ABSTRACT
Background
Iodine is essential for synthesizing thyroid hormones, but other micronutrients are also required for optimal thyroid function. However, there is a lack of data on combined micronutrient status in relation to thyroid hormones in pregnancy.
Objectives
We aimed to assess the joint associations of iodine, selenium, and zinc status with plasma concentrations of thyroid hormones and thyroid-stimulating hormone (TSH) in pregnancy.
Methods
We included 531 pregnant women (aged 22–40 y) participating in a Swedish birth cohort who provided blood and spot urine samples in gestational weeks 27–33 (mean: 29). Associations of urinary iodine concentration (UIC), plasma selenium concentration, and plasma zinc concentration (measured by inductively coupled plasma mass spectrometry) with plasma hormone concentrations [total and free thyroxine (tT4, fT4), total and free triiodothyronine (tT3, fT3), and TSH] were explored with Bayesian kernel machine regression (BKMR; n = 516; outliers excluded) and multivariable-adjusted linear regression (n = 531; splined for nonlinear associations).
Results
Median (IQR) micronutrient concentrations were 112 μg/L (80–156 μg/L) for UIC, 67 μg/L (58–76 μg/L) for plasma selenium, and 973 μg/L (842–1127 μg/L) for plasma zinc; the former 2 median values were below recommended concentrations (150 μg/L and 70 μg/L, respectively). Mean ± SD TSH concentration was 1.7 ± 0.87 mIU/L, with 98% < 4 mIU/L. BKMR showed a positive trend of joint micronutrient concentrations in relation to TSH. Plasma zinc was most influential for all hormones but tT3, for which plasma selenium was most influential. In adjusted linear regression models, zinc was positively associated with tT4, tT3, and TSH, and <1200 μg/L also with fT4 and fT3. Selenium was inversely associated with fT3, and <85 μg/L with tT3.
Conclusions
Pregnant women's plasma TSH concentrations in the early third trimester increased with increasing joint status of iodine, selenium, and zinc. Zinc and selenium were more influential than iodine for the hormone concentrations. Multiple micronutrients need consideration in future studies of thyroid hormone status.
Keywords: thyroxine (T4), triiodothyronine (T3), thyroid-stimulating hormone (TSH), micronutrients, pregnancy
Introduction
Adequate thyroid function is particularly important during pregnancy, because thyroid hormones are required for fetal development and both maternal and fetal metabolism (1). The fetus is totally dependent on maternal thyroid hormone production until the second trimester when fetal production is initiated (2, 3). Several micronutrients act in concert for optimal thyroid hormone production. Iodine is a constituent of the thyroid hormones thyroxine (T4), with 4 iodine atoms per hormone molecule, and triiodothyronine (T3), mainly formed from T4 by the removal of 1 iodine atom (deiodination). Prolonged iodine deficiency induces autoregulatory changes involving increased direct production of T3 in the thyroid at the expense of T4 production and is consequently characterized by reduced circulatory T4 and normal or even slightly elevated circulatory T3, and normal TSH concentrations (4). Importantly, the fetal brain derives a crucial part of its T3 by intracellular conversion of T4, and therefore maternal iodine deficiency may still influence fetal development despite maintained maternal thyroid function (4).
Selenium has several functions in thyroid hormone production and maintenance, but it is less studied than iodine. It is required for the selenoenzymes deiodinase types I, II, and III. Deiodinase types I and II convert T4 into T3 in tissues (5–7). Most of the circulating T3 is the product of deiodinase type I activity (8). Deiodinase type III inactivates T4 and T3 and it is highly expressed in the placenta where it regulates the transfer of thyroid hormone to the fetus (9). Selenium is also required for production of glutathione peroxidase, which protects the thyroid gland from oxidative stress produced during hormone synthesis (10, 11).
Zinc is an essential micronutrient involved in numerous enzymes and processes in the body, including maintenance of thyroid function, for example by regulating deiodinase activity (12–15). Studies in male rats have indicated that zinc positively influences circulating thyroid hormone concentrations (16, 17). However, observational studies in pregnant women are sparse, despite the fact that pregnancy-related zinc deficiency is common (18).
Severe gestational deficiency in both iodine and selenium combined has been associated with impaired mental and physical development (19), yet little is known concerning the combined impact of mild-to-moderate deficiencies on maternal thyroid hormones (6, 10). Most nutrition-related studies of thyroid function have focused on iodine alone, including 2 recent studies from Sweden (20, 21). In addition, we have recently reported that both iodine and selenium intakes in Swedish pregnant women are low (22). Therefore, we here aimed at elucidating the joint impact of iodine, selenium, and zinc status on thyroid hormone and TSH concentrations in pregnant women in northern Sweden.
Methods
Study population
This study included pregnant women from the birth cohort NICE (Nutritional impact on the Immunological maturation during Childhood in relation to the Environment), established between 2015 and 2018 in the catchment area of Sunderby Hospital in northern Sweden (23). The women were recruited around gestational week 18 (n = 655). For the present study we excluded twin pregnancies and second pregnancies of already-participating women, as well as 1 mother that withdrew from the study (Supplemental Figure 1). Of the 633 identified women, we in addition excluded those 1) with any form of thyroid dysfunction and/or who were prescribed thyroid-interfering drugs according to their pregnancy-related hospital records (n = 47), 2) who lacked a plasma sample (n = 37), or 3) who had extreme hormone concentrations (TSH > 20 mIU/L; n = 1). In total, we identified 531 women that had complete data on micronutrients, hormones, and covariates (Supplemental Figure 1).
The study was approved by the Regional Ethical Review Board, Umeå, Sweden. Women gave written consent after being informed of all study details and that participation was voluntary with the possibility to refrain at any time point.
Sample collection
Venous blood and spot urine samples were collected in gestational week 29 (range: 24–36 weeks of gestation) at local maternity health clinics (23, 24). Women were asked to fast for 8 h before the visit. As previously described in detail (23), blood samples were collected in EDTA-coated tubes (Becton Dickinson) and spot urine samples (midstream) were collected and transferred to polyethylene vials. Samples were transported cold to the hospital laboratory, where blood samples were centrifuged at 4°C and 2400 rpm (Hettich Rotina 420, Hettich Lab Technology, Tuttlingen, Germany) for 5 minutes and plasma divided into aliquots. Samples were stored at −80°C awaiting analysis.
Hormone analyses
Hormones were measured at the accredited laboratory at the Department of Clinical Chemistry, University Hospital of Malmö, Sweden, as previously described (25). Free and total T4 and T3 (fT4, tT4, fT3, and tT3, respectively) were all measured using an automated electrochemiluminescence immunoassay (ECLIA) on the Roche Cobas (Roche Diagnostics), consisting of a 2-step immunometric-competitive technique followed by chemiluminescent emission measurement. Plasma concentrations of TSH were measured using a 1-step sandwich method with ECLIA on the Roche Cobas (Roche Diagnostics).
Internal or transferable trimester-specific reference ranges for the hormone concentrations were not available. However, for TSH an upper reference limit of 4.0 mIU/L may be used as an indication of potential hypothyroidism (26).
Micronutrient analyses
We assessed selenium and zinc status by concentrations in plasma. Iodine status was evaluated by urinary iodine concentration (UIC; main route of excretion), adjusted to the mean specific gravity and measured with a refractometer (EUROMEX RD712 Clinical Refractometer) to compensate for urine dilution (27). UIC varies within and between days depending on intake (28) but is considered a valid intake biomarker on a population level (29). Micronutrient concentrations were measured at Karolinska Institutet using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700x, Agilent Technologies). Urine was diluted 1:10 in 0.1% ammonium hydroxide solution (30), whereas plasma was diluted 1:25 in alkali solution (31). The limit of detection (3 times the SD of blank concentrations) was 1.9 μg/L, 0.36 μg/L, and 3.7 μg/L for iodine, selenium, and zinc, respectively, and no concentration was below these limits. We included 2 commercial reference materials (serum or urine) in each run, and the obtained concentrations were in good agreement with the reference values (Supplemental Table 1).
Covariates
Hospital records were used to retrieve data on the women's age (y), first-trimester BMI (in kg/m2), parity (number of previous births), education (elementary school, high school, or university), pre- and early-pregnancy smoking (never, occasionally, or daily), and alcohol consumption (never, occasionally, or daily). Because thyroid hormones and TSH have been reported to vary by season (32, 33), the date of blood collection was categorized into 4 seasons: spring (March–May), summer (June–August), fall (September–November), and winter (December–February).
Statistical analyses
Statistical analyses were performed using the software Stata/IC 15.0 (StataCorp) and R 4.0.4 (34). P values < 0.05 were considered statistically significant, in combination with consistency and robustness of the results.
Potential confounders were identified with a directed acyclic graph (DAG) (Supplemental Figure 2). Covariates that were associated with both the micronutrients and the hormones were included in the DAG. Bivariate associations between micronutrients, hormones, and covariates were explored with either the Spearman rank correlation test, Mann–Whitney U test, or Kruskal–Wallis test. Gestational week at sampling was weakly correlated with TSH (ρ: −0.09; P = 0.045) but did not correlate with the thyroid hormones or micronutrients. Maternal prepregnancy smoking was associated only with fT3 and tT3, and alcohol intake was not associated with any of the hormones or micronutrients. In the DAG, the following variables were identified as potential confounders and therefore adjusted for in all the final models: parity (categorized as 0 and >0), maternal education (lower than university and university), and season at sampling (spring, summer, fall, and winter), besides mutual adjustment between the micronutrients.
Associations of all micronutrients combined and potential interactions between the micronutrients, in relation to the hormones, were explored with Bayesian kernel machine regression (BKMR), using the R package bkmr (35). BKMR can flexibly model the relations (linear or nonlinear) of multiple variables against an outcome by regressing the outcome on a flexible function of the exposure mixture components (here micronutrients) that is specified using a kernel function (36). We used centered and scaled micronutrient concentrations, applying variable selection and 50,000 iterations by the Markov chain Monte Carlo algorithm (which is used to fit the BKMR model). Because BKMR is sensitive to outliers, we removed UIC > 382 μg/L (n = 10) and plasma zinc > 1800 μg/L (n = 5), resulting in 516 included women. Applying variable selection allowed for each of the individual micronutrients to enter into the model so that the posterior inclusion probability (PIP) for each micronutrient could be estimated in relation to each outcome (36). The PIPs reflect the relative importance of the different micronutrients, where the highest PIP indicates the most important micronutrient for the given outcome. We used plots to assess the estimated association of a single micronutrient with each hormone outcome, adjusted for the other micronutrients as well as the selected covariates. The plots show the shape and direction of the estimated associations with pointwise 95% credible intervals. Similarly, we assessed the joint micronutrient–hormone associations in plots showing the estimated difference in the outcome (with 95% credible intervals) when all 3 micronutrients were held at different percentiles compared with when all 3 micronutrients were held at the 50th percentile. Lastly, to explore potential interactions between the 3 micronutrients in relation to the hormones, we plotted the micronutrient–hormone associations for each micronutrient fixing 1 of the other 2 micronutrients at different percentiles (i.e., the 10th, 50th, and 90th percentiles) while the third micronutrient was held at the 50th percentile. If the shape or direction of any micronutrient–hormone association were to differ between different concentrations of another micronutrient, this would indicate a potential interaction between the micronutrients in relation to the outcome.
Linear regression analyses of the mutually adjusted micronutrient–hormone associations included all 531 women. Plasma selenium and zinc were normally distributed, whereas urinary iodine was right-skewed and therefore log2-transformed. Models were adjusted for confounders defined in the DAG (Supplemental Figure 2). Linearity was explored with generalized additive models (GAMs). Nonlinear associations were suggested (p-gain < 0.05) for selenium with tT3 and for zinc with fT4 and fT3. The Akaike information criterion (AIC) was therefore compared between 1) models including 1 spline knot (assessing several positions) visually selected based on the GAM plots (Supplemental Figure 3A–C), 2) models including a quadratic micronutrient term, and 3) linear models. The spline models had the lowest AIC and were therefore applied to the nonlinear associations, with a spline knot at 85 μg/L for selenium and 1200 μg/L for zinc. The remaining associations were explored linearly.
Results
Background characteristics
Table 1 shows the general characteristics of the mothers. Only 1.7% (n = 9) of the included women had TSH concentrations >4.0 mIU/L, indicating that most women had normal thyroid function (26). None of the hormones changed noticeably within the gestational week range of sample collection. Both fT3 and tT3 were slightly higher among prepregnancy smokers than among never-smokers (mean: 4.3 compared with 4.0 pmol/L, P < 0.001; and 2.9 compared with 2.6 nmol/L, P < 0.001, respectively), and among women with an elementary or high school degree than among those with a university degree (4.1 compared with 4.0 pmol/L, P = 0.02; and 2.7 compared with 2.5 nmol/L, P = 0.006, respectively).
TABLE 1.
Background characteristics and concentrations of hormones and micronutrients among the studied mothers of the NICE (Nutritional impact on the Immunological maturation during Childhood in relation to the Environment) birth cohort in northern Sweden1
| Maternal characteristics | n | Median (2.5th–97.5th percentile) or % |
|---|---|---|
| Age, y | 531 | 30 (22–40) |
| Early-pregnancy weight, kg | 516 | 68 (52–108) |
| Height, cm | 531 | 167 (155–179) |
| Early-pregnancy BMI, kg/m2 | 516 | 24 (19–38) |
| Parity, nulliparous | 531 | 49 |
| Education, university | 531 | 70 |
| Prepregnancy smoking, yes | 529 | 6.6 |
| Prepregnancy alcohol intake, yes | 522 | 74 |
| Gestational week at sample collection | 492 | 29 (27–33) |
| Season at sampling, spring/summer/fall/winter | 531 | 30/27/20/22 |
| Plasma free T4, pmol/L | 531 | 12 (9.0–15) |
| Plasma total T4, nmol/L | 531 | 122 (88–167) |
| Plasma free T3, pmol/L | 531 | 4.1 (3.2–4.9) |
| Plasma total T3, nmol/L | 531 | 2.6 (1.8–3.5) |
| Plasma TSH, mIU/L | 531 | 1.6 (0.45–3.9) |
| Urinary iodine,2 μg/L | 531 | 112 (46–346) |
| Plasma selenium, μg/L | 531 | 67 (42–99) |
| Plasma zinc, μg/L | 531 | 973 (580–1606) |
TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, thyroxine.
Concentrations adjusted to the mean specific gravity of 1.017.
Median (IQR) UIC was 112 μg/L (80–156 μg/L), which is below the recommended range of 150–249 μg/L for population-based median UIC in pregnancy (29). Median (IQR) plasma selenium was 67 μg/L (58–76 μg/L), with 60% being <70 μg/L, the lower limit for sufficient glutathione peroxidase activity (37, 38). Median (IQR) plasma zinc was 973 μg/L (842–1127 μg/L), with only 1% (n = 6) being <500 μg/L, the proposed lower limit of the late-pregnancy reference interval (18). Supplemental Table 2 shows the correlations between the micronutrients (ρ: 0.04–0.30).
Iodine and selenium concentrations were slightly higher among women with a university education (median: 116 μg/L and 67 μg/L, respectively) than among women with an elementary or high school education (median: 107 μg/L and 65 μg/L, respectively; P < 0.05). Zinc concentrations in spring (median: 1018 μg/L) were significantly higher than in fall (935 μg/L; P = 0.001), and nulliparous women had higher plasma zinc (median: 1009 μg/L) than women with previous deliveries (960 μg/L; P = 0.001). There were no significant differences in micronutrient concentrations between the few women with elevated TSH (>4.0 mIU/L) and those with lower TSH.
BKMR
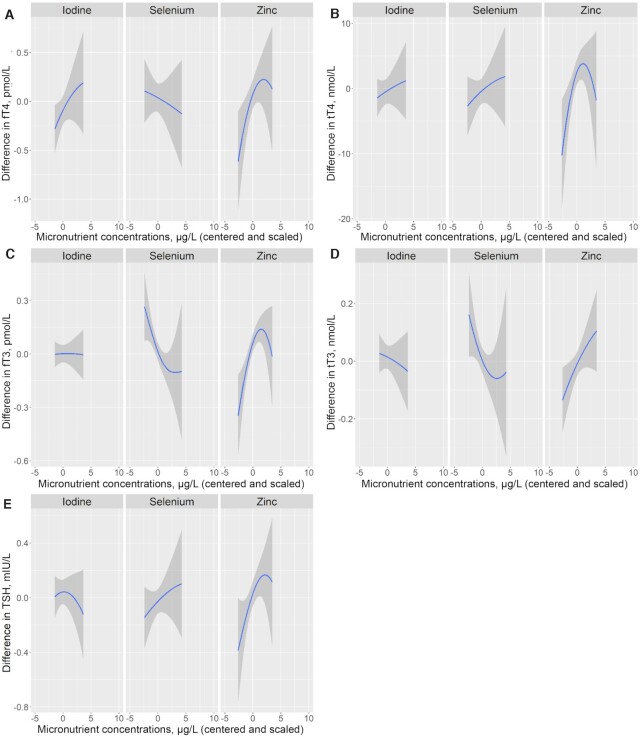
The PIPs, representing the relative importance of the micronutrients for the outcomes, are presented in Supplemental Table 3 for all BKMR models. Zinc showed the highest PIP for all hormones except tT3, for which selenium showed the highest probability. In the single micronutrient plots (adjusted for the other 2 micronutrients; Figure 1A–E), zinc was positively associated with all hormones. The plots showed that the associations of zinc with fT4, tT4, fT3, and TSH turned inverse above a certain zinc concentration (∼1570 μg/L, corresponding to ∼2.5 in the plots when centered and scaled), but the credible intervals were wide at these zinc concentrations and therefore the directions of the associations were uncertain. Selenium was inversely associated with fT3 and tT3 (leveling off roughly around the selenium concentration of 103 μg/L, corresponding to ∼2.5 in the plot when centered and scaled).
FIGURE 1.
Single micronutrient–hormone relations, with 95% credible intervals, of centered and scaled maternal concentrations of urinary iodine (μg/L), plasma selenium (μg/L), and plasma zinc (μg/L) with maternal concentrations of fT4 (pmol/L) (A), tT4 (nmol/L) (B), fT3 (pmol/L) (C), tT3 (nmol/L) (D), and TSH (mIU/L) (E) in pregnant women early in the third trimester participating in the NICE (Nutritional impact on the Immunological maturation during Childhood in relation to the Environment) study (n = 516), estimated by Bayesian kernel machine regression. The exposure–response relation for each micronutrient is controlled for the other 2 nutrients by fixing them at their median concentration. All models (A–E) were also adjusted for parity (0 and >0), maternal education (lower than university and university), and season at sampling (spring, summer, fall, and winter). fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine.
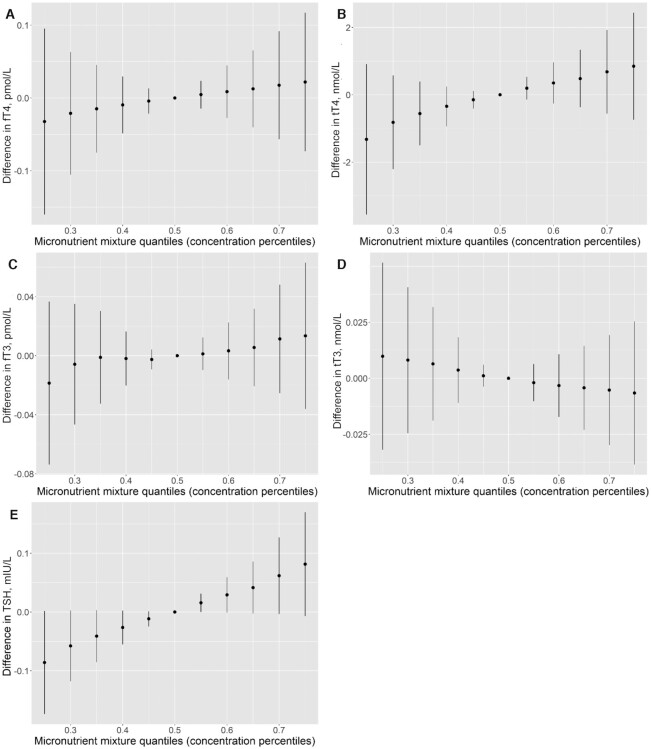
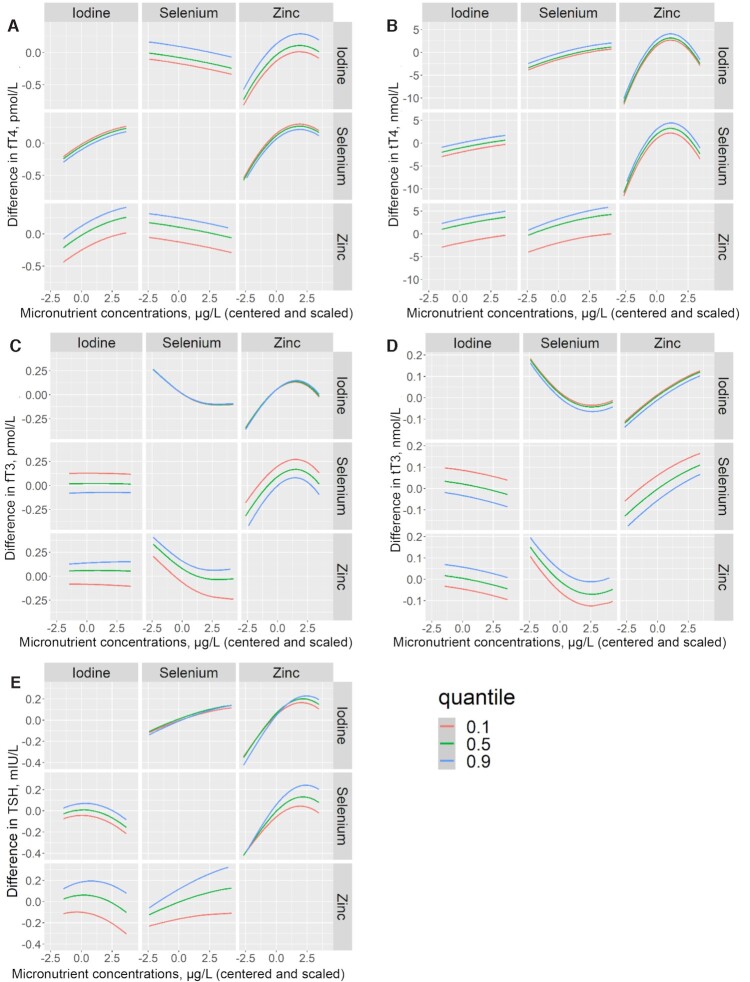
There was a positive joint trend of the micronutrients in relation to TSH, whereas no obvious trend was observed in relation to the other thyroid hormones (Figure 2A–E). The positive association between zinc and TSH was slightly more pronounced at higher selenium concentrations. Otherwise, there was no indication of micronutrient interactions (Figure 3A–E).
FIGURE 2.
Joint associations of urinary iodine (μg/L), plasma selenium (μg/L), and plasma zinc (μg/L) with fT4 (A), tT4 (B), fT3 (C), tT3 (D), and TSH (E) in pregnant women early in the third trimester participating in the NICE (Nutritional impact on the Immunological maturation during Childhood in relation to the Environment) study (n = 516), estimated by Bayesian kernel machine regression. The figures show the estimated difference in the outcomes, with 95% credible intervals, when all 3 micronutrients are held at different percentiles compared with when all 3 micronutrients are held at their median concentration (50th percentile). For all models (A–E), the maternal micronutrient concentrations were centered and scaled, and all models were adjusted for parity (0 and >0), maternal education (lower than university and university), and season at sampling (spring, summer, fall, and winter). fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine.
FIGURE 3.
Exposure–response function for centered and scaled concentrations of urinary iodine (μg/L), plasma selenium (μg/L), and plasma zinc (μg/L) against maternal fT4 (A), tT4 (B), fT3 (C), tT3 (D), and TSH (E) in pregnant women early in the third trimester participating in the NICE (Nutritional impact on the Immunological maturation during Childhood in relation to the Environment) study (n = 516), estimated by Bayesian kernel machine regression. In each graph (A–E), the middle left panel shows the estimated micronutrient–hormone relation for urinary iodine when plasma selenium is fixed at the 10th (red line), 50th (green line), and 90th (blue line) percentiles and plasma zinc is fixed at the 50th percentile. The bottom left panel shows the relation for iodine when zinc is held at the 10th, 50th, and 90th percentiles and selenium is fixed at the 50th percentile. The top middle panel shows the relation for selenium when iodine is fixed at the 10th, 50th, and 90th percentiles and zinc is fixed at the 50th percentile. The bottom middle panel shows the relation for selenium when zinc is fixed at the 10th, 50th, and 90th percentiles and iodine is held at the 50th percentile. The top right panel shows the relation for zinc when iodine is fixed at the 10th, 50th, and 90th percentiles and selenium is held at the 50th percentile. The middle right panel shows the relation for zinc when selenium is fixed at the 10th, 50th, and 90th percentiles and iodine is fixed at the 50th percentile. For all models (A–E) the maternal micronutrient concentrations were centered and scaled, and all models were adjusted for parity (0 and >0), maternal education (lower than university and university), and season at sampling (spring, summer, fall, and winter). fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine.
Linear regression analyses
In the regression models (Table 2), plasma selenium was inversely associated with fT3 [−0.19 SD in fT3 per IQR increase in selenium (18 μg/L)]. The association of selenium and tT3 was nonlinear (Supplemental Figure 3C), with an inverse association below the spline knot of 85 μg/L (−0.25 SD per 18-μg/L increase in selenium). Plasma zinc was nonlinearly associated with fT4 and fT3 (Supplemental Figure 3A, B), with positive associations observed below the spline knot of 1200 μg/L with both fT4 [0.24 SD per IQR increase in zinc (283 μg/L)] and fT3 (0.34 SD per 283-μg/L increase in zinc). Further, plasma zinc was linearly and positively associated with tT4, tT3, and TSH (0.14, 0.16, and 0.14 SD, respectively, per 283-μg/L increase in zinc).
TABLE 2.
Multivariable-adjusted regression analyses of maternal UIC (μg/L; log2-transformed), P-Se (μg/L; splined for nonlinear associations), and P-Zn (μg/L; splined for nonlinear associations) with plasma hormone concentrations early in the third trimester1
| Adjusted regression models | ||||
|---|---|---|---|---|
| Plasma hormones | n | B | 95% CI | P |
| fT4, pmol/L | ||||
| UIC, μg/L (log2) | 531 | 0.14 | (−0.041, 0.33) | 0.13 |
| P-Se, μg/L | 531 | −0.004 | (−0.014, 0.006) | 0.46 |
| P-Zn < 1200 μg/L | 434 | 0.0013 | (0.0005, 0.0022) | 0.003 |
| P-Zn > 1200 μg/L | 97 | −0.0009 | (−0.002, 0.0003) | 0.15 |
| tT4, nmol/L | ||||
| UIC, μg/L (log2) | 531 | 1.3 | (−1.0, 3.5) | 0.28 |
| P-Se, μg/L | 531 | 0.063 | (−0.057, 0.18) | 0.30 |
| P-Zn, μg/L | 531 | 0.010 | (0.003, 0.016) | 0.005 |
| fT3, pmol/L | ||||
| UIC, μg/L (log2) | 531 | −0.020 | (−0.070, 0.031) | 0.45 |
| P-Se, μg/L | 531 | −0.005 | (−0.007, −0.002) | 0.001 |
| P-Zn < 1200 μg/L | 434 | 0.001 | (0.0003, 0.001) | <0.001 |
| P-Zn > 1200 μg/L | 97 | −0.0001 | (−0.0004, 0.0002) | 0.65 |
| tT3, nmol/L | ||||
| UIC, μg/L (log2) | 531 | −0.011 | (−0.062, 0.039) | 0.66 |
| P-Se < 85 μg/L | 465 | −0.006 | (−0.009, −0.003) | 0.001 |
| P-Se > 85 μg/L | 66 | 0.009 | (−0.0001, 0.017) | 0.052 |
| P-Zn, μg/L | 531 | 0.0003 | (0.0001, 0.0004) | 0.001 |
| TSH, mIU/L | ||||
| UIC, μg/L (log2) | 531 | −0.021 | (−0.12, 0.081) | 0.68 |
| P-Se, μg/L | 531 | 0.004 | (−0.002, 0.009) | 0.17 |
| P-Zn, μg/L | 531 | 0.0004 | (0.0001, 0.0007) | 0.006 |
All models included mutual adjustment between the micronutrients and additional adjustment for parity (0 and >0), maternal education (lower than university and university), and season of sampling (spring, summer, fall, and winter). fT3, free triiodothyronine; fT4, free thyroxine; P-Se, plasma selenium; P-Zn, plasma zinc; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine; UIC, urinary iodine concentration.
Discussion
To our knowledge, this is the first study evaluating the joint association of iodine, selenium, and zinc concentrations with thyroid hormones during pregnancy. Unexpectedly, zinc was most influential for the plasma concentrations of fT4, tT4, fT3, and TSH in the BKMR analyses, showing positive associations with all of them. Selenium was the most important micronutrient for tT3 (inverse association). Our findings suggested that higher concentrations of all 3 micronutrients increased maternal TSH.
The women included in this study had no known thyroid-related diagnosis and essentially all (98%) had TSH concentrations <4.0 mIU/L, indicative of normal thyroid function (26). They had generally low concentrations of iodine and selenium (22), which is in line with studies of pregnant women in other parts of Sweden (20, 39–41). On the other hand, only 1% (n = 6) had plasma zinc concentrations <500 μg/L [proposed lower limit of the late-pregnancy reference interval (18)]. Still, plasma zinc was the micronutrient that was most influential for the hormone concentrations, being positively associated with T3, T4, as well as TSH. Thyroid hormone control involves a fine-tuned feedback regulation, keeping circulating T3 concentrations fairly constant (7). The fact that all hormones increased with zinc may suggest that the increase in TSH was not due to the feedback loop of low T4 or T3. TSH production in the pituitary gland is driven by hypothalamic thyrotropin-releasing hormone (TRH), for which zinc is essential (13). Further, zinc is involved in the utilization of iodine in the thyroid, which would increase T4 and T3 concentrations, as well as in the transcription factors governing the synthesis of thyroid hormones (12, 13). Zinc may also be involved in regulation of type I and type II deiodinases (13), which increase the conversion of T4 to T3, the active form of the hormone in tissues. However, plasma zinc did not correlate with the T3:T4 ratio (data not shown). Lastly, zinc has been implicated in regulating binding of T3 to its nuclear receptor (13). There are few previous studies on zinc status and thyroid function in pregnancy. In a large (n = 2041) Dutch study (42), first-trimester plasma zinc was positively associated with concurrent fT4, but not with TSH, which, however, is suppressed in early pregnancy (43). In experimental animal studies, zinc supplementation was found to increase circulating thyroid hormone concentrations (16), whereas zinc deficiency caused a decline in circulating thyroid hormones (17), possibly due to reduced TRH (17), or through direct effects on thyroid hormone synthesis (44).
We observed an inverse association of maternal plasma selenium with fT3 and tT3, the active form of the thyroid hormone. We observed no associations of selenium with T4, suggesting that the associations with the T3 concentrations were not related to the hormone production in the thyroid. They might instead be related to a selenium-dependent increase in type III deiodinase, which inactivates T3 (5). The expression of this selenoenzyme is increased during pregnancy, particularly in the placenta and fetal tissues (7). In support, pregnant mice fed a moderately selenium-deficient diet showed increased concentrations of T4 and T3, in combination with reduced placental expression of both type II and type III deiodinases (45). In the Dutch study aforementioned, maternal plasma selenium (59–103 μg/L) late in the first trimester was positively associated with TSH and inversely associated with fT4 (42), whereas T3 was not measured.
It is well known that iodine deficiency adversely affects maternal thyroid function and may lead to maternal hypothyroxinemia (3, 4, 26). We did not observe any significant associations between UIC and the hormones. Nevertheless, the tendency for a positive association with fT4 (P = 0.13) is in line with the reduced circulatory T4, with normal T3 and TSH, previously observed in individuals with iodine deficiency (4). The wide CIs and credible intervals may be the result of UIC being an uncertain marker of iodine status on an individual level. Most of the absorbed iodine that is not taken up by the thyroid gland is rapidly excreted in urine (28, 29), and most likely the women's iodine intake varied during the day, because iodized table salt and supplements containing iodine were the primary sources of iodine intake (22). Because the women were asked to fast before urine collection, the measured UIC, although likely rather stable, might in fact have underestimated the amount of iodine available to the thyroid, which is largely supplied by the peak plasma concentrations after a meal or supplementation. Another reason for weak associations might be that fT4 was measured by automated immunoassays, which often results in lower hormone concentrations in the third trimester (26). However, we did not observe any change in either fT4 or tT4 across the gestational week range.
The main strength of this study is the assessment of 3 thyroid-associated micronutrients, measured using highly sensitive ICP-MS methods, and their associations with several thyroid-related hormones. A limitation of the study is that only ∼10% of all women delivering at Sunderby Hospital during the entire recruitment period (>6000) participated in the study (46). However, although self-selection bias has been shown for some lifestyle factors in the NICE cohort, this has not skewed pregnancy outcomes, or influenced the impact of certain well-known lifestyle parameters on pregnancy outcomes (46). Further, the UIC and plasma selenium concentrations were similar to those previously reported for pregnant women in southern and central Sweden (20, 39–41). Thus, we believe that the results are representative of the pregnant women in the population. Notably, we measured maternal hormone concentrations early in the third trimester, when fetal production of thyroid hormone has been initiated and the maternal thyroid function may have less influence on fetal development than in early pregnancy. However, the drastic changes in TSH and T4 in early pregnancy may obscure potential associations with nutritional factors. Another limitation is that hemodilution during pregnancy may lead to lower concentrations of micronutrients being measured in blood from pregnant women (47). It is therefore possible that the selenium status was slightly underestimated in the present study. For plasma zinc, however, we compared the present concentrations to a proposed reference limit specific for pregnant women in the third trimester, based on data from the NHANES in the United States (18). Another limitation is that we did not obtain any measurements of autoimmune thyroid disease, such as thyroid peroxidase or thyroglobulin antibody tests, and therefore we could not establish reliable normal reference ranges for the thyroid hormones among the present women. Also, underlying autoimmunity may influence the impact of the micronutrients on thyroid hormone production. However, women with any notation in their pregnancy-related hospital records regarding thyroid disease, including autoimmune disease, were excluded from the present analyses. Further, we did not measure any marker of iron status, e.g., serum ferritin, which may also influence thyroid function (12, 48), although iron status often changes in the third trimester. Lastly, this cross-sectional observational study does not demonstrate causality, and we cannot exclude the possibility that residual or unmeasured confounding influenced our findings.
In conclusion, the present findings support that other micronutrients besides iodine influence thyroid function in pregnancy. Particularly, zinc was positively associated with TSH and the thyroid hormones, whereas selenium was inversely associated with T3. In addition, there was a positive trend between joint micronutrient status and TSH concentrations. Further studies on potential consequences of joint micronutrient concentrations for both maternal and child health are warranted.
Supplementary Material
Acknowledgments
We thank the participant mothers as well as all the local nurses and technical personnel assisting in the recruitment of the study individuals, sample handling, and analyses. The authors’ responsibilities were as follows—KG, MV, MB, AS, AS-S, AEW, and MK: designed the research; KG, MV, and MB: conducted the research; KG: analyzed the data; KG and MV: wrote the paper; MK: had primary responsibility for the final content; and all authors: read, reviewed, and approved the final manuscript.
Notes
Supported by Swedish Research Council grant 2017-01172 (to A-SS), Swedish Research Council Formas grants 2018-02275 (to MK) and 2019-01007 (to MV), Swedish Research Council Forte grant 2018-00485 (to AEW), and Karolinska Institutet. Funding bodies were not involved in the study design; in the collection, analysis, and interpretation of data; or in writing the manuscript.
Author disclosures: the authors report no conflicts of interest.
Supplemental Figures 1–3 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AIC, Akaike information criterion; BKMR, Bayesian kernel machine regression; DAG, directed acyclic graph; ECLIA, electrochemiluminescence immunoassay; fT3, free triiodothyronine; fT4, free thyroxine; GAM, generalized additive model; ICP-MS, inductively coupled plasma mass spectrometry; NICE, Nutritional impact on the Immunological maturation during Childhood in relation to the Environment; PIP, posterior inclusion probability; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine; T3, triiodothyronine; T4, thyroxine; UIC, urinary iodine concentration.
Contributor Information
Klara Gustin, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Marie Vahter, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Malin Barman, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden; Food and Nutrition Science, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden.
Bo Jacobsson, Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Obstetrics and Gynecology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Helena Skröder, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Helena Filipsson Nyström, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Endocrinology, Specialized Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden; Wallenberg's Centre for Molecular and Translational Medicine, Sahlgrenska University Hopsital, Gothenburg, Sweden.
Anna Sandin, Department of Clinical Science, Pediatrics, Sunderby Research Unit, Umeå University, Umeå, Sweden.
Ann-Sofie Sandberg, Food and Nutrition Science, Department of Biology and Biological Engineering, Chalmers University of Technology, Gothenburg, Sweden.
Agnes E Wold, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Maria Kippler, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
References
- 1. Mullur R, Liu Y-Y, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obregon MJ, Calvo RM, Escobar del Rey F, Morreale de Escobar G. Ontogenesis of thyroid function and interactions with maternal function. Endocr Dev. 2007;10:86–98. [DOI] [PubMed] [Google Scholar]
- 3. Zimmermann MB. The importance of adequate iodine during pregnancy and infancy. World Rev Nutr Diet. 2016;115:118–24. [DOI] [PubMed] [Google Scholar]
- 4. Obregon MJ, Escobar del Rey F, Morreale de Escobar G. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid. 2005;15(8):917–29. [DOI] [PubMed] [Google Scholar]
- 5. Luongo C, Dentice M, Salvatore D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol. 2019;15(8):479–88. [DOI] [PubMed] [Google Scholar]
- 6. Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008;52(11):1235–46. [DOI] [PubMed] [Google Scholar]
- 7. Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GWet al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev. 2019;40(4):1000–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsen PR, Zavacki AM. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur Thyroid J. 2012;1(4):232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang SA. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid. 2005;15(8):875–81. [DOI] [PubMed] [Google Scholar]
- 10. Arthur JR, Beckett GJ, Mitchell JH. The interactions between selenium and iodine deficiencies in man and animals. Nutr Res Rev. 1999;12(1):55–73. [DOI] [PubMed] [Google Scholar]
- 11. Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48(3):756–75. [DOI] [PubMed] [Google Scholar]
- 12. O'Kane SM, Mulhern MS, Pourshahidi LK, Strain JJ, Yeates AJ. Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutr Rev. 2018;76(6):418–31. [DOI] [PubMed] [Google Scholar]
- 13. Severo JS, Morais JBS, de Freitas TEC, Andrade ALP, Feitosa MM, Fontenelle LCet al. The role of zinc in thyroid hormones metabolism. Int J Vitam Nutr Res. 2019;89(1–2):80–8. [DOI] [PubMed] [Google Scholar]
- 14. Wada L, King JC. Effect of low zinc intakes on basal metabolic rate, thyroid hormones and protein utilization in adult men. J Nutr. 1986;116(6):1045–53. [DOI] [PubMed] [Google Scholar]
- 15. Yamauchi K. The interaction of zinc with the multi-functional plasma thyroid hormone distributor protein, transthyretin: evolutionary and cross-species comparative aspects. Biometals. 2021;34(3):423–37. [DOI] [PubMed] [Google Scholar]
- 16. Baltaci AK, Mogulkoc R, Kul A, Bediz CS, Ugur A. Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology. 2004;195(1):69–75. [DOI] [PubMed] [Google Scholar]
- 17. Morley JE, Gordon J, Hershman JM. Zinc deficiency, chronic starvation, and hypothalamic-pituitary-thyroid function. Am J Clin Nutr. 1980;33(8):1767–70. [DOI] [PubMed] [Google Scholar]
- 18. International Zinc Nutrition Consultative Group (IZiNCG), Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JCInternational Zinc Nutrition Consultative Group (IZiNCG) et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25(1 Suppl 2):S99–203. [PubMed] [Google Scholar]
- 19. Vanderpas JB, Contempre B, Duale NL, Goossens W, Bebe N, Thorpe Ret al. Iodine and selenium deficiency associated with cretinism in northern Zaire. Am J Clin Nutr. 1990;52(6):1087–93. [DOI] [PubMed] [Google Scholar]
- 20. Levie D, Derakhshan A, Shu H, Broeren MAC, de Poortere RA, Peeters RPet al. The association of maternal iodine status in early pregnancy with thyroid function in the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy study. Thyroid. 2019;29(11):1660–8. [DOI] [PubMed] [Google Scholar]
- 21. Manousou S, Eggertsen R, Hulthén L, Filipsson Nyström H. A randomized, double-blind study of iodine supplementation during pregnancy in Sweden: pilot evaluation of maternal iodine status and thyroid function. Eur J Nutr. 2021;60(6):3411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stråvik M, Gustin K, Barman M, Skröder H, Sandin A, Wold AEet al. Infant iodine and selenium status in relation to maternal status and diet during pregnancy and lactation. Front Nutr. 2021;8:733602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barman M, Murray F, Bernardi AI, Broberg K, Bölte S, Hesselmar Bet al. Nutritional impact on Immunological maturation during Childhood in relation to the Environment (NICE): a prospective birth cohort in northern Sweden. BMJ Open. 2018;8(10):e022013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gustin K, Barman M, Stråvik M, Levi M, Englund-Ögge L, Murray Fet al. Low-level maternal exposure to cadmium, lead, and mercury and birth outcomes in a Swedish prospective birth-cohort. Environ Pollut. 2020;265(Pt B):114986. [DOI] [PubMed] [Google Scholar]
- 25. Gustin K, Barman M, Skröder H, Jacobsson B, Sandin A, Sandberg A-Set al. Thyroid hormones in relation to toxic metal exposure in pregnancy, and potential interactions with iodine and selenium. Environ Int. 2021;157:106869. [DOI] [PubMed] [Google Scholar]
- 26. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou Cet al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–89. [DOI] [PubMed] [Google Scholar]
- 27. Nermell B, Lindberg AL, Rahman M, Berglund M, Persson LA, El Arifeen Set al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106(2):212–18. [DOI] [PubMed] [Google Scholar]
- 28. Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr. 2008;99(S3):S2–S9. [DOI] [PubMed] [Google Scholar]
- 29. WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10(12A):1606–11. [DOI] [PubMed] [Google Scholar]
- 30. Rydbeck F, Bottai M, Tofail F, Persson LA, Kippler M. Urinary iodine concentrations of pregnant women in rural Bangladesh: a longitudinal study. J Exposure Sci Environ Epidemiol. 2014;24(5):504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu Y, Kippler M, Harari F, Grander M, Palm B, Nordqvist Het al. Alkali dilution of blood samples for high throughput ICP-MS analysis—comparison with acid digestion. Clin Biochem. 2015;48(3):140–7. [DOI] [PubMed] [Google Scholar]
- 32. Mahwi TO, Abdulateef DS. Relation of different components of climate with human pituitary-thyroid axis and FT3/FT4 ratio: a study on euthyroid and SCH subjects in two different seasons. Int J Endocrinol. 2019:2762978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang D, Cheng X, Yu S, Qiu L, Lian X, Guo Xet al. Data mining: seasonal and temperature fluctuations in thyroid-stimulating hormone. Clin Biochem. 2018;60:59–63. [DOI] [PubMed] [Google Scholar]
- 34. R Core Team . R: a language and environment for statistical computing[Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Accessed 2022 April 15]. Available from: https://www.R-project.org/. [Google Scholar]
- 35. Bobb JF. Bayesian Kernel Machine Regression. R package version 0.2.0[Internet]. 2017. [Accessed 2022 April 15]. Available from: https://cran.r-project.org/package=bkmr.
- 36. Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar Met al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Institute of Medicine . Vitamin C, vitamin E, selenium and carotenoids. Washington (DC): National Academy Press; 2000. [Google Scholar]
- 38. European Food Safety Authority (EFSA) . Scientific opinion on Dietary Reference Values for selenium. EFSA J. 2014;12(10):3846. [Google Scholar]
- 39. Björnberg KA, Vahter M, Grawé KP, Berglund M. Methyl mercury exposure in Swedish women with high fish consumption. Sci Total Environ. 2005;341(1–3):45–52. [DOI] [PubMed] [Google Scholar]
- 40. Manousou S, Andersson M, Eggertsen R, Hunziker S, Hulthén L, Filipsson Nyström H. Iodine deficiency in pregnant women in Sweden: a national cross-sectional study. Eur J Nutr. 2020;59(6):2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osman K, Åkesson A, Berglund M, Bremme K, Schutz A, Ask Ket al. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33(2):131–8. [DOI] [PubMed] [Google Scholar]
- 42. Pop V, Krabbe J, Maret W, Rayman M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br J Nutr. 2021;125(1):71–8. [DOI] [PubMed] [Google Scholar]
- 43. Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. 2017;13(10):610–22. [DOI] [PubMed] [Google Scholar]
- 44. Gupta RP, Verma PC, Garg SL. Effect of experimental zinc deficiency on thyroid gland in guinea-pigs. Ann Nutr Metab. 1997;41(6):376–81. [DOI] [PubMed] [Google Scholar]
- 45. Hofstee P, Bartho LA, McKeating DR, Radenkovic F, McEnroe G, Fisher JJet al. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J Physiol. 2019;597(23):5597–617. [DOI] [PubMed] [Google Scholar]
- 46. Englund-Ögge LE, Murray F, Modzelewska D, Lundqvist R, Nilsson S, Carré Het al. Maternal characteristics and pregnancy outcomes in the NICE birth cohort: an assessment of self-selection bias. J Matern Fetal Neonatal Med. 2022; Jan 3 (Epub ahead of print; doi: 10.1080/14767058.2021.2011854). [DOI] [PubMed] [Google Scholar]
- 47. Ferrer E, Alegría A, Barberá R, Farré R, Lagarda MJ, Monleon J. Whole blood selenium content in pregnant women. Sci Total Environ. 1999;227(2–3):139–43. [DOI] [PubMed] [Google Scholar]
- 48. Zimmermann MB, Köhrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12(10):867–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.