long-term effects on serum immunoglobulins. Arthritis Care Res (Hoboken) 2017;69:857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niederfellner G, Lammens A, Mundigl O. et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood 2011;118:358–67. [DOI] [PubMed] [Google Scholar]
- 27. Mossner E, Brunker P, Moser S. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee CS, Ashton-Key M, Cogliatti S. et al. Expression of the inhibitory Fc gamma receptor IIB (FCGR2B, CD32B) on follicular lymphoma cells lowers the response rate to rituximab monotherapy (SAKK 35/98). Br J Haematol 2015;168:145–8. [DOI] [PubMed] [Google Scholar]
- 29. Horvat M, Kloboves Prevodnik V, Lavrencak J, Jezersek Novakovic B.. Predictive significance of the cut-off value of CD20 expression in patients with B-cell lymphoma. Oncol Rep 2010;24:1101–7. [PubMed] [Google Scholar]
- 30. Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther 2013;15(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheema GS, Roschke V, Hilbert DM, Stohl W.. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001;44:1313–9. [DOI] [PubMed] [Google Scholar]
- 32. Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA.. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology (Oxford) 2005;44:1542–5. [DOI] [PubMed] [Google Scholar]
- 33. Leandro MJ, Edwards JC, Cambridge G.. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis 2002;61:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vital EM, Rawstron AC, Dass S. et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis Rheum 2011;63:603–8. [DOI] [PubMed] [Google Scholar]
- 35. Diaz-Lagares C, Croca S, Sangle S. et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev 2012;11:357–64. [DOI] [PubMed] [Google Scholar]
- 36. Lech M, Anders HJ.. The pathogenesis of lupus nephritis. J Am Soc Nephrol 2013;24:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen Y, Sun CY, Wu FX. et al. Association of intrarenal B-cell infiltrates with clinical outcome in lupus nephritis: a study of 192 cases. Clin Dev Immunol 2012;2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zickert A, Lannfelt K, Schmidt Mende J, Sundelin B, Gunnarsson I.. Resorption of immune deposits in membranous lupus nephritis following rituximab vs conventional immunosuppressive treatment. Rheumatology (Oxford) 2021;60:3443–50. [DOI] [PubMed] [Google Scholar]
- 39. Lim SH, Vaughan AT, Ashton-Key M. et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011;118:2530–40. [DOI] [PubMed] [Google Scholar]
- 40. Golay J, Lazzari M, Facchinetti V. et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 2001;98:3383–9. [DOI] [PubMed] [Google Scholar]
- 41. van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB.. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res 2006;12:4027–35. [DOI] [PubMed] [Google Scholar]
- 42. He B, Chadburn A, Jou E. et al. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 2004;172:3268–79. [DOI] [PubMed] [Google Scholar]
- 43. Kraaij T, Arends EJ, van Dam LS. et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant 2021;36:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merrill JT, Neuwelt CM, Wallace DJ. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author.