ABSTRACT
Background
Nonalcoholic fatty liver disease (NAFLD) among Latinos is partially attributed to a prevalent C>G polymorphism in the patatin-like phospholipase 3 (PNPLA3) gene. Cross-sectional analyses in Latino children showed the association between dietary sugar and liver fat was exacerbated by GG genotype. Pediatric feeding studies show extreme sugar restriction improves liver fat, but no prior trial has examined the impact of a clinical intervention or whether effects differ by PNPLA3 genotype.
Objectives
We aimed to test effects of a clinical intervention to reduce dietary sugar compared with standard dietary advice on change in liver fat, and secondary-endpoint changes in liver fibrosis, liver enzymes, and anthropometrics; and whether effects differ by PNPLA3 genotype (assessed retrospectively) in Latino youth with obesity (BMI ≥ 95th percentile).
Methods
This parallel-design trial randomly assigned participants (n = 105; mean baseline liver fat: 12.7%; mean age: 14.8 y) to control or sugar reduction (goal of ≤10% of calories from free sugar) for 12 wk. Intervention participants met with a dietitian monthly and received delivery of bottled water. Changes in liver fat, by MRI, were assessed by intervention group via general linear models.
Results
Mean free sugar intake decreased in intervention compared with control [11.5% to 7.3% compared with 13.9% to 10.7% (% energy), respectively; P = 0.02], but there were no significant effects on liver outcomes or anthropometrics (Pall > 0.10), and no PNPLA3 interactions (Pall > 0.10). In exploratory analyses, participants with whole-body fat mass (FM) reduction (mean ± SD: −1.9 ± 2.4 kg), irrespective of randomization, had significant reductions in liver fat compared with participants without FM reduction (median: −2.1%; IQR: −6.5% to −0.8% compared with 0.3%; IQR: −1.0% to 1.1%; P < 0.001).
Conclusions
In Latino youth with obesity, a dietitian-led sugar reduction intervention did not improve liver outcomes compared with control, regardless of PNPLA3 genotype. Results suggest FM reduction is important for liver fat reduction, confirming clinical recommendations of weight loss and a healthy diet for pediatric NAFLD.
This trial was registered at clinicaltrials.gov as NCT02948647.
Keywords: nonalcoholic fatty liver disease, PNPLA3 genotype, sugar, Latino, adolescents
Introduction
The prevalence of pediatric nonalcoholic fatty liver disease (NAFLD) has doubled over the past 20 y (1). This is concerning given that NAFLD is a risk factor for future cardiometabolic disease (1, 2) and progression of liver disease (3). Treatment guidelines for pediatric NAFLD focus on weight loss through increasing physical activity and improving dietary intake, owing to the strong association between obesity and NAFLD (4). However, current guidelines do not support one specific diet over another, and it remains unclear which dietary factors should be targeted to have the greatest impact on liver fat owing to the sparsity of available data (4). Despite this, reduction in added sugar intake, especially through reducing consumption of sugar-sweetened beverages (SSBs), is a promising strategy to reduce liver fat in children, primarily due to its significant effect on adiposity in large pediatric randomized controlled trials (RCTs) (4–6).
To our knowledge, only 2 trials have directly assessed the therapeutic potential of reducing added sugar intake to treat pediatric NAFLD, and both were controlled feeding trials providing all foods to participants with almost complete restriction of sugar intake. First, in an 8-wk RCT of adolescent boys with NAFLD (n = 40), restricting free sugar intake to <3% of total calories significantly reduced liver fat content (from 25% to 17% liver fat) compared with a control group who maintained their typical diet (from 21% to 20% liver fat) (7). A second trial in 41 adolescent boys and girls with obesity and metabolic syndrome found that reducing fructose intake to 4% of total calories for 9 d significantly reduced liver fat as compared with baseline (from 7.2% to 3.8% liver fat) (8). Although results from these 2 efficacy studies suggest that sugar restriction may be an effective strategy for reducing liver fat, they used extreme sugar reduction (3%–4% of total calories) and were conducted under tightly controlled feeding conditions, which may not be sustainable or directly translate to clinical care. Therefore, it is important to determine whether a sugar reduction intervention that utilizes services more commonly accessible to patients in a real-world clinical setting, such as nutrition education, can also effectively reduce liver fat.
Latino children are particularly predisposed to NAFLD (9, 10), with an ≤4-fold increase in NAFLD risk compared with their non-Latino peers (11). Although the rise of pediatric NAFLD in Latinos is partially attributable to the higher prevalence of obesity among this ethnic group (12, 13), the disparity may also result from the high prevalence of an amino acid substitution (rs738409; C>G; Ile148Met) in the patatin-like phospholipase 3 (PNPLA3) gene (14, 15). On average, liver fat is 2 times higher in children who are GG for the PNPLA3 variant than in children who are GC or CC (14). Therefore, it is particularly relevant to investigate strategies to reduce liver fat that are effective in this target population.
This 12-wk RCT examined the efficacy of a dietitian-led sugar reduction intervention relative to a control group receiving handouts with general diet advice, on liver fat in Latino adolescents with obesity and suspected NAFLD. Based on a previously observed nutrigenetic interaction between the PNPLA3 GG variant and dietary sugar on liver fat content (16), we also hypothesized that the effect of sugar reduction on reducing liver fat would be more pronounced in individuals with the GG genotype. Secondary outcomes also reported include liver fibrosis, liver enzymes, and anthropometric variables.
Methods
Recruitment and enrollment
Recruitment took place from October 2016 through March 2020, when recruitment ended owing to COVID-19-related restrictions. Our primary recruitment strategy was based on referrals from pediatric gastroenterology clinics at the University of Southern California (USC) and Children's Hospital Los Angeles (CHLA), as well as community centers and health fairs in the greater Los Angeles area. Interested potential participants underwent either a telephone screen with a study coordinator or an in-person screen with the referring health professional to assess initial eligibility. Final eligibility was assessed by a pediatrician who conducted a medical examination and confirmed participants’ eligibility and health status before enrollment and randomization at the baseline assessment. All eligible and enrolled participants signed youth assents and their parents signed informed consents before study initiation. The USC and CHLA institutional review boards approved this study. This trial was registered at clinicaltrials.gov on 28 October, 2016 (NCT02948647) before enrolling the first study participant.
We enrolled Latino adolescents (11–18 y of age) with obesity (BMI ≥ 95th percentile for age and sex). Our recruitment strategy aimed to identify children with elevated liver fat by enrolling adolescents with obesity and recruiting from clinics focused on treating NAFLD. Overall, 73% of enrolled participants with a baseline MRI scan had a liver fat fraction ≥ 5.5%, meeting the clinical criteria for NAFLD (17). For the purposes of this study, children were defined as Latino if they identified as such and both parents and all 4 grandparents reported origins in Cuba, the Dominican Republic, Mexico, Puerto Rico, or South or Central America (98% of subjects had origins in Mexico or Central America). Siblings were allowed to enroll in the study, with a total of 12 sibling pairs recruited and enrolled. Key exclusion criteria included 1) diagnosis of any disease known to influence insulin action and secretion (including type 1 and type 2 diabetes); 2) involvement in a weight loss, exercise, or dietary intervention within 6 mo before participation; 3) use of medication known to influence body composition or fat distribution, insulin resistance, gut function, or lipid profiles; 4) history of renal/liver disease or any disease affecting liver fibrosis and steatosis; 5) diagnosis/current treatment of celiac disease, inflammatory bowel disease, or other major gastrointestinal issues; 6) any other disease that could compromise the immune system; 7) current pregnancy; 8) current smoking (>1 cigarette in the past week or ≥200 cigarettes or electronic cigarettes in their lifetime) or use of other recreational drugs; and 9) consumption of alcohol on a regular basis (40 g/d).
Study design
This parallel-design randomized controlled dietary intervention trial was conducted at the Saban Research Institute at CHLA and USC in Los Angeles, CA. After completing a baseline assessment, participants were randomly assigned to either a sugar reduction intervention group (intervention group) or a control group receiving handouts with general diet advice (control group) for 12 wk. The randomization was performed using a stratified, blocked randomization schema developed by the study statistician (WJM); randomization was stratified by sex, with block sizes of 4. Participants were enrolled and randomly assigned to the intervention arms by a study coordinator who was not involved in the assessment of any outcome measures or data analysis of study outcomes. Participants completed a postintervention assessment 12–16 wk after being randomly assigned to their study arm. Eight participants included in the analysis completed their postintervention assessment >16 wk after randomization, because of postponement due to COVID-19-related scheduling challenges. These 8 participants averaged 163 d between the baseline and postintervention assessments. At both the pre- and postintervention assessments, trained staff collected fasting blood; measured body weight, height, waist circumference, and hip circumference using standardized protocols (18, 19); and performed an abdominal MRI/magnetic resonance elastography (MRE) scan and a whole-body DXA scan. Assessments were conducted at the Diabetes and Obesity Research Institute clinical facility at USC.
Study diets
Participants randomly assigned to the control group received a packet of handouts ∼1 wk after their baseline assessment, that contained general dietary advice based on the 2015 Dietary Guidelines for Americans (20) and the USDA's MyPlate (21). The control participants did not receive any other form of support or dietary guidance. Participants randomly assigned to the intervention group, accompanied by their parent or caregiver, met 3 times over the 12-wk intervention period with a bilingual registered dietitian nutritionist (RDN) to discuss principles of healthy eating, with a focus on reducing free sugar intake (intake of added sugar and sugar from SSBs). During weeks where participants assigned to the intervention group did not meet with the RDN, they received a check-in phone call. During in-person sessions, the RDN provided nutrition education, sharing the most common sources of free sugars (SSBs as well as sweets, treats, and desserts) and teaching how to read nutrition labels. Participants assigned to the intervention group were asked to monitor their free sugar intake and were provided with a free sugar intake maximum in grams per day. This value was set at 10% of daily calories from free sugar, based on recommendations by the 2015–2020 Dietary Guidelines for Americans (20), and was estimated using the participant's age and sex. In addition, intervention participants were encouraged to replace SSBs with water as the preferred choice, or 0-calorie drinks. To help displace the consumption of SSBs, bottled water was delivered weekly to the homes of intervention participants. All participants, regardless of randomization, were asked to continue their typical physical activity. Neither the intervention nor the control participants were provided with any specific total daily calorie targets, and caloric restriction was not emphasized during the intervention period for either of the intervention or control groups. All control and intervention materials were provided in both English and Spanish, and all study staff interacting with participants were bilingual (English/Spanish).
Liver fat, liver fibrosis, and liver enzymes
Liver fat was assessed from an abdominal MRI scan and liver fibrosis was measured by MRE at the USC Radiology imaging center using a 3.0 Tesla GE Scanner. Liver fat content was determined using the previously validated 3D iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) method (22–25). The abdominal MRI scan covered the top of the liver to the L5 vertebra, using 8–9 contiguous axial slabs, each acquired during a single 20-s breath-hold. We used 6-echo T2*IDEAL reconstruction with fat spectrum modeling. The liver was manually segmented from the volume data using Slice-O-Matic (Tomovision). Fat volume fraction and fat mass (FM) fraction were computed on a voxel-by-voxel basis and averaged over each segmented organ. Liver fibrosis was assessed by measuring liver stiffness via MRE from an abdominal scan using a 3.0 Tesla GE scanner equipped with the Mayo Clinic MRE apparatus, and synchronized motion-encoded MRE sequence, based on published validation studies (26, 27). Imaging parameters were 60 Hz acoustic vibration, echo time (TE) = 20 ms; repetition time (TR) = 50 ms; field of view (FOV) = 30–48 cm; matrix 256 × 64; phase offset = 4, bandwith (BW) = 31 KHz. Imaging was performed during 10- to 20-s breath-holds. All imaging analyses were performed by an MRI physicist, who was blinded to randomization. The liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in plasma by the Norris laboratory using spectrophotometry with a Cobas c501.
Anthropometrics
Body weight, waist circumference, and hip circumference were measured by a registered nurse or phlebotomist using standardized procedures (18, 19). Body FM and lean mass were measured by a whole-body DXA scan using a Hologic QDR 5400 densitometer (Hologic, Inc.). Visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAAT) were measured from an abdominal MRI scan using the 3D IDEAL method described already.
Dietary intake and physical activity
Dietary intake was assessed using a total of 6 unannounced 24-h dietary recalls: 3 recalls taken ±1 wk from the baseline assessment and 3 recalls taken ±1 wk from the postintervention assessment, including 2 weekdays and 1 weekend day at each time point. The procedures used in the 1986–1989 USDA Continuing Survey of Food Intakes of Individuals were followed and all recalls were collected in a personal interview using a standardized “multiple pass” protocol. Data were collected and compiled using the Nutrition Data System for Research (versions 2016–2019, University of Minnesota). Physical activity was assessed at both baseline and postintervention assessments through a 3-d physical activity recall that was based on the validated Previous Day Physical Activity Recall (28). These data were used to calculate a physical activity factor (kcal · kg−1 · h−1) using metabolic equivalents.
PNPLA3 genotyping
PNPLA3 genotyping was done retrospectively after all study participants completed the trial. Genomic DNA was extracted from whole blood using DNeasy kits (Cat # 69506, Qiagen). Genotyping of the PNPLA3 rs738409 polymorphism (C444G; Ile148Met) was determined by restriction fragment length polymorphism (RFLP) analysis, with modifications from previously described methods (29). Briefly, PCR amplification was carried out with forward primer 5′-TGGGCCTGAAGTCCGAGGGT-3 and reverse primer 5′-CCGACACCAGTGCCCTGCAG-3′. The PCR reaction included 2.5 mM MgCl2, 0.2 mM of each deoxyribose nucleotide triphosphate (dNTP), 0.5 μM of each primer, 20 ng genomic DNA, and 1.25 U Taq polymerase in a total volume of 25 μL. Cycling conditions were 95°C for 2 mins, 10 cycles of 94°C denaturation for 1 min and 68°C annealing/extension for 2 min, followed by 25 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 45 s, with a final extension at 72°C for 5 min. Of the 333-bp PCR product, 15 μL was then incubated with 8 U of the restriction endonuclease NlaIII in CutSmart® buffer at 37°C for 4 h. The digested PCR products were run on a 2% agarose gel and visualized with ethidium bromide. The G creates a restriction site for NlaIII that generates 200-bp and 133-bp fragments. Thus, the presence of only the uncut 333-bp product indicates the homozygous CC genotype, the presence of only the 200-bp and 133-bp fragments indicates GG homozygotes, and the presence of all 3 fragments indicates CG heterozygosity.
Statistical analysis
We aimed to randomly assign 120 participants, with the goal of having ≥96 participants with complete MRI scans at both clinic visits, for a sample size of 96 in our primary analysis of change in liver fat. Sample size estimates were computed for change in liver fat fraction using NQuery Advisor 3.0 (Statsols). Power was computed to test the treatment (intervention compared with control) by genotype (GG compared with CC/CG) interaction in a 2-factor ANOVA, based on the assumption from our previous studies (30) that 50% of the participants would be GG and 50% would be either GC or CC for the PNPLA3 genotype. Based on data from a previous lifestyle intervention and preliminary data from our group, we used the following to estimate study power in a 2-factor ANOVA: mean treatment reduction (change) in liver fat of 4% in the GG stratum; mean treatment reduction (change) in liver fat of 1% in the CC/CG stratum; SD of change in liver fat of 3%. Using these parameters, a sample size of 96 would give us 98% power to detect a treatment main effect of 2.5% reduction in liver fat and 68% power to detect the genotype-by-treatment interaction. Post hoc power calculations using the sample size included in our primary analysis of liver fat (n = 81) and the parameters used in our original sample size calculations aforementioned indicated that we had 96% power to detect a main effect (of 2.5% reduction in liver fat) and 60% power to detect an interaction with a reduction of 4% units of liver fat fraction in the GG intervention group even if there was a 1% reduction in liver fat in the GC/CC intervention group.
Statistical analyses were performed using R statistical package version 4.0.3 (31) and RStudio version 1.4.1106 (RStudio, Inc.) (32), with the 2-sided level of significance set to P < 0.05 for all analyses. All variables were assessed for normality and logarithmic transformations were performed on all variables that were not normally distributed. Per CONSORT guidelines, baseline characteristics were visualized by randomization arm to check the size of any chance imbalances (33). The primary trial outcome was change (post minus pre) in liver fat; secondary trial outcomes were similarly calculated. Secondary outcomes included changes in liver fibrosis, liver enzymes, weight, BMI, waist circumference, hip circumference, VAT, SAAT, total fat both in kilograms and as a percentage of total body weight, and lean mass both in kilograms and as a percentage of total body weight. The main effects of the intervention were evaluated using general linear models, with change in outcome as the dependent variable, adjusting for the outcome variable at baseline (raw model). We subsequently ran an adjusted model that included covariates for sex, change in BMI (we did not adjust for change in BMI for models testing anthropometric outcomes), and change in physical activity (adjusted model). To determine whether the treatment effect differed by PNPLA3 genotype (GG compared with CC/CG), we added an interaction term of genotype-by-treatment arm. The main analysis included those individuals with complete data for the outcome of interest. In an additional analysis, we also used generalized estimating equations (GEEs) to adjust for clustering of sibling pairs who were enrolled into the study and had complete data at both baseline and postintervention assessments (7 sibling pairs). For our primary outcome of change in liver fat, we subsequently ran the following sensitivity analyses: 1) using the “Mice” package in R we used multiple imputation to generate complete intent-to-treat data sets (n = 5) that included all participants enrolled and randomly assigned, with summarization of treatment effects over the repeated analyses (34); 2) we ran a sensitivity analysis limiting the sample to those participants with a baseline free sugar intake ≥ 10% of total calories; 3) we ran a sensitivity analysis limiting the sample to those participants with a baseline liver fat fraction ≥ 5.5%; and 4) we ran a sensitivity analysis limiting the sample to those participants whose final clinic visit occurred before March of 2020, removing participants who were on the intervention for longer than the prespecified 12- to 16-wk intervention window as a result of COVID-related restriction. In the analysis of dietary data, additional subjects were removed because they may have received dietary advice before completing their baseline dietary assessment. Specifically, this resulted in removing 3 subjects from the dietary analysis of subjects included in our primary analysis of liver fat, and 4 subjects from the dietary analysis of all subjects randomly assigned with both baseline and postintervention assessments.
In exploratory analyses of pooled data, we assessed the impact of reducing sugar intake and reducing FM, irrespective of randomization, on liver fat, liver fibrosis, and liver enzymes. To assess the effect of reducing total sugar intake (TS) on our outcomes of interest, we split our data set into those with a reduction in total sugar over the intervention period [TS; TSPost (% of energy) − TSPre (% of energy) < 0] and those with no change or an increase in TS [TSPost (% of energy) − TSPre (% of energy) ≥ 0] during the intervention period, regardless of study diet assignment. This variable replaced the diet intervention variable in the general linear models described already. Similarly, in an exploratory analysis, we also assessed the effect of reducing FM on our outcomes of interest. We split our data set into those with a reduction in whole-body FM (FMPost − FMPre < 0) and those with no change or an increase in FM (FMPost − FMPre ≥ 0) during the intervention period, regardless of study diet assignment. This variable then replaced the diet intervention variable in the general linear models described already.
Results
Description of participants and adverse events
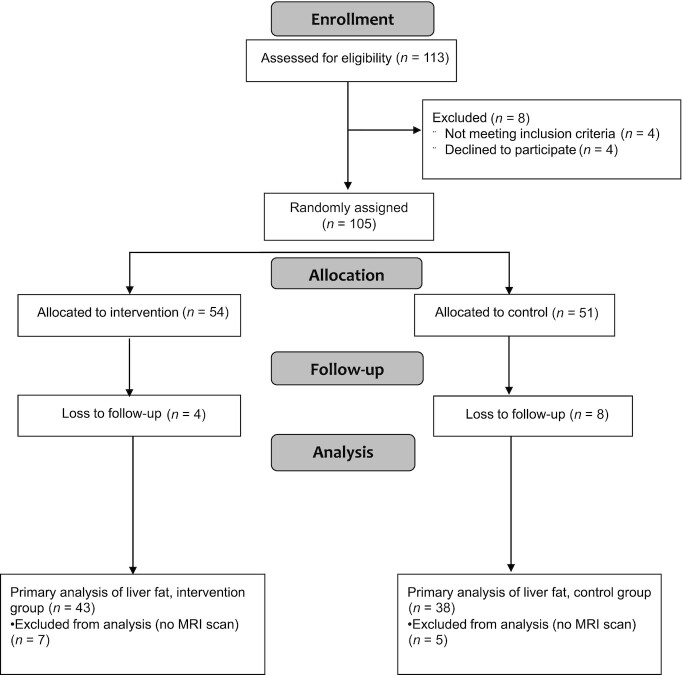
The trial was conducted between October 2016 and March 2020, when recruitment ended early owing to COVID-19-related restrictions. A total of 113 potential participants were assessed for eligibility by a pediatrician with 105 participants ultimately enrolled into and randomly assigned in the trial (72% GG and 28% GC/CC PNPLA3 genotype). Participants were randomly assigned to 1 of 2 diet groups: 54 to the intervention group with a dietitian-led sugar reduction intervention and 51 to the control group receiving handouts with general diet advice (Figure 1). A total of 12 participants were excluded from the analyses of outcome variables of interest because these participants dropped out before the postintervention assessment. An additional 12 participants were excluded from the primary analyses of change in liver fat, because these participants did not undergo MRI scans at both the baseline and postintervention assessments. Of those participants who completed the study, participants randomly assigned to the control group and intervention group averaged 98 d (IQR: 91–102 d) and 84 d (IQR: 81–93 d), respectively, between the baseline assessment and postintervention assessment. Table 1 shows the baseline characteristics for the primary endpoint, change in liver fat, stratified by intervention group. Supplemental Table 1 shows baseline characteristics for all participants enrolled into and randomly assigned in the trial. No adverse events were reported, related or unrelated to the intervention, during the conduct of the trial.
FIGURE 1.
Flow diagram of participant recruitment, enrollment, intervention allocation, follow-up, and analysis for the primary outcome, change in liver fat.
TABLE 1.
Baseline characteristics of study participants by study arm in the primary analysis of change in liver fat1
| Variable | Intervention (n = 43) | Control (n = 38) |
|---|---|---|
| Age, y | 15.2 [13.2–16.5] | 14.5 [12.8–17.2] |
| Male sex, % | 20 (46.5) | 18 (47.4) |
| Tanner stage2 ≥ 4 | 30 (69.8) | 22 (59.5) |
| PNPLA3 GG genotype, % | 28 (65.1) | 28 (73.7) |
| Liver fat, % | 8.9 [5.5–14.9] | 11.2 [5.4–19.2] |
| Liver fibrosis3 | 2.3 [2.2–2.5] | 2.4 [2.3–2.5] |
| AST,4 IU/L | 20.0 [16.0–26.0] | 23.0 [19.0–33.5] |
| ALT,4 IU/L | 23.5 [16.2–36.8] | 29.0 [16.0–43.0] |
| Body weight, kg | 88.4 [73.6–98.5] | 88.1 [71.8–104.1] |
| BMI, kg/m2 | 32.1 [29.4–36.8] | 33.3 [30.0–36.9] |
| Waist circumference, cm | 102.0 [95.3–109.5] | 103.5 [97.1–117.2] |
| Hip circumference, cm | 110.5 [105.0–120.8] | 112.0 [104.0–120.0] |
| DXA total fat, % | 42.8 [37.3–45.9] | 44.2 [41.5–47.2] |
| DXA total fat, kg | 34.1 [29.1–43.1] | 36.8 [32.0–46.1] |
| DXA lean mass, kg | 47.3 [40.9–56.4] | 48.2 [36.9–54.2] |
| SAAT, L | 7.5 [5.2–9.0] | 7.2 [6.1–8.3] |
| VAT, L | 1.7 [1.3–2.1] | 1.9 [1.2–2.3] |
| Total energy intake,5 kcal/d | 1487 [1224–1898] | 1488 [1246–1727] |
| Total sugar intake,5 %E | 18.3 ± 6.7 | 20.5 ± 6.5 |
| Added sugar intake,5 %E | 11.2 ± 6.2 | 13.1 ± 5.7 |
| Free sugar intake,5 %E | 11.5 ± 6.2 | 13.9 ± 6.7 |
| Physical activity, MET-h/wk | 57.2 [54.8–65.0] | 59.3 [53.8–73.6] |
n = 81. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables and n (%) for categorical variables. ALT, alanine aminotransferase; AST, aspartate aminotransferase; MET, metabolic equivalent of task; PNPLA3, patatin-like phospholipase 3; SAAT, subcutaneous abdominal adipose tissue; VAT, visceral adipose tissue; %E, percentage of total energy intake.
Sample size: intervention, n = 43; control, n = 37.
Sample size: intervention, n = 42; control, n = 37.
Sample size: intervention, n = 42; control, n = 38.
Sample size: intervention, n = 43; control, n = 34.
Intervention adherence and dietary intakes
At baseline, there was no significant difference in free sugar intake between the control and intervention groups (P = 0.35, data not shown). However, at the postintervention assessment, those randomly assigned to the intervention group consumed (mean ± SD) 7.0% ± 4.5% of energy from free sugars, which was significantly less than the 10.6% ± 5.8% of energy from free sugars consumed by the control group (P = 0.003). Of those individuals who were randomly assigned to the intervention group and had dietary data at both baseline and postintervention assessments (n = 49), 81.6% reduced their free sugar intake, with 71.4% successfully meeting the intervention target of a free sugar intake ≤ 10% of total calories (data not shown). Intervention participants who successfully met the free sugar intake target (n = 35) averaged 4.7% ± 2.8% of energy from free sugar, whereas intervention participants who did not meet the free sugar intake goal (n = 14) averaged 12.8% ± 2.0% of energy from free sugar at the postintervention assessment. Of those individuals who were randomly assigned to the control group and had dietary data at both baseline and postintervention assessments (n = 39), 61.5% reduced their free sugar intake, with 51.3% meeting the threshold of a free sugar intake ≤ 10% of total calories (data not shown). Overall, intervention participants tended to meet the free sugar intake target of ≤10% of total calories more often than control participants (P = 0.06). In addition, in alignment with the design of our study, participants generally remained weight-stable with no significant change in their total energy intake (P = 0.67) or weight (P = 0.48) over the intervention period (data not shown).
There were significant dietary changes in the intervention group compared with the control group (Table 2) among participants included in our primary analysis for the outcome of change in liver fat, with complete diet data (n = 76). The intervention group had a significantly greater reduction in energy-adjusted TS (P = 0.02) and free sugar intake (P = 0.02) than the control group. There was also a trend for a greater decrease in energy-adjusted added sugar intake (P = 0.07) in the intervention group than in the control group. There were no significant differences in the intakes of total energy or nutrient-adjusted carbohydrate, fiber, fat, SFAs, MUFAs, PUFAs, or protein between the intervention and control groups (PAll > 0.10). Similar dietary changes were observed when expanding our analysis to include all individuals who were randomly assigned with complete diet (n = 88), with the exception that the trend for a difference in added sugar intake between the intervention and control groups became significant (P = 0.02) (Supplemental Table 2).
TABLE 2.
Dietary intakes at baseline and the change in intakes during the intervention period, based on unannounced 24-h dietary recalls, for the participants in the primary analysis of change in liver fat with complete diet data1
| Intervention (n = 42) | Control (n = 34) | ANCOVA | GEEs | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Raw | Adjusted | Adjusted | |
| Energy intake, kcal/d | 1495 [1225–1906] | −95 ± 514 | 1488 [1246–1727] | −113 ± 461 | 0.47 | 0.47 | 0.30 |
| Carbohydrates, %E | 49.2 [44.5–54.3] | −2.4 ± 8.9 | 48.0 [45.9–55.6] | −1.0 ± 12.7 | 0.51 | 0.60 | 0.63 |
| Total sugar, %E | 18.5 ± 6.7 | −4.2 ± 7.5 | 20.3 ± 6.5 | −2.4 ± 9.4 | 0.02 | 0.02 | 0.02 |
| Added sugars, %E | 11.2 ± 6.2 | −3.4 ± 7.0 | 13.1 ± 5.7 | −2.8 ± 7.8 | 0.06 | 0.07 | 0.03 |
| Free sugars, %E | 11.5 ± 6.2 | −4.2 ± 6.4 | 13.9 ± 6.9 | −3.4 ± 8.8 | 0.02 | 0.02 | <0.01 |
| Fiber, g/d | 14.1 [10.1–18.7] | −1.5 [−4.1 to 3.4] | 13.3 [8.4–16.0] | 0.0 [−5.6 to 4.0] | 0.29 | 0.23 | 0.20 |
| Fiber, %E | 3.5 [2.7–4.2] | 0.3 ± 2.1 | 3.4 [2.7–4.1] | 0.1 ± 1.6 | 0.49 | 0.44 | 0.29 |
| Fat, %E | 34.5 ± 6.5 | 0.7 ± 6.9 | 31.8 ± 4.9 | 1.4 ± 9.8 | 0.11 | 0.11 | 0.10 |
| SFAs, %E | 11.7 [9.5–13.3] | −0.1 ± 4.0 | 9.9 [8.7–12.5] | 1.2 ± 4.0 | 0.69 | 0.64 | 0.93 |
| MUFAs, %E | 12.3 [10.6–14.3] | 0.3 ± 3.4 | 10.6 [8.4–12.3] | 0.5 ± 4.7 | 0.11 | 0.12 | 0.16 |
| PUFAs, %E | 8.6 ± 2.2 | 0.0 ± 3.1 | 7.9 ± 2.6 | 0.1 ± 3.7 | 0.35 | 0.36 | 0.27 |
| Protein, %E | 16.4 ± 4.7 | 1.7 ± 4.7 | 17.8 ± 4.4 | −0.2 ± 4.9 | 0.17 | 0.20 | 0.20 |
n = 76. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables. Raw: change adjusted for baseline value. Adjusted: raw model adjusted for sex, change in BMI (except the outcome energy intake), and change in physical activity. GEE analysis of adjusted model, further adjusting for sibling clusters (7 sibling pairs). GEE, generalized estimating equation; %E, percentage of total energy intake.
Liver fat, liver fibrosis, and liver enzymes
Changes in liver fat fraction did not differ between the control (median: −1.1%; IQR: −3.7%, 0.5%) and intervention groups (median: −0.8%; IQR: −3.7%, 0.5%) (adjusted P = 0.50) (Figure 2A, Supplemental Table 3). This was the case in both the unadjusted and adjusted models controlling for sex, change in BMI, and change in physical activity (Supplemental Table 3). In sensitivity analyses, there were also no differences when samples were limited to 1) participants with a baseline free sugar intake ≥ 10% of total calories, 2) participants with a baseline liver fat fraction ≥ 5.5%, 3) participants who completed the study before March 2020, or 4) running the analysis on complete imputed data sets (adjusted P > 0.10 for all analyses, data not shown). In addition, there were no differential changes in liver fibrosis between the intervention and control groups as measured by MRE (adjusted P = 0.31), nor differential changes in the liver enzymes AST (adjusted P = 0.71) and ALT (adjusted P = 0.68) (Figure 2B–D). There was also no intervention-by-PNPLA3 genotype interaction observed for change in liver fat (P = 0.87; intervention PNPLA3 genotype: GG, n = 28 and GC/CC, n = 15; control PNPLA3 genotype: GG, n = 28 and GC/CC, n = 10), liver fibrosis (P = 0.70), AST (P = 0.78), or ALT (P = 0.98) (Supplemental Table 3). Consistent outcomes were obtained using GEE analysis of changes in liver fat, liver fibrosis, AST, and ALT (PAll > 0.10) (Supplemental Table 3).
FIGURE 2.
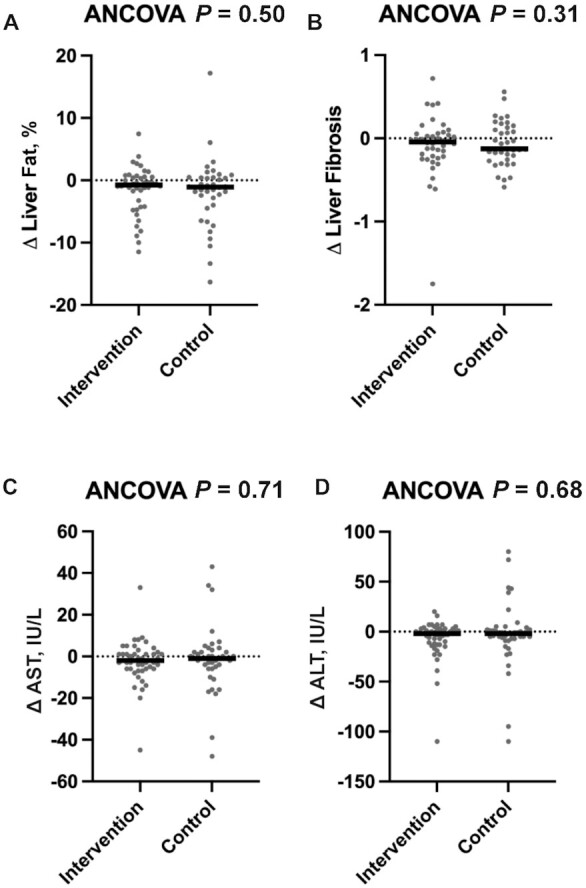
Intervention to reduce free sugar intake does not affect liver health as measured by liver fat, liver fibrosis, and liver enzymes. Changes in (A) liver fat fraction and (B) liver fibrosis (intervention group, n = 43; control group, n = 38) and changes in liver enzymes including (C) AST and (D) ALT (intervention group, n = 49; control group, n = 38) for the primary analysis. Outcome variables are represented as the change variable calculated as the postintervention value minus the value at baseline. Each participant's change variable is represented by a solid dot. The medians are represented by horizontal bars. P values for the ANCOVA adjusted for the baseline value of the outcome of interest, sex, change in BMI, and change in physical activity are displayed at the top of each boxplot. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Anthropometrics
There was no significant effect of the intervention on changes in any anthropometric outcome including body weight (adjusted P = 0.69), BMI (adjusted P = 0.47), waist circumference (adjusted P = 0.15), hip circumference (adjusted P = 0.93), percentage FM or FM in absolute kilograms (adjusted P = 0.88 and P = 0.71, respectively), lean mass as measured in absolute kilograms (P = 0.12), or the FM:lean mass ratio (adjusted P = 0.78) as measured by DXA. The intervention and control groups also did not differ in terms of changes in SAAT (P = 0.16) or VAT (P = 0.78) as measured by MRI (Table 3). Consistently, there were no effects of the intervention on any anthropometric outcome in GEE analyses (PAll > 0.10) (Table 3).
TABLE 3.
The effect of the intervention on anthropometric outcomes for all participants randomly assigned with data at both the baseline and postintervention assessments1
| Intervention (n = 50) | Control (n = 43) | ANCOVA | GEEs | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Raw | Adjusted | PNPLA3 interaction | Adjusted | |
| Weight, kg | 88.4 [73.4–100.2] | 0.6 [−1.0 to 2.4] | 90.2 [74.2–105.7] | 0.8 [−1.1 to 3.4] | 0.86 | 0.69 | 0.35 | 0.73 |
| BMI, kg/m2 | 32.2 [29.3–37.6] | 0.1 [−1.0 to 0.6] | 34.2 [30.4–38.0] | 0.0 [−0.7 to 0.7] | 0.68 | 0.47 | 0.25 | 0.52 |
| Waist circumference, cm | 103.3 [96.2–110.4] | −1.3 ± 5.4 | 106.3 [97.4–118.9] | −0.3 ± 6.2 | 0.21 | 0.15 | 0.42 | 0.16 |
| Hip circumference, cm | 110.7 [105.2–121.1] | −0.2 [−2.5 to 2.0] | 114.2 [105.2–122.7] | 0.5 [−1.2 to 2.4] | 0.91 | 0.93 | 0.31 | 0.86 |
| SAAT,2 L | 7.5 [5.2–9.0] | 0.0 [−0.7 to 0.5] | 7.2 [6.1–8.3] | 0.2 [−0.5 to 0.7] | 0.17 | 0.16 | 0.34 | 0.15 |
| VAT,2 L | 1.7 [1.3–2.1] | 0.0 [−0.2 to 0.2] | 1.9 [1.2–2.3] | 0.0 [−0.2 to 0.2] | 0.92 | 0.78 | 0.28 | 0.79 |
| Total fat, % | 43.0 [37.6–46.2] | 0.4 ± 2.8 | 44.6 [41.7–48.2] | 0.4 ± 3.5 | 0.77 | 0.88 | 0.73 | 0.88 |
| Total fat, kg | 34.5 [30.2–43.2] | 0.2 [−1.5 to 1.9] | 37.5 [32.4–48.4] | 0.2 [−0.9 to 1.9] | 0.63 | 0.71 | 0.59 | 0.68 |
| Lean mass, % | 54.5 [51.6–59.8] | −0.2 ± 2.4 | 52.9 [49.4–55.6] | 0.3 ± 2.5 | 0.45 | 0.61 | 0.55 | 0.59 |
| Lean mass, kg | 46.8 [40.0–56.4] | 0.1 ± 2.1 | 48.2 [39.0–54.0] | 0.8 ± 2.7 | 0.17 | 0.12 | 0.13 | 0.67 |
| Fat:lean ratio | 0.8 ± 0.2 | 0.0 ± 0.1 | 0.9 ± 0.2 | 0.0 ± 0.1 | 0.64 | 0.78 | 0.89 | 0.74 |
n = 93. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables. Raw: adjusted for baseline value of the outcome of interest. Adjusted: raw model adjusted for sex and change in physical activity. GEE analysis of adjusted model, further adjusting for sibling clusters (7 sibling pairs). PNPLA3 interaction: assessment of the intervention × PNPLA3 genotype interaction in the adjusted model. GEE, generalized estimating equation; PNPLA3, patatin-like phospholipase 3; SAAT, subcutaneous abdominal adipose tissue; VAT, visceral adipose tissue.
Sample size: intervention, n = 43; control, n = 38.
Changes in liver outcomes as a function of change in TS
In exploratory analyses of pooled data, we examined liver outcomes in all participants who successfully reduced sugar intake (n = 54) relative to participants without sugar reduction (n = 22), regardless of intervention assignment (Supplemental Table 4). There was no significant difference in the change in any liver-related outcome between participants who reduced TS and those who did not (Figure 3, Supplemental Figure 1, Supplemental Table 5) and no interaction by PNPLA3 genotype (9, Supplemental Figure 2).
FIGURE 3.
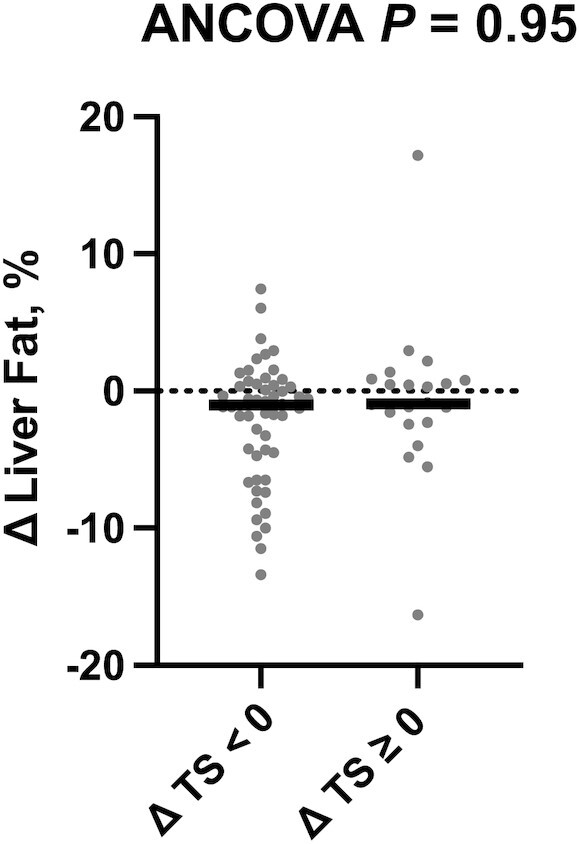
Reduction of TS does not affect liver fat fraction. Changes in liver fat fraction by change in sugar intake group (ΔTS < 0 group, n = 54; ΔTS ≥ 0 group, n = 22). The outcome variable is represented as the change variable calculated as the postintervention value minus the value at baseline. Each participant's change variable is represented by a solid dot. The median is represented by a horizontal bar. The P value for the ANCOVA adjusted for the baseline value of the outcome of interest, sex, and change in physical activity is displayed at the top of the boxplot. TS, total sugar intake.
Changes in liver outcomes as a function of change in FM
In exploratory analyses of pooled data, we examined liver outcomes in those participants who had a decrease in total whole-body FM (n = 38) relative to those without FM reduction (n = 43), regardless of intervention assignment (Supplemental Table 6). Participants who reduced FM over the course of the study had a decrease in liver fat fraction as compared with those who did not reduce their FM (adjusted P < 0.001) (Figure 4, Supplemental Table 7). Specifically, participants who decreased their FM averaged a 2.1% (IQR: −6.5% to −0.8%) reduction in liver fat fraction over the course of the study, whereas participants without FM reduction had a 0.3% (IQR: −1.0% to 1.1%) increase in liver fat fraction over the course of the study. This finding remained significant after removing outliers (changes in liver fat > 2 SD from the mean) (P = 0.04, data not shown). There were no significant differential changes in AST, ALT, or liver fibrosis between those who did or did not reduce FM during the intervention period. There was also no interaction by PNPLA3 genotype observed for any liver outcome (PAll > 0.3) (Supplemental Figure 2). Models that simultaneously assessed the impact of changes in FM (reduction compared with no reduction) and changes in TS (reduction compared with no reduction) on reducing liver fat further confirmed that reducing FM was significantly associated with reductions in liver fat (P < 0.01), whereas there was no association between reducing TS and changes in liver fat content (P = 0.99). In addition, there was no interaction between reducing FM and reducing TS on liver fat reduction (P = 0.58, data not shown).
FIGURE 4.
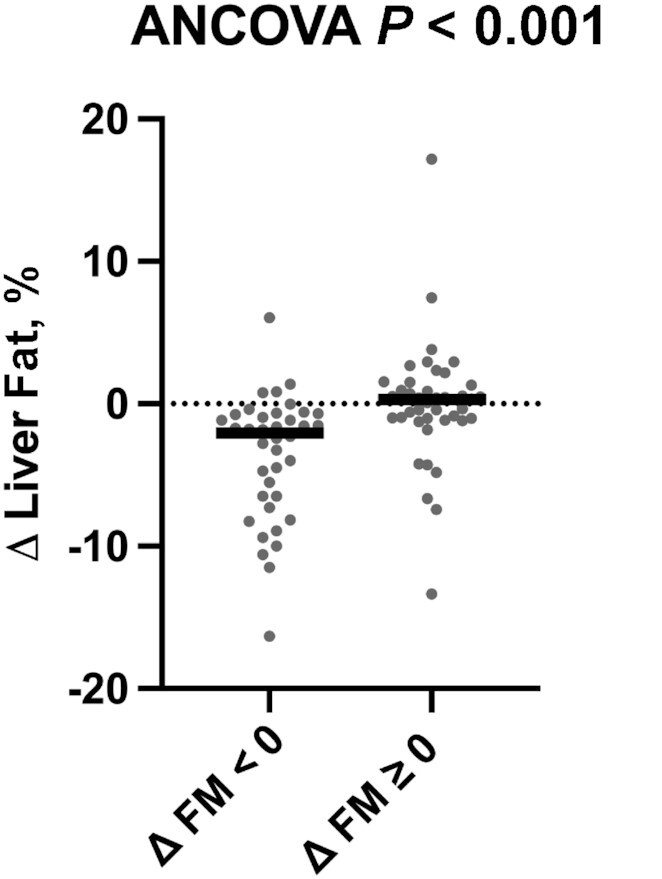
Reducing FM reduces liver fat. Changes in liver fat fraction by change in FM group (Δ FM < 0 group, n = 38; Δ FM ≥ 0 group, n = 43). The outcome variable is represented as the change variable calculated as the postintervention value minus the value at baseline. Each participant's change variable is represented by a solid dot. The median is represented by a horizontal bar. The P value for the ANCOVA adjusted for the baseline value of the outcome of interest, sex, and change in physical activity is displayed at the top of the boxplot. FM, fat mass.
Table 4 presents dietary intakes of participants in the FM reduction group compared with those without FM reduction, who were included in the analysis of change in liver fat. Changes in total energy intake or energy-adjusted intakes of carbohydrate, total sugar, added sugar, insoluble fiber, fat, SFAs, MUFAs, PUFAs, and protein did not differ between participants with or without FM reduction (PAll > 0.10). There was a trend for a difference in energy-adjusted and total soluble fiber intake (P = 0.05 and P = 0.06, respectively), with a greater decrease in soluble fiber intake in the group that did not reduce their FM than in the group that decreased their FM over the intervention. Similar results were obtained in analyses that included all participants who completed the postintervention assessment with complete diet data (Supplemental Table 8). However, the trends for differences in energy-adjusted and total soluble fiber intake became significant (P = 0.02 and P = 0.04, respectively). In addition, those who reduced their FM had a significant increase in total fiber intake as a percentage of energy compared with the group that did not reduce their FM (P = 0.02). Further, there was also a trend for an increase in insoluble fiber intake as a percentage of energy in the participants with FM reduction compared with those without (P = 0.05). Additionally, there was a trend for an increase in MUFA intake in the group without FM reduction as compared with the group that decreased their FM (P = 0.07).
TABLE 4.
Dietary intakes at baseline and change in intakes during the intervention period, based on unannounced 24-h dietary recalls, for participants who reduced their FM during the intervention period compared with those without FM reduction, included in the analysis of change in liver fat1
| Δ FM (kg) < 0 (n = 35) | Δ FM (kg) ≥ 0 (n = 41) | ANCOVA | GEEs | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Postintervention | Baseline | Postintervention | Raw | Adjusted | Adjusted | |
| Energy intake, kcal/d | 1615 [1254–1922] | −173 ± 514 | 1425 [1224–1739] | −44 ± 463 | 0.45 | 0.44 | 0.30 |
| Carbohydrates, %E | 48.4 [43.8–51.8] | 0.0 ± 10.7 | 49.7 [45.3–56.0] | −3.3 ± 10.6 | 0.15 | 0.18 | 0.18 |
| Total sugar, %E | 19.2 ± 6.4 | −2.7 ± 7.9 | 19.4 ± 6.9 | −4.0 ± 8.9 | 0.46 | 0.42 | 0.35 |
| Added sugars, %E | 12.1 ± 5.9 | −3.8 ± 6.5 | 12.1 ± 6.3 | −2.6 ± 8.0 | 0.32 | 0.28 | 0.28 |
| Free sugars, %E | 12.0 ± 6.2 | −3.6 ± 6.8 | 13.1 ± 7.0 | −4.1 ± 8.2 | 0.70 | 0.65 | 0.72 |
| Fiber, g/d | 14.1 [10.1–17.5] | 0.8 [−4.3 to 4.7] | 13.2 [8.9–18.9] | −1.6 [−5.1 to 2.6] | 0.09 | 0.15 | 0.13 |
| Fiber, %E | 3.5 [2.7–4.2] | 0.6 ± 1.7 | 3.5 [2.8–4.1] | −0.1 ± 2.0 | 0.07 | 0.10 | 0.11 |
| Soluble fiber, g/d | 4.0 [2.9–4.5] | 0.1 [−1.6 to 2.1] | 3.9 [2.5–5.1] | −0.5 [−2.0 to 0.9] | 0.03 | 0.06 | 0.03 |
| Soluble fiber, %E | 1.0 [0.8–1.2] | 0.1 [−0.2 to 0.6] | 1.0 [0.7–1.3] | 0.0 [−0.5 to 0.2] | 0.03 | 0.05 | 0.02 |
| Insoluble fiber, g/d | 10.1 [7.1–12.0] | −1.0 [−2.9 to 3.2] | 8.7 [5.6–12.1] | −1.3 [−2.8 to 1.8] | 0.27 | 0.35 | 0.31 |
| Insoluble fiber, %E | 2.3 [1.8–2.9] | 0.3 [−0.5 to 0.9] | 2.3 [1.9–3.0] | −0.2 [−0.8 to 0.6] | 0.10 | 0.13 | 0.13 |
| Fat, %E | 34.0 ± 5.9 | −0.7 ± 8.1 | 32.6 ± 5.9 | 2.5 ± 8.2 | 0.30 | 0.32 | 0.28 |
| SFAs, %E | 11.9 [9.9–13.4] | −0.1 ± 3.6 | 10.3 [8.0–12.6] | 0.9 ± 4.3 | 0.85 | 0.81 | 0.88 |
| MUFAs, %E | 11.4 [10.1–14.3] | −0.4 ± 3.9 | 12.0 [8.6–12.9] | 1.1 ± 4.0 | 0.16 | 0.17 | 0.10 |
| PUFAs, %E | 8.3 ± 2.3 | −0.4 ± 2.6 | 8.2 ± 2.5 | 0.5 ± 3.9 | 0.17 | 0.17 | 0.14 |
| Protein, %E | 16.7 ± 4.6 | 0.8 ± 5.1 | 17.2 ± 4.6 | 0.9 ± 4.8 | 0.93 | 0.99 | 0.94 |
n = 76. Values are means ± SDs or medians [IQRs] for nonnormally distributed variables. Raw: change adjusted for baseline value. Adjusted: raw model adjusted for sex, change in BMI (except the outcome energy intake), and change in physical activity. GEE analysis of adjusted model further adjusting for sibling clusters. FM, fat mass; GEE, generalized estimating equation; %E, percentage of total energy intake.
Discussion
Contrary to our primary hypothesis, a dietitian-led clinical intervention focused on reducing free sugar intake to ≤10% of total calories did not differentially decrease liver fat, liver fibrosis, liver enzymes, or alter body composition compared with general dietary advice in Latino adolescents with obesity and suspected NAFLD. Although sugar intake reduction was greater in the intervention group than in the control, both groups reduced their sugar intake and the difference between groups was only marginal. Therefore, the difference in sugar intake between the intervention and control groups may have been too small to produce meaningful differences in our outcomes of interest. In addition, our secondary analyses indicate that reduction in TS, independent of randomization, did not drive reductions in liver fat, liver fibrosis, or liver enzymes. Our analysis confirms that FM reduction is important to reduce liver fat.
Our findings differ from those of 2 previous intervention trials that investigated the effect of severe sugar restriction on liver fat content in pediatric populations (7, 8). Schwimmer et al. (7) randomly assigned 11- to 16-y-old boys with NAFLD (n = 40) to either a controlled-feeding intervention group with a free sugar intake < 3% of total calories, or a control group that maintained their typical diet for 8 wk. Schwarz et al. (8) tested the effect of a controlled-feeding intervention with a maximum fructose intake of 4% of total calories for 9 d as compared with their typical diet in 9- to 18-y-old boys and girls with obesity (n = 41). In both trials, the sugar restriction led to significant decreases in liver fat relative to control. There are some key differences between these trials and the trial reported here, which may explain the discrepancy in outcomes. The interventions in both Schwimmer et al. and Schwarz et al. had more extreme levels of sugar reduction (3%–4% compared with 10%) than our intervention. Further, to achieve this level of sugar reduction, both trials were designed as proof-of-concept efficacy studies and provided all foods to participants using tightly controlled feeding conditions. In contrast, our intervention took place in a real-world clinical setting where participants were guided by a dietitian to implement their intervention and make dietary choices independently at their homes. Therefore, our trial may not have found an effect of sugar reduction on liver fat and related outcomes because the behavioral change in sugar intake was too small. However, it remains unclear whether more extreme levels of sugar reduction, such as those prescribed in Schwimmer et al. and Schwarz et al., could be achieved in a real-world clinical setting where all foods are not provided. In addition, the control group in our trial was based on standard of care, where participants received materials describing a healthy diet. In contrast, the control groups in Schwimmer et al. and Schwarz et al. continued to consume their typical diet. In this way, our trial specifically focuses on whether a dietary sugar reduction intervention reduces liver fat beyond the current standard of care. Our results indicate that a dietitian-led intervention to reduce free sugar intake to ≤10% of total calories does not lead to greater reductions in liver fat than general healthful diet advice, at least in a real-world clinical setting with our study population.
One key finding from exploratory analyses in our trial is that reducing FM, regardless of changes in sugar intake or intervention group, is important to decrease liver fat. This finding is consistent with other lifestyle intervention trials assessing treatment strategies for pediatric NAFLD (35–43). These interventions included a variety of components such as caloric restriction (35, 36, 38, 40), carbohydrate restriction (35, 37), sugar restriction (39), fat restriction (37–39), healthful dietary pattern (41–43), exercise (35–42), psychological therapy (39, 42), and treatment sessions with a registered dietitian (35, 37, 41–43). Taken together, these findings suggest that a reduction in body fat, regardless of interventional approach, is key to reducing liver fat. Therefore, future treatment strategies could focus on identifying which FM-reducing dietary interventions are most feasible and sustainable for various pediatric populations with NAFLD.
The lack of significant interactions between PNPLA3 genotype and intervention on our outcomes of interest was not consistent with previous nutrigenetic evidence demonstrating higher liver fat accumulation among individuals with the GG genotype and high dietary sugar intake (16). These results may partially be explained by differences in PNPLA3 genotyping techniques. Specifically, our prior work used the TaqMan technique (44, 45), whereas the current trial used an RFLP analysis (29), which led to a larger number of participants being identified with the GG PNPLA3 genotype. The higher distribution of the GG PNPLA3 variant in our study population paired with the slight reduction in recruitment due to COVID-19-related challenges may have underpowered our ability to detect a PNPLA3 genotype-by-intervention interaction, because only 28% of participants were identified with the GC or CC PNPLA3 genotype. This may have been especially true because sugar reduction was even observed in the control group, further decreasing the potential for detecting an interaction with PNPLA3 genotype. However, the P values for a PNPLA3-by-intervention interaction in our analyses did not come close to approaching the threshold for a trend, suggesting that our null results were unlikely a result of the higher distribution of GG PNPLA3 participants in our study population. Nonetheless, to our knowledge, our study is the first to assess whether reducing sugar intake differentially affects liver health and anthropometric-related variables as a function of PNPLA3 genotype.
Some additional limitations to our study include the following. NAFLD status was not determined before study enrollment, which may have decreased our ability to detect an intervention effect. However, it is important to note that results from a sensitivity analysis including only participants with elevated liver fat at baseline (liver fat fraction ≥ 5.5%) were consistently null. It is also important to note that this sensitivity analysis was not defined a priori and, therefore, had a higher probability of a spurious result. In addition, ∼25% of participants already met the intervention free sugar intake target of ≤10% of total calories before study enrollment. Although this lowered our ability to determine whether reducing sugar intake itself affects our outcomes of interest, our study still provides important insight into the effectiveness of our intervention from a public health perspective given that the free sugar intake of our participants is representative of our target population. Therefore, limiting our population to those above the intervention free sugar threshold during recruitment could have arbitrarily inflated the effectiveness of the intervention from a real-world clinical perspective, the primary objective of this trial.
We also highlight several important strengths of our study. For example, liver fat and liver fibrosis were assessed by MRI and MRE, respectively. To our knowledge, a direct assessment of how sugar reduction affects liver fibrosis in a pediatric population has not previously been reported. Furthermore, we had high intervention compliance, with 81.6% of intervention participants reducing their free sugar intake and 71.4% meeting the goal of a free sugar intake maximum of 10% of total calories. In addition, we statistically controlled for changes in BMI and confirmed results with various sensitivity analyses. Limitations of this study include a lack of generalizability of the results to populations other than pediatric populations of Latino descent, and reliance on 24-h dietary recalls to assess dietary intake and intervention compliance, which can be subject to recall bias and underreporting. Lastly, we predominantly recruited from pediatric gastroenterology clinics and therefore cannot rule out whether participants received information on and implemented healthful dietary and lifestyle practices before study enrollment.
In conclusion, our study indicates that a dietary intervention implemented in a real-world clinical setting focused on promoting a reduction in free sugar intake to ≤10% of total calories did not affect liver fat, liver fibrosis, liver enzymes, or anthropometric variables more significantly than general healthful diet advice in Latino adolescents with obesity and elevated liver fat. Further, there was no intervention-by-PNPLA3 genotype interaction observed for any outcome of interest. However, our results do provide evidence that reducing FM is important for liver fat reduction and confirm current clinical recommendations to lose weight and follow a healthy diet for pediatric patients with NAFLD. Future studies will need to focus on identifying sustainable interventions that can be implemented in a real-world clinical setting to reduce FM in pediatric populations with NAFLD.
Supplementary Material
Acknowledgments
We also owe many thanks to the staff of the University of Southern California Diabetes and Obesity Research Institute. The authors’ responsibilities were as follows—KAS and RBJ are co-first authors of this manuscript; RBJ, PKB, JFP, TLA, JF, HA, RK, KSN, FRS, GH, S-JS, WJM, and MIG: designed the research; YC and CR: conducted the research; RBJ, KAS, WJM, and TAP: analyzed the data; RBJ, PKB, JFP, TLA, JF, and HH: contributed to the interpretation of the results; JAH and ZC: performed the PNPLA3 genotyping; KAS and MIG: wrote the paper; MIG: has primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by NIH/National Institute on Minority Health and Health Disparities (NIMHD) grants R01 MD010358 (to MIG) and UL1TR001855 (to MIG). PKB was supported in part by NIH grant K99 HD098288. S-JS was supported by NIMHD Obesity Health Disparities Research Center grant U54MD000502, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01HD092483, National Cancer Institute (NCI) grant R01CA258222, and the Hope Warschaw Center for Integrated Research in Cancer and Lifestyle Award.
Author disclosures: MIG is a scientific advisor for YUMI foods and receives book royalties from Penguin Random House for Sugarproof. All other authors report no conflicts of interest.
Supplemental Tables 1–8 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHLA, Children's Hospital Los Angeles; FM, fat mass; GEE, generalized estimating equation; MRE, magnetic resonance elastography; NAFLD, nonalcoholic fatty liver disease; PNPLA3, patatin-like phospholipase 3; RCT, randomized controlled trial; RDN, registered dietitian nutritionist; RFLP, restriction fragment length polymorphism; SAAT, subcutaneous abdominal adipose tissue; SSB, sugar-sweetened beverage; TS, total sugar intake; USC, University of Southern California; VAT, visceral adipose tissue.
Contributor Information
Kelsey A Schmidt, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Roshonda B Jones, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Claudia Rios, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Yesica Corona, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Paige K Berger, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Jasmine F Plows, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Tanya L Alderete, Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA.
Jennifer Fogel, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Hailey Hampson, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA; Department of Pediatrics, University of Southern California, Los Angeles, CA, USA.
Jaana A Hartiala, Department of Population and Public Health Sciences, University of Southern California, Los Angeles, CA, USA.
Zhiheng Cai, Department of Biochemistry & Molecular Medicine, University of Southern California, Los Angeles, CA, USA.
Hooman Allayee, Department of Population and Public Health Sciences, University of Southern California, Los Angeles, CA, USA; Department of Biochemistry & Molecular Medicine, University of Southern California, Los Angeles, CA, USA.
Krishna S Nayak, Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, USA; Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, USA.
Frank R Sinatra, Department of Pediatrics, University of Southern California, Los Angeles, CA, USA.
Gregory Harlan, Department of Pediatrics, University of Southern California, Los Angeles, CA, USA.
Trevor A Pickering, Department of Population and Public Health Sciences, University of Southern California, Los Angeles, CA, USA.
Sarah-Jeanne Salvy, Cancer Research Center for Health Equity, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Wendy Jean Mack, Department of Population and Public Health Sciences, University of Southern California, Los Angeles, CA, USA.
Rohit Kohli, Department of Pediatrics, Children's Hospital Los Angeles, Los Angeles, CA, USA; Division of Gastroenterology and Hepatology, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Michael I Goran, Department of Pediatrics, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, CA, USA.
Data Availability
An anonymized data set including all data described in the article, code book, and analytic code will be made available upon request to the principal investigator (MIG).
References
- 1. Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SAet al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi Ket al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. [DOI] [PubMed] [Google Scholar]
- 3. Pierantonelli I, Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103(1):e1–e13. [DOI] [PubMed] [Google Scholar]
- 4. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli Ret al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–406. [DOI] [PubMed] [Google Scholar]
- 6. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SKet al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367(15):1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KEet al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA. 2019;321(3):256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarz J-M, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VWet al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162(3):496–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. [DOI] [PubMed] [Google Scholar]
- 11. Rehm JL, Connor EL, Wolfgram PM, Eickhoff JC, Reeder SB, Allen DB. Predicting hepatic steatosis in a racially and ethnically diverse cohort of adolescent girls. J Pediatr. 2014;165(2):319–25.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JCet al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. [DOI] [PubMed] [Google Scholar]
- 14. Goran MI, Walker R, Le K-A, Mahurkar S, Vikman S, Davis JNet al. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes. 2010;59(12):3127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LAet al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davis JN, Lê K-A, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJet al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2010;92(6):1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy Set al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. [DOI] [PubMed] [Google Scholar]
- 18. Westat Inc . National Health and Nutrition Examination Survey III body measurements (anthropometry) manual. Rockville, MD: Westat Inc; 1988. [Google Scholar]
- 19. Lohman GT, Roche AF, Martorell R. Anthropometric standardization reference manual. Chicago, IL: Human Kinetics Books; 1988. [Google Scholar]
- 20. US Department of Health and Human Services (US DHHS), USDA . 2015–2020 Dietary Guidelines for Americans[Internet]. 8th ed. Washington (DC): US DHHS and USDA; 2015. Available from: https://health.gov/dietaryguidelines/2015/guidelines/. Accessed December 1st, 2016. [Google Scholar]
- 21. USDA . ChooseMyPlate.gov website. Washington (DC): USDA. Available from: http://www.myplate.gov, Accessed December 1st, 2016. [Google Scholar]
- 22. Hu HH, Nayak KS. Quantification of absolute fat mass using an adipose tissue reference signal model. J Magn Reson Imaging. 2008;28(6):1483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu HH, Kim H-W, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring). 2010;18(4):841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CBet al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24(7):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9(1):79–93. [DOI] [PubMed] [Google Scholar]
- 26. Feldstein AE, Wieckowska A, Lopez AR, Liu Y-C, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JBet al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26(3):347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29(1):138–43. [DOI] [PubMed] [Google Scholar]
- 29. Dutta A. A new PCR-RFLP method for diagnosing PNPLA3 rs738409 polymorphism. WebmedCentral GENETICS. 2012.3(7):WMC002928, 10.9754/journal.wmc.2012.002928 [DOI] [Google Scholar]
- 30. Walker RW, Sinatra F, Hartiala J, Weigensberg M, Spruijt-Metz D, Alderete TLet al. Genetic and clinical markers of elevated liver fat content in overweight and obese Hispanic children. Obesity (Silver Spring). 2013;21(12):E790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R Development Core Team . R: a language and environment for statistical computing. 2011; Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32. RStudio Team . RStudio: integrated development environment for R. Boston, MA: PBC; 2021. [Google Scholar]
- 33. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al.; CONSORT . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 34. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Soft. 2011;45(3):1–67. [Google Scholar]
- 35. Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola Det al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44(2):458–65. [DOI] [PubMed] [Google Scholar]
- 36. Grønbæk H, Lange A, Birkebæk NH, Holland-Fischer P, Solvig J, Hørlyck Aet al. Effect of a 10-week weight loss camp on fatty liver disease and insulin sensitivity in obese Danish children. J Pediatr Gastroenterol Nutr. 2012;54(2):223–8. [DOI] [PubMed] [Google Scholar]
- 37. Ramon-Krauel M, Salsberg SL, Ebbeling CB, Voss SD, Mulkern RV, Apura MMet al. A low-glycemic-load versus low-fat diet in the treatment of fatty liver in obese children. Child Obes. 2013;9(3):252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang C-L, Liang L, Fu J-F, Zou C-C, Hong F, Xue J-Zet al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14(10):1598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. 2009;94(6):437–42. [DOI] [PubMed] [Google Scholar]
- 40. Vajro P, Fontanella A, Perna C, Orso G, Tedesco M, De Vincenzo A. Persistent hyperaminotransferasemia resolving after weight reduction in obese children. J Pediatr. 1994;125(2):239–41. [DOI] [PubMed] [Google Scholar]
- 41. Pozzato C, Verduci E, Scaglioni S, Radaelli G, Salvioni M, Rovere Aet al. Liver fat change in obese children after a 1-year nutrition-behavior intervention. J Pediatr Gastroenterol Nutr. 2010;51(3):331–5. [DOI] [PubMed] [Google Scholar]
- 42. Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, Rijcken TH, Korevaar JC, van Aalderen WMet al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96(7):669–74. [DOI] [PubMed] [Google Scholar]
- 43. Tock L, Prado WL, Caranti DA, Cristofalo DM, Lederman H, Fisberg Met al. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol. 2006;18(12):1241–5. [DOI] [PubMed] [Google Scholar]
- 44. Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14(5–6):143–9. [DOI] [PubMed] [Google Scholar]
- 45. Livak KJ. SNP genotyping by the 5′-nuclease reaction. Methods Mol Biol. 2003;212:129–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An anonymized data set including all data described in the article, code book, and analytic code will be made available upon request to the principal investigator (MIG).