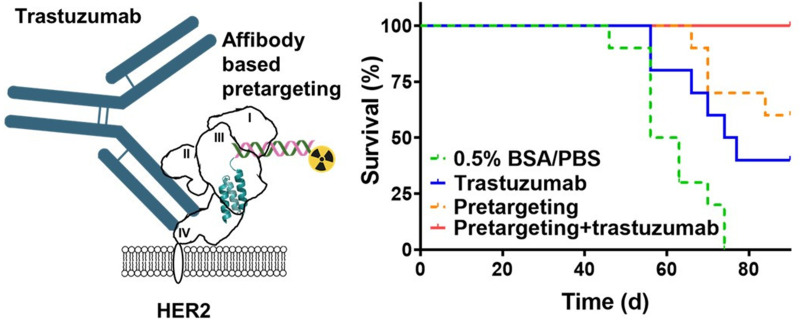
Visual Abstract
Keywords: trastuzumab, PNA pretargeting, Affibody molecules, radionuclide therapy, HER2
Abstract
Treatment of patients with human epidermal growth factor receptor 2 (HER2)–expressing tumors using the monoclonal antibody trastuzumab increases survival. The Affibody-based peptide nucleic acid (PNA)–mediated pretargeted radionuclide therapy has demonstrated efficacy against HER2-expressing xenografts in mice. Structural studies suggest that Affibody molecules and trastuzumab bind to different epitopes on HER2. The aim of this study was to test the hypothesis that a combination of PNA-mediated pretargeted radionuclide therapy and trastuzumab treatment of HER2-expressing xenografts can extend survival compared with monotherapies. Methods: Mutual interference of the primary pretargeting probe ZHER2:342-SR-HP1 and trastuzumab in binding to HER2-expressing cell lines was investigated in vitro. Experimental therapy evaluated the survival of mice bearing HER2-expressing SKOV-3 xenografts after treatment with vehicle, trastuzumab only, pretargeting using Affibody-PNA chimera ZHER2:342-SR-HP1 and complementary probe 177Lu-HP2, and combination of trastuzumab and pretargeting. The ethical permit limited the study to 90 d. The animals’ weights were monitored during the study. After study termination, samples of liver and kidneys were evaluated by a veterinary pathologist for toxicity signs. Results: The presence of a large molar excess of trastuzumab had no influence on the affinity of ZHER2:342-SR-HP1 binding to HER2-expressing cells in vitro. The affinity of trastuzumab was not affected by a large excess of ZHER2:342-SR-HP1. The median survival of mice treated with trastuzumab (75.5 d) was significantly longer than the survival of mice treated with a vehicle (59.5 d). Median survival of mice treated with pretargeting was not reached by day 90. Six mice of 10 in this group survived, and 2 had complete remission. All mice in the combination treatment group survived, and tumors in 7 mice had disappeared at study termination. There was no significant difference between animal weights in the different treatment groups. No significant pathologic alterations were detected in livers and kidneys of treated animals. Conclusion: Treatment of mice bearing HER2-expressing xenografts with the combination of trastuzumab and Affibody-mediated PNA-based radionuclide pretargeting significantly increased survival compared with monotherapies. Cotreatment was not toxic for normal tissues.
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor overexpressed in about 20%–30% of breast cancer (1), 18% of gastric and gastroesophageal cancer (2), and 9%–32% of ovarian carcinoma cases (3). The humanized monoclonal antibody trastuzumab binds to domain IV in the extracellular part of HER2. HER2-targeted therapy using trastuzumab together with nontargeted chemotherapy is a standard combination for treatment of patients with HER2-positive breast, gastric, and gastroesophageal cancers (4–6). However, resistance to trastuzumab treatment is often developed despite preserved HER2 expression (7).
In vitro studies have demonstrated that treatment with trastuzumab increases the sensitivity of breast cancer cell lines to radiation (8,9). Clinical data suggest that adding trastuzumab to local adjuvant radiotherapy significantly reduces the risk of breast cancer locoregional recurrence (10,11). It would also be attractive to combine radiation therapy and trastuzumab medication in a systemic treatment of disseminated HER2-expressing cancers. HER2-targeted radionuclide therapy might be a solution for such a combination treatment. However, the straightforward use of monoclonal antibodies labeled with cytotoxic radionuclides is problematic for the treatment of solid tumors (12,13). The slow clearance of bulky antibodies from the circulation results in high exposure of the radiosensitive bone marrow, which prevents sufficient delivery of radionuclides to tumors. The use of smaller targeting agents such as Affibody (Affibody AB) molecules (7 kDa) might be an alternative. Safety, tolerability, and excellent targeting of HER2-expressing breast cancer metastases using Affibody molecules have been confirmed in clinics (14). However, direct application of radiolabeled Affibody molecules for radionuclide therapy is excluded because of their high reabsorption in the kidneys (15). To avoid renal reabsorption, an Affibody-based pretargeting approach was investigated, and peptide nucleic acid (PNA)–mediated pretargeting appeared to be the most efficient of the tested strategies (16). PNAs are synthetic DNA analogs with charge-neutral and flexible peptide-like backbones (Supplemental Fig. 1A; supplemental materials are available at http://jnm.snmjournals.org) and are highly stable in human serum and cellular extracts (17). The pretargeting system is based on 2 complementary 15-meric PNA probes: the primary HP1 probe and the secondary HP2 probe (18). The secondary HP2 probe contains both a DOTA chelator and tyrosine, which enable labeling with a variety of radiometals and radioiodine (16,19). The primary HP1 probe is site-specifically conjugated to the HER2-targeting Affibody molecule ZHER2:342, forming the ZHER2:342-SR-HP1 Affibody-PNA chimera. A DOTA chelator is also incorporated into ZHER2:342-SR-HP1, which enables labeling of this agent for preclinical development and for imaging within theranostic applications (20). The sequences of ZHER2:342-SR-HP1 and HP2 are shown in Supplemental Figure 1B. This Affibody molecule–based PNA-mediated approach reduced renal uptake 70-fold compared with direct targeting using 177Lu-labeled parental Affibody molecules (16). A schematic illustration of the PNA-mediated pretargeted therapy approach is shown in Supplemental Figure 1C. Experimental therapy using ZHER2:342-SR-HP1/177Lu-HP2 pretargeting significantly increased the median survival of mice bearing HER2-expressing xenografts without bone marrow and renal toxicity (21).
Importantly, structural studies (22) have shown that the epitope of the anti-HER2 Affibody binding to HER2 is distant from the epitope of trastuzumab (Fig. 1). This distance creates a precondition for independent binding of trastuzumab and the primary probe without mutual interference and should enable cotreatment using trastuzumab and Affibody-mediated pretargeting.
FIGURE 1.
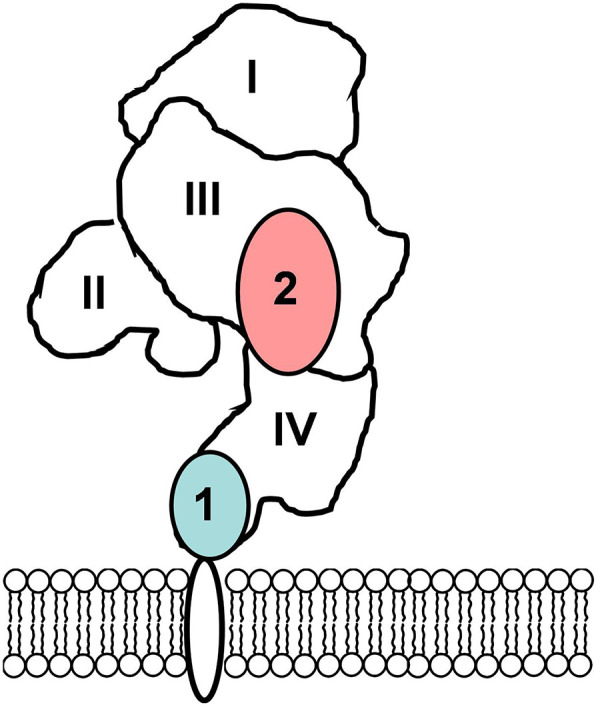
Epitopes for binding of trastuzumab (1) and ZHER2 Affibody molecule (2) to extracellular domain of HER2 (22).
The goal of this study was to test the hypothesis that Affibody-mediated PNA-based pretargeting cotreatment can improve the survival of mice with HER2-expressing xenografts treated with trastuzumab. We evaluated interference between the binding of trastuzumab and ZHER2:342-SR-HP1 to HER2-expressing cancer cell lines in vitro. Further, we compared the effect on tumor growth and survival of mice treated by cotargeting with the effects of treatment with trastuzumab and pretargeting alone.
MATERIALS AND METHODS
Carrier-free 177LuCl3 was purchased from Curium. HER2-expressing ovarian cancer SKOV-3 and breast cancer BT474 cells were purchased from the American Type Culture Collection. Cells were cultured at 37°C in 5% CO2 in RPMI medium (Flow Laboratories) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin.
Radiolabeling
The PNA probes (HP1 and HP2) and the HER2-binding Affibody were produced, purified, and characterized (Supplemental Figs. 2–5) as described earlier (18). The primary probe ZHER2:342-SR-HP1 was prepared from the HP1 probe and the Affibody molecule using an optimized conjugation protocol (23). The secondary probe HP2 was radiolabeled with 177Lu using a previously described method (23). For in vitro experiments, the primary probe ZHER2:342-SR-HP1 was labeled with 177Lu and trastuzumab was labeled with 125I as previously described (24,25).
In Vitro Studies
To check whether trastuzumab and ZHER2:342-SR-HP1 interfere with each other’s binding to HER2-expressing cell lines in vitro, the following assay was performed. Cells were seeded into cell culture dishes with a density of 106 cells per dish. Four sets of 3 dishes were used for each conjugate. For blocking, nonlabeled ZHER2:342-SR-HP1, trastuzumab, or the control anti-VEGF antibody bevacizumab (both from F. Hoffmann-La Roche) were added to sets of 3 dishes each to obtain concentrations of 200 nM. An equal volume of medium was added to the fourth set of dishes. After incubation at room temperature for 15 min, 177Lu-ZHER2:342-SR-HP1 or 125I-trastuzumab was added to all dishes to obtain a concentration of 1 nM. The cells were incubated for 1 h at 37°C. Thereafter, the cells were washed, the cells were detached by trypsin, and the cell-associated radioactivity was measured.
To evaluate the mutual interference of the targeting agents further, the affinity of their interaction with SKOV-3 cells in vitro was measured using a LigandTracer Yellow instrument (Ridgeview Instruments AB) as described earlier (19). Two concentrations of 177Lu-ZHER2:342-SR-HP1 (1 and 3 nM) were used to estimate an association rate. The measurements were performed either in the presence of trastuzumab (70 nM) or in its absence. The association rate of 125I-trastuzumab was measured with concentrations of 1 and 3 nM, in the presence of ZHER2:342-SR-HP1 (140 nM) or in its absence. The calculations of affinities and their visualization were performed using the InteractionMap software (Ridgeview Diagnostics AB). Experiments were performed in duplicate.
Experimental In Vivo Therapy
Animal experiments were performed in accordance with the national legislation for work with laboratory animals. Approval was granted by the Ethical Committee for Animal Research in Uppsala. According to the ethical permit, the therapy should not continue longer than 90 d.
Female BALB/c nu/nu mice (Scanbur) were subcutaneously (abdomen area) implanted with 107 SKOV-3 cells. The subcutaneous xenograft model was selected because it permits more exact tumor volume measurement than a disseminated tumor model. The mice were randomly divided into 4 groups of 10 animals each. Treatment started 1 wk after tumor implantation. The injected activity and number of injections were calculated using mouse dosimetry data (21) to provide the absorbed doses: approximately 100 Gy to tumor, 20 Gy to kidney, and 1 G to bone marrow. Group A (control) was subcutaneously injected with 100 μL of 0.5% bovine serum albumin in phosphate-buffered saline. Group B (trastuzumab treatment) was subcutaneously (neck area) injected with 6 injections of trastuzumab (4 mg/kg for 2 wk followed by 2 mg/kg weekly, that is, the same dosing as in clinics (26)). Group C (radionuclide pretargeting treatment) was intravenously injected with 100 μg (7.6 nmol) of ZHER2:342-SR-HP1 and 16 h later with 3.5 μg (0.68 nmol/16 MBq) of 177Lu-HP2 in a 100-μL solution containing 0.5% bovine serum albumin and 4 mg of Gelofusine (Hausmann Laboratories Ltd.) once a week. In total, 6 injections were performed. Group D (cotreatment) was treated with pretargeting (same as group C) in combination with trastuzumab (the same as group B). The tumor volumes at the start of treatment were 91± 25, 99 ± 42, 91 ± 34, and 97 ± 30 mm3, for mice treated with 0.5% bovine serum albumin/phosphate-buffered saline, trastuzumab, pretargeting, and combined trastuzumab and pretargeting, respectively (no significant difference in ANOVA, Supplemental Fig. 6A).
Throughout the experiment, tumor volumes and body weights were monitored twice per week. The tumor volumes were determined by caliper measurement of the largest longitudinal (length) and transverse (width) diameter and calculated by the following formula: tumor volume = ½ [length (mm)] × [width (mm)]2.
The animals were euthanized when tumors reached a size of 1,000 mm3 or became ulcerated, or if an animal’s weight dropped by more than 10% during 1 wk or by more than 15% since the study began. Ninety days after treatment started, all animals were euthanized using xylazine/ketamine anesthesia. After euthanasia, tumor, kidneys, and liver were excised for subsequent histologic and immunohistochemistry evaluations. The evaluations were performed at the Swedish National Veterinary Institute. The samples were formalin-fixed and paraffin-embedded. Tumor sections (4 μm) were stained with hematoxylin and eosin for histologic evaluation and using the HercepTest Kit (Agilent) to determine HER2 expression.
Imaging During Experimental Therapy
To confirm efficient tumor pretargeting, SPECT/CT imaging of 2 mice from group C and 2 mice from group D was performed 24 h after every injection of 177Lu-HP2. Imaging was performed using a nanoScan SPECT/CT device (Mediso Medical Imaging Systems) under sevoflurane anesthesia as described earlier (21).
Statistical Analysis
Data were analyzed by an unpaired, 2-tailed t test or 1-way ANOVA with Bonferroni adjustment for multiple comparisons. Survival data were analyzed by a log-rank test. Tumor ulceration was considered to be identical to the tumor size endpoint in the test. Prism (version 7.03 for Microsoft Windows; GraphPad Software) was used to determine significant statistical differences (P < 0.05).
RESULTS
Radiolabeling
The correct molecular weights of the primary and secondary probes were confirmed using mass spectrometry (Supplemental Figs. 2 and 3). The purity of the probes was over 95% (Supplemental Figs. 4 and 5). Radiolabeling of HP2 with 177Lu was achieved in high yields (>95%) at a maximum molar activity of 23.5 MBq/nmol.
In Vitro Studies
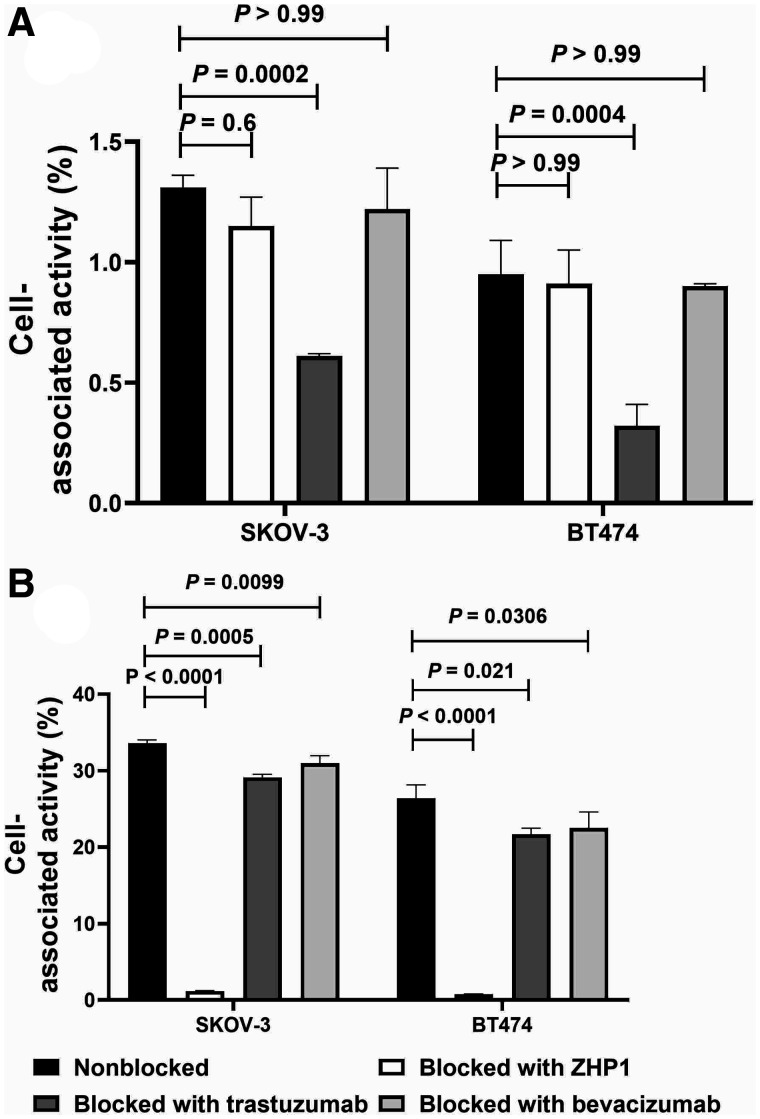
The results of the mutual blocking assay of trastuzumab and ZHER2:342-SR-HP1 to HER2-expressing cell lines are presented in Figure 2. The cell-associated radioactivity of 125I-trastuzumab for both HER2-expressing cell lines was significantly (P < 0.005) decreased when HER2 receptors were saturated with nonlabeled trastuzumab. No significant difference in binding was observed when cells were saturated with the primary agent ZHER2:342-SR-HP1 or bevacizumab (Fig. 2A). The cell-associated radioactivity of 177Lu-ZHER2:342-SR-HP1 to both HER2-expressing cell lines was significantly (P < 0.0001) decreased when HER2 receptors were saturated with ZHER2:342-SR-HP1. The binding of 177Lu-ZHER2:342-SR-HP1 was also significantly decreased by pretreatment with trastuzumab (P < 0.05). However, the decrease was much smaller in this case and was at the same level after treatment with the control anti-VEGF antibody bevacizumab (Fig. 2B).
FIGURE 2.
In vitro binding specificity of 125I-trastuzumab (A) and 177Lu-ZHER2:342-SR-HP1 (B) on HER2-expressing SKOV-3 and BT-474 cells. For both experiments, 1 nM of labeled conjugate and 200 nM of blocking agents were used. Data are presented as average value from 3 samples ± SD. ZHP1 = ZHER2:342-SR-HP1.
The data concerning the effect of trastuzumab presence on the binding affinity of 177Lu-ZHER2:342-SR-HP1 to HER2 receptors are presented in Table 1 and Supplemental Figure 7. The data concerning the influence of ZHER2:342-SR-HP1 presence on the binding affinity of 125I-trastuzumab to HER2 receptors are presented in Table 2 and Supplemental Figure 8. No interference of trastuzumab presence on the binding affinity of 177Lu-ZHER2:342-SR-HP1, or of ZHER2:342-SR-HP1 presence on the binding affinity of 125I-trastuzumab, to HER2 receptors was observed.
TABLE 1.
Association Rate (ka), Dissociation Rate (kd), and Equilibrium Dissociation (KD) Constants for Interaction Between 177Lu-ZHER2:342-SR-HP1 and HER2-Expressing SKOV-3 Cells in Presence and Absence of Trastuzumab Determined Using Interaction Map Analysis of LigandTracer Sensorgrams
| Parameter | ka [(1/M × s) × 104] | kd [(1/s) × 10−6] | KD (pM) |
|---|---|---|---|
| 177Lu-ZHER2:342-SR-HP1 only | 3.5 ± 0.4 | 2.6 ± 0.6 | 78 ± 28 |
| 177Lu-ZHER2:342-SR-HP1 with trastuzumab | 3.5 ± 0.3 | 2.90 ± 0.04 | 85 ± 7 |
TABLE 2.
Association Rate (ka), Dissociation Rate (kd), and Apparent Equilibrium Dissociation (KD) Constants for Interaction Between 125I-Trastuzumab and HER2-Expressing SKOV-3 Cells in Presence and Absence of ZHER2:342-SR-HP1 Determined Using Interaction Map Analysis of LigandTracer Sensorgrams
| Parameter | ka [(1/M × s) × 105] | kd [(1/s) × 10−6] | KD (pM) |
|---|---|---|---|
| 125I-trastuzumab only | 5.6 ± 0.0 | 2 ± 0.7 | 3.7 ± 1.2 |
| 125I-trastuzumab with ZHER2:342-SR-HP1 | 4.5 ± 0.2 | 1.9 ± 0.1 | 4.4 ± 0.7 |
Experimental In Vivo Therapy
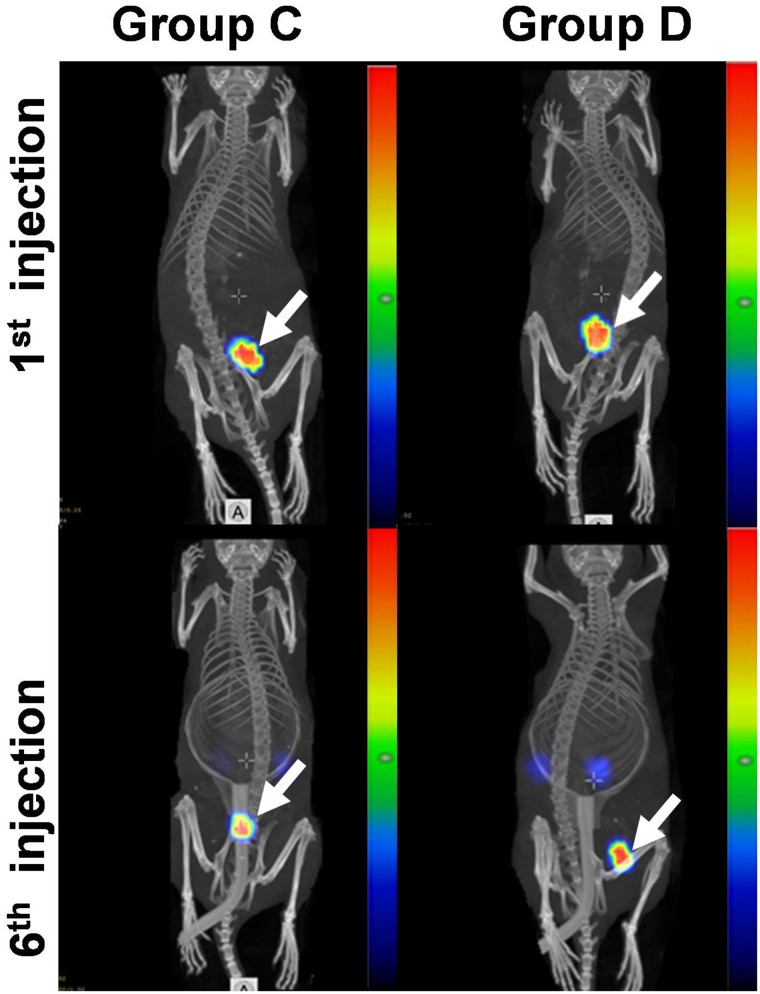
SPECT/CT imaging (Fig. 3) demonstrated efficient delivery of 177Lu to tumors. The tumor uptake in mice treated with both pretargeting and trastuzumab was on the same level as in mice treated with pretargeting alone (Supplemental Fig. 9). Six pretargeted treatment cycles did not decrease the maximum uptake of 177Lu-HP2 in tumors (Supplemental Fig. 9).
FIGURE 3.
Representative SPECT/CT images (maximum-intensity projections) of mouse from treatment group C and mouse from treatment group D. Imaging was performed 24 h after first injection and sixth injection of 177Lu-HP2. Arrows point at tumors. Linear relative scale (arbitrary units normalized to a maximum count rate) is applied.
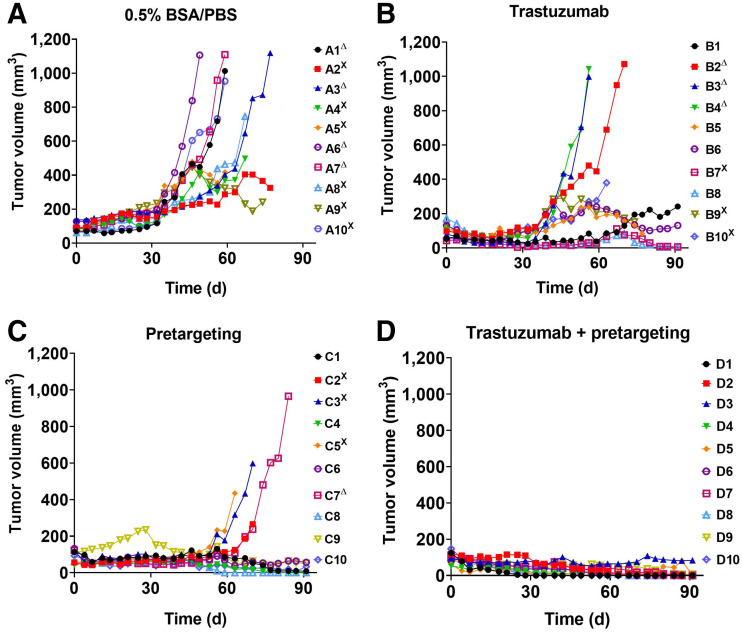
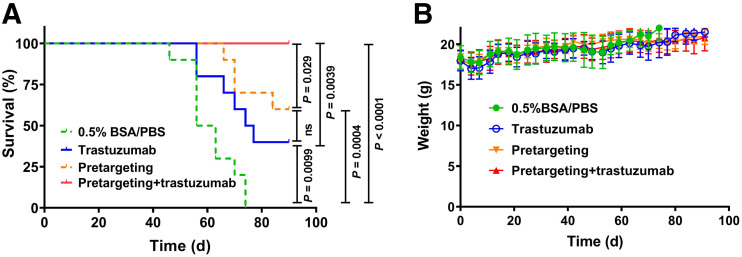
Information concerning tumor growth, body weight, and survival in different groups of animals is presented in Figures 4 and 5. All treatment modalities slowed the tumor growth rate. Seven days after the first injection, the average tumor volume in all treatment groups was significantly smaller than the average volume in the control group (group A) (Supplemental Fig. 6B). The median survival was the shortest in the control group (59.5 d), and all animals in this group were euthanized by day 74 (Fig. 5A). The treatment with trastuzumab alone (group B) significantly increased (P < 0.05) the median survival to 75.5 d (Fig. 5A). Two of 4 surviving mice had very small, less than 10 mm3, tumors. Pretargeted monotherapy (group C) was somewhat more efficient than monotherapy with trastuzumab. The median survival in group C was not reached within the permitted experiment time, but there was no significant difference between survival in groups C and D (Figs. 4 and 5). Tumors in 2 of 6 surviving mice disappeared completely at the time of euthanasia, and 1 mouse had a tumor smaller than 10 mm3. The combination of pretargeting and trastuzumab treatment (group D) was the most efficient, according to the results of the log-rank test (Fig. 5A). All mice survived until study termination. Seven mice had complete remission at that moment, and 2 mice had a tumor smaller than 10 mm3. Still, the small tumors contained viable tumor cells undergoing mitosis (Supplemental Fig. 10). Although tumors from groups A, B, and C retained HER2 expression on a 3+ level, the remaining tumors from group D had heterogeneous HER2 expression (Supplemental Fig. 11).
FIGURE 4.
Experimental therapy with tumor volume growth curves for individual mice in each group. SKOV-3 cells were subcutaneously implanted into belly of nude BALB/c nu/nu-mice. Mice were treated with 0.5% bovine serum albumin/phosphate-buffered saline (BSA/PBS) (control) (A), trastuzumab only (B), pretargeting only (C), and combination of trastuzumab and pretargeting (D). ΔMice were euthanized when volume of subcutaneous xenografts exceeded 1,000 mm3. XMice were euthanized when bleeding ulcers on xenografts were observed.
FIGURE 5.
(A) Survival of BALB/C nu/nu-mice with SKOV-3 xenografts treated with pretargeting plus trastuzumab, pretargeting alone, trastuzumab alone, or 0.5% bovine serum albumin in phosphate-buffered saline (0.5% BSA/PBS). (B) Average animal weight during therapy. Data are presented as average value ± SD. BSA = bovine serum albumin; PBS = phosphate-buffered saline.
When the outcomes were categorized as no response (animals were euthanized before the study endpoint), response (animals with visible tumors survived until the study endpoint), and remission (no visible tumors at the study endpoint), a Pearson χ2 test revealed significant differences among all groups (Supplemental Fig. 12).
The therapy was well tolerated. The average weight of the animals in the treatment groups did not differ significantly (ANOVA, P < 0.05) from the average weight in the control group at any time point (Fig. 5B). According to the histopathology evaluation, there was no evidence of renal toxicity in the treatment groups. No structures of the renal parenchyma in the treated animals differed from these structures in the kidneys of mice in the control group (Supplemental Fig. 13). In some livers of mice from the treatment groups, scattered mitoses were found among the hepatocytes, a finding that could indicate an ongoing regenerative activity (Supplemental Fig. 14). In the livers of 2 mice from the combined-treatment group, infiltrates of mononuclear leukocytes were observed in a few portal areas. According to the pathologist, these changes were very subtle.
DISCUSSION
Targeted therapies offer the advantage of a specific antitumor effect and minimize systemic toxicity. Therefore, there is an apparent trend to increase the targeted component in the treatment, such as by combining trastuzumab and pertuzumab treatment already in the first-line therapy of advanced breast cancer (27). In addition, the combination of 2 different therapeutics with different mechanisms of action has a potential to suppress resistance development (28). The use of radionuclide targeting is an attractive addition to trastuzumab because this antibody sensitizes the tumor to irradiation. Such a combination requires an absence of mutual interference of targeting agents in the binding to HER2. Trastuzumab does not interfere with binding of the small monomeric form of the anti-HER2 Affibody in vitro or in vivo (29). However, coupling of HP1 makes the ZHER2:342-SR-HP1 chimera bulkier, and interference could not be excluded. The results of the binding test (Fig. 2A) showed that there was no significant decrease in cell-associated radioactivity of 125I-trastuzumab when HER2-expressing cancer cells were pretreated with a large molar excess of ZHER2:342-SR-HP1. There was a small but significant reduction of cell-bound radioactivity of 177Lu-ZHER2:342-SR-HP1 when cells were pretreated with an excess of trastuzumab. However, treatment with the control antibody bevacizumab (which does not bind to HER2) had a similar effect. Thus, this phenomenon should not be associated with the blocking of 177Lu-ZHER2:342-SR-HP1 binding to the target. The measurement by LigandTracer did not show any impact of trastuzumab presence on the affinity of 177Lu-ZHER2:342-SR-HP1 binding to HER2-expressing cells (Table 2; Supplemental Fig. 7) or of ZHER2:342-SR-HP1 presence on the affinity of 125I-trastuzumab binding (Table 2; Supplemental Fig. 8). No influence of trastuzumab treatment on pretargeted delivery of 177Lu to tumors was observed by SPECT imaging performed during therapy (Fig. 3; Supplemental Fig. 9). The level of uptake was approximately the same with and without trastuzumab. Therefore, it is obvious from these experiments that the primary agent and trastuzumab are binding to different sites on HER2 receptors without mutual interference.
The experimental therapy data (Figs. 4 and 5) suggested that the tested hypothesis is correct. Trastuzumab as a monotherapy extended the median survival of mice from 59.5 d (vehicle-injected control) to 75.5 d. The median survival for mice treated with pretargeted radionuclide therapy was not reached within the permitted time of the therapy experiment but was apparently longer than 90 d. This treatment resulted in complete disappearance of tumors in 2 animals of 10, whereas residual tumors remained in all mice treated with trastuzumab. The combination therapy was the most efficient. It prevented tumor growth or ulceration more effectively than did pretargeting therapy alone (Fig. 4). There was complete remission in 7 animals of 10, and not a single animal had to be euthanized. A heterogeneous HER2 expression in the remaining tumors from this group indicates that downregulation of HER2 or a selection of clones with low HER2 expression might be a mechanism of resistance (or at least a reason for incomplete remission). At the same time, no severe toxicity was detected in any of the treatment groups. Noteworthy, SKOV-3 is appreciably more radioresistant than 2 other cell lines with high HER2 expression, SK-BR-3 and BT-474 (30), and can be considered the worst-case model for radionuclide therapy.
A precondition for a successful combination treatment is an additive effect of the drugs on tumors but different toxicity profiles. This requirement was fulfilled in the proposed therapy. No toxicity of anti-HER2 Affibody molecules was detected in rodents (31). No Affibody-caused toxicity has been found in clinical studies (14), nor has PNA toxicity has been found in clinics (32). The only toxicity in the pretargeted radionuclide therapy is expected from the radionuclide, and the kidneys are expected to be the dose-limiting organ (21). The most severe side effect of trastuzumab is cardiotoxicity (33). Hence, the toxicity profiles of the proposed therapeutics are different. Pathology investigation did not reveal any serious side effects in this study. HER2-targeted therapy remains an area of intensive development (34). As far as we know, our approach is the only one that does not rely on radioresistance of mice but keeps the dose limits to both kidneys and bone marrow within limits accepted in clinics.
CONCLUSION
We have shown that combined injection of the monoclonal antibody trastuzumab with Affibody-mediated PNA-based pretargeting significantly increased the median survival in mice bearing HER2-expressing tumors compared with trastuzumab only. This makes the combination of trastuzumab and pretargeting a promising candidate for clinical translation.
DISCLOSURE
This work has been supported by grants from the Swedish Cancer Society, Swedish Research Council, and Swedish Agency for Innovation VINNOVA. Vladimir Tolmachev and Anna Orlova are members of the Technical Advisory Board of Affibody AB (Solna, Sweden) and have shares in this company. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does the addition of PNA-mediated Affibody-based pretargeted therapy increase the efficacy of trastuzumab treatment of HER2-expressing xenografts?
PERTINENT FINDINGS: The combination of PNA-mediated Affibody-based radionuclide pretargeting and trastuzumab therapy has a stronger antitumor effect than either modality alone. The combination treatment is not associated with any additional toxicity.
IMPLICATIONS FOR PATIENT CARE: Data from this study support further development of combination targeting therapy, which might improve the survival of patients with HER2-expressing cancer.
REFERENCES
- 1. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 2. Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22:4619–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meden H, Kuhn W. Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor. Eur J Obstet Gynecol Reprod Biol. 1997;71:173–179. [DOI] [PubMed] [Google Scholar]
- 4. Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. [DOI] [PubMed] [Google Scholar]
- 5. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003;2:1113–1120. [PubMed] [Google Scholar]
- 9. Sato S, Kajiyama Y, Sugano M, et al. Monoclonal antibody to HER-2/neu receptor enhances radiosensitivity of esophageal cancer cell lines expressing HER-2/neu oncoprotein. Int J Radiat Oncol Biol Phys. 2005;61:203–211. [DOI] [PubMed] [Google Scholar]
- 10. Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. 2012;118:1982–1988. [DOI] [PubMed] [Google Scholar]
- 11. Peterson DJ, Truong PT, Sadek BT, et al. Locoregional recurrence and survival outcomes by type of local therapy and trastuzumab use among women with node-negative, HER2-positive breast cancer. Ann Surg Oncol. 2014;21:3490–3496. [DOI] [PubMed] [Google Scholar]
- 12. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartholomä MD. Radioimmunotherapy of solid tumors: approaches on the verge of clinical application. J Labelled Comp Radiopharm. 2018;61:715–726. [DOI] [PubMed] [Google Scholar]
- 14. Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 Affibody PET/CT. Theranostics. 2016;6:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortin MA, Orlova A, Malmström PU, Tolmachev V. Labelling chemistry and characterization of [90Y/177Lu]-DOTA-ZHER2:342-3 Affibody molecule, a candidate agent for locoregional treatment of urinary bladder carcinoma. Int J Mol Med. 2007;19:285–291. [PubMed] [Google Scholar]
- 16. Altai M, Membreno R, Cook B, Tolmachev V, Zeglis BM. Pretargeted imaging and therapy. J Nucl Med. 2017;58:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Demidov VV, Potaman VN, Frank-Kamenetskii MD, et al. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994;48:1310–1313. [DOI] [PubMed] [Google Scholar]
- 18. Westerlund K, Honarvar H, Tolmachev V, Eriksson Karlström A. Design, preparation, and characterization of PNA-based hybridization probes for affibody molecule-mediated pretargeting. Bioconjug Chem. 2015;26:1724–1736. [DOI] [PubMed] [Google Scholar]
- 19. Honarvar H, Westerlund K, Altai M, et al. Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics. 2016;6:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vorobyeva A, Westerlund K, Mitran B, et al. Development of an optimal imaging strategy for selection of patients for affibody-based PNA-mediated radionuclide therapy. Sci Rep. 2018;8:9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westerlund K, Altai M, Mitran B, et al. Radionuclide therapy of HER2-expressing human xenografts using an affibody molecule-based PNA-mediated pretargeting: in vivo proof-of-principle. J Nucl Med. 2018;59:1092–1098. [DOI] [PubMed] [Google Scholar]
- 22. Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsén L, Härd T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci USA. 2010;107:15039–15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Altai M, Westerlund K, Velletta J, et al. Evaluation of affibody molecule-based PNA-mediated radionuclide pretargeting: development of an optimized conjugation protocol and 177Lu labeling. Nucl Med Biol. 2017;54:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Tano H, Oroujeni M, Vorobyeva A, et al. Comparative evaluation of novel [177Lu]Lu-labelled PNA conjugates for affibody mediated PNA-based pretargeting. Cancers (Basel). 2021;13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westerlund K, Vorobyeva A, Mitran B, et al. Site-specific conjugation of recognition tags to trastuzumab for peptide nucleic acid-mediated radionuclide HER2 pretargeting. Biomaterials. 2019;203:73–85. [DOI] [PubMed] [Google Scholar]
- 26. Vogel CL, Cobleigh MA, Tripathy D, et al. First-line Herceptin® monotherapy in metastatic breast cancer. Oncology. 2001;61(suppl 2):37–42. [DOI] [PubMed] [Google Scholar]
- 27. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2736–2740. [DOI] [PubMed] [Google Scholar]
- 28. Saputra EC, Huang L, Chen Y, Tucker-Kellogg L. Combination therapy and the evolution of resistance: the theoretical merits of synergism and antagonism in cancer. Cancer Res. 2018;78:2419–2431. [DOI] [PubMed] [Google Scholar]
- 29. Orlova A, Tolmachev V, Pehrson R, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67:2178–2186. [DOI] [PubMed] [Google Scholar]
- 30. Steffen AC, Göstring L, Tolmachev V, Palm S, Stenerlöw B, Carlsson J. Differences in radiosensitivity between three HER2 overexpressing cell lines. Eur J Nucl Med Mol Imaging. 2008;35:1179–1191. [DOI] [PubMed] [Google Scholar]
- 31. Ahlgren S, Orlova A, Wållberg H, et al. Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J Nucl Med. 2010;51:1131–1138. [DOI] [PubMed] [Google Scholar]
- 32. Cohen M. The efficacy of a peptide-nucleic acid solution (Reticulose) for the treatment of hepatitis A and hepatitis B: a preliminary controlled human clinical trial. J R Soc Health. 1992;112:266–270. [DOI] [PubMed] [Google Scholar]
- 33. Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altunay B, Morgenroth A, Beheshti M, et al. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48:1371–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]