ABSTRACT
Background
Consuming live microbes in foods may benefit human health. Live microbe estimates have not previously been associated with individual foods in dietary databases.
Objectives
We aimed to estimate intake of live microbes in US children (aged 2–18 y) and adults (≥19 y) (n = 74,466; 51.2% female).
Methods
Using cross-sectional data from the NHANES (2001–2018), experts assigned foods an estimated level of live microbes per gram [low (Lo), <104 CFU/g; medium (Med), 104–107 CFU/g; or high (Hi), >107 CFU/g]. Probiotic dietary supplements were also assessed. The mean intake of each live microbe category and the percentages of subjects who ate from each live microbe category were determined. Nutrients from foods with live microbes were also determined using the population ratio method. Because the Hi category comprised primarily fermented dairy foods, we also looked at aggregated data for Med or Hi (MedHi), which included an expanded range of live microbe–containing foods, including fruits and vegetables.
Results
Our analysis showed that 52%, 20%, and 59% of children/adolescents, and 61%, 26%, and 67% of adults, consumed Med, Hi, or MedHi foods, respectively. Per capita intake of Med, Hi, and MedHi foods was 69, 16, and 85 g/d for children/adolescents, and 106, 21, and 127 g/d for adults, respectively. The proportion of subjects who consumed live microbes and overall per capita intake increased significantly over the 9 cycles/18-y study period (0.9–3.1 g/d per cycle in children across categories and 1.4 g/d per cycle in adults for the Med category).
Conclusions
This study indicated that children, adolescents, and adults in the United States steadily increased their consumption of foods with live microbes between the earliest (2001–2002) and latest (2017–2018) survey cycles. Additional research is needed to determine the relations between exposure to live microbes in foods and specific health outcomes or biomarkers.
Keywords: NHANES, fermented food, probiotics, live dietary microbes, International Scientific Association for Probiotics and Prebiotics, ISAPP
Introduction
Ingested microorganisms are increasingly recognized for their potential positive contributions to human health (1). There is strong evidence that probiotics, defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on their host” (2), are able to affect intestinal and systemic diseases and conditions (3). Epidemiologic and intervention studies on fermented foods containing live microbes (for example, yogurt and kimchi) have also indicated that consumption of those foods can improve metabolic and immune health (4–6). These observations are consistent with, and expand upon, the “old friends hypothesis” which states that exposure to commensal or nonharmful microbes in foods is an important, beneficial source of microbial stimuli for the immune system (7). Such immune regulatory activities may affect contemporary chronic immune, metabolic, and other “lifestyle” diseases linked to Western diets (8).
However, links between human health and ingested live microorganisms in whole diets have yet to be directly investigated. With few exceptions, studies on fermented foods have not separated health outcomes resulting from the contributions of live microorganisms in fermented foods from the effects of those foods as a whole (9). Moreover, living microbes are found not just in fermented foods, but also in a wide range of other foods. Although fermented foods that are not processed to remove or inactivate microbes frequently harbor >107 cells/g (10), microbial cell numbers can range from 106 to 108 CFU/g on raw, unpeeled fruits and vegetables (11–13). These cell quantities contrast with shelf-stable, processed foods that are commercially sterile or pasteurized and contain very low levels of viable microorganisms (<104 CFU/g) (14–16). Similarly, refrigerated pasteurized foods including milk (17) and deli meats (18) contain low cell numbers, at least before spoilage. Unlike nutrients such as proteins, carbohydrates, and fats, the microbial contents of foods are not available in dietary composition databases.
To address this gap in knowledge, we recently outlined steps to rigorously test the hypothesis that the regular consumption of safe, live microbes confers health-promoting properties that affect disease risk (19). We noted the need to use existing dietary databases and to conduct new prospective and randomized controlled trials to determine if there are quantifiable health benefits from consuming living microbes (19).
Whereas nutrient intakes from foods and beverages are available in dietary surveys such as What We Eat in America (WWEIA; the dietary component of the NHANES), numbers of live microbes must first be estimated for the most common foods and beverages eaten in America. Thus, our primary aim was to assess the number of live microbes that are consumed in the diet by estimating amounts in foods and beverages reported by NHANES participants, a necessary first step in estimating exposure of microbes from the diet.
Methods
Data set
NHANES is a large, ongoing, nationally representative, cross-sectional survey of the noninstitutionalized civilian population designed to monitor the nutritional, dietary, and health status of Americans. Currently, the data are continuously collected and released every 2 y by the National Center for Health Statistics (NCHS) of the CDC. Participants are selected using a complex, stratified, multistage cluster sampling probability design. Data are collected via an in-home interview for demographic and basic health information and a comprehensive diet and health examination in a mobile examination center. Detailed descriptions of the subject recruitment, survey design, and data collection procedures are available online (20). Because NHANES survey cycles are conducted using consistent state-of-the-art techniques and standardized procedures, extremely large data sets (>60,000 subjects) can be obtained by combining multiple cycles of data. All data obtained from this study are publicly available (20). The NHANES protocol was approved by the NCHS Ethics Review Board and all participants or proxies provided signed written informed consent. This study was a secondary data analysis that lacked personal identifiers and, therefore, was exempt from additional approvals by Institutional Review Boards.
Participants
Data from children (age 2–18 y) and adults (age ≥ 19 y) participating in 9 NHANES cycles (2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018) were extracted for the current analysis. However, participants with unreliable data, incomplete 24-h dietary recalls, and pregnant or lactating females were excluded (n = 10,163). The final sample size was 74,466 children and adults: 37,856 (51.2%) females and 36,610 (48.8%) males. Importantly, all surveys were conducted in the United States and reflect dietary habits only of US respondents. In addition, the US focus also affected how estimates were determined, because many fermented foods produced in the United States are heat-treated.
Dietary intakes
Dietary intakes were estimated using data from 24-h dietary recall which were collected through in-person interviews that asked participants detailed information about all foods and beverages, including amounts consumed on the previous day (midnight to midnight). Complete descriptions of the dietary interview methods for NHANES are provided elsewhere (20). Energy and nutrients for each food and beverage consumed were determined using the NHANES cycle–specific Food and Nutrient Database for Dietary Studies of the USDA (21, 22).
Estimating viable microbial cell numbers in foods and beverages
The estimated quantities of live microbes (per gram) for 9388 food codes contained in 48 subgroups in the NHANES database were determined by 4 experts in the field (MLM, MES, RH , and CH). Because of the expected variation in the numbers of living microorganisms in each food type, the foods were assigned to categories with ranges defined as low (Lo; <104 CFU/g), medium (Med; 104–107 CFU/g), or high (Hi; >107 CFU/g) levels of live microbes. These levels of Lo, Med, and Hi were chosen to reflect the approximate numbers of viable microbes expected to be present in pasteurized foods (<10 4 CFU/g), fresh fruits and vegetables eaten unpeeled (104–107 CFU/g), and unpasteurized fermented foods and probiotic supplements (>107 CFU/g).
As a first step, food subgroups estimated to contain only food codes having <104 CFU/g were identified by 3 individuals (MLM, MES, and RH) ( Supplemental Table 1). For these assessments, experts relied on reported values in the primary literature (10–15, 17, 18, 23–32), authoritative reviews (33), or inferred values based on known effects of food processing (for example, pasteurization) on microbial viability. Next, the remaining 6317 food codes contained in 25 food subgroups were assessed by the experts working in teams of 2. Team 1 (RH and CH) and Team 2 (MLM and MES) assessed 2856 and 3461 food codes, respectively, comprising subgroups indicated in Supplemental Table 1. Assignments were based on professional knowledge of the field and by reviewing primary publications that assessed the indicated foods. Disagreement between the 2 experts occurred for ∼150 food codes (Team 1) and ∼250 food codes (Team 2). For Team 1, most disagreements were for cheese and formulated foods such as salads, sandwiches, or dips containing cheese or cultured dairy foods. These were resolved by consulting the literature (34), the relative expected weight of the live microbe–containing food compared with the other ingredients (for example, bread), and the use of preservation methods (for example, pasteurization). For example, a sandwich containing processed American cheese was categorized in the Lo category. Sandwiches containing cheese and other major components (for example, steak) were similarly categorized in the Lo category. Cheddar cheese and general cheese sandwiches were labeled as Med, to take into account the weight of the bread and other potential condiments (for example, mayonnaise). For Team 2, 24 of these food codes were described as “pickled” fruits or vegetables. Reviewers subsequently reconciled these differences by consultation within and between the teams (MLM, MES, RH, and CH) and by external consultation with Fred Breidt, USDA Agricultural Research Service Microbiologist, Food Science and Market Quality and Handling Research Unit, Raleigh, NC.
Although “pickled” fruits or vegetables could be acidified and not fermented, the food descriptions were inadequate to distinguish between these 2 possibilities. The experts agreed to assume these were fermented or partially fermented in the case of refrigerated and non-heat-treated products and assigned all such pickled foods to Med. For the last step, intakes of Med and Hi categories were determined by linking microbe definitions to food codes. A fourth category was also developed, MedHi, consisting of an aggregate of consumers of foods from Med, Hi, or both Med and Hi categories.
Probiotic dietary supplements
Intakes of probiotic supplements were estimated using the dietary supplement questionnaire that was administered in person during the NHANES interview to assess use of vitamins, minerals, botanicals, and other dietary supplements over the past 30 d (20). The dietary supplement questionnaire queries the consumption frequency, duration, and dosage for each supplement reported. Interviewers ask participants to show all containers for the dietary supplements reported so that the dietary supplement name and other information can be entered into the survey collection tablet. The NCHS maintains a dietary supplements database that contains product label information obtained from manufacturers of dietary supplements reported in NHANES so that serving sizes, ingredients, and amounts are available for all dietary supplements reported by NHANES participants. All dietary supplements that included “probiotic” or a specific microbial genus or species (e.g., Bifidobacterium, Lactobacillus acidophilus) were included as a probiotic dietary supplement. For analysis purposes, only the percentage of the population using a probiotic, rather than the amount consumed, was determined. Probiotic supplements were not included in food analyses.
Statistics
All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc.). NHANES survey weights, strata, and primary sampling units were used in all calculations to adjust for oversampling of certain groups, nonresponse by some selected sample persons, and to adjust for the complex sample design of NHANES to ensure nationally representative estimates. Mean (± SE) amount consumed within the Med, Hi, and MedHi categories and the percentage of consumers with at least some consumption in those categories were determined and linear regression was used to estimate the average per-cycle linear change in intake (reported as β) across the 9 NHANES cycles under study, the P value for which is reported as a trend test (P-trend). The nutrient contributions (% ± SE) from the score categories Med, Hi, and MedHi were determined using the population ratio method.
Results
Assignment of Lo, Med, and Hi designations to food codes
Supplemental Table 2 lists the NHANES food codes and the assigned categories. Out of the 9388 food codes in the NHANES database, 8954 were estimated to contain low numbers of living microbes (<104 CFU/g). Processed foods that ordinarily are heat-treated (milk; prepared meat; pork, poultry, and seafood dishes; sauces and gravies) were considered to have very low levels of microorganisms and were assigned to Lo. Likewise, raw meat, pork, poultry, and seafood were assumed to be cooked before consumption and also were assigned to Lo (the exception being the few such foods stipulated as being consumed raw). Uncooked mixed salads, such as tuna, macaroni, and beef salad, and sushi, were assumed to be composed primarily of low-CFU/g components, and were assigned to Lo. Fresh fruit and vegetables peeled before consumption were assigned to the Lo category.
The top 2 foods assigned to Med were those composed of fresh vegetables and fruits: 41% and 39%, respectively. Fresh fruit juices, such as fruit smoothies, were assigned to Med. Beverages, condiments, and sauces provided >10% of the foods in Med. Some fermented foods (for example, miso and sauerkraut) were assigned to Med.
Fermented dairy products comprised the majority of foods assigned to Hi (Supplemental Table 2). Yogurts and other cultured milks were assigned to Hi unless present as a constituent of other foods. Codes containing a large content of fermented foods (such as yogurt or sour cream) were assigned to Hi. Most cheeses were assigned to Hi, except long-aged cheese (e.g., Parmesan) (10), pizza-type cheeses that are typically heated before consumption, and American (processed) cheese, which is a pasteurized product. Cheese-containing sandwiches (unless made with American cheese or heated) were assigned to Hi. Foods containing cheese as a minor component were assigned to either Lo or Med, depending on their relative quantity in the food product.
Consumption of live microbes
The numbers of individuals consuming live microbes and their per capita intakes were examined based on the food code assignments of Lo, Med, and Hi. In order to span the range of foods with live microbes, we also analyzed MedHi. Approximately 52%, ∼20%, and ∼59% of children/adolescents (age 2–18 y), and ∼61%, ∼26%, and ∼67% of adults (age ≥ 19 y) were consumers of Med, Hi, and MedHi, respectively (Table 1). Only 0.81% of children/adolescents (age 2–18 y) and 2.30% of adults (age ≥ 19 y) were consumers of probiotic supplements (Table 1). Per capita intake of Med, Hi, and MedHi was 69, 16, and 85 g/d, respectively, for children/adolescents (age 2–18 y), and 106, 21, and 127 g/d, respectively, for adults (age ≥ 19 y) (Table 2). Approximately 12% more children (age 2–8 y) than adolescents (age 9–18 y) and 8% more older adults (age ≥ 51 y) than younger adults (age 19–50 y) were consumers of MedHi, and these groups had ∼7% and ∼14% higher intakes of MedHi foods, respectively.
TABLE 1.
Percentages of all subjects by age group with intake of Med, Hi, and MedHi foods and consuming a probiotic supplement1
| Age, y | n | Med | Hi | MedHi | Probiotic supplement |
|---|---|---|---|---|---|
| ≥2 | 74,466 | 59.0 ± 0.5 | 24.4 ± 0.4 | 65.1 ± 0.5 | 1.95 ± 0.15 |
| 2–18 | 28,375 | 52.3 ± 0.6 | 20.3 ± 0.6 | 59.2 ± 0.6 | 0.81 ± 0.14 |
| 2–8 | 11,626 | 55.0 ± 0.9 | 23.8 ± 0.8 | 63.1 ± 0.8 | 1.29 ± 0.24 |
| 9–18 | 16,749 | 50.5 ± 0.7 | 18.0 ± 0.6 | 56.4 ± 0.7 | 0.47 ± 0.12 |
| ≥19 | 46,091 | 61.0 ± 0.5 | 25.7 ± 0.5 | 67.0 ± 0.5 | 2.30 ± 0.17 |
| 19–50 | 25,071 | 58.6 ± 0.6 | 25.8 ± 0.5 | 64.6 ± 0.6 | 1.70 ± 0.17 |
| ≥51 | 21,020 | 64.5 ± 0.6 | 25.6 ± 0.6 | 70.3 ± 0.6 | 3.16 ± 0.30 |
Values are % ± SE of consumers. Sex-combined data from NHANES 2001–2018. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Med, estimated to contain 104–107 CFU/g; Hi, estimated to contain >107 CFU/g; MedHi, estimated to contain >104 CFU/g.
TABLE 2.
Per capita intake for specific age groups of Med, Hi, and MedHi foods1
| Age, y | n | Med, g/d | Hi, g/d | MedHi, g/d |
|---|---|---|---|---|
| ≥2 | 74,466 | 97.1 ± 1.6 | 20.0 ± 0.5 | 117 ± 2.0 |
| 2–18 | 28,375 | 69.0 ± 1.5 | 16.4 ± 0.5 | 85.4 ± 1.7 |
| 2–8 | 11,626 | 66.2 ± 1.8 | 22.5 ± 1.0 | 88.8 ± 2.2 |
| 9–18 | 16,749 | 70.9 ± 1.9 | 12.3 ± 0.6 | 83.2 ± 2.0 |
| ≥19 | 46,091 | 106 ± 2.0 | 21.2 ± 0.6 | 127 ± 2.0 |
| 19–50 | 25,071 | 98.2 ± 2.0 | 20.5 ± 0.7 | 119 ± 2.0 |
| ≥51 | 21,020 | 117 ± 2.0 | 22.1 ± 0.8 | 139 ± 3.0 |
Values are mean ± SEM. Sex-combined data from NHANES 2001–2018. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Hi, estimated to contain >107 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g.
Trends of live microbe consumption
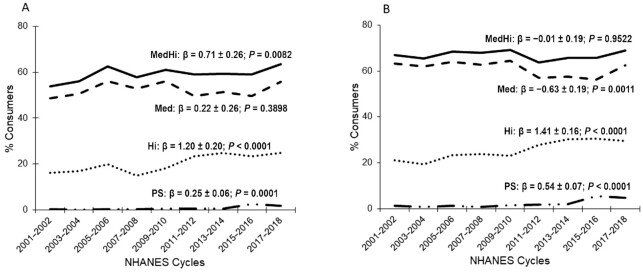
The fraction (percentage unit change/cycle) of children/adolescents (age 2–18 y) consuming live microbe–containing foods significantly increased from 2001–2002 to 2017–2018 for score categories Hi (β = 1.20 ± 0.20, P-trend < 0.0001) and MedHi (β = 0.71 ± 0.26, P-trend = 0.0082) but did not change for score category Med (P-trend = 0.3898) (Figure 1). The percentage of adults (age ≥ 19 y) consuming foods in specific live microbe categories significantly increased for Hi (β = 1.41 ± 0.16, P-trend < 0.0001), significantly decreased for Med (β = −0.63 ± 0.19, P-trend = 0.0011), and did not change for MedHi (P-trend = 0.9522) during the 18 y under study (Figure 1). The proportion of participants consuming probiotic supplements also increased significantly over the 18 y (β = 0.25 ± 0.06, P-trend = 0.0001 for age 2–18 y; β = 0.54 ± 0.07, P-trend < 0.0001 for age ≥ 19 y) (Figure 1).
FIGURE 1.
Percentages of children (age 2–18 y; n = 28,373) (A) and adults (age ≥ 19 y; n = 45,088) (B) with intake of live microbe–containing foods (Med, Hi, and MedHi) and percentages of subjects consuming a PS by NHANES study periods. Sex-combined data. All β and P values represent regression coefficients and significance for change over time. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Hi, estimated to contain >107 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g; PS, probiotic supplement.
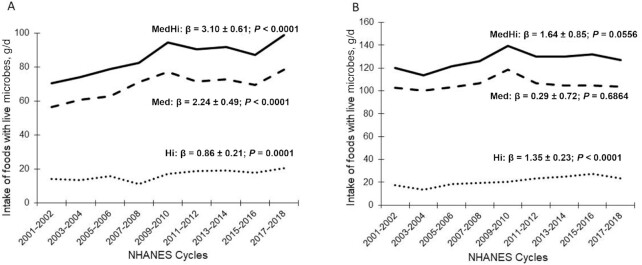
Per capita intake (g/d per cycle) significantly increased for Med (β = 2.24 ± 0.49, P-trend < 0.0001), Hi (β = 0.86 ± 0.21, P-trend = 0.0001), and MedHi (β = 3.10 ± 0.61, P-trend < 0.0001) for children/adolescents (age 2–18 y) over the last 9 cycles of NHANES (Figure 2). For adults (age ≥ 19 y), per capita intake significantly increased for Hi (β = 1.35 ± 0.23, P-trend < 0.0001), but did not change for Med (P-trend = 0.6864) and MedHi (P-trend = 0.0556), over the last 9 cycles of NHANES (Figure 2).
FIGURE 2.
Per capita intake of live microbe–containing foods (Med, Hi, and MedHi) for children (age 2–18 y; n = 28,373) (A) and adults (age ≥ 19 y; n = 45,088) (B) by NHANES study period. Sex-combined data. All β and P values represent regression coefficients and significance for change over time. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Hi, estimated to contain >107 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g.
Food groups providing live microbes
Vegetables, fruits, and milk and dairy were the top 3 food groups contributing live microbes to the diet. Over 85% of food codes assigned Med or Hi contained vegetables, fruits, or milk and dairy. Vegetables and fruits were the top 2 sources of Med foods, providing 41% and 39%, respectively, whereas milk and dairy provided >93% of Hi foods (Table 3). Beverages, and condiments and sauces ranked next after vegetables, fruits, and milk and dairy, providing >9% of MedHi foods and >10% of Med foods. Fats and oils provided ∼5% of Hi foods (Table 3).
TABLE 3.
Contribution of WWEIA main food groups to intake of Med, Hi, and MedHi foods1
| Med | Hi | MedHi | ||||
|---|---|---|---|---|---|---|
| WWEIA food groups (40) | Amount, g/d | % Daily2 | Amount, g/d | % Daily2 | Amount, g/d | % Daily2 |
| Vegetables | 39.9 ± 0.8 | 41.1 ± 0.5 | 0.05 ± 0.01 | 0.24 ± 0.04 | 39.9 ± 0.8 | 34.1 ± 0.4 |
| Fruit | 37.9 ± 0.7 | 39.0 ± 0.4 | 0 | 0 | 37.9 ± 0.7 | 32.3 ± 0.4 |
| Milk and dairy | 3.86 ± 0.12 | 3.97 ± 0.12 | 18.7 ± 0.5 | 93.4 ± 0.4 | 22.6 ± 0.5 | 19.3 ± 0.3 |
| Beverages | 6.13 ± 0.43 | 6.31 ± 0.42 | 0 | 0 | 6.13 ± 0.43 | 5.23 ± 0.35 |
| Condiments and sauces | 4.79 ± 0.16 | 4.94 ± 0.16 | 0.19 ± 0.03 | 0.96 ± 0.14 | 4.98 ± 0.17 | 4.26 ± 0.14 |
| Mixed dishes | 1.79 ± 0.14 | 1.84 ± 0.14 | 0 | 0 | 1.79 ± 0.14 | 1.53 ± 0.11 |
| Sugars | 1.32 ± 0.08 | 1.36 ± 0.08 | 0.03 ± 0.01 | 0.15 ± 0.05 | 1.35 ± 0.08 | 1.15 ± 0.07 |
| Snacks and sweets | 1.33 ± 0.10 | 1.37 ± 0.10 | 0 | 0 | 1.33 ± 0.10 | 1.13 ± 0.08 |
| Fats and oils | 0 | 0 | 0.97 ± 0.06 | 4.85 ± 0.29 | 0.97 ± 0.06 | 0.83 ± 0.05 |
| Protein foods | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.08 ± 0.03 | 0.39 ± 0.16 | 0.15 ± 0.04 | 0.13 ± 0.03 |
| Other | 0.06 ± 0.01 | 0.06 ± 0.01 | 0 | 0 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| Baby foods and formulas | 0 | 0 | 0.005 ± 0.003 | 0.02 ± 0.02 | 0.005 ± 0.003 | 0.004 ± 0.003 |
| Grains | 0 | 0 | 0 | 0 | 0 | 0 |
| Alcoholic beverages | 0 | 0 | 0 | 0 | 0 | 0 |
| Water | 0 | 0 | 0 | 0 | 0 | 0 |
Sex-combined data from children and adults age ≥ 2 y (n = 74,461) from NHANES 2001–2018. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Hi, estimated to contain >107 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g; WWEIA, What We Eat in America.
Represents the percentage of the total amount of Med, Hi, and MedHi foods consumed using the population ratio method.
Nutrient contribution by live microbe–containing foods
Foods estimated to contain >104 CFU/g live microbes (i.e., MedHi foods) provided >5% of daily nutrients obtained from foods for dietary fiber (9.1%), total sugars (5.6%), calcium (8.7%), phosphorus (5.6%), potassium (6.4%), vitamin A (9.9%), vitamin C (10.3%), and vitamin K (14.8%) in children/adolescents (age 2–18 y), and protein (5.4%), total fat (5.2%), dietary fiber (10.7%), total sugars (6.9%), calcium (12.6%), copper (5.4%), magnesium (5.6%), phosphorus (7.1%), potassium (8.4%), zinc (5.6%), vitamin A (15.3%), riboflavin (5.4%), vitamin C (16.2%), vitamin E (5.5%), and vitamin K (25.2%) in adults (age ≥ 19 y) (Table 4). Foods estimated to contain >104 CFU/g live microbes also provided 3.7% and 4.5% of daily energy in children/adolescents (age 2–18 y) and adults (age ≥ 19 y), respectively (Table 4).
TABLE 4.
Nutrient contribution of MedHi food codes among children (age 2–18 y) and adults (age ≥ 19 y)1
| Children (n = 28,373) | Adults (n = 46,088) | |||
|---|---|---|---|---|
| Nutrient | Amount | % Daily | Amount | % Daily |
| Energy, kcal/d | 72.7 ± 1.4 | 3.73 ± 0.07 | 98.3 ± 1.7 | 4.54 ± 0.08 |
| Carbohydrate, g/d | 9.41 ± 0.22 | 3.63 ± 0.08 | 11.5 ± 0.2 | 4.43 ± 0.08 |
| Protein, g/d | 3.03 ± 0.07 | 4.41 ± 0.10 | 4.44 ± 0.09 | 5.36 ± 0.10 |
| Total fat, g/d | 2.91 ± 0.08 | 3.99 ± 0.10 | 4.33 ± 0.09 | 5.20 ± 0.11 |
| Dietary fiber, g/d | 1.22 ± 0.03 | 9.10 ± 0.19 | 1.79 ± 0.3 | 10.7 ± 0.10 |
| Total sugars, g/d | 7.15 ± 0.18 | 5.63 ± 0.14 | 8.06 ± 0.16 | 6.87 ± 0.14 |
| Added sugars, tsp eq/d | 0.31 ± 0.01 | 1.63 ± 0.07 | 0.32 ± 0.01 | 1.72 ± 0.06 |
| Calcium, mg/d | 87.7 ± 2 | 8.69 ± 0.18 | 120 ± 2 | 12.6 ± 0.2 |
| Copper, mg/d | 0.04 ± 0.001 | 4.37 ± 0.09 | 0.07 ± 0.001 | 5.35 ± 0.09 |
| Iron, mg/d | 0.24 ± 0.01 | 1.69 ± 0.04 | 0.46 ± 0.01 | 3.07 ± 0.05 |
| Magnesium, mg/d | 10.0 ± 0.2 | 4.33 ± 0.08 | 16.9 ± 0.3 | 5.64 ± 0.08 |
| Phosphorus, mg/d | 70.1 ± 1.5 | 5.59 ± 0.12 | 98.1 ± 1.9 | 7.12 ± 0.12 |
| Potassium, mg/d | 139 ± 3 | 6.36 ± 0.13 | 226 ± 4 | 8.38 ± 0.13 |
| Selenium, μg/d | 2.21 ± 0.06 | 2.36 ± 0.06 | 3.40 ± 0.08 | 3.01 ± 0.07 |
| Sodium, mg/d | 93.9 ± 2.4 | 3.06 ± 0.07 | 149 ± 3 | 4.16 ± 0.08 |
| Zinc, mg/d | 0.44 ± 0.01 | 4.22 ± 0.09 | 0.66 ± 0.02 | 5.60 ± 0.14 |
| Vitamin A, RE/d | 58.0 ± 2.1 | 9.89 ± 0.32 | 97.1 ± 2.4 | 15.3 ± 0.3 |
| Thiamin, mg/d | 0.03 ± 0.001 | 2.01 ± 0.04 | 0.05 ± 0.001 | 3.21 ± 0.06 |
| Riboflavin, mg/d | 0.08 ± 0.002 | 4.02 ± 0.08 | 0.12 ± 0.002 | 5.35 ± 0.10 |
| Niacin, mg/d | 0.23 ± 0.01 | 1.09 ± 0.03 | 0.45 ± 0.01 | 1.78 ± 0.04 |
| Folate, DFE, μg/d | 11.0 ± 0.3 | 2.11 ± 0.05 | 25.0 ± 0.5 | 4.64 ± 0.08 |
| Vitamin B-6, mg/d | 0.05 ± 0.001 | 3.19 ± 0.07 | 0.10 ± 0.002 | 4.65 ± 0.08 |
| Vitamin B-12, μg/d | 0.18 ± 0.005 | 3.78 ± 0.10 | 0.26 ± 0.01 | 4.92 ± 0.11 |
| Vitamin C, mg/d | 8.18 ± 0.3 | 10.3 ± 0.3 | 13.7 ± 0.3 | 16.2 ± 0.3 |
| Vitamin D, μg/d | 0.15 ± 0.005 | 2.71 ± 0.08 | 0.20 ± 0.01 | 4.25 ± 0.11 |
| Vitamin E (ATE), mg/d | 0.24 ± 0.01 | 3.68 ± 0.09 | 0.46 ± 0.01 | 5.53 ± 0.10 |
| Vitamin K, μg/d | 8.86 ± 0.43 | 14.8 ± 0.6 | 27.8 ± 0.7 | 25.2 ± 0.5 |
| Total choline,2 mg/d | 7.75 ± 0.19 | 3.10 ± 0.08 | 12.8 ± 0.3 | 3.81 ± 0.08 |
Values are mean ± SE. Sex-combined data from NHANES 2001–2018. Hi and Med were categories assigned to food codes; MedHi represented aggregated consumers of foods from Med, Hi, or both Med and Hi. Hi, estimated to contain >107 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g. ATE, alpha tocopherol equivalents; DFE, dietary folate equivalents; RE, retinol equivalents.
n for total choline was 20,781 and 36,376 for children and adults, respectively.
Discussion
We previously proposed the need to interrogate nationally representative databases to determine if there are quantifiable benefits from consuming live dietary microbes (19). In this study, we completed the first step in this process by estimating the numbers of live microbes present in foods as well as the number of live microbes consumed. Our cross-sectional analysis examined dietary data across 9 NHANES cycles and showed that >50% of children and adults were consumers of Hi foods. In addition, in general, the proportions of children and adults who consumed live microbes and overall per capita intake increased significantly from 2001–2002 to 2017–2018.
To our knowledge, only 1 prior study has attempted to enumerate the numbers of microbes consumed in different diets (35). Examination of 3 meal plans showed that 2 of the plans provided <107 CFU/d, whereas the third meal plan, which included yogurt, provided >109 CFU/d. These results contrast with the levels of ingested microbes (between 108 and 1012 CFU/d) from fermented foods and probiotics estimated by Derrien and van Hylckama Vlieg (36). Our results build significantly upon these studies by estimating the number of live microbes present in all foods contained in a major dietary database and using that estimate to determine the approximate number of live microbes consumed by children and adults in the US population. We showed that the estimated intakes of foods with live microbes were seemingly low (∼85 and 127 g/d for children and adults, respectively) despite consumer interest in fermented foods. These estimates are similar to those observed in the study by Lang et al. (35), indicating that the average diet lacks consistent sources of fermented foods.
In our analysis, fruits, vegetables, and fermented dairy were the top 3 food groups providing live microbes to the diet. The finding that fermented dairy products were one of the primary sources of live microbes was not surprising because of their association with health-promoting microorganisms since the beginning of the 20th century. In contrast, fruits and vegetables were the main sources of live microbes in this study despite pre- and postharvest approaches to minimizing bacterial load for food safety purposes. Although previous work has reported that the microbial contents of fruits and vegetables were often <106 CFU/g (23), the amounts consumed by average US children and adults make these food groups an important source of live microbes. Beyond providing live microbes, the data also showed that these foods contributed a meaningful amount of key nutrients that are lacking in the diets of children and adults, including 3 of the 4 nutrients of public health concern—calcium, fiber, and potassium—as defined by the Dietary Guidelines Advisory Committee (37). Indeed, the most recent Dietary Guidelines for Americans noted that both children and adults do not meet the recommendations for fruits, vegetables, and dairy (38). Therefore, simply meeting dietary recommendations for these food groups would increase the numbers of live microbes and amounts of essential nutrients consumed in the diet. Further, it is reasonable to hypothesize based on the evidence presented here that a recommendation for specific fermented foods within the fruit, vegetable, and dairy food groups would lead to greater intakes of safe, beneficial microbes as well.
The strengths of this study include the use of a large nationally representative sample that included both children and adults, for whom live microbe consumption was estimated over a long period of time. Another strength of the present study was that we examined whole-food consumption, based on detailed 24-h dietary recall, to estimate the numbers of live microbes in foods and in the diet.
However, we also recognize the challenges of our approach. A major limitation of this study is the use of a cross-sectional study design, which cannot be used to determine cause and effect nor the effects of temporal changes in diet over time. The dietary intake data in NHANES are also self-reported relying on memory and are potentially subject to reporting bias. Moreover, although we accounted for a number of covariates in our statistical models, residual confounding cannot be ruled out. Further, our analysis is specific to the United States; categorization of foods and the intake of live microbes may be very different in other places of the world.
Further, another limitation was that we used a general approach to estimating the numbers of microbes present on food by assigning defined levels (<104, 104–107, and >107 CFU/g) to each food based on previous studies and expert opinion. Although this enabled us to make an estimate of microbes in the diet, we appreciate the imprecision of these values. We also recognize that the present analysis lacks specificity with regard to the total numbers and types of dietary microbes. The types and numbers of live microbes present in food—which include bacteria, yeasts, and molds—will vary depending on the food, the extent of food processing that has occurred, and, if refrigerated, the length of time it is stored before consumption. For example, all fermented foods not processed postfermentation to eliminate or kill microbes were placed in the “Hi” category, but even within individual food groups (e.g., yogurt) the numbers of live microbes can vary greatly. In addition, the specific microbial species present differ across food types. Whereas most of the microbes in yogurt and cultured dairy products are members of the lactic acid bacteria, microbes present on the surfaces of fruits and vegetables are more diverse and typically include bacteria from the Pseudomonadota phylum (formerly Proteobacteria). Moreover, for pasteurized foods, ready-to-eat meats, and ready-to-eat vegetables, growth of microbes (including lactic acid and non–lactic acid bacteria) can occur during storage at retail or in the home. Thus, it is possible that the products scored as Low (<104 CFU/g) could have approached >107 CFU/g by the time of consumption. Nonetheless, the positive contribution of these latter organisms to human health has not been extensively investigated with the exception of the probiotic Escherichia coli Nissle (39). Likewise, microorganisms can also die during storage, so even foods considered as High may drop below 107 CFU/g over time. However, given that the actual numbers of live microbes present in fermented foods are rarely reported on food labels nor are they available in NHANES, this represented the most reliable way to estimate intakes at the present time.
In conclusion, this study showed that children and adults have steadily increased their consumption of foods with live microbes over an 18-y period of time. Linking food or nutrients with health requires the ability to estimate the amount of the food or nutrient of interest consumed by the test population. The same is true of live microbes. Assessing the intake of live microbes in diets is critical for determining the relation between live microbes and health. This study represents the first estimate of the numbers of live microbes in foods and diets consumed by US children and adults. Future work should analyze the data by age, sex, and race to understand live microbe consumption and to explore if there are differences between subgroups of the US population. Future research is also needed that examines the relations between the consumption of live microbes in foods and specific health outcomes or biomarkers. These additional studies will further elucidate the role dietary microbes play in health and help move us closer to making science-based dietary recommendations on live microbes.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CH, RH, MLM, and MES: assigned categories to all NHANES food codes; VLF: conducted the NHANES analysis, wrote most of the Results and Methods sections, and developed most of the figures and tables; MES: prepared Supplemental Table 1, wrote part of the Methods section and the key takeaways, and compiled the paper; MLM: wrote the introduction; JG: wrote the abstract; CJC: wrote the discussion with help from JLS; MES and MLM: did extensive editing; and all authors: developed the concept of the project, edited the paper, and read and approved the final manuscript.
Notes
The International Scientific Association for Probiotics and Prebiotics (ISAPP) funded the NHANES analysis, which was conducted by Nutrition Impact, Battle Creek, MI. The ISAPP had no role in the design, implementation, analysis, or interpretation of the data.
Author disclosures: CH serves in the nonpaid position of member of the ISAPP board of directors. DM has consulted for Bayer and Howaru, has done legal work for VSL#3, and serves in the nonpaid position of president of the ISAPP board of directors. CJC serves as Vice President of Nutrition Research, National Dairy Council. DJT has served as a statistical consultant for IFF and Synbiotic Health, and serves in the nonpaid position of member of the ISAPP board of directors. MES has been compensated for consulting, giving presentations, or serving on advisory boards for Associated British Foods, Bayer, Bill and Melinda Gates Foundation, Bloom, California Dairy Research Foundation, Cargill, Church & Dwight, Danone North America, Danone Research, Fairlife, GlaxoSmithKline, Kerry, Mead Johnson, Omnibiotic/Allergosan, Pepsico, Probi, Sanofi, Trouw Nutrition, Winclove Probiotics, and Yakult. MLM serves in the nonpaid position of vice president of the ISAPP board of directors. RH has received recent grants or honoraria from Mead Johnson Nutrition, Pharmavite, and Danone; is a co-owner of Synbiotic Health; and serves in the nonpaid position of member of the ISAPP board of directors. VLF serves as Senior Vice-President of Nutrition Impact, provides food and nutrition consulting services for food and beverage companies, and also conducts analyses of NHANES data for members of the food industry. All other authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Hi, estimated to contain >107 CFU/g; Lo, estimated to contain <104 CFU/g; Med, estimated to contain 104–107 CFU/g; MedHi, estimated to contain >104 CFU/g; NCHS, National Center for Health Statistics.
Contributor Information
Maria L Marco, Department of Food Science and Technology, University of California Davis, Davis, CA, USA.
Robert Hutkins, Department of Food Science and Technology, University of Nebraska–Lincoln, Lincoln, NE, USA.
Colin Hill, APC Microbiome Ireland, University College Cork, Cork, Ireland; School of Microbiology, University College Cork, Cork, Ireland.
Victor L Fulgoni, III, Nutrition Impact, LLC, Battle Creek, MI, USA.
Christopher J Cifelli, National Dairy Council, Rosemont, IL, USA.
Jaime Gahche, Office of Dietary Supplements, NIH, Bethesda, MD, USA.
Joanne L Slavin, Department of Food Science and Nutrition, University of Minnesota, St. Paul, MN, USA.
Daniel Merenstein, Department of Family Medicine, Georgetown University School of Medicine, Washington, DC, USA.
Daniel J Tancredi, Department of Pediatrics, University of California Davis School of Medicine, Sacramento, CA, USA.
Mary E Sanders, International Scientific Association for Probiotics and Prebiotics, Centennial, CO, USA.
References
- 1. Roselli M, Natella F, Zinno P, Guantario B, Canali R, Schifano Eet al. Colonization ability and impact on human gut microbiota of foodborne microbes from traditional or probiotic-added fermented foods: a systematic review. Front Nutr. 2021;8:689084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot Bet al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 3. Merenstein DJ, Sanders ME, Tancredi DJ. Probiotics as a Tx resource in primary care. J Fam Pract. 2020;69(3):E1–E10. [PubMed] [Google Scholar]
- 4. Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11(8):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: a systematic review. Nutr Rev. 2021;79(5):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FBet al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rook GA. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margina D, Ungurianu A, Purdel C, Tsoukalas D, Sarandi E, Thanasoula Met al. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int J Environ Res Public Health. 2020;17(11):4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, De Vuyst Let al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. 2021;18(3):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezac S, Kok CR, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeddi MZ, Yunesian M, Gorji ME, Noori N, Pourmand MR, Khaniki GRJ. Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J Health Popul Nutr. 2014;32(3):391–9. [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston LM, Jaykus LA, Moll D, Martinez MC, Anciso J, Mora Bet al. A field study of the microbiological quality of fresh produce. J Food Prot. 2005;68(9):1840–7. [DOI] [PubMed] [Google Scholar]
- 13. Valentin-Bon I, Jacobson A, Monday SR, Feng PC. Microbiological quality of bagged cut spinach and lettuce mixes. Appl Environ Microbiol. 2008;74(4):1240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Institute of Food Technologists . Evaluation and definition of potentially hazardous foods. A Report of the Institute of Food Technologists for the Food and Drug Administration of the United States Department of Health and Human Services. [Internet]. Silver Spring, MD: FDA; 2001[cited 3 July, 2020]. Available from: https://www.fda.gov/files/food/published/Evaluation-and-Definition-of-Potentially-Hazardous-Foods.pdf. [Google Scholar]
- 15. Feroz F, Shimizu H, Nishioka T, Mori M, Sakagami Y. Bacterial and fungal counts of dried and semi-dried foods collected from Dhaka, Bangladesh, and their reduction methods. Biocontrol Sci. 2016;21(4):243–51. [DOI] [PubMed] [Google Scholar]
- 16. Alp D, Bulantekin Ö. The microbiological quality of various foods dried by applying different drying methods: a review. Eur Food Res Technol. 2021;247:1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziyaina M, Govindan BN, Rasco B, Coffey T, Sablani SS. Monitoring shelf life of pasteurized whole milk under refrigerated storage conditions: predictive models for quality loss. J Food Sci. 2018;83(2):409–18. [DOI] [PubMed] [Google Scholar]
- 18. Montville R, Schaffner DW. Statistical distributions describing microbial quality of surfaces and foods in food service operations. J Food Prot. 2004;67(1):162–7. [DOI] [PubMed] [Google Scholar]
- 19. Marco ML, Hill C, Hutkins R, Slavin J, Tancredi DJ, Merenstein Det al. Should there be a recommended daily intake of microbes?. J Nutr. 2020;150(12):3061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CDC . National Health and Nutrition Examination Survey. [Internet]. Atlanta, GA: CDC; 2021[cited 9 December, 2021]. Available from: http://www.cdc.gov/nchs/nhanes/. [Google Scholar]
- 21. Haytowitz DB, Ahuja JKC, Wu Z, Somanchi M, Nickle M, Nguyen QAet al. USDA National Nutrient Database for Standard Reference, Legacy Release. [Internet]. Beltsville, MD: USDA Agricultural Research Service; 2019[cited 9 December, 2021]. Available from: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release. [Google Scholar]
- 22. USDA Agricultural Research Service (ARS) . USDA Food and Nutrient Database for Dietary Studies. [Internet]. Beltsville, MD: USDA ARS; 2021[cited 9 December, 2021]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/. [Google Scholar]
- 23. Becker B, Stoll D, Schulz P, Kulling S, Huch M. Microbial contamination of organically and conventionally produced fresh vegetable salads and herbs from retail markets in Southwest Germany. Foodborne Pathog Dis. 2019;16(4):269–75. [DOI] [PubMed] [Google Scholar]
- 24. Dees MW, Lysøe E, Nordskog B, Brurberg MB. Bacterial communities associated with surfaces of leafy greens: shift in composition and decrease in richness over time. Appl Environ Microbiol. 2015;81(4):1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4(4):CD004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson CR, Randolph KC, Osborn SL, Tyler HL. Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 2013;13(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol Infect. 2009;137(3):307–15. [DOI] [PubMed] [Google Scholar]
- 28. Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019;17(6):383–90. [DOI] [PubMed] [Google Scholar]
- 29. Tournas VH, Katsoudas E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int J Food Microbiol. 2005;105(1):11–17. [DOI] [PubMed] [Google Scholar]
- 30. Yu AO, Leveau JHJ, Marco ML. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ Microbiol Rep. 2020;12(1):16–29. [DOI] [PubMed] [Google Scholar]
- 31. Zwielehner J, Handschur M, Michaelsen A, Irez S, Demel M, Denner EBet al. DGGE and real-time PCR analysis of lactic acid bacteria in bacterial communities of the phyllosphere of lettuce. Mol Nutr Food Res. 2008;52(5):614–23. [DOI] [PubMed] [Google Scholar]
- 32. Ranieri ML, Huck JR, Sonnen M, Barbano DM, Boor KJ. High temperature, short time pasteurization temperatures inversely affect bacterial numbers during refrigerated storage of pasteurized fluid milk. J Dairy Sci. 2009;92(10):4823–32. [DOI] [PubMed] [Google Scholar]
- 33. Roberts TA, Cordier J-L, Gram L, Tompkin RB, Pitt JI, Gorris LGMet al., editors. Micro-organisms in foods 6: microbial ecology of food commodities. 2nd ed. London, United Kingdom: Springer; 2005. [Google Scholar]
- 34. Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM. Recent advances in cheese microbiology. Int Dairy J. 2001;11(4–7):259–74. [Google Scholar]
- 35. Lang JM, Eisen JA, Zivkovic AM. The microbes we eat: abundance and taxonomy of microbes consumed in a day's worth of meals for three diet types. PeerJ. 2014;2:e659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23(6):354–66. [DOI] [PubMed] [Google Scholar]
- 37. USDA and US Department of Health and Human Services (DHHS) . Dietary Guidelines for Americans, 2020–2025. [Internet]. 9th ed. Washington (DC): USDA and US DHHS; 2020[cited 21 October, 2021]. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. [Google Scholar]
- 38. Dietary Guidelines Advisory Committee . Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. [Internet]. Washington (DC): USDA Agricultural Research Service; 2020[cited 21 October, 2021]. Available from: https://www.dietaryguidelines.gov/2020-advisory-committee-report. [Google Scholar]
- 39. Sonnenborn U. Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 2016;363(19):fnw212. [DOI] [PubMed] [Google Scholar]
- 40. USDA Agricultural Research Service (ARS) . What We Eat in America. [Internet]. Beltsville, MD: USDA ARS; 2021[cited 4 March, 2021]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.