ABSTRACT
Background
Specialized pro-resolving mediators (SPMs), synthesized from PUFAs, resolve inflammation and return damaged tissue to homeostasis. Thus, increasing metabolites of the SPM biosynthetic pathway may have potential health benefits for select clinical populations, such as subjects with obesity who display dysregulation of SPM metabolism. However, the concentrations of SPMs and their metabolic intermediates in humans with obesity remains unclear.
Objectives
The primary objective of this study was to determine if a marine oil supplement increased specific metabolites of the SPM biosynthetic pathway in adults with obesity. The second objective was to determine if the supplement changed the relative abundance of key immune cell populations. Finally, given the critical role of antibodies in inflammation, we determined if ex vivo CD19 + B-cell antibody production was modified by marine oil intervention.
Methods
Twenty-three subjects [median age: 56 y; BMI (in kg/m2): 33.1] consumed 2 g/d of a marine oil supplement for 28–30 d. The supplement was particularly enriched with 18-hydroxyeicosapentaenoic (HEPE), 14-hydroxydocosahexaenoic acid (14-HDHA), and 17-HDHA. Blood was collected pre- and postsupplementation for plasma mass spectrometry oxylipin and fatty acid analyses, flow cytometry, and B-cell isolation. Paired t-tests and Wilcoxon tests were used for statistical analyses.
Results
Relative to preintervention, the supplement increased 6 different HEPEs and HDHAs accompanied by changes in plasma PUFAs. Resolvin E1 and docosapentaenoic acid–derived maresin 1 concentrations were increased 3.5- and 4.7-fold upon intervention, respectively. The supplement did not increase the concentration of D-series resolvins and had no effect on the abundance of immune cells. Ex vivo B-cell IgG but not IgM concentrations were lowered postsupplementation.
Conclusions
A marine oil supplement increased select SPMs and their metabolic intermediates in adults with obesity. Additional studies are needed to determine if increased concentrations of specific SPMs control the resolution of inflammation in humans with obesity. This trial was registered at clinicaltrials.gov as NCT04701138.
Keywords: obesity, omega-3, polyunsaturated fatty acids, specialized pro-resolving mediators, inflammation, antibody
Introduction
Specialized pro-resolving mediators (SPMs) are highly potent oxylipins synthesized from n-3 and n-6 PUFAs. SPMs have a critical role in resolving inflammation and returning damaged tissues to homeostasis (1, 2). SPMs are synthesized from the long chain n-3 PUFAs EPA, docosapentaenoic acid (DPA), DHA, and the n-6 PUFA arachidonic acid (AA) (3, 4). SPMs are broadly categorized as resolvins, protectins, and maresins (Mars), as well as lipoxins derived from AA and EPA (3, 4). There is considerable interest in increasing the amounts of these metabolites in humans across a range of diseases that are associated with chronic inflammation, such as obesity. One approach toward increasing circulating concentrations of SPMs is to administer marine oils that are enriched in metabolites of either the SPM biosynthetic pathways or their parent compounds.
There is debate on the bioavailability of SPMs in humans, particularly in response to marine oil intervention (5). This debate may stem from the analysis of differing human populations. Some laboratories have demonstrated that key circulating SPMs are not detectable in healthy humans but are present in response to inflammation. For instance, a study suggested that SPMs must be <0.1 nM in healthy individuals, as these metabolites were not detectable (6). Interestingly, SPMs were measurable in response to peritonitis or septic shock, suggesting that SPM concentrations are increased in the context of chronic inflammation (6, 7). Some studies have demonstrated that SPMs are robustly increased in healthy humans upon marine oil supplementation (8–10). Issues with SPM detection may also be related to methodological and/or analytical issues (11).
SPM bioavailability has not been effectively established in adults with obesity who are taking marine oil supplements. This population is of interest as obesity can drive deficiencies in SPMs and their metabolic intermediates (4, 12–17). Importantly, obesity and its comorbidities are associated with chronic inflammation that contributes to insulin resistance, hepatic steatosis, cardiovascular diseases, and poor responses to infections and vaccinations (4, 18–24). Notably, our research group and others have shown that SPMs and their metabolic intermediates can improve a range of obesity-related complications in mouse models (3, 12, 18, 20, 25–27). However, it remains unknown whether SPMs are sufficiently elevated in humans with obesity in response to a marine oil supplement that contains metabolites of the SPM biosynthetic pathway.
The primary objective of this study was to determine if a change from baseline in select PUFA-derived oxylipins occurs after 1 mo of supplementation in adults with obesity who are taking a marine oil supplement that contains several oxylipins but is particularly enriched in 14-hydroxydocosahexaenoic acid (14-HDHA), 17-HDHA, and 18-hydroxyeicosapentaenoic acid (HEPE). The secondary objective was to determine if 1 mo of supplementation modified the abundance of circulating peripheral blood mononuclear cells (PBMCs) from baseline. The tertiary objective was to determine if 1 mo of supplementation changed antibody production compared with baseline. The rationale for focusing on antibodies was based on our previous work and the work of others, which have shown that SPM intermediates control antibody concentrations (12, 13, 24, 28). For instance, we previously demonstrated that oxylipins of the DHA-derived SPM pathway could lower circulating proinflammatory IgG2c in obese mice (13). To address these objectives, we conducted a clinical trial in which subjects with obesity consumed 2 g/d of an enriched marine oil supplement for 1 mo.
Subjects and Methods
Study design and subject recruitment
We conducted a nonrandomized uncontrolled clinical trial (registered at clinicaltrials.gov as NCT04701138) in adults with obesity [BMI 30–40 (in kg/m2)]. All procedures and study protocols were approved according to the University of North Carolina's Institutional Review Board (IRB) guidelines and regulations. We recruited and enrolled 24 subjects (n = 13 females, 11 males), with 1 male subject as a loss to follow-up. Therefore, the study consisted of 23 participants with obesity (n = 13 females, 10 males) aged 50–65 y. We recruited only postmenopausal females to reduce confounding effects of estrogen on lipid metabolism during supplementation. Patients were also excluded if they had diagnosed type 1 or type 2 diabetes, had consumed n-3 PUFA supplements in the 3 mo prior to enrollment, regularly consumed >2 servings/wk of fatty fish (based on self-report), had an allergy to fish/shellfish, or took any of the following medications: anticoagulants, estrogen, testosterone, nonsteroidal anti-inflammatory drugs, asthma medications, or immunosuppressant therapy. Individuals with autoimmune disease, liver disease, coagulopathy, uncontrolled hypothyroidism, and/or active malignancy were also excluded. Eligible participants were recruited from the University of North Carolina's Family Medicine Center in Chapel Hill. Written informed consent was obtained from every subject. The baseline characteristics of the participants are shown in Table 1. The median age was 56 y, 56.5% of subjects were female, and approximately two-thirds identified as white or Caucasian (69.57%). At baseline, the median BMI was 33.1. The study from IRB approval through collecting blood samples from the last enrolled subject lasted 4 mo.
TABLE 1.
Subject demographics at baseline1
| Characteristic | Value |
|---|---|
| Age, y | 56 [52–61] |
| Female sex, n (%) | 13 (56.52) |
| Race, n (%) | |
| White or Caucasian | 16 (69.57) |
| Black or African American | 5 (21.74) |
| Asian | 1 (4.35) |
| Other | 1 (4.35) |
| Weight, kg | 96.4 [87.3–112] |
| Height, cm | 170.2 [160–177.8] |
| BMI | 33.1 [31–36] |
Values are medians [IQRs] or n (%). Total n data of 23 subjects are represented in the table.
All participants received the marine oil supplement (SPM Active) provided by Metagenics. Participants were advised to take 4 capsules once daily of SPM Active for 4 wk (28–30 d). Two capsules contained 1 g active fractionated marine lipid concentrate that contains 14-HDHA, 17-HDHA, and 18-HEPE. The oxylipin and fatty acid composition of SPM Active has been previously described (8). We confirmed that these metabolites, in addition to several other oxylipins, were present in the capsules (Supplemental Table 1). Based on our analyses, the supplement provided 76.12 μg of 14-HDHA, 32.41 μg of 17-HDHA, and 100.96 μg of 18-HEPE per capsule for a total daily dose of 837.96 μg of 14-HDHA + 17-HDHA + 18-HEPE. We also measured the concentrations of the major fatty acids in the capsules (Supplemental Table 2).
The study required 2 face-to-face visits and 2 phone contacts. Subject compliance was assessed via phone by the study coordinator. Participants were required to fast for 8 h prior to each visit. Fasting blood samples were drawn by the laboratory staff at the clinic from which patients were recruited. The first visit consisted of a height and weight measurement, followed by a baseline blood draw (presupplementation), and the second face-to-face visit concluded the study with a second blood draw (postsupplementation). Each subject took their last dose of the supplement the morning of their second blood draw visit. We monitored subject health status via phone contact at the end of the second and third week of the 4-wk supplementation period. No subjects reported adverse events or changes in health status related to the supplement. During each phone contact, subjects were asked health questions related to COVID-19 to ensure they had not contracted the coronavirus. In addition, none of the subjects received the COVID-19 vaccine during supplementation. Any subjects who did receive the COVID-19 vaccine prior to supplementation were enrolled at least 4 wk after their completed vaccine doses.
Plasma lipidomics
A blood sample from each subject was collected into a heparin-coated plasma tube and then centrifuged at 900 × g for 10 min at 4°C. The upper plasma layer was then collected and frozen at –80°C for lipidomic analysis by the Lipidomics Core Facility of Wayne State University following previously published protocols (10, 29–31). Metabolites that were under the detection limit for >80% of the samples were not imputed and were excluded from the analysis. All other missing metabolite values were imputed with 0.0001 ng/mL. Quantitation limits were ∼0.0005 ng on the column. Variation in the internal standard quantitation method was typically 3–5% CV. All samples for lipidomics, including the fatty acid analyses described below, were processed and analyzed as one batch to avoid methodological issues.
Plasma fatty acid analyses
Plasma fatty acids were extracted and analyzed using a Perkin Elmer 680/600S GC-MS as previously described (32).
PBMC isolation and sample handling
PBMCs were separated using StemCell SepMate PBMC Isolation tubes (catalog no. 86450) with a gradient separation using the StemCell Lymphoprep solution (catalog no. 07851) followed by centrifugation (1200g for 10 min at 23oC. The top layer of plasma was discarded without disturbing the buffy coat layer containing the PBMCs. The PBMC cell layer was collected and washed with sterile fluorescence-activated cell sorting buffer (2% fetal bovine serum in 1X PBS). Next, the PBMCs were treated with ammonium-chloride-potassium lysis buffer to remove any residual RBC contamination. The PBMCs were then transferred to cryogenic vials for storage. When thawing PMBCs, the protocol described in the Accucell package insert was utilized. After thawing, samples were placed in the incubator for a 16-h rest period prior to any experiments.
PBMC flow cytometry analysis
All PBMCs were thawed and rested for 16 h prior to any flow cytometry analyses. Following the rest, each PBMC sample was split in half for staining with panel 1 and panel 2 fluorophores. Panel 1 samples were stained with the following fluorophore-tagged antibodies obtained from either BioLegend or BD: Zombie Fixable UV, CD19 (BUV737), CD38 (BV421), CD27 (APC), HLA-DR (FITC), CD23 (PE-Cy7), CD24 (PE), CD3 (BV570), CD56 (BV650), CD16 (PerCP-Cy5.5), and CD14 (APC-Cy7). Panel 2 samples were stained with the following fluorophore-tagged antibodies obtained from BioLegend: Zombie Fixable UV, CD3 (BV570), CD4 (APC-Cy7), CD8 (PerCP-Cy5.5), HLA-DR (FITC), and CD56 (BV711). The BD brilliant stain buffer (catalog no. 563794) was used in all staining steps to prevent interactions between the various brilliant violet dyes. Samples were fixed with 1% paraformaldehyde for 12 h overnight at 4°C. The following PBMC subsets were adapted from the optimized multicolor immunofluorescence panels (OMIP-024 and OMIP-069) and analyzed using a BD LSRII flow cytometer: CD3-CD19+ (B cells), CD19 + CD38 + CD27 + HLA-DR+(plasmablasts and plasma cells), CD19 + CD38-CD27-CD23+ (follicular B cells), CD19 + CD24hiCD38+ (B-regulatory cells), CD14 + CD16-HLA-DR+ (classical monocytes), CD14 + CD16 + HLA-DR+ (intermediate monocytes), CD14dimCD16 + HLA-DR+ (nonclassical monocytes), NK cell subsets (CD3-CD56hiCD16-, CD3-CD56dimCD16-, CD3-CD56hiCD16+, CD3-CD56dimCD16+, CD3-CD56-CD16+), CD3 + CD4+ (CD4 + T cells), CD3 + CD8+ (CD8 + T cells), CD3 + CD56+ (NKT cells), CD3 + CD4 + HLA-DR+ (activated CD4 + T cells), CD3 + CD8 + HLA-DR+ (activated CD8 + T cells), CD3 + CD4 + HLA-DR- (nonactivated CD4 + T cells), and CD3 + CD8 + HLA-DR- (nonactivated CD8 + T cells) (33, 34). All data were analyzed in FlowJo and R v.4.0.3. Gates were drawn from fluorescence minus one control.
B-cell isolation/expansion
The Miltenyi Biotec's human B-Cell isolation kit II (catalog no. 130-091-151) was used for B cell isolation from PBMCs. Cell counts were performed following isolation and samples were plated in 12-well, flat-bottom plates (Genesee Scientific catalog no. 25-106) at a density of 0.3 × 106 cells per well. For each subject, there were 4 wells: presupplement stimulated, presupplement unstimulated, postsupplement stimulated, and postsupplement unstimulated. Stimulated wells were treated with 16 μL CD40L and 0.4 μL IL4 (modeling T-dependent activation of B cells) as described in the Miltenyi Biotec human B-cell expansion kit (catalog no. 130-106-196) protocol. In addition, each subject's serum (100 μL) was combined with StemMACS media (1.9 mL), and then added to the stimulants for each sample well. Unstimulated wells were only plated with StemMACS expansion media and the subject's serum. Media exchanges were performed on day 7 and day 10. Cell supernatants were collected on day 14 and stored at –80°C for ELISA analysis.
ELISA analysis
Abcam's Human IgG ELISA kit (ab195215) and Human IgM ELISA kit (ab137982) were utilized to measure antibody concentrations from the supernatants of unstimulated and stimulated B cells. For the IgG ELISA, stimulated samples were diluted 1:100,000 and unstimulated samples were diluted 1:10. For the IgM ELISA, stimulated samples were diluted 1:100,000, and unstimulated samples were diluted 1:10,000. Plates were read at 450 nm using an automated plate reader (BMG Labtech FLUOstar Omega).
Statistics
Statistical analyses
All data were analyzed in R v.4.0.3. First, each dataset and supplementation time point (pre/post) was assessed for normality and heteroscedasticity via the Shapiro-Wilks test and Bartlett test, respectively. If the assumptions of normality and heteroscedasticity were met, a paired 2-sided Student t-test was preformed between the pre- and postsupplementation participants. If the assumption of heteroscedasticity failed, a paired 2-sided Welch t-test was performed. Lastly, if the data failed both assumptions of normality and heteroscedasticity, a paired Wilcoxon test was performed. All outcomes in all the datasets underwent a Benjamini–Hochberg (BH) P-value adjustment with a false discovery rate of 10% to adjust for type 1 errors from multiple hypothesis testing. A BH-adjusted P value < 0.1 was considered significant. Subject ID (SPM-16) was removed from all analyses due to loss to follow-up. Sample ID (SPM-21) was removed from all B-cell isolation and stimulation studies (ELISA data) due to PBMC cell death upon sample freezing. In addition, a Spearman correlation analysis was performed between baseline BMI and each fatty acid.
Sample size justification
The primary aim of the study was powered to measure an increase in 18-HEPE, 17-HDHA, and 14-HDHA. Changes in remaining metabolites were exploratory. At a family-wise error rate of 5% with a 2-sided alpha and an effect size of 1.4 controlling for testing of the 3 metabolites mentioned above, n = 9 provided more than 80% power to detect a 50%-fold change increase in metabolite concentrations within each subject given a paired 2-sided t-test. We assumed a similar effect size for both males and females and doubled the sample size (n = 18, 9 per sex). To account for possible loss to follow-up, we increased the sample size from 18 to 24 (n = 12 male + 12 female). Power analyses were conducted assuming a paired t-test on pre- and posttreatment metabolite concentrations for the 3 prespecified metabolites. Expected variability of within-subject differences in metabolite concentrations was assessed from a previous dataset in which the median and SD were calculated across all metabolites (35).
Results
Marine oil supplementation increases plasma EPA-, DPA-, and DHA-derived metabolites
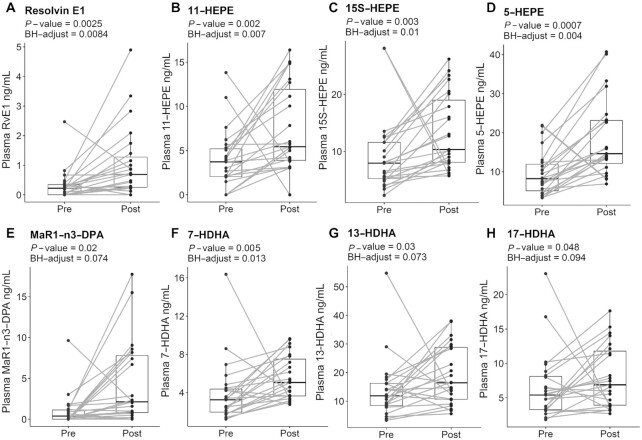
MS-based plasma lipidomic analyses (Figure 1, Supplemental Figure 1) revealed a significant increase in EPA-derived metabolites such as resolvin E1 (RvE1; BH P = 0.008) with a 3.5-fold increase (Figure 1A), 11-hydroxy-EPA (11-HEPE, BH P = 0.007) with a 2-fold increase (Figure 1B), 15S-hydroxy-EPA (15S-HEPE, BH P = 0.01) with a 1.5-fold increase (Figure 1C), and 5-hydroxy-EPA (5-HEPE, BH P = 0.004) with a 2-fold increase (Figure 1D) in response to supplementation relative to baseline. In addition, DPA- and DHA-derived mediators were also significantly elevated postsupplementation relative to baseline, such as DPA-derived MaR1 (MaR1-n3-DPA; BH P = 0.074) with a 4.7-fold increase (Figure 1E), 7-hydroxy-DHA (7-HDHA, BH P = 0.013) with a 1.5-fold increase (Figure 1F), 13-hydroxy-DHA (13-HDHA, BH P = 0.073) with a 1.4-fold increase (Figure 1G), and 17-hydroxy-DHA (17-HDHA, BH P = 0.094) with a 1.2-fold increase (Figure 1H). EPA-, DPA-, and DHA-derived metabolites that were measured in the samples but were not statistically significant between pre/postsupplementation included 12-hydroxy-EPA (12-HEPE), 18-hydroxy-EPA (18-HEPE), 5S,6R-dihydroxy eicosatetraenoic acid [5(S),6(R)-DiHETE], 5S,15S-dihydroxy EPA [5(S),15(S)-DiHEPE], 14-hydroxy DHA (14-HDHA), 4-hydroxy DHA (4-HDHA), DPA-derived resolvin D5 (RvD5-n3-DPA), resolvin D1 (RvD1), and resolvin D2 (RvD2) (Supplemental Figure 2).
FIGURE 1.
Marine oil supplement increases EPA-, DPA- and DHA-derived metabolites in adults with obesity. EPA-derived metabolites: RvE1 (A), 11-HEPE (B), 15S-HEPE (C), and 5-HEPE (D). DPA-derived: MaR1-n3-DPA (E). DHA-derived metabolites: 7-HDHA (F), 13-HDHA (G), and 17-HDHA (H); n = 23 for all metabolites. P values and BH-adjusted P values are denoted underneath the header of each metabolite. Dots represent individual pre- and postsupplementation values, and the box plot represents median and IQR limits. DHA, docosahexaenoic acid; DPA; docosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; MaR1, maresin 1; SPM, specialized pro-resolving mediator.
Marine oil supplementation exerts differential effects on plasma AA-derived metabolites
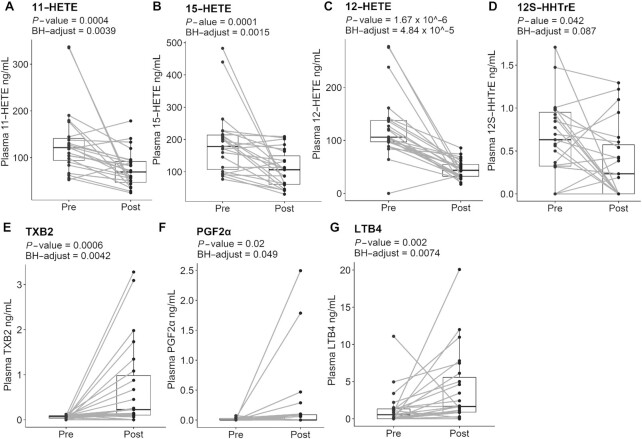
Subjects had some decreased plasma AA-derived oxylipins postsupplementation relative to baseline. Those included hydroxyeicosatetraenoic acid (HETE) 11-HETE (BH P = 0.0039) with a 1.7-fold decrease (Figure 2A), 15-HETE (BH P = 0.0015) with a 1.7-fold decrease (Figure 2B), 12-HETE (BH P = 4.84 × 10–5) with a 3-fold decrease (Figure 2C), and 12S-hydroxy heptadecatrienoic acid (12S-HHTrE) (BH P = 0.087) with a 1.9-fold decrease (Figure 2D) compared with presupplementation. We also found significantly upregulated AA-derived mediators postsupplementation, such as thromboxane B2 (TXB2, BH P = 0.0042) with a 10.7-fold increase (Figure 2E), prostaglandin F2α (PGF2α, BH P = 0.049) with a 17.7 increase (Figure 2F), and leukotriene B4 (LTB4, BH P = 0.0074) with a 2.8-fold increase (Figure 2G). AA-derived metabolites that were measured in the samples but were not statistically significant between pre- and postsupplementation included 5S,12S-dihydroxy eicosatetraenoic acid [5(S),12(S)-DiHETE], prostaglandin E2 (PGE2), PGD2, lipoxin A4 (LXA4), and 5-HETE (Supplemental Figure 3).
FIGURE 2.
Marine oil supplement differentially influences AA-derived metabolites in adults with obesity. AA-derived metabolites: 11-HETE (A), 15-HETE (B), 12-HETE (C), 12S-HHTrE (D), TXB2 (E), PGF2α (F), and LTB4 (G); n = 23 for all metabolites. P values and BH-adjusted P values are denoted underneath the header of each metabolite. Dots represent individual pre- and postsupplementation values, and the box plot represents median and IQR limits. AA, arachidonic acid; HETE, hydroxyeicosatetraenoic acid; LTB4, leukotriene B4; PGF2α, prostaglandin F2α; SPM, specialized pro-resolving mediator; TXB2, thromboxane B2; 12S-HHTrE, 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid.
Plasma fatty acid analyses
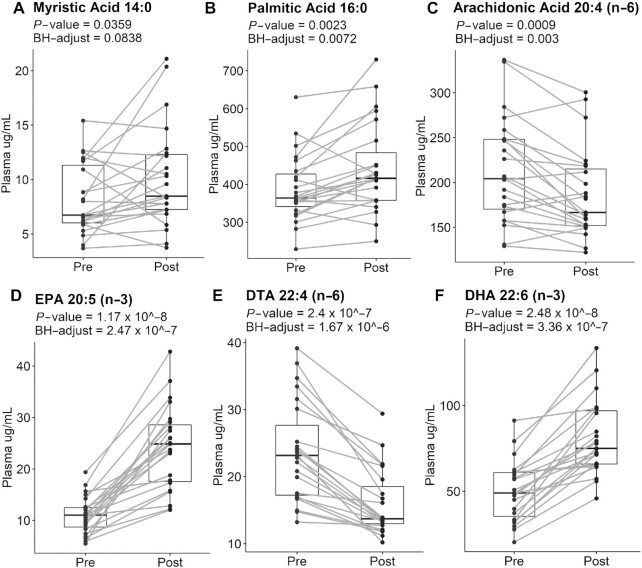
Plasma fatty acid analyses revealed changes in response to the supplement when comparing postsupplementation to baseline (Figure 3). Myristic (14:0) (Figure 3A) and palmitic (16:0) (Figure 3A) acid concentrations were slightly increased by 1.2- and 1.1-fold, respectively, upon supplementation. AA concentrations were decreased by 0.9-fold (Figure 3C) upon supplementation relative to baseline. EPA (20:5) concentrations were increased upon supplementation by 2.2-fold compared with preintervention (Figure 3D). DTA (22:4n-6) concentrations (Figure 3E) were decreased by 0.7-fold upon supplementation relative to baseline. The concentration of DHA (Figure 3F) was increased by 1.6-fold compared with baseline. DPA (22:5n-3) concentrations were not modified in response to the supplement (data not shown). In addition, we found no correlation with the concentration of these fatty acids with baseline BMI (data not shown).
FIGURE 3.
Marine oil supplement increases the concentration of EPA and DHA while lowering AA in adults with obesity. Plasma concentration of 14:0 (A), 16:0 (B), 20:4(n-6) (C), 20:5(n-3) (D), 22:4(n-6) (E), and 22:6(n-3) (F); n = 23 for all fatty acids. P values and BH-adjusted P values are denoted for each fatty acid. Dots represent individual pre- and postsupplementation values, and the box plot represents median and IQR limits. AA, arachidonic acid; DHA, docosahexaenoic acid; SPM, specialized pro-resolving mediator.
Flow cytometry analyses reveal no differences in abundance of resting PBMCs pre- and postsupplementation
We conducted a flow cytometry analysis of the resting PBMCs pre- and postsupplementation to determine if marine oil supplementation could impact the abundance of key immune cell populations in circulation, as in the gating strategies shown in panel 1 (Supplemental Figure 4A) and panel 2 (Supplemental Figure 4B). When analyzing both the absolute counts and frequencies of these cell populations, we found no significant differences between pre- and postsupplementation (Supplemental Figures 4C and D).
IgG but not IgM antibody concentrations decrease upon activation
Finally, we assessed whether marine oil supplementation affects antibody concentrations. The rationale was driven by previous work from our laboratory and others to show that SPMs and their metabolic intermediates control antibody concentrations (12, 13, 24, 36). Unstimulated (Figure 4A) and stimulated (Figure 4B) B cells did not produce significantly differing amounts of IgM pre- compared with postsupplementation. Similarly, unstimulated B cells did not produce significantly different amounts of IgG (Figure 4C). However, stimulated B cells produced significantly lower concentrations of IgG postsupplementation (Figure 4D), with P = 0.003 and BH-adjusted P = 0.01.
FIGURE 4.
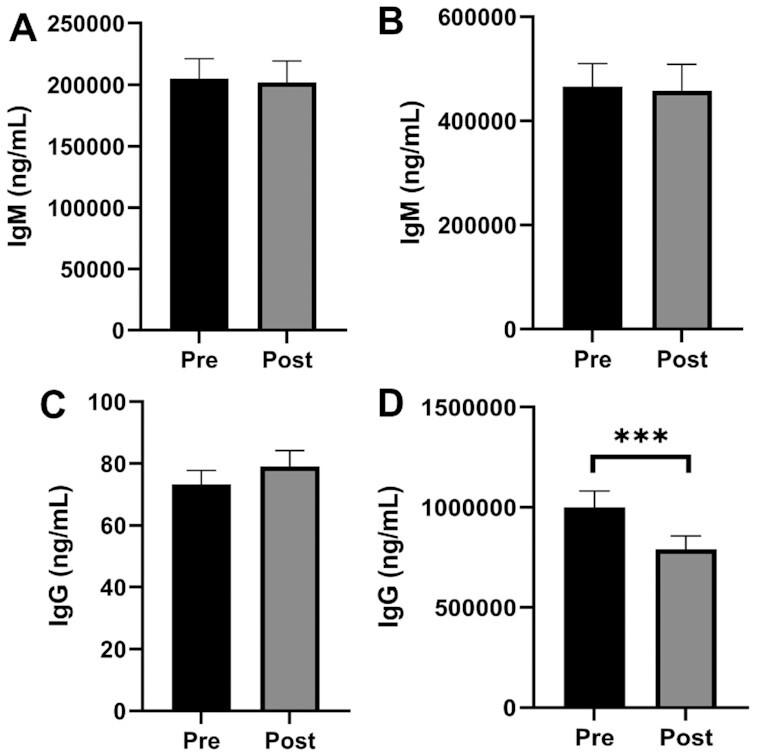
Ex vivo IgG but not IgM concentrations are decreased postsupplementation with a marine oil supplement in adults with obesity. Unstimulated (A) and stimulated B-cell IgM production (B). Unstimulated (C) and stimulated (D) B-cell IgG production. P value is denoted by *** < 0.005. Bar plots are presented as mean ± SEM; n = 22.
Discussion
Adults with obesity present with a dysregulated SPM signature that may contribute to chronic low-grade inflammation and thereby a wide range of health complications (25). The impaired SPM signature in adults with obesity is mirrored in preclinical studies. Several laboratories have reported that mice that were obese in response to a high-fat diet or genetically obese show significantly lower concentrations of SPMs or their metabolic intermediates across differing tissues than lean mice (4, 12–17). Therefore, increasing the concentration of circulating SPMs and their metabolic intermediates with specific dietary or pharmacological supplements may have a therapeutic role in preventing or treating conditions associated with obesity. In this study related to obesity, we investigated the plasma lipidomic signature in response to a marine oil supplement, which was particularly enriched in 14-HDHA, 17-HDHA, and 18-HEPE.
We found that the marine oil supplement significantly increased some oxylipins of the SPM biosynthetic pathway. Notably, RvE1 concentrations were increased 3.5-fold. RvE1 is of interest as it has an emerging role in controlling glucose homeostasis in mice (17, 37, 38). In addition, high RvE1 concentrations are associated with plaque regression in coronary artery disease patients and exerts protective effects in murine models of atherosclerosis (7, 17, 39–41). Interestingly, we did not find an increase in the concentration of D-series resolvins upon intervention, although several DHA-derived metabolites were increased. This could be due to an inability of adults with obesity to generate D-series resolvins. Previous work demonstrated that n-3 PUFA supplementation with aspirin led an increase in EPA-derived SPMs but not DHA-derived SPMs in subjects with the metabolic syndrome (42). There is also evidence that adults with morbid obesity have impaired 15-lipoxygenase (LOX) activity, a reduction in 5-LOX expression, and decreased 5-LOX serine-271 phosphorylation (25). There may be a possibility that a higher dose of the marine oil supplement or a longer intervention time could lead to increased concentrations of D-series resolvins and other oxylipins of the SPM family.
The supplement, despite being enriched in 14-HDHA, 17-HDHA, and 18-HEPE, only led to an increase in 17-HDHA concentrations upon supplementation, relative to baseline, but had no effect on the concentration of 14-HDHA or 18-HEPE. The oxylipin 18-HEPE may have converted to RvE1, which was significantly elevated with supplementation as described above. Thus, it is possible that a higher dose of 14-HDHA, 17-HDHA, and 18-HEPE may be needed to elevate these oxylipins in adults with obesity. Interestingly, the supplement contained some HETEs, which were lowered in response to the marine oil supplement. Furthermore, since several other n-3 PUFA-derived oxylipins besides 14-HDHA, 17-HDHA, and 18-HEPE were increased upon supplementation relative to baseline, it is possible that the elevation in oxylipins may be a direct consequence of the other oxylipins that were also in the supplement (8). In addition, EPA, DHA, and other fatty acids in the supplement may also contribute to changes in oxylipins.
The supplement decreased some HETEs, which are synthesized from AA. There is a need to further investigate how oxylipins of the SPM family and their parent compounds decrease HETE concentrations, as these molecules may have an important role in the pathogenesis of obesity. For instance, 20-HETE interferes with insulin signaling in mouse models of obesity (43). In humans, increasing concentrations of 5-, 11-, and 15-HETE are positively associated with BMI and serum leptin (44). We also observed a significant increase in several AA-derived metabolites. The results with AA-derived metabolites were generally consistent with a previous clinical trial testing the same supplement on PUFA-derived metabolites in healthy subjects (8). In this previous study, the same marine oil supplement increased LTB4 concentrations (8). It is interesting that the supplement lowered plasma concentrations of arachidonic acid, even though the supplement contained a small amount of AA. We acknowledge that other fatty acids and oxylipins in the marine oil supplement could also impact the concentration of SPMs and their metabolic intermediates.
We observed an increase in TXB2 and PGF2α in response to the marine oil supplement. The underlying mechanism by which a marine oil supplement would increase the concentration of these eicosanoids is unclear. The changes in these eicosanoids could be linked to the effects of select drugs (45). There is considerable variability in the literature on how marine oil supplements may influence differing AA-derived oxylipins, which warrants further investigation. For instance, fish oil supplementation can increase TXB2 and PGF2α, whereas other studies show fish oil can lower some of these eicosanoids in either circulation or upon LPS stimulation of PBMCs, whereas other studies suggest no effects of fish oil on some of these metabolites (46–48).
A notable outcome, albeit highly exploratory, was that ex vivo B-cell stimulation led to a decrease in IgG concentrations in culture upon intervention. This may mimic the resolution phase of the stimulation response, by which antibody production is lowered to the homeostatic state. The reduction in antibody concentrations was consistent with the literature reporting that some SPM pathway markers can lower the concentration of antibodies, whereas others increase antibody concentrations (49). We previously reported that a cocktail of metabolites including 17-HDHA, which was modestly elevated with the subjects in this study, could lower the concentration of circulating IgG2c concentrations in obese mice (13). IgG2c is of interest since it is proinflammatory and contributes toward insulin resistance (50). We have also previously reported that a fish oil concentrate could lower IgM but not IgG concentrations upon ex vivo activation for 3 d with a mixture of agonists to stimulate the Toll-like receptor 9 agonist and B-cell receptor (35). Therefore, future studies will need to discriminate how differing PUFA-derived metabolites, which are either increased or decreased with marine oil supplementation, may manipulate antibody concentrations using a wide range of stimulation protocols that target different signaling pathways.
There are several limitations to this study. We did not use a placebo control and thereby did not conduct a randomized clinical trial. Thus, there is potential for selection bias as this was not a randomized study (51). Also, we were not powered to discriminate between females and males. There are likely sex differences in oxylipins within the population with obesity. Previous studies show strong sex differences in the concentration of PUFA-derived metabolites, including SPMs in mice and humans (13, 52–54). The study did not test for the concentration of metabolites of the SPM biosynthetic pathway in subjects that are younger with obesity, which may be a good population to intervene with to prevent some of the later complications of obesity. Furthermore, the study design did not allow us to entirely blind the samples; therefore, future studies should be designed as double-blind randomized placebo-controlled clinical trials. Another limitation is that we were not powered to study inflammatory biomarkers in circulation, which would be an area for future investigation. Subsequent studies incorporating adults who are not obese will be an important comparison to establish whether any increases in these metabolites in adults with obesity with intervention rescues metabolite concentrations to amounts similar to those adults without obesity.
This study did not account for heterogeneity in the population with obesity, which may explain why not every individual had the same set of detectable oxylipins and the same response to the intervention (55). Heterogeneity may be driven by differences in microbiome profiles, food intake, baseline metabolic status, and ethnic differences (55). These measures should be considered in future trials. As an example, food recalls or FFQs can be used to account for differences in food intake between subjects and for each subject during the course of the intervention. Moreover, we did not account for host genetics as there are well-identified polymorphisms in the biosynthetic pathways for differing oxylipins, including SPMs (4). A recent study, which underscores this point, demonstrated that the APOE4 genotype controls the concentration of specific oxylipins (56).
In summary, the findings from this study show that 17-HDHA, but not 14-HDHA or 18-HEPE, are increased in response to supplementation relative to baseline. In addition, some SPMs such as RvE1 and other oxylipins, notably HEPEs, are significantly increased in adults with obesity upon intervention with a marine oil supplement that is particularly enriched with 14-HDHA, 17-HDHA, and 18-HEPE. We also observed that many SPMs were not increased, particularly D-series resolvins, whereas other select DHA-derived metabolites were increased upon intervention. In addition, AA-derived metabolites were both increased and decreased with the marine oil supplement. Collectively, these results provide a framework for futures studies on the use of a marine oil supplement to examine the effects of how SPMs and their metabolic intermediates control varying aspects of inflammation and immunity, including antibody concentrations, in subjects with obesity.
Supplementary Material
Acknowledgments
We thank the UNC Family Medicine center and UNC Family Medicine laboratory staff Jennifer Willson, Isaiah Beyah, Francesca Carney, and Brittney McCoy who were instrumental to our study. We also thank the UNC Department of Nutrition for their support on this project.
The authors’ responsibilities were as follows—AEA: designed/performed research, conducted experiments, analyzed data, and wrote/edited the manuscript; JR: performed research, conducted experiments, analyzed data, and wrote/edited parts of the manuscript; NB: performed research and conducted experiments; ST: recruited subjects, obtained consent, and wrote/edited parts of the manuscript; CD: performed research; MB: performed research; SS: conducted experiments, analyzed data; JIF: performed research and analyzed data; KM: conducted lipidomic analyses and edited the manuscript; SK: recruited subjects; EB: recruited subjects, designed research, acquired funding, and edited parts of the manuscript; SRS: designed research, acquired funding, and wrote/edited parts of the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by NIH CTSA grant UL1TR002489 (subaward 550KR242033 from the North Carolina Translational and Clinical Sciences Institute to SRS and EB) and by Metagenics Inc (SRS). This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program under grant no. 1650116 to AEA.
Author disclosures: SRS has received support for organizing conferences and laboratory research from several industry groups related to omega-3 fatty acids, which include Metagenics and the Wiley Companies. All other authors report no conflicts of interest.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This study was supported in part by National Center for Research Resources, National Institutes of Health Grant S10RR027926. Metagenics provided the dietary supplements and funding as a sponsor for this clinical study. Metagenics was not involved in the study design; collection, management, analysis, and interpretation of data; writing of the manuscript; and the decision to submit the manuscript for publication.
Supplemental Tables 1–2 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
AEA-S and JR contributed equally to this work.
Abbreviations used: AA, arachidonic acid; BH, Benjamini–Hochberg; DPA, docosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; LOX, lipoxygenase; MaR, maresin; PBMC, peripheral blood mononuclear cell; PG, prostaglandin; RvE1, resolvin E1; SPM, specialized pro-resolving mediator
Contributor Information
Abrar E Al-Shaer, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Jennifer Regan, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Nicole Buddenbaum, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sonum Tharwani, The University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA.
Catie Drawdy, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Madeline Behee, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Selin Sergin, Department of Food Science and Human Nutrition, College of Agriculture and Natural Resources and College of Osteopathic Medicine, Michigan State University, East Lansing, MI, USA.
Jenifer I Fenton, Department of Food Science and Human Nutrition, College of Agriculture and Natural Resources and College of Osteopathic Medicine, Michigan State University, East Lansing, MI, USA.
Krishna Rao Maddipati, Department of Pathology, Bioactive Lipids Research Program, Wayne State University, Detroit, MI, USA.
Shawn Kane, The University of North Carolina at Chapel Hill Family Medicine Center, Chapel Hill, NC, USA.
Erik Butler, The University of North Carolina at Chapel Hill Family Medicine Center, Chapel Hill, NC, USA.
Saame Raza Shaikh, Department of Nutrition, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; The University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA.
Data Availability
Data described in the manuscript and analytic code will be made publicly and freely available without restriction at https://github.com/abrar-alshaer
References
- 1. Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2015;7(2):a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–84. [DOI] [PubMed] [Google Scholar]
- 4. Al-Shaer AE, Buddenbaum N, Shaikh SR. Polyunsaturated fatty acids, specialized pro-resolving mediators, and targeting inflammation resolution in the age of precision nutrition. Biochim Biophys Acta. 2021;1866(7):158936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skarke C, Alamuddin N, Lawson JAet al. , Bioactive products formed in humans from fish oils. J Lipid Res. 2015;56(9):1808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kutzner L, Rund KM, Ostermann AIet al. Development of an optimized LC-MS method for the detection of specialized pro-resolving mediators in biological samples. Front Pharmacol. 2019;10:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomez EA, Colas RA, Souza PRet al. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat Commun. 2020;11(1):5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Souza PR, Marques RM, Gomez EAet al. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ Res. 2020;126(1):75–90. [DOI] [PubMed] [Google Scholar]
- 9. Schaller MS, Chen M, Colas RAet al. Treatment with a marine oil supplement alters lipid mediators and leukocyte phenotype in healthy patients and those with peripheral artery disease. J Am Heart Assoc. 2020;9(15):e016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norris PC, Skulas-Ray AC, Riley Iet al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 2018;8(1):18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Donnell V, Schebb NH, Milne GLet al. Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Zenodo. Published online 2021. doi: 10.5281/zenodo.5766267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosaraju R, Guesdon W, Crouch MJet al. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J Immunol. 2017;198(12):4738–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crouch MJ, Kosaraju R, Guesdon Wet al. Frontline science: a reduction in DHA-derived mediators in male obesity contributes toward defects in select B cell subsets and circulating antibody. J Leukocyte Biol. 2019;106(2):241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neuhofer A, Zeyda M, Mascher Det al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62(6):1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spite M, Hellmann J, Tang Yet al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187(4):1942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lainez NM, Jonak CR, Nair MGet al. Diet-Induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol. 2018;9:1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pal A, Al-Shaer AE, Guesdon Wet al. Resolvin E1 derived from eicosapentaenoic acid prevents hyperinsulinemia and hyperglycemia in a host genetic manner. FASEB J. 2020;34(8):10640–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González-Périz A, Horrillo R, Ferré Net al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23(6):1946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López-Vicario C, Alcaraz-Quiles J, García-Alonso Vet al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci. 2015;112(2):536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White PJ, St-Pierre P, Charbonneau Aet al. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med. 2014;20(6):664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brennan EP, Mohan M, Andrews D, Bose M, Kantharidis P. Specialized pro-resolving mediators in diabetes: novel therapeutic strategies. Clin Sci (Colch). 2019;133(21):2121–41. [DOI] [PubMed] [Google Scholar]
- 22. Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Börgeson E, Johnson AMF, Lee YSet al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 2015;22(1):125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramon S, Baker SF, Sahler JMet al. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant?. J Immunol. 2014;193(12):6031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Vicario C, Titos E, Walker MEet al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 2019;33(6):7072–83. [DOI] [PubMed] [Google Scholar]
- 26. Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25(7):2399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189(5):2597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189(2):1036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maddipati KR, Romero R, Chaiworapongsa Tet al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014;28(11):4835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Markworth JF, Vella L, Lingard BSet al. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol 2013;305(11):R1281–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maddipati KR, Zhou S-L. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 2011;94(1-2):59–72. [DOI] [PubMed] [Google Scholar]
- 32. Jain R, Ezeamama AE, Sikorskii Aet al. Serum n-6 fatty acids are positively associated with growth in 6-to-10-year old Ugandan children regardless of HIV Status—a cross-sectional study. Nutrients. 2019;11(6):1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park LM, Lannigan J, Jaimes MC. OMIP-069: forty-color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Cytometry Part A. 2020;97(10):1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moncunill G, Han H, Dobaño C, McElrath MJ, De Rosa SC. OMIP-024: pan-leukocyte immunophenotypic characterization of PBMC subsets in human samples. Cytometry Part A. 2014;85(12):995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guesdon W, Kosaraju R, Brophy Pet al. Effects of fish oils on ex vivo B-cell responses of obese subjects upon BCR/TLR stimulation: a pilot study. J Nutr Biochem. 2018;53:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A₄ modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol. 2014;44(2):357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arita M, Ohira T, Sun Y-P, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and Chemr23 to regulate inflammation. J Immunol. 2007;178(6):3912–7. [DOI] [PubMed] [Google Scholar]
- 38. Al-Shaer AE, Pal A, Shaikh SR. Resolvin E1-ChemR23 axis regulates the hepatic metabolic and inflammatory transcriptional landscape in obesity at the whole genome and exon level. Front Nutr. 2021;8:799492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sima C, Montero E, Nguyen Det al. ERV1 overexpression in myeloid cells protects against high fat diet induced obesity and glucose intolerance. Sci Rep. 2017;7(1):12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laguna-Fernandez A, Checa A, Carracedo Met al. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation. 2018;138(16):1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welty FK, Schulte F, Alfaddagh A, Elajami TK, Bistrian BR, Hardt M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B4. FASEB J. 2021;35(4):e21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barden AE, Mas E, Croft KD, Phillips M, Mori TA. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am J Clin Nutr. 2015;102(6):1357–64. [DOI] [PubMed] [Google Scholar]
- 43. Gilani A, Agostinucci K, Hossain Set al. 20-HETE interferes with insulin signaling and contributes to obesity-driven insulin resistance. Prostaglandins Other Lipid Mediat. 2021;152:106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pickens CA, Sordillo LM, Zhang C, Fenton JI. Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism. 2017;70:177–91. [DOI] [PubMed] [Google Scholar]
- 45. Kidson-Gerber G, Weaver J, Gemmell R, Prasan AM, Chong BH. Serum thromboxane B2 compared to five other platelet function tests for the evaluation of aspirin effect in stable cardiovascular disease. Heart, Lung and Circ. 2010;19(4):234–42. [DOI] [PubMed] [Google Scholar]
- 46. Vedin I, Cederholm T, Freund-Levi Yet al. Reduced prostaglandin F2 alpha release from blood mononuclear leukocytes after oral supplementation of omega3 fatty acids: the OmegAD study. J Lipid Res. 2010;51(5):1179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murphy KJ, Galvin K, Kiely M, Morrissey PA, Mann NJ, Sinclair AJ. Low dose supplementation with two different marine oils does not reduce pro-inflammatory eicosanoids and cytokines in vivo. Asia Pac J Clin Nutr. 2006;15(3):418–24. [PubMed] [Google Scholar]
- 48. Zulyniak MA, Perreault M, Gerling C, Spriet LL, Mutch DM. Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism. 2013;62(8):1107–13. [DOI] [PubMed] [Google Scholar]
- 49. Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, Sime PJ. Key roles for lipid mediators in the adaptive immune response. J Clin Invest. 2018;128(7):2724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winer DA, Winer S, Chng MHY, Shen L, Engleman EG. B lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell Mol Life Sci. 2014;71(6):1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: including non-randomized studies on intervention effects | cochrane training. [Internet] Available from: https://training.cochrane.org/handbook/current/chapter-24. Published February 2021. Accessed January 29, 2022. [Google Scholar]
- 52. Yaeger MJ, Reece SW, Kilburg-Basnyat Bet al. Sex differences in pulmonary eicosanoids and specialized pro-resolving mediators in response to ozone exposure. Toxicol Sci. 2021;183(1):170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rathod KS, Kapil V, Velmurugan Set al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 2016;127(1):169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins, Leukotrienes Essent Fatty Acids. 2017;117:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Largen PG, French JE, Moustaid-Moussa Net al. Synergizing mouse and human studies to understand the heterogeneity of obesity. Adv Nutr. 2021;12:2023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saleh RNM, West AL, Ostermann AI, Schebb NH, Calder PC, Minihane AM. APOE genotype modifies the plasma oxylipin response to omega-3 polyunsaturated fatty acid supplementation in healthy individuals. Front Nutrition. 2021;8:723813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript and analytic code will be made publicly and freely available without restriction at https://github.com/abrar-alshaer