ABSTRACT
Background
Validation of the EAT–Lancet reference diet (ELR-diet), recently proposed by the EAT–Lancet Commission, within the context of real-life studies is necessary to elucidate its feasibility, nutritional value, sustainability, and health effects.
Objectives
We aimed to develop a dietary index (DI) score to measure adherence to the ELR-diet. We further aimed to study the association between the DI score and 1) nutritional characteristics, 2) indicators of ecological sustainability, and 3) anthropometric markers and biomarkers for cardiometabolic health.
Methods
A DI score was constructed by comparing the categories defined by the ELR-diet with the dietary data of 2–5 sets of 3-d weighed dietary records from DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study participants (n = 298; ≥15 y of age). Prospective associations between the DI score and risk markers (anthropometric and cardiometabolic) in young adulthood (≥18 y old) were investigated using multivariate linear regression.
Results
Adherence to the DI score components was considerable (majority > 50%), but varied within the population (2%–100%). The highest tertile of the DI score was inversely associated with the intake of protein (tertile 3 compared with tertile 1: 13.5 compared with 14.5 energy %), added sugars (10.5 compared with 12.4 energy %), and cholesterol (100 compared with 116 mg/1000 kcal), but positively associated with fiber intake (10.0 compared with 8.82 g/1000 kcal) (all P < 0.05). The DI score was inversely associated with greenhouse-gas emissions (tertile 1 compared with tertile 3: 6.48 compared with 5.85 kg of carbon dioxide equivalents/2500 kcal; P < 0.001) and land use (8.24 compared with 7.16 m2 × y/2500 kcal; P < 0.001). Inverse associations between the DI score and anthropometric markers during young adulthood were observed (e.g., BMI: tertile 1 compared with tertile 3: 22.9 compared with 21.9 kg/m2; P = 0.03) (all P < 0.05). No associations between the DI score and cardiometabolic risk markers were found (all P ≥ 0.05).
Conclusions
Adherence to the ELR-diet was associated with favorable nutritional characteristics and reduced environmental impact. Adherence to the DI score in adolescence was also beneficial with respect to anthropometric markers in early adulthood, although not for further cardiometabolic risk markers.
Keywords: sustainability, EAT–Lancet, dietary index, anthropometry, cardiovascular disease risk markers
Introduction
Food systems are one of the most important underlying drivers in the global syndemic of malnutrition and climate change (1). According to the Global Burden of Disease study, the number of deaths attributable to dietary risks increased from 8 to 11 million worldwide between 1990 and 2017, with cardiovascular diseases (CVDs) being the leading causes of diet-related deaths (2). Equally important, the transition toward westernized diets has contributed to environmental degradation (3), with food systems now responsible for ∼11% of global greenhouse-gas emissions (GHGEs). This is predominantly through agricultural activities, which are conducted on 40% of all arable land (4). Hence, shifts toward healthy and sustainable diets are needed.
Existing food-based dietary guidelines (FBDGs) are typically based on scientific studies focused on diet–disease relations. Yet few countries have incorporated aspects of environmental sustainability into the derivation of these guidelines (5). However, although most FBDGs emphasize the consumption of plant-based foods, and aim to establish dietary patterns which align human health goals with environmental sustainability, clear scientific targets must be established (6). Therefore, in 2019 the EAT–Lancet Commission proposed global scientific targets for healthy diets and sustainable food systems. These targets defined a safe operating space for dietary intake while simultaneously considering overall human and planetary health. In the end, a reference diet was proposed, including recommended food intake ranges for 8 food groups. This was the EAT–Lancet reference diet (ELR-diet) (6).
This framework was not designed or intended to provide a plan for translating the proposed global targets to a national or subnational level (6). Therefore, the next milestone required was to investigate how definitions of a set of global targets translated to smaller scales (6). As a result, the evaluation of the ELR-diet in the context of real-life studies has shed light on its feasibility, nutritional value, and effect on human health (7). In this process, important steps are the calculation of the adherence to the ELR-diet across populations, evaluation of its nutritional value, investigation of its associations with specific health outcomes, and identifying indications of its environmental sustainability and impact. Comparisons of the ELR-diet with dietary guidelines from the USA (8), Italy (7), India (9), and Denmark (10) have been reported. Moreover, adherence to the ELR-diet has been investigated in relation to environmental indicators (including GHGEs) in French adults (11). Ultimately, the diet was found to be associated with lower risks of ischemic heart disease and diabetes, yet no clear associations with mortality and stroke were found in the English European Prospective Investigation into Cancer and Nutrition (EPIC)-Oxford cohort (12). In addition, in a Swedish population, the ELR-diet was found to be associated with a lower risk of mortality (including all-cause, cancer, and cardiovascular mortality) (13).
To our knowledge, no study has evaluated adherence to the ELR-diet using individual-level data from Germany, or its associations with specific health outcomes. The German Nutrition Society has reported that the current FBDGs are mostly correspondent with the ELR-diet (14). However, according to the EAT–Lancet Commission, regional and local adaptation must be carefully considered in order to successfully transition to healthier diets and sustainable food systems (6). Therefore, the aim of this study was to develop a dietary index (DI) score to measure the adherence to the ELR-diet, using data from the DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study. In addition, our aim was to analyze the cross-sectional association of this DI score with nutritional characteristics, GHGEs, and land use (LU), and the longitudinal association of the DI score with anthropometric markers and risk biomarkers for cardiometabolic health, specifically including concentrations of plasma lipids and plasma glucose, and blood pressure.
Methods
Study design
The DONALD study is based on an ongoing cohort in Dortmund, Germany. Since 1985, every year 35–40 infants aged 3 mo have been newly recruited to the cohort. The overall aim of the DONALD study is to examine the complex relations between nutritional intake, metabolism, and growth, from infancy into adulthood (15). Annual examinations take place during childhood and adolescence. These include 3-d weighted dietary records (WDRs), anthropometric measurements, lifestyle interviews, and medical examinations. From the age of 18 y onward, participants are in addition invited to provide fasting blood samples for analyses. A detailed description of the DONALD study can be found elsewhere (16). Written informed consent was obtained from all parents and adult participants. This study has been performed in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of the University of Bonn, Germany (ethics applications: 098/06 and 185/20).
Study participants
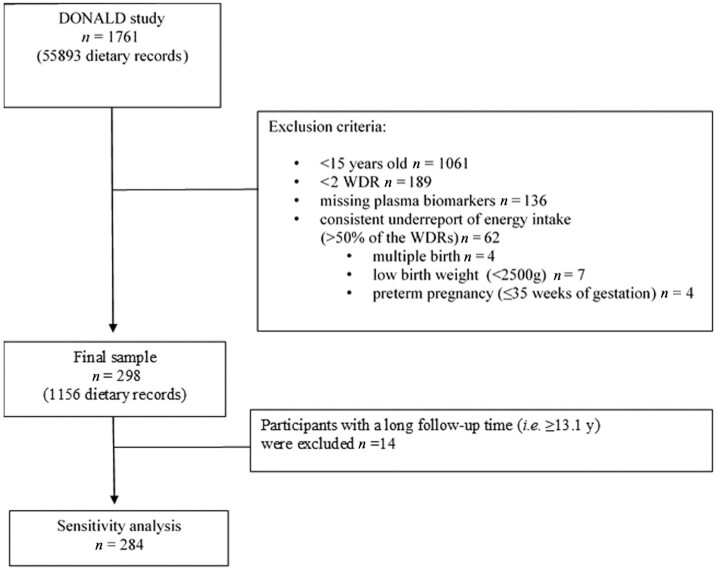
As of January 2021, a total of 1761 participants were enrolled in the ongoing DONALD cohort. For this study, participants with the following characteristics were included: 1) ≥15 y old at dietary intake assessment, 2) ≥2 WDRs available (to take into account habitual intake), 3) 1 adult fasting blood sample available, 4) born full-term (36–42 weeks of gestation), 5) singleton birth, and 6) a birth weight ≥ 2500 g. Participants who potentially underreported their energy intake by providing more than half of the available WDR with a reported energy intake not matching the basal metabolic rate were excluded. The basal metabolic rate was calculated based on Schofield's sex- and age-specific equations (17) and Goldberg's cutoff limits for energy intake (18). A final sample of n = 298 was included in the analysis, with 2–5 WDRs per participant (Figure 1).
FIGURE 1.
Flowchart of the study population. DONALD, Dortmund Nutritional and Anthropometric Longitudinal Designed; WDR, 3-d weighted dietary record.
Dietary assessment and construction of the DI score
In the WDR, detailed information on food and drink intake was recorded. This included recipes and convenience food products. All composite food items were deconstructed to their individual ingredients (16). Foods were grouped according to the 18 food components included in the ELR-diet (Supplemental Table 1). Modifications were made for whole grains, peanuts, and tree nuts; and intake of all grains was considered with a minimum intake of whole grain, in a manner similar to methods used in previous investigations (12). Because the intake of peanuts and all nuts was low in the DONALD study, they were combined into all nuts.
The mean of all WDRs available was used (≥2 WDRs; range: 2–5) for each participant, in order to calculate their individual habitual DI score. In detail, the mean of each component for each individual WDR was calculated and standardized to 2500 kcal (by multiplying the mean of the food component by 2500 kcal and dividing the product by the individual energy intake), with an energy intake of 2500 kcal/d used as the basis for the ELR-diet (6). Next, the mean intake for each food component was determined for each participant by calculating the arithmetic mean from each of their total WDRs. Then, 1 point was assigned when the average food component intake was in line with the proposed recommendations (6) (Supplemental Table 1); otherwise, 0 points were assigned. Finally, the overall score was computed by summing the individual points of each food component for each person. Thus, the DI score ranged from 0 (low adherence) to 18 (high adherence) points.
Indicators of environmental sustainability
All reported food items were linked to the environmentally Sustainable, Healthy, Affordable, Reliable, and Preferred diets (SHARP) indicators database (19). The European SHARP indicators database includes information on GHGEs and LU for food items from life-cycle analyses. The SHARP indicators database was used to assess the environmental impacts of individual-level diets (20), with GHGEs expressed as kg of carbon dioxide equivalents (kgCO2eq) per kg of food item, and LU expressed as m2 × y/kg food item (20). The individual daily GHGEs (kgCO2eq/d) and daily LU [m2 × y/(kg food item × d)] for each participant's habitual diet were calculated as described earlier.
Anthropometric measures and biomarkers for cardiometabolic health
The young adult anthropometric markers used in the study included body weight (BW), BMI (in kg/m2), waist circumference (WC), body fat percentage (%BF), and fat-free mass index (FFMI). Anthropometric measurements were conducted by trained nurses utilizing standard procedures, which have been described elsewhere (16). Cardiometabolic risk markers included plasma concentrations of total cholesterol, LDL and HDL cholesterol, triglycerides (TGs), fasting plasma glucose (FPG), systolic blood pressure, and diastolic blood pressure (DBP). Fasting blood samples were retrieved, centrifuged at 4°C for 15 min, stored at −18°C, and analyzed as previously described (21). For a small number of participants, some anthropometric and cardiometabolic risk markers were unavailable (i.e., FFMI and %BF, n = 17; FPG, n = 2; LDL cholesterol, n = 6; HDL cholesterol, n = 4; TGs, n = 10; and blood pressure, n = 4).
Assessment of covariates
Low birth weight (22), preterm birth (23), and educational attainment (24) have been previously associated with cardiometabolic disorders later in life. Therefore, this study considered early-life factors, including birth weight (g) and pregnancy duration (wk), which were obtained from standardized German pregnancy documents. In addition, educational attainment data related to school and higher education histories of participants and their parents were acquired through questionnaires.
Statistical analysis
A significantly higher DI score was observed among females. Therefore, sex-specific tertiles were used for analyses. Thus, participants within the first tertile (n = 99) had a median score of 9 points (range: 7–9 for males; 6–10 for females). Participants within the second tertile (n = 98) had a median score of 10 points (males) or 11 points (females). Participants classified in the highest tertile (n = 101) had a median score of 11 points (males) or 12 points (females) (range: 11–14 for males; 12–15 for females). Energy intake, dietary fiber, added sugar, micronutrients, plant protein and animal to plant protein (AP) ratio, PUFAs, alcohol, and cardiometabolic risk markers were log-transformed before analysis. Missing data for 5 covariates ( n = 5, 1.68% of all data) were imputed using a multiple imputation process, which resulted in the creation of 10 imputed databases. The duration (i.e., follow-up time) between the assessment of the mean habitual DI score and assessment of the outcome in young adulthood was calculated as the difference in age between the 2 assessments.
Differences across the DI score tertiles were tested using linear regression for continuous variables and the chi-square test for categoric variables. Age-adjusted linear regression was used to investigate the cross-sectional association between the DI score and the nutritional characteristics. The cross-sectional association between the DI score and GHGEs or LU was examined using linear regression. The prospective associations between the DI score and anthropometric and cardiometabolic risk markers in adulthood were investigated using multivariable linear regression models. In addition to a crude model (model 1), adjusted models were constructed to include potential confounders. Model 2 was adjusted for age and sex; whereas, model 3 was adjusted for age, sex, birth weight, gestational age, socioeconomic factors (i.e., participant and parental school and higher education attainment), follow-up time, and total energy intake [i.e., mean of all dietary records (kcal/d)], using the standard multivariate method.
Post hoc sensitivity analyses were conducted to analyze whether a long follow-up time might have had an effect on the results. Therefore, participants with follow-up times between the latest WDR provided and the outcome assessments >90th percentile (≥13.1 y) were excluded. In addition, an alternative version of the DI score was compiled, which was standardized to 2500 kcal/d for males and 2200 kcal/d for females. This accounted for the fact that females generally have lower energy requirements.
All analyses were conducted using SAS software (version 9.4; SAS Institute). A P value ≤ 0.05 was considered statistically significant. In addition, we considered multiple testing by holding the false discovery rate at 5%.
Results
Fifty-two percent of the study population was male, and the averaged median age at the time of assessment of the habitual diet was 16.7 y. Participants were 18.1 y old at outcome assessment and the median follow-up time between the first dietary assessment and the outcome assessment was 3.01 y (range: 1.58–32.2 y). On average, 3 WDRs were used for the calculation of the DI score. Male participants had a slightly lower mean DI score (10.15 points; range: 7–14 points) than young females (10.76 points; range: 6–15 points), but there was no statistically significant difference between the sexes (P = 0.12). Furthermore, baseline characteristics were similar across DI score tertiles (Table 1).
TABLE 1.
General characteri stics of the 298 study participants across tertiles of the DI score1
| Tertiles of the DI score | ||||
|---|---|---|---|---|
| Characteristics | Tertile 1 (n = 99) | Tertile 2 (n = 98) | Tertile 3 (n = 101) | P value2 |
| DI score, male | 9 (7–9) | 10 (10–10) | 11 (11–14) | <0.001 |
| DI score, female | 9 (6–10) | 11 (11–11) | 12 (12–15) | <0.001 |
| Sex, male | 47 (30.3) | 47 (30.3) | 61 (39.4) | 0.116 |
| Age at first dietary assessment, y | 15.1 (15.0–16.9) | 15.1 (15.0–17.1) | 15.1 (15.0–16.3) | 0.383 |
| Mean age at DI assessment, y | 16.7 (15.6–26.1) | 16.8 (15.5–21.8) | 16.7 (16.0–21.4) | 0.819 |
| Age at outcome assessment, y | 18.2 (18.0–47.3) | 18.2 (17.9–36.8) | 18.1 (17.9–35.4) | 0.276 |
| Follow-up time,3 y | 3.01 (1.90–32.2) | 2.99 (1.60–20.7) | 3.01 (1.70–19.9) | 0.247 |
| Education status | ||||
| School education | 94 (33.5) | 92 (32.7) | 95 (33.8) | 0.941 |
| Higher education | 35 (38.5) | 30 (32.9) | 26 (28.6) | 0.337 |
| Parental school education | 55 (30.7) | 58 (32.4) | 66 (36.9) | 0.359 |
| Parental higher education | 33 (28.5) | 39 (33.6) | 44 (37.9) | 0.325 |
| Early-life factors | ||||
| Birth weight, g | 3440 (2570–4660) | 3575 (2680–4670) | 3490 (2550–4600) | 0.361 |
| Pregnancy duration, wk | 40 (37–42) | 40 (36–42) | 40 (37–42) | 0.464 |
Values are median (range) for continuous variables and n (%) for categoric variables, unless otherwise indicated. DI, dietary index.
P values for trend were calculated using a linear model for continuous variables and the chi-square test for categoric variables.
Time between the first dietary assessment and the outcome assessment.
The majority of the population adhered well (>50%) to 11 of the 18 components, with the intakes of fish; dry beans, lentils, and peas; and soy foods being almost fully in line (>98% adherence) with the recommendations proposed by the EAT–Lancet Commission. However, <10% of the population consumed pork (8.72%), added sugar (1.68%), all nuts (1.01%), and butter (0.34%), varying from the recommendations (Table 2). The average total energy intake of the 298 participants was 2261 kcal/d (range: 1365–3872 kcal/d). The intakes of energy, as well as the intakes of carbohydrates, fat, and saturated fats, were similar across the DI score tertiles (all P > 0.05). However, participants within the highest DI score tertile consumed significantly less total protein (13.5 compared with 14.5 EN%; P < 0.001), less animal protein (19.2 compared with 23.0 g/1000 kcal; P < 0.001), and higher amounts of plant protein (14.2 compared with 12.8 g/1000 kcal; P < 0.001), than did participants classified as in the lowest DI score tertile. Likewise, the AP ratio was found to be lower in the third tertile than in the first tertile (tertile 1: 1.75; 95% CI: 1.56, 1.95 compared with tertile 3: 1.25; 95% CI: 1.12, 1.39; P < 0.001). In addition, the DI score was inversely associated with added sugars (10.5 compared with 12.4 EN%; P = 0.005) and cholesterol (100 compared with 116 mg/1000 kcal; P < 0.001), as well as positively associated with total intake of fiber (10.0 compared with 8.82 g/1000 kcal; P < 0.001), fiber from grains (4.55 compared with 3.97 g/1000 kcal; P = 0.004), insoluble- (6.89 compared with 6.08 g/1000 kcal; P < 0.001), and soluble fiber (3.41 compared with 2.97 g/1000 kcal; P < 0.001) (Table 3). Moreover, significant differences were observed across the DI score tertiles in the intake of some fatty acids including linoleic acid (18:2n–6; P = 0.007), arachidonic acid (20:4n–6; P < 0.001), α-linolenic acid (18:3n–3; P = 0.001), and ω-6 to ω-3 fatty acid ratio (P < 0.001), but not all, e.g., EPA (20:5n–3; P = 0.95) and DHA (22:6n–3; P = 0.39). Intakes of micronutrients, including magnesium (P = 0.012), vitamin E (P = 0.001), total folic acid (P = 0.018), and vitamin K (P = 0.018), were significantly higher in the third tertile than in the first tertile (Table 3).
TABLE 2.
Adherence to the individual food components included in the DI score among 298 participants from the DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study1
| Food component | All (n = 298) | Male (n = 155) | Female (n = 143) |
|---|---|---|---|
| Whole grains and all grains2 | 289 (96.9) | 149 (96.1) | 140 (97.9) |
| Tubers or starchy vegetables | 243 (81.5) | 127 (81.9) | 116 (81.1) |
| Vegetables | 81 (27.2) | 29 (18.7) | 52 (36.4) |
| Fruits | 163 (54.7) | 75 (48.4) | 88 (61.5) |
| Dairy foods | 219 (73.5) | 107 (69.0) | 112 (78.3) |
| Beef and lamb | 134 (44.9) | 58 (37.4) | 76 (53.2) |
| Pork | 26 (8.7) | 8 (5.2) | 18 (12.6) |
| Chicken and other poultry | 266 (89.3) | 138 (89.0) | 128 (89.5) |
| Eggs | 195 (65.4) | 105 (67.7) | 90 (62.9) |
| Fish3 | 295 (98.9) | 154 (99.4) | 141 (98.6) |
| Dry beans, lentils, and peas2 | 298 (100.0) | 155 (100.0) | 143 (100.0) |
| Soy foods | 298 (100.0) | 155 (100.0) | 143 (100.0) |
| All nuts4 | 3 (1.01) | 1 (0.7) | 2 (1.4) |
| Palm oil | 265 (88.9) | 135 (87.1) | 130 (90.9) |
| Unsaturated oils | 76 (25.5) | 40 (25.8) | 36 (25.2) |
| Butter | 1 (0.3) | 1 (0.7) | 0 (0.0) |
| Lard or tallow | 256 (85.9) | 133 (85.8) | 123 (86.0) |
| All sweeteners | 5 (1.7) | 4 (2.6) | 1 (0.7) |
Values are n (%).
Whole grain and beans, lentils, and peas as dry, raw weight.
Includes fish and shellfish.
Includes tree nuts and peanuts.
TABLE 3.
Nutritional characteristics across tertiles of the DI score among 298 participants from the DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study1
| Tertiles of the DI score2 | ||||
|---|---|---|---|---|
| Nutritional characteristics | Tertile 1 (n = 99) | Tertile 2 (n = 98) | Tertile 3 (n = 101) | P-trend2,3 |
| Energy, kcal/d | 2165 (2078, 2256) | 2195 (2107, 2288) | 2275 (2184, 2369) | 0.226 |
| Carbohydrate, EN% | 50.3 (49.3, 51.3) | 51.0 (50.0, 52.0) | 51.1 (50.2, 52.1) | 0.435 |
| Protein, EN% | 14.5 (14.1, 14.8) | 13.5 (13.1, 13.8) | 13.5 (13.2, 13.9) | <0.001 |
| Fat, EN% | 33.7 (32.8, 34.6) | 33.8 (32.9, 34.8) | 33.5 (32.6, 34.5) | 0.906 |
| Added sugar, EN% | 12.4 (11.4, 13.4) | 12.4 (11.4, 13.4) | 10.5 (9.80, 11.4) | 0.005 |
| Total fiber,4 g/1000 kcal | 8.82 (8.40, 9.26) | 8.89 (8.47, 9.34) | 10.0 (9.57, 10.5) | <0.001 |
| Fiber from grains,5 g/1000 kcal | 3.97 (3.74, 4.21) | 4.08 (3.85, 4.34) | 4.55 (4.29, 4.82) | 0.004 |
| Animal protein, g/1000 kcal | 23.0 (22.1, 23.9) | 20.3 (19.4, 21.2) | 19.2 (18.3, 20.1) | <0.001 |
| Saturated fats, g/1000 kcal | 16.7 (16.2, 17.2) | 16.7 (16.2, 17.2) | 16.2 (15.6, 16.7) | 0.268 |
| Cholesterol, mg/1000 kcal | 116 (111, 122) | 108 (102, 113) | 100 (95, 106) | <0.001 |
| Plant protein, g/1000 kcal | 12.8 (12.4, 13.2) | 13.0 (12.6, 13.5) | 14.2 (13.7, 14.6) | <0.001 |
| AP ratio | 1.75 (1.56, 1.95) | 1.53 (1.37, 1.71) | 1.25 (1.12, 1.39) | <0.001 |
| Polyunsaturated fats, g/1000 kcal | 5.11 (4.89, 5.33) | 5.18 (4.96, 5.40) | 5.58 (5.36, 5.80) | 0.007 |
| Linoleic acid (18:2n–6), g/d | 9.06 (8.57, 9.58) | 9.35 (8.84, 9.89) | 10.3 (9.70, 10.8) | 0.007 |
| Arachidonic acid (20:4n–6), mg/d | 119 (107, 132) | 101 (90.8, 112) | 86.6 (78.1, 96.1) | <0.001 |
| α-Linolenic acid (18:3n–3), g/d | 1.37 (1.29, 1.45) | 1.46 (1.38, 1.55) | 1.60 (1.51, 1.69) | 0.001 |
| EPA (20:5n–3), mg/d | 47.9 (38.4, 59.9) | 47.1 (37.7, 58.8) | 49.6 (39.8, 61.7) | 0.946 |
| DHA (22:6n–3), mg/d | 83.6 (70.4, 99.4) | 79.8 (67.1, 94.9) | 94.2 (79.4, 112) | 0.386 |
| n–6:n–3 PUFA ratio | 0.08 (0.08, 0.09) | 0.07 (0.06, 0.07) | 0.05 (0.05, 0.06) | <0.001 |
| Sodium, g/d | 2.78 (2.63, 2.94) | 2.76 (2.62, 2.91) | 2.76 (2.62, 2.91) | 0.978 |
| Potassium, g/d | 2.89 (2.77, 3.03) | 2.86 (2.73, 2.99) | 3.04 (2.91, 3.18) | 0.138 |
| Calcium, g/d | 0.96 (0.90, 1.03) | 0.96 (0.90, 1.02) | 1.03 (0.97, 1.10) | 0.179 |
| Magnesium, mg/d | 316 (300, 332) | 320 (304, 337) | 350 (332, 368) | 0.012 |
| Phosphorus, g/d | 1.30 (1.24, 1.37) | 1.28 (1.22, 1.34) | 1.36 (1.29, 1.43) | 0.183 |
| Iron, mg/d | 12.2 (11.6, 12.8) | 11.6 (11.0, 12.2) | 12.7 (12.1, 13.4) | 0.058 |
| Zinc, mg/d | 10.8 (10.2, 11.4) | 10.6 (9.98, 11.2) | 11.3 (10.7, 11.9) | 0.256 |
| Iodine, μg/d | 97.0 (90.5, 104) | 88.7 (82.8, 95.1) | 95.6 (89.3, 102) | 0.159 |
| Vitamin A,6 mg/d | 1.23 (1.13, 1.33) | 1.22 (1.13, 1.32) | 1.28 (1.19, 1.39) | 0.639 |
| Vitamin E,7 mg/d | 12.2 (11.5, 12.9) | 12.9 (12.2, 13.8) | 14.4 (13.6, 15.3) | 0.001 |
| Thiamin, mg/d | 1.15 (1.07, 1.23) | 1.14 (1.07, 1.23) | 1.19 (1.11, 1.28) | 0.647 |
| Riboflavin, mg/d | 1.52 (1.42, 1.63) | 1.48 (1.38, 1.59) | 1.54 (1.43, 1.64) | 0.742 |
| Vitamin B-6, mg/d | 1.74 (1.62, 1.87) | 1.77 (1.65, 1.89) | 1.84 (1.71, 1.97) | 0.549 |
| Total folic acid, μg/d | 371 (349, 395) | 375 (352, 398) | 423 (398, 450) | 0.004 |
| Vitamin D, μg/d | 1.54 (1.39, 1.70) | 1.70 (1.53, 1.88) | 1.82 (1.64, 2.01) | 0.071 |
| Vitamin K, μg/d | 82.2 (72.8, 92.7) | 88.6 (78.5, 99.9) | 105 (92.9, 118) | 0.018 |
| Vitamin B-12, μg/d | 4.64 (4.22, 5.10) | 4.40 (4.00, 4.83) | 4.44 (4.04, 4.87) | 0.705 |
| Alcohol, g/d | 0.45 (0.27, 0.72) | 0.69 (0.42, 1.12) | 0.93 (0.58, 1.50) | 0.105 |
Values are adjusted mean estimates (95% CIs) obtained from linear regression. AP ratio, animal to plant protein ratio; DI, dietary index; EN%, percentage of total energy intake.
Adjusted for age.
P value for trend across tertiles of the dietary index.
Total fiber intake.
Intake of fiber attributed to intake of grains.
Vitamin A equivalents.
Vitamin E activity including tocotrienols.
In this population, the mean ± SD GHGEs associated with dietary intake were 6.14 ± 1.03 kg CO2eq/2500 kcal and the mean ± SD LU was 7.66 ± 1.53 m2 × y/2500 kcal. An inverse association between the DI score and GHGEs and LU (both P < 0.0001) was observed (Table 4).
TABLE 4.
Associations between indicators of environmental sustainability and the DI score measuring the adherence to the EAT–Lancet reference diet in 298 participants from the DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study1
| Tertiles of the DI score | ||||||
|---|---|---|---|---|---|---|
| Indicators of environmental sustainability | β (95% CI)2 | P value2 | Tertile 1 (n = 99) | Tertile 2 (n = 98) | Tertile 3 (n = 101) | P-trend3 |
| Greenhouse gas emissions (kgCO2eq/2500 kcal) | −0.22 (−0.30, −0.14) | <0.001 | 6.48 (6.28, 6.67) | 6.08 (5.88, 6.28) | 5.85 (5.66, 6.05) | <0.001 |
| Land use (m2 × y/2500 kcal) | −0.40 (−0.52, −0.29) | <0.001 | 8.24 (7.95, 8.52) | 7.61 (7.32, 7.90) | 7.16 (6.87, 7.44) | <0.001 |
Values are crude mean estimates (95% CIs) obtained from linear regression. DI, dietary index; kgCO2eq, kilogram of carbon dioxide equivalents.
Linear model including the DI score as a continuous variable.
P value for trend across tertiles of the DI score.
The average BMI of the participants was 22.6 kg/m2. Inverse associations between the DI score during adolescence and BW (P = 0.002), BMI (P = 0.004), FFMI (P = 0.034), WC (P = 0.022), and BF% (P = 0.049) during young adulthood were observed. Participants within the lowest DI score tertile had a statistically significantly higher BMI (22.9; 95% CI: 22.0, 23.9) than participants classified within the highest DI score tertile (21.9; 95% CI: 20.9, 22.8; P-trend = 0.030) (Table 5). After correction for multiple testing, the inverse associations for BW (P = 0.009) and BMI (P = 0.015) remained statistically significant (data not shown). No additional cardiometabolic risk markers were associated with the DI score. Sex-stratified analyses revealed that inverse associations of the DI score with continuous BW (P = 0.036) and continuous BMI (P = 0.020) were found for males. Differences in DBP across DI score tertiles were observed only in female participants (P = 0.039) (Supplemental Figures 1 and 2). Similar results were observed in sensitivity analyses which excluded participants with longer follow-up times (Supplemental Table 2). When an alternate DI score was used which had been standardized to 2500 kcal/d for males and 2200 kcal/d for females, the findings remained similar and led to the same conclusions (data not shown).
TABLE 5.
Prospective associations between adherence to the EAT–Lancet reference diet during adolescence measured by a DI score and anthropometric and cardiometabolic risk markers during young adulthood in 298 participants from the DONALD (Dortmund Nutritional and Anthropometric Longitudinal Designed) study1
| Tertiles of the DI score2 | |||||||
|---|---|---|---|---|---|---|---|
| Risk markers3 | n 4 | β (95% CI)5 | P value3,5 | Tertile 1 | Tertile 2 | Tertile 3 | P-trend3 |
| BW, kg | 298 | ||||||
| Model 1 | 0.97 (0.95, 0.98) | <0.001 | 70.9 (68.3, 73.6) | 70.4 (67.8, 73.1) | 68.7 (66.2, 71.3) | 0.480 | |
| Model 2 | 0.98 (0.97, 0.99) | 0.004 | 70.5 (67.9, 73.1) | 70.5 (68.0, 73.1) | 69.1 (66.6, 71.6) | 0.659 | |
| Model 3 | 0.98 (0.97, 0.99) | 0.002 | 70.5 (67.3, 73.8) | 69.4 (66.4, 72.7) | 67.6 (64.6, 70.7) | 0.117 | |
| BMI, kg/m2 | 298 | ||||||
| Model 1 | 0.98 (0.97, 0.99) | <0.001 | 22.8 (22.2, 23.5) | 22.4 (21.8, 23.0) | 21.7 (21.1, 22.3) | 0.045 | |
| Model 2 | 0.98 (0.97, 0.99) | 0.004 | 22.7 (22.1, 23.3) | 22.4 (21.9, 23.0) | 21.8 (21.3, 22.4) | 0.104 | |
| Model 3 | 0.99 (0.97, 0.99) | 0.004 | 22.9 (22.0, 23.9) | 22.5 (21.6, 23.5) | 21.9 (20.9, 22.8) | 0.030 | |
| FFMI, kg/m2 | 281 | ||||||
| Model 1 | 0.98 (0.97, 0.99) | <0.001 | 17.1 (16.7, 17.6) | 17.0 (16.5, 17.5) | 17.0 (16.6, 17.5) | 0.925 | |
| Model 2 | 0.99 (0.99, 0.99) | 0.039 | 17.1 (16.6, 17.6) | 17.0 (16.6, 17.5) | 17.1 (16.6, 17.5) | 0.980 | |
| Model 3 | 0.99 (0.99, 0.99) | 0.034 | 16.9 (16.3, 17.5) | 16.8 (16.2, 17.4) | 16.6 (16.1, 17.2) | 0.514 | |
| WC, cm | 298 | ||||||
| Model 1 | 0.98 (0.97, 0.99) | <0.001 | 77.1 (75.4, 78.8) | 76.5 (74.8, 78.2) | 75.1 (73.5, 76.8) | 0.265 | |
| Model 2 | 0.99 (0.98, 0.99) | 0.025 | 76.7 (75.1, 78.4) | 76.6 (74.9, 78.2) | 75.4 (73.8, 76.9) | 0.451 | |
| Model 3 | 0.99 (0.98, 0.99) | 0.022 | 77.1 (74.8, 79.4) | 76.3 (74.1, 78.6) | 74.9 (72.7, 77.1) | 0.089 | |
| Body fat, % | 281 | ||||||
| Model 1 | 1.01 (0.98, 1.04) | 0.423 | 23.2 (21.5, 24.9) | 22.1 (20.4, 23.9) | 20.1 (18.6, 21.8) | 0.040 | |
| Model 2 | 0.98 (0.96, 0.99) | 0.038 | 22.9 (21.3, 24.7) | 22.2 (20.5, 23.9) | 20.3 (18.8, 21.9) | 0.071 | |
| Model 3 | 0.98 (0.96, 0.99) | 0.049 | 25.1 (22.6, 27.9) | 24.2 (21.8, 26.8) | 22.9 (20.6, 25.5) | 0.149 | |
| FPG, mg/dL | 296 | ||||||
| Model 1 | 0.99 (0.98, 1.00) | 0.158 | 90.3 (88.3, 92.4) | 92.9 (90.8, 95.0) | 90.7 (88.7, 92.8) | 0.176 | |
| Model 2 | 0.99 (0.99, 1.00) | 0.667 | 90.3 (88.3, 92.3) | 92.9 (90.9, 95.1) | 90.8 (88.8, 92.8) | 0.166 | |
| Model 3 | 0.99 (0.99, 1.01) | 0.647 | 92.1 (88.9, 95.4) | 94.7 (91.5, 98.1) | 92.2 (89.0, 95.5) | 0.138 | |
| Plasma total cholesterol, mg/dL | 298 | ||||||
| Model 1 | 1.01 (0.99, 1.03) | 0.197 | 162 (156, 169) | 164 (158, 171) | 158 (152, 164) | 0.379 | |
| Model 2 | 1.00 (0.99, 1.02) | 0.70 | 161 (155, 167) | 165 (158, 171) | 159 (153, 165) | 0.428 | |
| Model 3 | 1.00 (0.99, 1.02) | 0.747 | 161 (151, 171) | 165 (155, 175) | 160 (150, 170) | 0.467 | |
| Plasma LDL cholesterol, mg/dL | 292 | ||||||
| Model 1 | 1.01 (0.99, 1.04) | 0.365 | 87.8 (82.6, 93.5) | 88.1 (82.7, 93.8) | 87.3 (82.1, 92.8) | 0.979 | |
| Model 2 | 1.01 (0.98, 1.03) | 0.565 | 87.2 (82.0, 92.7) | 88.2 (82.9, 93.9) | 87.8 (82.7, 93.3) | 0.967 | |
| Model 3 | 1.01 (0.98, 1.03) | 0.616 | 86.6 (78.6, 95.4) | 87.6 (79.6, 96.5) | 87.4 (79.4, 96.2) | 0.981 | |
| Plasma HDL cholesterol, mg/dL | 294 | ||||||
| Model 1 | 1.02 (1.00, 1.04) | 0.029 | 55.6 (52.9, 58.4) | 57.3 (54.6, 60.3) | 54.5 (51.9, 57.2) | 0.357 | |
| Model 2 | 1.01 (0.99, 1.02) | 0.561 | 55.5 (52.8, 58.2) | 57.4 (54.6, 60.3) | 54.6 (52.1, 57.4) | 0.371 | |
| Model 3 | 1.00 (0.99, 1.02) | 0.676 | 56.4 (52.5, 60.6) | 58.7 (54.7, 63.0) | 56.5 (52.7, 60.7) | 0.394 | |
| Plasma TGs, mg/dL | 288 | ||||||
| Model 1 | 1.00 (0.97, 1.03) | 0.899 | 82.6 (75.2, 90.8) | 79.6 (72.5, 87.4) | 80.4 (73.4, 88.1) | 0.848 | |
| Model 2 | 0.99 (0.96, 1.04) | 0.954 | 82.4 (75.0, 90.6) | 79.7 (72.5, 87.5) | 80.6 (73.5, 88.3) | 0.876 | |
| Model 3 | 1.00 (0.96, 1.04) | 0.999 | 76.5 (65.8, 89.0) | 74.4 (64.2, 86.3) | 74.9 (64.5, 86.8) | 0.911 | |
| SBP, mm Hg | 294 | ||||||
| Model 1 | 0.99 (0.99, 1.00) | 0.204 | 114 (112, 116) | 114 (112, 116) | 115 (113, 117) | 0.573 | |
| Model 2 | 1.00 (0.99, 1.01) | 0.877 | 114 (112, 116) | 114 (112, 116) | 115 (113, 117) | 0.547 | |
| Model 3 | 1.00 (0.99, 1.01) | 0.925 | 114 (111, 117) | 114 (111, 117) | 115 (112, 118) | 0.739 | |
| DBP, mm Hg | 294 | ||||||
| Model 1 | 0.99 (0.99, 1.01) | 0.467 | 73.1 (71.3, 74.9) | 71.9 (70.2, 73.7) | 73.8 (72.0, 75.6) | 0.336 | |
| Model 2 | 1.00 (0.99, 1.00) | 0.962 | 72.9 (71.2, 74.7) | 71.9 (70.2, 73.7) | 73.9 (72.2, 75.7) | 0.292 | |
| Model 3 | 0.99 (0.98, 1.01) | 0.873 | 73.2 (70.4, 75.9) | 71.9 (69.2, 74.7) | 73.7 (70.9, 76.6) | 0.330 | |
Values are adjusted mean estimates (95% CIs) obtained from linear regression unless otherwise indicated. BW, body weight; DBP, diastolic blood pressure; DI, dietary index; FFMI, fat-free mass index; FPG, fasting plasma glucose; SBP, systolic blood pressure; TG, triglyceride; WC, waist circumference.
Numbers of participants within each tertile of the DI score with available respective anthropometric and cardiometabolic risk markers are as follows: BW, BMI, WC, and plasma total cholesterol: n = 99, 98, and 101; FFMI and body fat: n = 96, 92, and 93; FPG: n = 98, 97, and 101; plasma LDL cholesterol: n = 98, 94, and 100; plasma HDL cholesterol: n = 99, 95, and 100; plasma TGs: n = 94, 95, and 99; SBP and DBP: n = 98, 96, and 100, for tertiles 1, 2, and 3, respectively.
Model 1 = crude; model 2 = adjusted for age and sex (continuous DI score only); model 3 = adjusted for age, sex (continuous DI score only), total energy intake, follow-up time, birth weight, pregnancy duration, parental school and higher education, and participant school and higher education.
Total number of participants with available anthropometric and cardiometabolic risk markers.
β estimates (95% CIs) from a linear model including the DI score as a continuous variable.
Discussion
The participants of this study included adolescents and young adults, and it was observed in the prospective analysis that a higher DI score was inversely associated with anthropometric markers (BW, BMI, FFMI, WC, and %BF). Moreover, the observed associations with a more favorable dietary intake and lower environmental impact confirm the validity of the ELR-diet concept.
To our knowledge, few studies have investigated the ELR-diet with respect to nutritional or health associations (7, 9–12). Knuppel et al. (12) and Stubbendorff et al. (13) evaluated the associations with major health outcomes and mortality, respectively, in middle-aged populations, and other studies have evaluated the ELR-diet in relation to existing FBDGs in Italy (7), the USA (8), and Denmark (10). Thus, this is not only the first study to have investigated the ELR-diet in a German cohort including adolescents (median age: 16.7 y), but also the first to have investigated the DI score and its associations with nutritional characteristics, indicators of environmental sustainability, and risk markers for cardiometabolic health.
These results regarding the nutritional characteristics of the DONALD study are in agreement with previous findings. The observed associations of a higher DI score with lower added sugar and higher fiber intakes are consistent with key dietary priorities for cardiometabolic health (25). However, in this study, a higher DI score was not found to be associated with energy intake, or with fat and carbohydrate intake. This likely reflected the relatively low adherence by study cohort members to certain individual components, in particular intake of vegetables, pork, butter, and unsaturated oils. Regardless, a higher DI score was found to be associated with lower intake of total and animal proteins, which may be the result of the relatively high adherence (≥44.9%) with respect to sources of protein from meat (e.g., chicken and other poultry, and beef and lamb), and eggs. Interestingly, the amount of animal protein consumed was higher in the first tertile than in the third tertile (23 compared with 19 g/1000 kcal; P < 0.001). Likewise, the AP ratio was found to be higher in the first than in the third tertile (1.75; 95% CI: 1.56, 1.95 compared with 1.25; 95% CI: 1.12, 1.39; P < 0.001), and it has been shown that a better AP ratio may have beneficial effects on cardiometabolic risk factors (26).
Moreover, diets lower in GHGEs have been shown to be higher in plant-based protein-rich foods, and as such might prove beneficial for the environment (27).
In addition, this study's results are similar to those from the NutriNet-Santé study, which showed that adherence to the ELR-diet may lead to reduced negative environmental impacts (11). Moreover, a pooled analysis including 443,991 participants from the EPIC-cohort suggested that cobenefits for human health and the environment could be achieved synergistically by adhering to diets that are based on the ELR-diet (28).
Although no association was observed between the DI score and cardiometabolic risk markers, these results are of primary interest because being overweight and being obese are well-known risk factors for CVD (29). Hence, considering that BMI is an intermediate risk marker, adherence to the DI score may be beneficial for the prevention of CVD and other chronic diseases in later life. Consistent with this study, an inverse association was observed between the “EAT-Lancet score” and BMI in cross-sectional analyses conducted in a subsample of the EPIC-Oxford study (12).
It is possible that the ELR-diet has limitations in its ability to fully capture how diets potentially lower the risk of disease in specific, regional scenarios, such as seen here. One possible explanation for this may be the low observed variance of the DI score (range: 6–15 points). This is supported by previous results from the DONALD study, which indicated that dietary factors were associated with various health outcomes (30, 31) in younger adulthood. One potential pitfall to this explanation may be that the ELR-diet allows 0 consumption of some food components for which meta-analyses have demonstrated positive health effects (i.e., fish, legumes, soy, and nuts) (32). Indeed, Hanley-Cook et al. (33) have already argued that positive scoring of nonconsumption (i.e., 0 g/d) for all nutrient-dense food components, in a dietary score based on the ELR-diet, should be avoided. In the present study, the methodological implication of this was evident for fish; dry beans, lentils, and peas; and soy foods—where these were consumed very rarely, but were scored with >98.9% adherence. This was the result of a considerable number (27.9%, 21.1%, and 40.9%, respectively) of nonconsumers. Local investigations are highly warranted, because this German investigation and others (8, 10) have identified peculiarities specific to the populations under investigation. For instance, excess consumption of animal protein may not be a problem in India (9) as it may be in other regions. Finally, it was noticed that some food components may be unrepresented in the ELR-diet, such as beverages and processed foods. In fact, Sharma et al. (9) have already included an additional component in their diet score to consider the intake of processed foods. Interestingly, some food items such as tea, coffee, chocolate, and alcohol were not considered in the ELR-diet, yet coffee and tea have been found to be associated with lower risk of stroke and dementia (34), and an inverse association between CVD risk and moderate coffee consumption has been reported in a meta-analysis of 36 studies (35). However, it has been shown that ∼20% of daily GHGEs can be attributed to beverages. For adults, coffee, tea, and alcoholic beverages are the major contributors to daily GHGEs (36), and it has been suggested that tea, coffee, and alcohol should be replaced with more sustainable alternatives (37). Therefore, it would be worthwhile to consider these beverages in a reference diet which considers both human and planetary health.
Similar to the limited number of studies (7, 10) which have investigated the ELR-diet, some methodological issues were encountered when developing and applying the DI score in the DONALD study. Two modifications were necessary to construct the DI score within this German cohort. First, whole grain intake is 1 of the 8 food groups included in the ELR-diet (6); whereas, for the DI score, intake of all grains was taken into account. This is because the German FBDG refers to intake of all grains and a recommended fiber intake of 30 g/d, and considers intake from various fiber sources. To emphasize intake of fiber and whole grain, a conditional minimum intake of fiber from both grains and whole grains was applied (Supplemental Table 1), which was similar to a previous approach (12). Second, in contrast to previous studies calculating indexes based on the ELR-diet using an FFQ (12) or a 24-h recall (33), dietary data in the DONALD study were derived by multiple WDRs. This resulted in very detailed dietary data which allowed distinctions to be made between the meat components beef and lamb, and pork; and between 4 subgroups of added fats.
The strength of this study lies in the prospective design with a mean follow-up time of 5.64 y. In addition, the ELR-diet recommendation was adopted precisely in association with a dietary assessment consisting of multiple WDRs and recipe deconstruction, as well as the incorporation of 4 subgroups addressing added fats in the DI score. Furthermore, a variety of data related to socioeconomic factors were prospectively collected as well, and this allowed for adjustments to be made to address potential confounders. Lastly, the DI score was related to indicators of environmental sustainability.
The present study has several limitations which should be noted. First, there is the relatively young age of the population; the majority of study participants might have been too young to present characteristics of CVDs. However, this was the first study examining a younger population. Nonetheless, participants from the DONALD study that were ≥15 y of age were included, because their recommended energy intake is ∼2500 kcal (38), which is the basis for different isocaloric dietary scenarios that were used by the EAT–Lancet Commission (6). In addition, the ELR-diet is focused at generally healthy individuals 2 y of age and older. Second, the sample size was modest (n = 298) in comparison with the EPIC-Oxford study (n = 46,069) (12). Finally, participants in the DONALD study were quite homogeneous in terms of their educational and socioeconomic status (16), which might limit the generalizability. This may have minimized residual confounding, yet it cannot be excluded. Moreover, because of the homogeneous population, it was not possible to fully investigate diversity, yet the population was stratified by sex to gain some indication of that factor. Lastly, GHGEs and LU were investigated, but no additional environmental indicators were examined.
Outlook
The full range of benefits and potential issues faced when adopting global targets and adapting them to a national or subnational level which are specific to a population under investigation may only manifest when diverse populations are examined. Hence, further studies are warranted from various locations, examining different age groups and using various dietary assessment tools. These future studies will likely improve the understanding of the peculiarities of the ELR-diet and its corresponding implications for environment and health.
In conclusion, few studies have evaluated a proposed planetary health diet within the context of real-life studies. This study used data from a German cohort study, then constructed a DI score based on the ELR-diet for sustainable food systems (6), and then found that the adherence to the DI score varied across food components and was associated with lower environmental impacts. Moreover, the observed inverse association with BMI and BW established modifiable intermediate risk markers for CVD and other chronic diseases. This indicated that adherence to the DI score may be beneficial for disease prevention in later life.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—RMV, C-AS, and UN: designed the research; UA and KvdL: conducted the research; RMV, KO, and C-AS: analyzed the data; RMV and C-AS: wrote the paper; UN: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by German Federal Ministry of Education and Research grant 01EA1809A (to UN) and the Ministry of Science and Research of North Rhine Westphalia, Germany.
Author disclosures: The authors report no conflicts of interest.
The funders had no role in the study design, data collection, analysis, interpretation, or writing of the manuscript.
Supplemental Tables 1 and 2 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
RMV and C-AS contributed equally to this work
Abbreviations used: AP, animal to plant protein; BW, body weight; CVD, cardiovascular disease; DBP, diastolic blood pressure; DI, dietary index; DONALD, Dortmund Nutritional and Anthropometric Longitudinal Designed; ELR-diet, EAT–Lancet reference diet; EPIC, European Prospective Investigation into Cancer and Nutrition; FBDG, food-based dietary guideline; FFMI, fat-free mass index; FPG, fasting plasma glucose; GHGE, greenhouse-gas emission; kgCO2eq, kilogram of carbon dioxide equivalents; LU, land use; SHARP, Sustainable, Healthy, Affordable, Reliable, and Preferred diets; TG, triglyceride; WC, waist circumference; WDR, 3-d weighted dietary record; %BF, percentage body fat.
Contributor Information
Rebeca Montejano Vallejo, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Christina-Alexandra Schulz, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Karen van de Locht, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Kolade Oluwagbemigun, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Ute Alexy, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Ute Nöthlings, Nutritional Epidemiology, Department of Nutrition and Food Sciences, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Data Availability
Data described in the article, code book, and analytic code of this study will be made available upon request. DONALD study data are available upon reasonable request for research questions within the scope of the DONALD study and which are consistent with the legal and ethical standard practices of the DONALD study. Requests should be addressed to epi@uni-bonn.de.
References
- 1. Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JRet al. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet. 2019;393(10173):791–846. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark MA, Springmann M, Hill J, Tilman D. Multiple health and environmental impacts of foods. Proc Natl Acad Sci U S A. 2019;116(46):23357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fanzo J, Bellows AL, Spiker ML, Thorne-Lyman AL, Bloem MW. The importance of food systems and the environment for nutrition. Am J Clin Nutr. 2021;113(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bechthold A, Boeing H, Tetens I, Schwingshackl L, Nöthlings U. Perspective: food-based dietary guidelines in Europe—scientific concepts, current status, and perspectives. Adv Nutr. 2018;9(5):544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen Set al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 7. Tucci M, Martini D, Del Bo C, Marino M, Battezzati A, Bertoli Set al. An Italian-Mediterranean dietary pattern developed based on the EAT-Lancet reference diet (EAT-IT): a nutritional evaluation. Foods. 2021;10(3):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackstone NT, Conrad Z. Comparing the recommended eating patterns of the EAT-Lancet Commission and Dietary Guidelines for Americans: implications for sustainable nutrition. Curr Dev Nutr. 2020;4(3):nzaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma M, Kishore A, Roy D, Joshi K. A comparison of the Indian diet with the EAT-Lancet reference diet. BMC Public Health. 2020;20(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lassen AD, Christensen LM, Trolle E. Development of a Danish adapted healthy plant-based diet based on the EAT-Lancet reference diet. Nutrients. 2020;12(3):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kesse-Guyot E, Rebouillat P, Brunin J, Langevin B, Allès B, Touvier Met al. Environmental and nutritional analysis of the EAT-Lancet diet at the individual level: insights from the NutriNet-Santé study. J Cleaner Prod. 2021;296:126555. [Google Scholar]
- 12. Knuppel A, Papier K, Key TJ, Travis RC. EAT-Lancet score and major health outcomes: the EPIC-Oxford study. Lancet. 2019;394(10194):213–14. [DOI] [PubMed] [Google Scholar]
- 13. Stubbendorff A, Sonestedt E, Ramne S, Drake I, Hallström E, Ericson U. Development of an EAT-Lancet index and its relation to mortality in a Swedish population. Am J Clin Nutr. 2022;115(3):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The German Nutrition Society . Vollwertige Ernährung nach den Empfehlungen der DGE ist auch ökologisch nachhaltig. DGE Info. 2019:82–7. [Google Scholar]
- 15. Buyken AE, Alexy U, Kersting M, Remer T. [The DONALD cohort. An updated overview on 25 years of research based on the DOrtmund Nutritional and Anthropometric Longitudinally Designed study]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(6–7):875–84. [DOI] [PubMed] [Google Scholar]
- 16. Kroke A, Manz F, Kersting M, Remer T, Sichert-Hellert W, Alexy Uet al. The DONALD study. History, current status and future perspectives. Eur J Nutr. 2004;43(1):45–54. [DOI] [PubMed] [Google Scholar]
- 17. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 18. Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WAet al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45(12):569–81. [PubMed] [Google Scholar]
- 19. European Food Safety Authority (EFSA) . The food classification and description system FoodEx 2 (revision 2). EFSA Support Publ. 2015;12(5):804E. [Google Scholar]
- 20. Mertens E, Kaptijn G, Kuijsten A, van Zanten H, Geleijnse JM, van ’t Veer P. SHARP-Indicators Database towards a public database for environmental sustainability. Data Brief. 2019;27:104617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oluwagbemigun K, Buyken AE, Alexy U, Schmid M, Herder C, Nöthlings U. Developmental trajectories of body mass index from childhood into late adolescence and subsequent late adolescence–young adulthood cardiometabolic risk markers. Cardiovasc Diabetol. 2019;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith CJ, Ryckman KK, Barnabei VM, Howard BV, Isasi CR, Sarto GEet al. The impact of birth weight on cardiovascular disease risk in the Women's Health Initiative. Nutr Metab Cardiovasc Dis. 2016;26(3):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli H-M, Miettola S, Hovi Pet al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181(11):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kubota Y, Heiss G, MacLehose RF, Roetker NS, Folsom AR. Association of educational attainment with lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities study. JAMA Intern Med. 2017;177(8):1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azemati B, Rajaram S, Jaceldo-Siegl K, Haddad EH, Shavlik D, Fraser GE. Dietary animal to plant protein ratio is associated with risk factors of metabolic syndrome in participants of the AHS-2 Calibration Study. Nutrients. 2021;13(12):4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose D, Heller MC, Willits-Smith AM, Meyer RJ. Carbon footprint of self-selected US diets: nutritional, demographic, and behavioral correlates. Am J Clin Nutr. 2019;109(3):526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laine JE, Huybrechts I, Gunter MJ, Ferrari P, Weiderpass E, Tsilidis Ket al. Co-benefits from sustainable dietary shifts for population and environmental health: an assessment from a large European cohort study. Lancet Planet Health. 2021;5(11):e786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJet al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krupp D, Shi L, Remer T. Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int. 2014;85(1):204–10. [DOI] [PubMed] [Google Scholar]
- 31. Goletzke J, Herder C, Joslowski G, Bolzenius K, Remer T, Wudy SAet al. Habitually higher dietary glycemic index during puberty is prospectively related to increased risk markers of type 2 diabetes in younger adulthood. Diabetes Care. 2013;36(7):1870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwingshackl L, Knuppel S, Michels N, Schwedhelm C, Hoffmann G, Iqbal Ket al. Intake of 12 food groups and disability-adjusted life years from coronary heart disease, stroke, type 2 diabetes, and colorectal cancer in 16 European countries. Eur J Epidemiol. 2019;34(8):765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanley-Cook GT, Argaw AA, de Kok BP, Vanslambrouck KW, Toe LC, Kolsteren PWet al. EAT–Lancet diet score requires minimum intake values to predict higher micronutrient adequacy of diets in rural women of reproductive age from five low- and middle-income countries. Br J Nutr. 2021;126(1):92–100. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Yang H, Li S, Li W-d, Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: a cohort study in the UK Biobank. PLoS Med. 2021;18(11):e1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Temme EH, Toxopeus IB, Kramer GF, Brosens MC, Drijvers JM, Tyszler Met al. Greenhouse gas emission of diets in the Netherlands and associations with food, energy and macronutrient intakes. Public Health Nutr. 2015;18(13):2433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rippin HL, Cade JE, Berrang-Ford L, Benton TG, Hancock N, Greenwood DC. Variations in greenhouse gas emissions of individual diets: associations between the greenhouse gas emissions and nutrient intake in the United Kingdom. PLoS One. 2021;16(11):e0259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The German Nutrition Society . Energie. Bonn, Germany: Deutsche Gesellschaft für Ernährung; 2015[cited 21 October, 2021]. Available from: https://www.dge.de/wissenschaft/referenzwerte/energie/?L=0. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code of this study will be made available upon request. DONALD study data are available upon reasonable request for research questions within the scope of the DONALD study and which are consistent with the legal and ethical standard practices of the DONALD study. Requests should be addressed to epi@uni-bonn.de.