Abstract
Objectives
JDM is an inflammatory myopathy characterized by prominent vasculopathy. AECAs are frequently detected in inflammatory and autoimmune diseases. We sought to determine whether AECAs correlate with clinical features of JDM, and thus serve as biomarkers to guide therapy or predict outcome.
Methods
Plasma samples from 63 patients with JDM, 49 patients with polyarticular JIA and 40 juvenile healthy controls were used to detect anti-heat shock cognate 71 kDa protein (HSC70) autoantibodies, a newly identified AECA, in ELISA assays. Clinical features were compared between JDM patients with and without anti-HSC70 autoantibodies.
Results
Anti-HSC70 autoantibodies were detected in 35% of patients with JDM, in 0% of patients with JIA (P < 0.0001) and in 0% of healthy donors (P < 0.0001). Both the presence of cutaneous ulcers (59% vs 17%, P < 0.002) and the use of wheelchairs and/or assistive devices (64% vs 27%, P < 0.007) were strongly associated with anti-HSC70 autoantibodies in JDM. High scores on the severity of myositis damage measures at the time of measurement of anti-HSC70 autoantibodies and an increased number of hospitalizations were also associated with anti-HSC70 autoantibodies. Intravenous immunoglobulin therapy was used more often in anti-HSC70 autoantibody-positive patients.
Conclusion
Anti-HCS70 autoantibodies are detected frequently in children with JDM and are novel myositis-associated autoantibodies correlating with disease severity.
Keywords: JDM, vasculopathy, anti-endothelial cell antibodies, heat shock cognate 71 kDa protein, myositis-associated autoantibodies
Rheumatology key messages.
Anti-HSC70 autoantibodies were detected in 35% of North American JDM patients by ELISA using plasma.
Anti-HSC70-positive patients with JDM show a marked increased tendency to develop cutaneous ulcers.
The presence of anti-HSC70 autoantibodies significantly correlates with use of IVIG therapy in JDM patients.
Introduction
JDM is the most common form of inflammatory myopathy in children [1]. Although relatively rare, JDM is associated with significant morbidity and, in some cases, mortality [2, 3]. One of the striking pathological features of involved tissues in JDM is the presence of prominent vascular and perivascular inflammation. In many cases, this inflammation is accompanied by thrombosis and vessel occlusion, which contributes directly to the observed clinical features [4, 5]. We recently reported that children with JDM have detectable autoantibodies to multiple antigens on human endothelial cells [6]. Among these antigens were molecular chaperones, such as heat shock cognate 71 kDa protein (HSC70; also known as HSPA8). Autoantibodies against HSC70 are more commonly detected in children with active, untreated disease than in healthy children. We also reported that these AECAs are rare in children with juvenile idiopathic arthritis, another chronic autoimmune disease affecting children. This has raised the question of whether an autoimmune response directed to the vasculature may play a role in the pathobiology of JDM.
A broad spectrum of autoantibodies has been detected in children with JDM, and many of these autoantibodies are linked to specific clinical features [1, 7]. In this study, we sought to determine whether anti-HSC70 autoantibodies, an AECA, might correlate with specific clinical features of JDM, and thus serve as surrogate biomarkers to guide therapy or predict outcome.
Methods
Patients and plasma samples
Plasma of JDM patients under treatment (n = 63) were collected as part of natural history protocols at the National Institutes of Health (NIH) between 2003 and 2017. The median [interquartile range (IQR)] age of the patients with JDM (42 females and 21 males) was 11 (9–16) years. All except one patient without anti-HSC70 autoantibodies had the active disease at the time of measurement of the autoantibodies in JDM. Disease course was classified as monocyclic if the patient achieved remission without evidence of active disease within 2 years of diagnosis, based on the clinical features and laboratory testing; as polycyclic if the patient had recurrence of active disease after a definite clinical remission; and as chronic if active disease persisted for >2 years [8]. Active disease and remission were defined, respectively, as a physician global score of >0 and as clinically inactive disease for at least six continuous months of all treatment [9]. The presence or absence of symptoms at diagnosis in seven organ systems was examined using a total symptom score defined as the number of symptoms present divided by the number of symptoms evaluated (range, 0–1), as previously described [8]. For instance, pulmonary system score was evaluated using the five items (dysphonia, dyspnea on exertion, dyspnea at rest, interstitial lung disease, and pneumothorax or pneumomediastinum) or a minimum of four out of the five items and was determined based on the presence or absence of signs or symptoms prior to or at diagnosis. Severity of illness at onset, up to the time of diagnosis, was graded on a 4-point Likert scale as determined by the enrolling physician, and graded from mild to very severe disease activity [1]. The presence of ulceration was determined by pediatric and adult rheumatologists who enrolled patients into the study and was defined using the tools that we validated, including Myositis Disease Activity Assessment Tool and Cutaneous Assessment Tool. Plasma from patients with newly diagnosed, untreated (JIA; n = 49, 39 females and 10 males) and healthy children (HC; n = 40, 25 females and 15 males) were collected from children attending the General Pediatrics Clinics at the University of Oklahoma College of Medicine and the Women and Children’s Hospital of Buffalo. The median (IQR) age of the patients with JIA and HC was 11 (7–14) and 12 (9–15) years, respectively. Children with JIA had the extended oligoarticular or polyarticular phenotypes as assessed using accepted international criteria [10]. Written informed consent, and, where appropriate, assent, was obtained from all participants prior to obtaining samples. This study was approved by the ethics committee of St. Marianna University School of Medicine (no. 1710), the University of Oklahoma Institutional Review Board (no. 12134), University at Buffalo Institutional Review Board (IRB no. MOD 00002155) and the National Institutes of Health Institutional Review Board (05E-N-200, 94-E-0165, 03-E-0099 and 11-E-0072).
Anti-HSC70 autoantibody detection
ELISA were conducted as described previously [6]. To detect autoantibodies in plasma to heat shock cognate 71 kDa protein encoded by the HSPA8 gene, each well of an ELISA plate (Thermo Fisher Scientific, Waltham, MA, USA) was coated with 0.125 µg of HSC70 (StressMarq Biosciences Inc., Victoria, BC, Canada) in a carbonate buffer (50 mM sodium carbonate, pH 9.6). The plasma diluted 1:200 with 10% Block Ace was reacted with the coated recombinant proteins. Optical density at 490 nm was measured by a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). The mean plus 3 s.d. among the group of healthy donors was defined as 100 arbitrary binding units (AU) and was used as a cut-off point for determining reactivity to HSC70.
Myositis autoantibodies and HLA typing
Sera were tested for myositis-specific autoantibodies (MSAs) and myositis-associated autoantibodies (MAAs) using validated immunoprecipitation and immunoprecipitation–immunoblotting methods at the Oklahoma Medical Research Foundation laboratory [1]. Anti-Ro52 autoantibodies were detected using ELISA for Ro52 [11]. Genotyping of HLA Class II (HLA DRB1 and DQA1) alleles was performed in Caucasian anti-MDA5 autoantibody-positive JDM patients [12].
Statistics
Plasma levels of autoantibodies were summarized as medians and IQR. Categorical variables were presented as percentages and were compared by Fisher's exact test. Continuous variables were compared by the Mann–Whitney U-test and expressed as medians and IQR. The frequencies of specific HLA alleles were compared between the subject groups using the χ2 test or Fisher’s exact test. Because we conducted an exploratory study, a two-tailed P-value of <0.05 was considered statistically significant and not corrected for multiple testing. Statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA).
Results
Prevalence of anti-HSC70 autoantibodies in JDM compared with other rheumatic diseases and controls
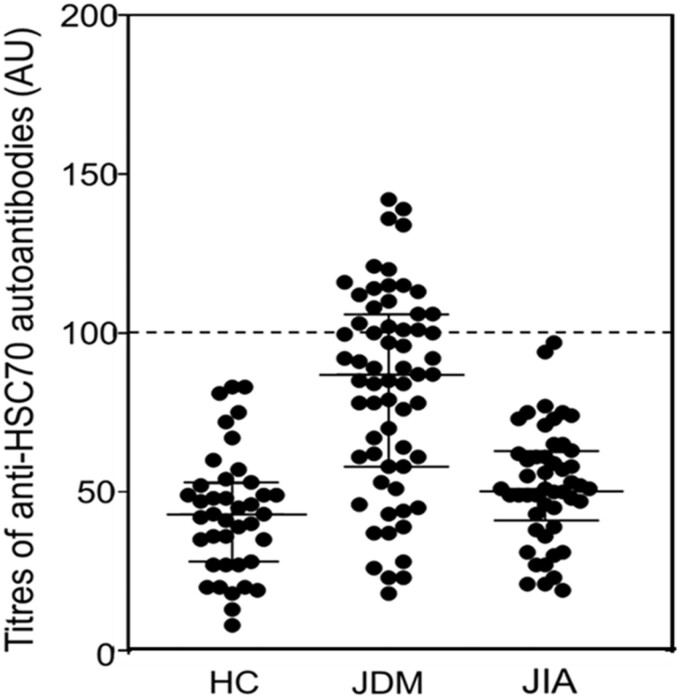
Significant differences in plasma levels and the frequency of autoantibodies to HSC70 were found between children with JDM and the other groups (Fig. 1).
Fig. 1.
Results of ELISAs for anti-HSC70 autoantibodies
The dashed line of 100 arbitrary units indicates the cut-off values for anti-HSC70 autoantibody positivity. The average plus 3 s.d. among the group of healthy donors defined as 100 arbitrary units was 0.603 (optical density). The continuous lines indicate median and interquartile range of anti-HSC70 autoantibody levels. Plasma samples from 63 patients with JDM, 49 patients with JIA, and 40 sex- and age-matched healthy children (HC) were used for ELISA assays. Anti-HSC70 autoantibodies were detected in 35% of patients with JDM, in 0% of patients with JIA, and in 0% of HC.
Autoantibodies to HSC70 were detected in 35% (22/63) of patients with JDM, in 0% (0/49) of patients with JIA (P < 0.0001) and in 0% (0/40) of HC (P < 0.0001). Median (IQR) levels of autoantibodies to HSC70 were 86.6 (59.4–104.6) AU for JDM, 51.2 (43.5–62.3) AU for JIA and 43.2 (28.0–52.0) AU for HC. The median plasma levels of anti-HSC70 autoantibodies were higher in children with JDM than in children with JIA (P < 0.0001) and in HC (P < 0.0001). The ELISA assay exhibited no matrix effects and was shown to be highly reproducible.
General features of JDM patients with and without anti-HSC70 autoantibodies
There were no significant differences in demographic characteristics, including age at diagnosis, age at enrolment in the NIH studies, gender, race, or delay to diagnosis (i.e. the time from onset of signs or symptoms of myositis to diagnosis), between JDM patients with and without anti-HSC70 autoantibodies (Table 1). Patients positive for anti-HSC70 autoantibodies more often had NT5C1A and/or anti-Ro52 autoantibodies (69% vs 29%, P < 0.02 and 43% vs 15%, P < 0.03, respectively). In contrast, there were no significant differences in the presence of any MSAs between JDM patients with and without anti-HSC70 autoantibodies. Furthermore, the presence of anti-HSC70 autoantibodies alone was more frequent in JDM patients with cutaneous ulcerations compared with that of anti-HSC70 autoantibodies co-occurring with anti-NT5C1A and/or anti-Ro52 autoantibodies [89% (8 out of 9) vs 38% (5 out of 13), P = 0.031].
Table 1.
General features of JDM patients with and without anti-HSC70 autoantibodies
| Anti-HSC70 positive (N = 22) | Anti-HSC70 negative (N = 41) | P-value | |
|---|---|---|---|
| Age at diagnosis, median (IQR), years | 8.6 (7.0–10.9) | 8.0 (6.3–12.4) | 0.8 |
| Age at enrolment, median (IQR), years | 10.7 (8.8–14.6) | 11.2 (8.6–15.8) | 0.9 |
| Delay to diagnosis, median (IQR), months | 3.5 (2.0–6.8) | 5.0 (2.0–8.3) (N = 40) | 0.3 |
| Follow-up, median (IQR), years | 2.8 (1.9–6.4) | 3.1 (1.1–7.1) | 0.8 |
| Female, n/N (%) | 12/22 (55) | 30/41 (73) | 0.2 |
| Race, n/N (%) | |||
| White | 14/22 (64) | 28/41 (68) | 0.8 |
| Black | 3/22 (14) | 4/41 (10) | 0.7 |
| Hispanic | 1/22 (5) | 2/41 (5) | 1.0 |
| Asian | 0/22 (0) | 1/41 (2) | 1.0 |
| Biracial or multiracial | 4/22 (18) | 6/41 (15) | 0.7 |
| Myositis-associated autoantibodies, n/N (%) | 15/22 (68) | 16/41 (39) | <0.04 |
| Anti-NT5C1A | 11/16 (69) | 10/35 (29) | <0.02 |
| Anti-Ro52 | 9/21 (43) | 6/40 (15) | <0.03 |
| Anti-Ro | 6/22 (27) | 4/41 (10) | 0.08 |
| Anti-U1RNP | 1/22 (5) | 2/41 (5) | 1.0 |
| Anti-PM-SCL | 0/22 (0) | 1/41 (2) | 1.0 |
| Myositis-specific autoantibodies, n/N (%) | 20/22 (91) | 34/41 (83) | 0.5 |
| Anti-p155/140 (TIF-1) | 7/22 (32) | 16/41 (39) | 0.8 |
| Anti-NXP-2 | 6/22 (27) | 11/41 (27) | 1.0 |
| Anti-MDA5 | 6/22 (27) | 3/41 (7) | 0.06 |
| Anti-Mi-2 | 1/22 (5) | 1/41 (2) | 1.0 |
| Anti-KS | 0/22 (0) | 1/41 (2) | 1.0 |
| Anti-HMGCR | 0/22 (0) | 1/41 (2) | 1.0 |
| Anti-Jo1 | 0/22 (0) | 1/41 (2) | 1.0 |
Categorical variables were analysed by Fisher's exact tests and are presented as count/total (percentage). Continuous variables were analysed by the Mann–Whitney U-test and are expressed as median (IQR [Q1–Q3]). Myositis-associated autoantibodies (n) include anti-Ro (10), anti-Ro52 (15), anti-NT5C1A (21), anti-U1RNP (3) and anti-polymyositis-scleroderma antibodies (1). Myositis-specific autoantibodies (n) include anti-p155/140 (23), anti-NXP-2 (17), anti-MDA5 (9), anti-Mi-2 (2), anti-KS (1), anti-HMGCR (1) and anti-Jo1 (1) autoantibodies. HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase; IQR: interquartile range; Jo1: anti-histidyl tRNA synthetase; MDA5: melanoma differentiation associated protein-5; NT5C1A: cytosolic 5'-nucleotidase 1A; NXP-2: nuclear matrix protein-2; PM-SCL: polymyositis-scleroderma; TIF-1: transcription intermediary factor 1.
Clinical features of JDM patients with and without anti-HSC70 autoantibodies
The presence of anti-HSC70 autoantibodies correlated with multiple clinical features of children with JDM. For example, cutaneous ulcers and malar erythema were associated with the presence of autoantibodies against HSC70 (59% vs 17%, P < 0.002 and 82% vs 54%, P < 0.04, respectively) (Table 2).
Table 2.
Clinical features of JDM patients with and without anti-HSC70 autoantibodies
| Clinical features, ever present | Anti-HSC70 positive (N = 22) | Anti-HSC70 negative (N = 41) | P-value |
|---|---|---|---|
| Skin involvement, n/N (%) | |||
| Cutaneous ulcer | 13/22 (59) | 7/41 (17) | <0.002 |
| Periungual capillary dilatation | 22/22 (100) | 38/41 (93) | 0.5 |
| Malar erythemaa | 18/22 (82) | 22/41 (54) | <0.04 |
| Oedema | 9/22 (41) | 7/41 (17) | 0.07 |
| Calcinosis | 12/22 (55) | 16/41 (39) | 0.3 |
| Systemic involvement, n/N (%) | |||
| Weight loss | 11/22 (50) | 21/41 (51) | 1.0 |
| Fever | 11/22 (50) | 7/41 (17) | <0.009 |
| Muscle involvement, n/N (%) | |||
| Distal weakness | 16/22 (73) | 27/41 (66) | 0.8 |
| Proximal and distal weakness | 16/22 (73) | 27/41 (66) | 0.8 |
| Muscle atrophy | 12/22 (55) | 16/41 (39) | 0.3 |
| Lung involvement, n/N (%) | |||
| Dyspnea on exertion | 13/22 (59) | 15/41 (37) | 0.1 |
| Dyspnea at rest | 1/22 (5) | 9/41 (22) | 0.1 |
| Interstitial lung disease | 4/22 (18) | 2/41 (5) | 0.2 |
| Joint involvement, n/N (%) | |||
| Arthralgia | 16/22 (73) | 33/41 (80) | 0.5 |
| Arthritis | 14/22 (64) | 24/41 (59) | 0.8 |
| Gastrointestinal involvement, n/N (%) | |||
| Regurgitation | 8/22 (36) | 11/41 (27) | 0.6 |
| Abdominal paina | 13/22 (59) | 13/39 (33) | 0.06 |
| Early total symptom score, median (IQR) | 0.33 (0.23–0.38) | 0.28 (0.20–0.35) | 0.2 |
| Early muscle score | 0.43 (0.29–0.57) | 0.43 (0.29–0.57) | 0.5 |
| Early joint score | 0.50 (0.50–1.0) | 0.50 (0.50–1.0) | 0.9 |
| Early cutaneous score | 0.38 (0.33–0.44) | 0.38 (0.31–0.44) | 0.9 |
| Early gastrointestinal score | 0.13 (0–0.25) | 0 (0–0.13) | 0.09 |
| Early pulmonary score | 0.20 (0–0.20) | 0 (0–0.20) | <0.04 |
| Early constitutional score | 0.38 (0.25–0.69) | 0.25 (0.25–0.5) | 0.6 |
| Muscle enzymes (highest value), median (IQR), IU/l | |||
| AST | 122 (48–202) | 85 (46–127) | 0.3 |
| ALT | 58 (38–107) | 54 (30–127) | 0.7 |
| LDHb | 488 (346–808) | 266 (207–377) | <0.002 |
| CK | 1154 (286–3537) | 800 (278–3082) | 0.7 |
| Severity at onset, median (IQR) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 1.0 |
Categorical variables were analysed by Fisher's exact tests and are presented as count/total (percentage). Continuous variables were analysed by the Mann–Whitney U-test and are expressed as median (IQR [Q1–Q3]).
In those with the history during the follow-up period after onset of first symptom.
All values of LDH were adjusted for the NIH upper limit of normal for LDH in adults, which is 226 U/l. ALT: alanine aminotransferase; AST: aspartate aminotransferase; CK: creatine kinase; IQR: interquartile range; LDH: lactate dehydrogenase.
Furthermore, a history of fever was also associated with the presence autoantibodies against HSC70 (50% vs 17%, P < 0.009). Higher median serum levels of lactate dehydrogenase, which is reported to be released from endothelial cells in response to endothelial cytotoxicity [13], were found in JDM children with anti-HSC70 autoantibodies (median 488 in those with anti-HSC70 vs 266 in those without, P < 0.002). JDM patients with anti-HSC70 autoantibodies also had higher early pulmonary scores (median 0.20 vs 0, P < 0.04); however, they did not have an increased frequency of interstitial lung disease or dyspnea.
Clinical course and medications used in JDM patients with and without anti-HSC70 autoantibodies
The median number of hospitalizations was higher in JDM patients with anti-HSC70 autoantibodies than in those without (median 2.0 vs 1.0, P < 0.04) (Table 3). Use of wheelchairs and/or assistive devices was associated with the presence of anti-HSC70 autoantibodies (64% vs 27%, P < 0.007), as was the use of wheelchairs in the observation period and at last follow-up (55% vs 22%, P < 0.02 and 18% vs 2%, P < 0.05, respectively). Lastly, JDM patients with anti-HSC70 autoantibodies had higher Myositis Damage Index severity of damage scores than those without at the time of measurement of the autoantibodies (median 8.0 vs 5.0, P = 0.01).
Table 3.
Disease outcomes and medications used in JDM patients with and without anti-HSC70 autoantibodies
| Disease outcomes and medications, ever present | Anti-HSC70 positive (N = 22) | Anti-HSC70 negative (N = 41) | P-value |
|---|---|---|---|
| Ever hospitalized, n/N (%) | 17/22 (77) | 24/41 (59) | 0.2 |
| Median number of hospitalizations, median (IQR) | 2.0 (0.5–4.5) (N = 19) | 1.0 (0–1.5) (N = 39) | <0.04 |
| Wheelchair/devicea use, n/N (%) | 14/22 (64) | 11/41 (27) | <0.007 |
| Wheelchair use, n/N (%) | 12/22 (55)c | 9/41 (22)c | <0.02 |
| 4/22 (18)d | 1/41 (2)d | <0.05 | |
| Disease activity at final assessment, median (IQR), median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 0.1 |
| Physician global activity scoreb, median (IQR) | 2.3 (1.5–3.9) | 2.5 (1.7–3.5) | 0.7 |
| Physician global damage assessmentb, median (IQR) | 2.2 (1.0–3.8) (N = 21) | 1.4 (0.7–2.7) (N = 30) | 0.07 |
| Myositis Damage Index severity of damage scoreb, median (IQR) | 8.0 (5.0–14.0) (N = 21) | 5.0 (2.0–7.0) (N = 29) | 0.01 |
| CHAQ scoreb, median (IQR) | 2.1 (1.0–4.7) | 2.6 (1.1–4.7) | 0.6 |
| MMT8 score (left)b, median (IQR) | 74.0 (66.0–80.0) (N = 21) | 72.5 (66.5–77.3) (N = 32) | 0.6 |
| MMT8 score (right)b, median (IQR) | 74.0 (66.0–80.0) (N = 21) | 75.0 (66.5–78.0) (N = 32) | 0.9 |
| CMAS scoreb, median (IQR) | 42.5 (34.8–48.3) (N = 20) | 44.0 (38.3–49.0) (N = 30) | 0.4 |
| Disease course, n/N (%) | |||
| Monocyclic course | 0/16 (0) | 2/27 (7) | 0.5 |
| Polycyclic course | 2/16 (13) | 3/27 (11) | 1.0 |
| Chronic continuous course | 14/16 (88) | 22/27 (81) | 0.7 |
| Response to treatment | |||
| Complete clinical response, n/N (%) | 1/22 (5) | 7/40 (18) | 0.2 |
| Remission, n/N (%) | 0/22 (0) | 1/40 (3) | 1.0 |
| Prednisone discontinuatione, n/N (%) | 0/22 (0) | 3/40 (8) | 0.5 |
| Total number of medications used, median (IQR) | 7.0 (5.3–8.0) (N = 22) | 6.0 (4.0–7.3) (N = 40) | 0.2 |
| Medications received, n/N (%) | |||
| Oral steroids | 22/22 (100) | 40/40 (100) | 1.0 |
| Intravenous pulsed steroids | 20/22 (91) | 30/40 (75) | 0.2 |
| Methotrexate | 21/22 (95) | 39/40 (98) | 1.0 |
| Intravenous immunoglobulin | 21/22 (95) | 29/40 (73) | <0.05 |
| Other DMARDs | 22/22 (100) | 39/40 (98) | 1.0 |
Categorical variables are analysed by Fisher's exact tests and are presented as count/total (percentage). Continuous variables are analysed by the Mann–Whitney U test and are expressed as median (IQR [Q1–Q3]).
Wheelchair, bed bound and other assistive devices are included.
Evaluation at the time of measurement of anti-HSC70 autoantibodies.
In the observation period.
At last follow-up.
Discontinuation of prednisone in first 12 months of treatment.
CHAQ: childhood HAQ; IQR: interquartile range; CMAS: Childhood Myositis Assessment Scale.
JDM patients with anti-HSC70 autoantibodies more often received IVIG compared with those without (95% vs 73%, P < 0.05). There was no association of the presence of anti-HSC70 autoantibodies with disease course, other medications or response to treatment (Table 3). It is notable that few patients with JDM achieved complete clinical response or remission in this study.
Differences in human leucocyte antigen allele associations in JDM patients with and without anti-HSC70 autoantibodies
A weak association was observed between HLA class I or II alleles and the presence of anti-HSC70 autoantibodies. The presence of autoantibodies against HSC70 was associated with the presence of HLA-C*04 [16.7% vs 0%, odds ratio (OR) not calculable, P = 0.04] and a decrease in the frequency of HLA-DPB1*0401 [17.7% vs 47.1%, OR 0.24 (95% CI: 0.07, 0.91), P = 0.04].
Discussion
The presence of prominent vascular and perivascular inflammation is a notable feature of JDM and contributes directly to the clinical manifestations of the disease [2, 4]. We have recently reported that, similar to other vasculitides, patients with JDM have autoantibodies directed against a broad range of antigens in and/or on endothelial cells [6]. We also found that autoantibodies to an endothelial cell antigen, heat shock protein cognate 70 (HSC70), were more common and detected in higher titres in children with newly diagnosed, untreated disease than in children who had achieved remission. Here we sought to explore clinical associations with anti-HSC70 autoantibodies and found that anti-HSC70 autoantibodies occur frequently in JDM and are associated with multiple features of disease severity.
We found that anti-HSC70 autoantibodies are seen in 35% of children with JDM, which is higher than what we previously observed [6] and may reflect a larger number of patients with severe disease in the current cohort or a difference in the ethnogeographic distribution of these autoantibodies in JDM patients. Importantly, the presence of anti-HSC70 autoantibodies was also associated with cutaneous ulceration, a manifestation of vasculopathy in JDM [5], raising the question of whether these autoantibodies may be associated with other forms of vasculopathy and/or vasculitis. However, there were no significant associations between anti-HSC70 autoantibodies and calcinosis, which may be driven by smouldering vascular disease in JDM [4, 14], nor was there an association with periungual capillary dilatation, a predominant vasculopathic feature, which was present in almost all of the JDM patients in our cohort. Furthermore, anti-HSC70 autoantibodies have also been observed in chronic inflammatory illnesses without vasculitis, for instance, myasthenia gravis [15].
Anti-HSC70 autoantibodies were also associated with multiple measures of disease severity in JDM, including use of wheelchairs and/or devices, number of hospitalizations and scores on the severity of myositis damage measures, which highlights the potential of anti-HSC70 autoantibodies as a predictor of disease severity and poor prognosis in JDM. Patients with anti-HSC70 autoantibodies were also more likely to have been treated with IVIG than patients who were autoantibody-negative. Although it is reported that cutaneous ulcers occur in patients with JDM whose disease is more resistant to immunosuppressive therapy [16], there was no significant difference between histories of cutaneous ulcers and the use of IVIG regardless of whether JDM patients had autoantibodies against HSC70 (data not shown). This suggests that patients with anti-HSC70 autoantibodies may show poor response to standard first-line measures of glucocorticoids plus MTX because of more tenacious disease independent of cutaneous ulceration. This finding raises the question of whether anti-HSC70 autoantibodies might be used as a biomarker to select patients likely to have a more severe or intractable disease. In addition, the question arises as to whether autoantibodies to HSC70 interfere with MTX therapy as HSC70 binds MTX and is involved in its transport into the cells [17, 18].
We report that anti-HSC70 autoantibodies are a novel MAA that are not associated with any particular MSA but co-occur frequently with the MAA anti-NT5C1A and anti-Ro52. We did not see a significant association with anti-MDA5 autoantibodies, even though anti-MDA5 positive patients frequently have cutaneous ulceration [19]. Anti-NT5C1A autoantibodies occur more often in patients with pulmonary symptoms at diagnosis and have the potential to be a predictor of severe disease [20]. Similarly, the presence of anti-Ro52 autoantibodies has relevance to interstitial lung disease and has the potential to be used as a predictor of disease severity and poorer prognosis [11]. Interestingly, anti-HSC70 autoantibodies are not associated with pulmonary manifestations of disease. Here, questions remain as to the association between anti-HSC70 autoantibodies and anti-Ro52 autoantibodies. Ro52 ELISA assay may be affected by matrix effects (i.e. high levels of IgG, cholesterol and rheumatoid factor) and/or lead to false positives in the presence of other autoantibodies, such as anti-SS-A/Ro, anti-SS-B/La and anti-Jo1 autoantibodies. These effects were not investigated in the prior study of Ro52 in juvenile myositis [11]. Future analyses utilizing direct comparison of these and other autoantibodies are required to determine which are the most reliable and clinically useful in predicting disease severity.
Our study also raises the question of the extent to which anti-HSC70 autoantibodies contribute to the observed pathology in JDM, and specifically to the vasculopathy. Complement and immunoglobin deposits in vessel walls are documented features of JDM [21] and of DM [21, 22], and it is tempting to speculate that autoantibodies directed to endothelial cells could directly contribute to the vascular injury. However, it is just as plausible that AECAs are formed in response to the vascular injury, while still playing a role in the ongoing inflammatory process. We are becoming increasingly aware that cellular and tissue damage by itself can drive autoantibody formation [23]. For example, in a mouse model of experimental myositis, tissue damage was caused by CD8+ T cells and type I interferons, but not by CD4+ T cells, B cells or autoantibodies, and anti-transcriptional intermediary factor 1 (TIF1)γ autoantibody production followed the development of experimental myositis [24]. Interestingly, HSC70 is also known to modulate viral infectivity and its presence correlates with virus-induced host immunoprotective responses [25]. As infectious diseases have also the potential to be a significant risk factor for JDM pathophysiology, further studies are needed to assess the roles of anti-HSC70 autoantibodies in infection events in JDM [26, 27].
It is reported that anti-HSC70 autoantibodies are detected in other autoimmune and inflammatory diseases, including Behçet's disease, systemic sclerosis, Kawasaki disease, inflammatory bowel diseases, myasthenia gravis and immune thrombocytopenic purpura [15, 28–32]. Associations with disease severity in other autoimmune diseases is currently unknown. For instance, in myasthenia gravis, decreased titres of anti-HSC70 autoantibodies have been associated with clinical improvement; however, the presence of anti-HSC70 autoantibodies was not correlated with disease severity [15]. To clarify whether anti-HSC70 autoantibodies are associated with clinical features and treatment response and/or are involved in the pathophysiology of other autoimmune and inflammatory diseases is important.
It is reported that HLA-C polymorphisms have shown independent associations with specific subsets of RA, specifically RA patients with vasculitis [33]. Among RA patients with vasculitis, the frequency of HLA-C*04 was significantly diminished. Furthermore, the incidence of granulomatosis with polyangiitis was significantly associated with HLA-DPB1*0401 allele frequency [34]. Thus, the presence of anti-HSC70 autoantibodies and their association with HLA alleles in other forms of vasculopathy may link these observations. These associations deserve further investigation. Since high throughput next generation genotyping has been implemented, HLA data at high resolution will be increasingly available and allow a more detailed analysis of potential associations with implicated HLA antigens in larger patient cohorts.
A limitation of the current study is our small patient sample size, few patients with inactive disease or remission, few patients with low disease severity and the small number of patients with specific co-morbidities such as retinitis or gastrointestinal ulcers. Thus, it was impossible to determine whether there was a true association between anti-HSC70 autoantibodies and these rare clinical complications. Furthermore, this is a retrospective study, which may be insufficient for biomarker studies. In addition, no correction for multiple testing was performed. Further studies using larger numbers of patients and understanding of JDM-related disease phenotypes, including other vasculitic symptoms in particular, and comparative studies between races could more clearly determine the clinical significance of anti-HSC70 autoantibodies in patients with JDM.
In conclusion, we have demonstrated that anti-HCS70 autoantibodies are detected in more than a third of children with JDM. These autoantibodies correlate with multiple measures of disease severity, suggesting that they may serve as useful clinical biomarkers to detect children who will require therapies to augment or complement standard first-line treatment with glucocorticoids and methotrexate.
Acknowledgements
The authors thank Peter D. Burbelo and Sarthak Gupta for their critical reading and suggestions on the manuscript and Sharon D. Adams for performing HLA allele typing.
Collaborators: Members of the Childhood Myositis Heterogeneity Collaborative Study Group who contributed to this project: Bita Arabshahi, Lilliana Barillas-Arias, Mara Becker, April Bingham, Ruy Carrasco, Victoria Cartwright, Rodolfo Curiel, Marietta M. DeGuzman, Barbara Anne Eberhard, Barbara S. Edelheit, Terri Finkel, Stephen W. George, Ellen A. Goldmuntz, William Hannan, Michael Henrickson, Adam M. Huber, Anna Jansen, James N. Jarvis, Lawrence Jung, Ildy M. Katona, Steven J. Klein, W Patrick Knibbe, Bianca A. Lang, Carol B. Lindsley, Gulnara Mamyrova, Linda Myers, Stephen R. Mitchell, Kabita Nanda, Terrance P. O’Hanlon, Murray H. Passo, Maria D. Perez, Donald A. Person, Linda I. Ray, Rafael F. Rivas-Chacon, Tova Ronis, Deborah Rothman, Adam Schiffenbauer, Bracha Shaham, David Sherry, Abigail Smukler, Matthew L. Stoll, Sangeeta H. Sule, Scott A. Vogelgesang, Rita Volochayev, Jennifer C. Wargula, Pamela Weiss.
R.K., L.G.R. and J.N.J. contributed to research design. T.P.O. and J.N.J. prepared blood samples. L.G.R., S.E.S. and P.N.F. collected and interpreted clinical data. R.K., T.S., S.E.S., I.N.T., M.E.T., M.A.T., A.L.M., W.A.F. and L.G.R. analysed data and interpreted data. W.A.F. contributed HLA antigen typing data. R.K., M.H., S.E.S., L.G.R. and J.N.J. wrote the paper. F.W.M., K.Y., L.G.R. and J.N.J. supervised the study. All authors have read and approved of the version of the paper as published.
Funding: This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Number JP16K10045 (R.K.). This work was also supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 (J.N.J.) to the University at Buffalo and the National Institute of Environmental Health Sciences (NIEHS; ZIAES101074 and ZIAES101081) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; ZIA AR041203), National Institutes of Health. This work was also supported by the National Institutes of Health Clinical Center under project ZIC CL002128 (W.A.F.) and the Cure JM Foundation (J.N.J., S.E.S., L.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement: I.T. is a consultant to the Oklahoma Medical Research Foundation Clinical Immunology Laboratory with regard to myositis autoantibody testing.
Contributor Information
Rie Karasawa, Department of Frontier Medicine, St. Marianna University School of Medicine, Kawasaki, Japan.
Kazuo Yudoh, Department of Frontier Medicine, St. Marianna University School of Medicine, Kawasaki, Japan.
Toshiko Sato, Department of Frontier Medicine, St. Marianna University School of Medicine, Kawasaki, Japan.
Megumi Tanaka, Department of Frontier Medicine, St. Marianna University School of Medicine, Kawasaki, Japan.
Mayumi Tamaki, Department of Frontier Medicine, St. Marianna University School of Medicine, Kawasaki, Japan.
Sara E Sabbagh, Muscle Disease Unit, Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD; Division of Rheumatology, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI.
Terrance P O’Hanlon, Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health (NIH), Bethesda, MD.
Payam Noroozi-Farhadi, Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health (NIH), Bethesda, MD.
Ira N Targoff, Oklahoma City VA Health Care System, University of Oklahoma Health Sciences Center, and Oklahoma Medical Research Foundation, Oklahoma City, OK.
Willy A Flegel, Department of Transfusion Medicine, NIH Clinical Center, National Institutes of Health (NIH), Bethesda, MD.
Andrew L Mammen, Muscle Disease Unit, Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD.
Frederick W Miller, Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health (NIH), Bethesda, MD.
Mark D Hicar, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences.
Lisa G Rider, Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health (NIH), Bethesda, MD.
James N Jarvis, Department of Pediatrics, Jacobs School of Medicine and Biomedical Sciences; Genetics, Genomics, & Bioinformatics Program, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, USA.
References
- 1. Shah M, Mamyrova G, Targoff IN. et al. ; Childhood Myositis Heterogeneity Collaborative Study Group. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine 2013;92:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banker BQ, Victor M.. Dermatomyositis (systemic angiopathy) of childhood. Medicine (Baltimore) 1966;45:261–89. [DOI] [PubMed] [Google Scholar]
- 3. Huber AM, Mamyrova G, Lachenbruch PA. et al. ; Childhood Myositis Heterogeneity Collaborative Study Group. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken) 2014;66:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crowe WE, Bove KE, Levinson JE, Hilton PK.. Clinical and pathogenetic implications of histopathology in childhood polydermatomyositis. Arthritis Rheum 1982;25:126–39. [DOI] [PubMed] [Google Scholar]
- 5. Gitiaux C, Antonio MD, Aouizerate J. et al. Vasculopathy-related clinical and pathological features are associated with severe juvenile dermatomyositis. Rheumatology (Oxford) 2016;55:470–9. [DOI] [PubMed] [Google Scholar]
- 6. Karasawa R, Tamaki M, Sato T. et al. Multiple target autoantigens on endothelial cells identified in juvenile dermatomyositis using proteomics. Rheumatology (Oxford) 2018;57:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rider LG, Shah M, Mamyrova G, Huber AM. et al. ; Childhood Myositis Heterogeneity Collaborative Study Group. The myositis autoantibody phenotypes of juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92:223–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Habers GE, Huber AM, Mamyrova G. et al. ; Childhood Myositis Heterogeneity Study Group. Association of myositis autoantibodies, clinical features, and environmental exposures at illness onset with disease course in Juvenile myositis. Arthritis Rheumatol 2016;68:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oddis CV, Rider LG, Reed AM. et al. ; International Myositis Assessment and Clinical Studies Group. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum 2005;52:2607–15. [DOI] [PubMed] [Google Scholar]
- 10. Petty RE, Southwood TR, Manners P. et al. ; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 11. Sabbagh S, Pinal-Fernandez I, Kishi T. et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis 2019;78:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Hanlon TP, Carrick DM, Arnett FC. et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1 and -DQA1 allelic profiles and motifs define clinicopathologic groups in Caucasians. Medicine (Baltimore) 2005;84:338– 49. [DOI] [PubMed] [Google Scholar]
- 13. Yu S, Xia T, Xuehong W, Hui F.. Vascular protection of salicin on IL-1β-induced endothelial inflammatory response and damages in retinal endothelial cells. Artif Cells Nanomed Biotechnol 2019;47:1995–2002. [DOI] [PubMed] [Google Scholar]
- 14. Hoeltzel MF, Oberle EJ, Robinson AB, Agarwal A, Rider LG.. The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Curr Rheumatol Rep 2014;16:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munakata S, Chen M, Aosai F. et al. The clinical significance of anti-heat shock cognate protein 71 antibody in myasthenia gravis. J Clin Neurosci 2008;15:158–65. [DOI] [PubMed] [Google Scholar]
- 16. Gupta A, Pilania RK, Prasad VD, Guleria S.. Extensive skin ulcers in a child with juvenile dermatomyositis. BMJ Case Rep 2018;bcr-2017-222915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu T, Dean A, Ashwini S. et al. Identification and characterization of a 66-68-kDa protein as a methotrexate-binding protein in murine leukemia L1210 cells. Cell Stress Chaperones 2013;18:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu T, Singh R, Rios Z. et al. Tyrosine phosphorylation of HSC70 and its interaction with RFC mediates methotrexate resistance in murine L1210 leukemia cells. Cancer Lett 2015;357:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tansley SL, Betteridge ZE, Gunawardena H. et al. ; UK Juvenile Dermatomyositis Research Group. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 2014;16:R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeker RM, Pinal-Fernandez I, Kishi T. et al. Anti-NT5C1A autoantibodies are associated with more severe disease in patients with juvenile myositis. Ann Rheum Dis 2018;77:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitaker JN, Engel WK.. Vascular deposits of immunoglobulin and complement in idiopathic inflammatory myopathy. N Engl J Med 1972;286:333–8. [DOI] [PubMed] [Google Scholar]
- 22. Lahoria R, Selcen D, Engel AG.. Microvascular alterations and the role of complement in dermatomyositis. Brain 2016;139:1891–903. [DOI] [PubMed] [Google Scholar]
- 23. Mackay IR, Natasha VL, Noel RR.. Cell damage and autoimmunity: a critical appraisal. J Autoimmun 2008;30:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okiyama N, Ichimura Y, Shobo M. et al. Immune response to dermatomyositis-specific autoantigen, transcriptional intermediary factor 1γ can result in experimental myositis. Ann Rheum Dis 2021;80:1201–8. [DOI] [PubMed] [Google Scholar]
- 25. Wang Z, Li Y, Yang X. et al. Mechanism and complex roles of HSC70 in viral infections. Front Microbiol 2020;11:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan L, O'Hanlon TP, Lai Z. et al. Gene expression profiles from disease discordant twins suggest shared antiviral pathways and viral exposures among multiple systemic autoimmune diseases. PLoS One 2015;10:e0142486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manlhiot C, Liang L, Tran D. et al. Assessment of an infectious disease history preceding juvenile dermatomyositis symptom onset. Rheumatology (Oxford) 2008;47:526–9. [DOI] [PubMed] [Google Scholar]
- 28. Zheng Z, Sohn S, Ahn KJ, Bang D, Cho SB.. Serum reactivity against herpes simplex virus type 1 UL48 protein in Behcet's disease patients and a Behcet's disease-like mouse model. Acta Derm Venereol 2015;95:952–8. [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto M, Sato S, Ihn H, Takehara K.. Autoantibodies to the heat-shock protein hsp73 in localized scleroderma. Arch Dermatol Res 1995;287:581–5. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Y, Cui J, Hu H. et al. Identification of a novel anti-heat shock cognate 71 kDa protein antibody in patients with Kawasaki disease. Mol Med Rep 2020;21:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jarjour WN, Jeffries BD, Davis JS IV. et al. Autoantibodies to human stress proteins. A survey of various rheumatic and other inflammatory diseases. Arthritis Rheum 1991;34:1133–8. [DOI] [PubMed] [Google Scholar]
- 32. Xiao C, Chen S, Yuan M. et al. Expression of the 60 kDa and 71 kDa heat shock proteins and presence of antibodies against the 71 kDa heat shock protein in pediatric patients with immune thrombocytopenic purpura. BMC Blood Disord 2004;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. A. Siegel RJ, Bridges SL Jr, Ahmed S.. HLA-C: an accomplice in rheumatic diseases. ACR Open Rheumatol 2019;1:571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richard AW, Alex JM, Sarah LM.. HLA allele variation as a potential explanation for the geographical distribution of granulomatosis with polyangiitis. Rheumatology (Oxford) 2015;54:359–62. [DOI] [PubMed] [Google Scholar]