ABSTRACT
Background
Omega-3 (n–3) PUFAs are recognized for triglyceride-lowering effects in people with dyslipidemia, but it remains unclear if n–3-PUFA intake influences lipoprotein profiles in older adults without hypertriglyceridemia.
Objectives
The objective was to determine the effect of n–3-PUFA supplementation on plasma lipoprotein subfractions in healthy older men and women in the absence of cardiovascular disease (CVD) or hypertriglyceridemia. This was a secondary analysis and considered exploratory.
Methods
Thirty young (20–35 y old) and 54 older (65–85 y old) men and women were enrolled in the study. Fasting plasma samples were collected. After baseline sample collection, 44 older adults were randomly assigned to receive either n–3-PUFA ethyl esters (3.9 g/d) or placebo (corn oil) for 6 mo. Pre- and postintervention plasma samples were used for quantitative lipoprotein subclass analysis using high-resolution proton NMR spectroscopy.
Results
The number of large, least-dense LDL particles decreased 17%–18% with n–3 PUFAs compared with placebo (<1% change; P < 0.01). The number of small, dense LDL particles increased 26%–44% with n–3 PUFAs compared with placebo (∼11% decrease; P < 0.01). The cholesterol content of large HDL particles increased by 32% with n–3 PUFAs and by 2% in placebo (P < 0.01). The cholesterol content of small HDL particles decreased by 23% with n–3 PUFAs and by 2% in placebo (P < 0.01).
Conclusions
Despite increasing abundance of small, dense LDL particles that are associated with CVD risk, n–3 PUFAs reduced total triglycerides, maintained HDL, reduced systolic blood pressure, and shifted the HDL particle distribution toward a favorable cardioprotective profile in healthy older adults without dyslipidemia. This study suggests potential benefits of n–3-PUFA supplementation to lipoprotein profiles in healthy older adults without dyslipidemia, which should be considered when weighing the potential health benefits against the cost and ecological impact of widespread use of n–3-PUFA supplements.
This trial was registered at clinicaltrials.gov as NCT03350906.
Keywords: aging, eicosapentaenoic acid, docosahexaneoic acid, triglyceride, lipoproteins, nuclear magnetic resonance
Introduction
The robust triglyceride-lowering effect of dietary omega-3 (n–3) PUFAs has been recognized for decades (1, 2), eventually leading to FDA approval of several pharmaceutical-grade n–3-PUFA formulations for the treatment of severe hypertriglyceridemia (3). The health benefits of n–3 PUFAs likely extend beyond individuals with very high triglycerides based on growing evidence that these bioactive lipids have anti-inflammatory effects (4, 5), are strong ligands for peroxisome proliferator–activated receptor (PPAR) nuclear hormone receptors (6), and incorporate into cellular membranes with promiscuity (7). Of particular relevance to healthy aging are the possibilities that n–3 PUFAs may alleviate insulin resistance and help prevent atherosclerotic cardiovascular disease (ASCVD), although both of these possibilities continue to be debated owing to heterogeneous and inconsistent findings in randomized controlled trials (8, 9). Nevertheless, age-related dyslipidemia and inflammation are major factors in the pathogenesis of several chronic conditions related to aging, and dietary n–3 PUFAs represent a potential component of a primary prevention strategy in older adults.
The potential health-promoting effects of dietary n–3 PUFAs in generally healthy older adults without hyperlipidemia or ASCVD have been examined as part of the quest to identify strategies to promote healthy aging. Published studies indicate that n–3-PUFA supplementation in generally healthy older adults improves markers of insulin sensitivity (10), improves key aspects of mitochondrial physiology (10, 11), enhances skeletal muscle protein synthesis (10, 12), and enhances strength gains with exercise training in older women (13). We recently (14) completed an open-label pilot study of n–3-PUFA supplementation in a small group of healthy older men and women and reported that, in addition to the expected reductions in total triglycerides, there were notable shifts in the composition of circulating lipoprotein particle subclasses suggestive of a potential antiatherogenic effect of n–3 PUFAs even in healthy, nonhypertriglyceridemic older adults. Despite some promising early evidence, there is continued ambiguity about whether n–3-PUFA supplementation is beneficial to healthy older adults without dyslipidemia or ASCVD, mainly because there are insufficient data to guide such recommendations. Additional considerations relate to cost, sustainability, environmental impact, and potential adverse effects. Following on earlier preliminary observations in open-label pilot studies, the objective here was to perform a secondary analysis of a randomized, double-blind, placebo-controlled study to determine the effect of n–3-PUFA supplementation on plasma lipoprotein subfractions in healthy older men and women in the absence of ASCVD or hypertriglyceridemia. Understanding the compositional changes in plasma lipoprotein subclasses through quantitative proton nuclear magnetic resonance (1H-NMR) may help gauge the benefit of n–3-PUFA supplementation in healthy older adults.
Methods
Participants and study design
All study (NCT03350906) procedures were approved by the Mayo Foundation Institutional Review Board (IRB; no. 17–004403) and conformed to principles outlined in the Declaration of Helsinki. Thirty young (mean ± SD: 27.7 ± 4.1 y old) men and women and 54 older (mean ± SD: 71.4 ± 4.4 y old) men and women were recruited from the southeast Minnesota area. The inclusion criteria were age of 20–35 y for the young group and 65–85 y for the older group. Participants were excluded if they reported regular use of ω-3 nutritional supplements, diabetes or fasting plasma glucose > 126 mg/dL, anemia (hemoglobin <11 g/dL for females and <12 g/dL for males), active coronary artery disease or history of unstable macrovascular disease, renal failure (serum creatinine > 1.5 mg/dL), active liver disease (aspartate aminotransferase > 144 IU/L or alanine aminotransferase > 165 IU/L), history of blood clotting disorders, anticoagulant therapy, international normalized ratio > 2.0, substance abuse, untreated or uncontrolled hypothyroidism, pregnancy or breastfeeding, or fish or shellfish allergy. After providing written informed consent, participants in the older age group were randomly assigned to placebo or n–3 PUFA based on a randomization table prepared by a statistician. Masking of investigators and participants to the group assignments was maintained by the Mayo Clinic Research Pharmacy and unblinding protocols were implemented by the pharmacy staff and the Mayo Clinic IRB. The pharmacy staff handled all aspects of medication procurement, storage, and dispensation to participants in accordance with the randomization table. A total of 25 older adults were randomly assigned to receive ω-3 fatty acid ethyl esters, sold commercially as Ocean Blue® Professional Omega-3 2100™. Participants were instructed to swallow 2 softgels with a morning meal and 2 softgels with an evening meal. Each softgel was formulated as 1000-mg liquid-filled capsules with ∼675 mg EPA (20:5n–3) and ∼300 mg DHA (22:6n–3) for a total dosage of 3.9 g/d for 6 mo. A total of 24 older adults were randomly assigned to the placebo group and instructed to swallow two 1000-mg softgels containing corn oil twice per day with morning and evening meals for 6 mo. A total of 5 participants dropped out after randomization (2 placebo, 3 n–3 PUFA; Supplemental Figure 1). Placebo and n–3-PUFA softgels were identical in appearance and both contained an orange flavoring. Participants received a 1-mo supply of softgels from the research pharmacy. Participants reported to the Clinical Research and Trials Unit after an overnight fast every 4 wk to obtain a prescription refill and for fasting blood samples to measure liver function and coagulation. Unused medication was returned to the research pharmacy to assess participant compliance. The participants in the young age group were studied as a comparator group and did not undergo the intervention. Fasting blood samples were collected from a peripheral vein before the intervention and again after 6 mo of placebo or n–3 PUFA. Whole blood was immediately processed to isolate plasma, which was stored at −80°C until analysis. Additional whole-blood specimens were used to isolate RBCs for measurement of EPA and DHA concentrations. After centrifugation (650 x g, 4°C, 10 minutes), packed erythrocytes were resuspended in cold 0.9% NaCl and centrifuged again, and this step was repeated twice before freezing at −80°C. Concentrations of EPA and DHA were measured against a standard curve on a triple quadrupole mass spectrometer coupled with an ultra-pressure liquid chromatography system (LC/MS), as previously described (15). Plasma glucose and insulin were measured in the clinical chemistry laboratory by a Cobas analyzer. Free-living physical activity was measured by accelerometer (Actigraph wGT3X-BT) over a 2-wk period. Supplemental Figure 1 provides a participant flow diagram. The lipoprotein particle analysis was not predeclared as a primary or secondary endpoint of the study and should be considered as exploratory post hoc analyses.
1H-NMR spectroscopy and sample preparation
We used high-resolution 1H-NMR spectroscopy for quantitative lipoprotein subclass analysis according to the Bruker B.I.-LISA lipoprotein platform standard operating procedure. Plasma samples were thawed on ice and mixed with Bruker VERBR plasma buffer [phosphate buffer pH 7.4 containing 0.1% 3-(trimethylsilyl)-2,2,3,3-tetradeutero propionic acid (TSP-d4)] in a 9:1 (vol:vol) ratio. Then, 300 μL plasma was mixed with 300 μL phosphate buffer and transferred to an Eppendorf tube, with 70 μL buffer subsequently added before mixing the mixture on a vortex for 20 s. The sample was then centrifuged at 2400 × g at 4°C for 10 min, and 600 μL of the supernatant was transferred to a 5-mm NMR tube. The NMR spectra were collected using a Bruker 600 MHz Avance III HD spectrometer with a BBI room temperature probe head and SampleJet auto sampler (Bruker Biospin). Proton spectra were acquired using a 1D NOESY pulse sequence with presaturation (noesygppr1d), over 32 total scans. Spectra were transferred to the Bruker Data Analysis server for automated remote analysis.
Statistical analysis
Owing to the exploratory nature of these secondary outcomes of the trial, no formal sample size estimates were performed a priori. Values are presented as means ± SDs. Unpaired t tests were used to evaluate the effect of aging on lipoprotein concentrations between young adults and the preintervention samples from older adults in both arms of the trial. After verifying normality, homogeneity, and linearity, 1-factor ANCOVA using baseline values as a covariate was used to compare the plasma lipoprotein concentrations of older adults in the 2 treatment groups. The estimated marginal mean differences (adjusted mean differences) and 95% CIs are reported. All results were analyzed using RStudio Team (2022) version 1.3.95.
Results
Participant characteristics
At baseline, before intervention, young and older adults were similar in height, weight, and BMI. Systolic blood pressure was significantly higher in older adults than in young adults with no difference in diastolic blood pressure (Table 1). Physical activity, assessed from average steps per day, was similar in young and older adults at baseline, although there was a nonsignificant trend (P = 0.089) for lower time spent in moderate-to-vigorous physical activity (MVPA) in older adults (Table 1). A total of 30 young and 54 older adults were included in these baseline comparisons. At the time of analysis, 44 of the older adults completed follow-up studies and were included in the analysis of placebo compared with n–3 PUFA. These participants were studied between April 2018 and August 2020. The placebo and n–3 PUFA groups were similar in age, BMI, and blood pressure. A total of 14 participants were receiving statin therapy (6 in the placebo group, 8 in the n–3 PUFA group). Treatment groups did not differ in baseline-adjusted average steps per day and MVPA after the intervention. Fasting glucose was significantly higher in older than in young adults at baseline (Table 1), but insulin and HOMA-IR were similar in young and older adults. After intervention, treatment groups did not differ in baseline-adjusted glucose, insulin, and HOMA-IR. The concentrations of EPA and DHA in RBCs increased significantly in the n–3 PUFA group but not in the placebo group.
TABLE 1.
Descriptive characteristics of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young vs. old | Old placebo (n = 22) | Old n–3 PUFA (n = 22) | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | P value | Baseline | Follow-up | Baseline | Follow-up | difference | 95% CI | P value | |
| Sex, F/M | 15/15 | 28/26 | 14/8 | 7/15 | ||||||
| Age, y | 27.7 ± 4.11 | 71.4 ± 4.40 | 71.0 ± 4.80 | 71.5 ± 4.90 | 72.3 ± 4.32 | 72.5 ± 4.23 | ||||
| Height, cm | 171 ± 8.56 | 169 ± 9.72 | 0.189 | 167 ± 10.8 | 167 ± 11.0 | 170 ± 10.3 | 170 ± 9.61 | 0.044 | −0.509, 0.598 | 0.872 |
| Weight, kg | 73.8 ± 11.4 | 74.7 ± 13.4 | 0.832 | 72.5 ± 13.5 | 72.5 ± 13.8 | 77.7 ± 13.2 | 78.2 ± 14.2 | 0.782 | −0.651, 2.21 | 0.277 |
| BMI, kg/m2 | 24.9 ± 2.70 | 26.2 ± 3.59 | 0.10 | 25.8 ± 3.62 | 25.9 ± 3.72 | 26.7 ± 3.70 | 27.0 ± 4.10 | 0.128 | −0.443, 0.698 | 0.653 |
| Heart rate, BPM | 67.5 ± 10.8 | 62.9 ± 8.17 | 0.03 | 64.0 ± 9.85 | 66.9 ± 11.3 | 61.7 ± 6.92 | 63.4 ± 10.3 | 1.40 | −3.42, 6.22 | 0.560 |
| SBP, mm Hg | 116 ± 10.1 | 129 ± 13.5 | <0.001 | 128 ± 15.7 | 132 ± 19.6 | 131 ± 11.5 | 126 ± 12.8 | 6.67 | −0.437, 13.8 | 0.065 |
| DBP, mm Hg | 72.1 ± 9.13 | 73.3 ± 9.95 | 0.57 | 74.2 ± 9.62 | 75.5 ± 13.1 | 71.7 ± 9.83 | 75.4 ± 9.90 | −2.37 | −7.85, 3.11 | 0.387 |
| RBC EPA, μM | 3.74 ± 1.82 | 7.56 ± 4.83 | 0.035 | 8.54 ± 4.45 | 6.82 ± 7.04 | 6.58 ± 5.10 | 38.1 ± 13.0 | −32.8 | −39.4, −26.2 | <0.001 |
| RBC DHA, μM | 59.2 ± 16.2 | 66.8 ± 25.4 | 0.122 | 72.2 ± 24.5 | 57.4 ± 13.6 | 61.5 ± 25.7 | 94.0 ± 18.9 | −39.2 | −48.1, −30.3 | <0.001 |
| Plasma glucose, mg/dL | 86.0 ± 7.48 | 93.5 ± 8.31 | <0.001 | 92.5 ± 8.87 | 95.1 ± 9.70 | 94.4 ± 7.81 | 98.2 ± 10.9 | −1.00 | −5.52, 3.51 | 0.655 |
| Plasma insulin, μIU/mL | 7.37 ± 3.33 | 7.90 ± 5.55 | 0.641 | 7.85 ± 5.85 | 9.43 ± 5.86 | 7.94 ± 5.38 | 11.0 ± 8.14 | −1.66 | −4.83, 1.51 | 0.298 |
| HOMA-IR | 1.59 ± 0.80 | 1.89 ± 1.52 | 0.326 | 1.87 ± 1.62 | 2.24 ± 1.63 | 1.90 ± 1.46 | 2.78 ± 2.44 | −0.536 | −1.40, 0.328 | 0.217 |
| Steps/d | 7540 ± 1900 | 7990 ± 3200 | 0.489 | 7660 ± 3320 | 6670 ± 3320 | 8320 ± 3110 | 7650 ± 3030 | 189 | −1150, 1530 | 0.777 |
| MVPA, min | 35.6 ± 19.9 | 27.2 ± 20.8 | 0.089 | 29.2 ± 24.7 | 23.8 ± 22.7 | 25.2 ± 16.2 | 23.6 ± 18.2 | −1.28 | −8.91, 6.34 | 0.735 |
Values are mean ± SD unless otherwise indicated. BPM, beats per minute; DBP, diastolic blood pressure; MVPA, moderate-to-vigorous physical activity; SBP, systolic blood pressure.
Main lipid parameters
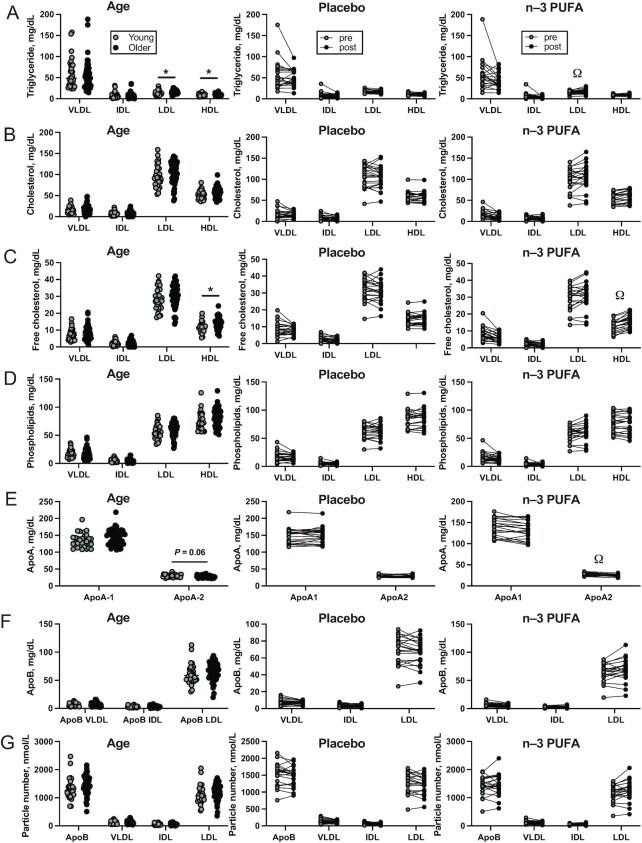
Before the intervention, older adults exhibited normal triglycerides and only modestly elevated total cholesterol, LDL cholesterol, HDL cholesterol, apoA-1, and apoB-100 compared with young adults (differences did not reach statistical significance), which is consistent with the overall good health of this cohort of older adults (Table 2). Lipoprotein particle numbers did not differ significantly between young and older adults with the exception of the large, least-dense LDL-1 which were more abundant in older adults than in young adults. Total triglycerides decreased by ∼24% in the n–3 PUFA group and by ∼6% on average in the placebo group (ANCOVA P = 0.004). The adjusted mean difference in total cholesterol, LDL cholesterol, and HDL cholesterol between treatment groups was not significant. The 2 most abundant protein components of HDL, apoA-1 and apoA-2, decreased to a greater extent in the n–3 PUFA group than in the placebo group. VLDL particle number decreased to a greater extent in n–3 PUFA than in the placebo group. The group treated with n–3 PUFAs exhibited a marked shift in the distribution of LDL particles. Compared with the placebo group, the n–3 PUFA group significantly decreased the number of large, less-dense LDL particles (LDL-1, LDL-2) and increased the number of small, denser LDL particles (LDL-5, LDL-6).
TABLE 2.
Main plasma lipoprotein parameters and particle number of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 PUFA vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Old n–3 PUFA (n = 22) | Old placebo (n = 22) | Young vs. old | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | Baseline | Follow-up | Baseline | Follow-up | P value | difference | 95% CI | P value | |
| TG, mg/dL | 98.7 ± 44.5 | 99.7 ± 41.9 | 101 ± 41.7 | 77.1 ± 20.9 | 100 ± 47.5 | 94.3 ± 22.2 | 0.923 | 17.3 | 5.90, 28.7 | 0.004 |
| Cholesterol, mg/dL | 178 ± 32.2 | 190 ± 43.2 | 188 ± 35.1 | 185 ± 35.9 | 195 ± 54.9 | 195 ± 36.4 | 0.164 | 6.65 | −12.6, 25.9 | 0.489 |
| LDL cholesterol, mg/dL | 96.4 ± 26.2 | 101 ± 28.8 | 99.5 ± 26.4 | 106 ± 29.8 | 104 ± 34.4 | 103 ± 27.7 | 0.484 | −5.98 | −18.1, 6.08 | 0.322 |
| HDL cholesterol, mg/dL | 51.8 ± 10.5 | 55.5 ± 14.2 | 54.4 ± 12.3 | 58.6 ± 13.5 | 57.1 ± 16.7 | 60.0 ± 13.9 | 0.208 | −0.499 | −6.15, 5.15 | 0.859 |
| ApoA-1, mg/dL | 137 ± 19.0 | 144 ± 25.5 | 143 ± 20.1 | 136 ± 19.3 | 146 ± 32.7 | 152 ± 24.9 | 0.183 | 14.9 | 3.56, 26.3 | 0.011 |
| ApoA-2, mg/dL | 29.1 ± 4.30 | 27.0 ± 5.20 | 27.3 ± 3.94 | 23.9 ± 3.05 | 26.9 ± 6.66 | 27.5 ± 5.39 | 0.063 | 3.74 | 1.21, 6.26 | 0.005 |
| ApoB-100, mg/dL | 72.0 ± 20.7 | 78.6 ± 19.4 | 78.0 ± 17.8 | 79.8 ± 20.5 | 80.3 ± 22.9 | 78.3 ± 15.9 | 0.149 | −3.02 | −11.2, 5.16 | 0.460 |
| Particle number | ||||||||||
| ApoB, nmol/L | 1310 ± 377 | 1430 ± 352 | 1420 ± 323 | 1450 ± 372 | 1460 ± 416 | 1420 ± 289 | 0.149 | −54.9 | −203, 93.7 | 0.460 |
| VLDL, nmol/L | 116 ± 51.1 | 129 ± 58.6 | 130 ± 57.7 | 103 ± 42.8 | 130 ± 65.9 | 121 ± 41.4 | 0.325 | 17.8 | 0.595, 35 | 0.043 |
| IDL, nmol/L | 51.0 ± 28.0 | 60.7 ± 29.1 | 59.1 ± 26.6 | 51.9 ± 30.8 | 64.5 ± 33.8 | 57.2 ± 29.5 | 0.143 | 2.49 | −13.4, 18.3 | 0.752 |
| LDL, nmol/L | 1110 ± 325 | 1190 ± 311 | 1170 ± 289 | 1250 ± 360 | 1220 ± 369 | 1200 ± 280 | 0.296 | −92.9 | −226, 40.2 | 0.166 |
| LDL-1, nmol/L | 211 ± 39.2 | 253 ± 62.1 | 243 ± 62.6 | 198 ± 51.6 | 271 ± 66.8 | 269 ± 60.7 | 0.001 | 52.9 | 29.4, 76.3 | <0.001 |
| LDL-2, nmol/L | 162 ± 36.6 | 176 ± 60.7 | 166 ± 53.9 | 138 ± 46.0 | 191 ± 69.4 | 194 ± 65.3 | 0.242 | 36.8 | 17.1, 56.4 | 0.001 |
| LDL-3, nmol/L | 178 ± 42.9 | 177 ± 60.0 | 169 ± 56.2 | 161 ± 55.3 | 186 ± 70.0 | 188 ± 65.8 | 0.956 | 14.2 | −7.7, 35.9 | 0.199 |
| LDL-4, nmol/L | 166 ± 63.3 | 154 ± 68.5 | 153 ± 70.2 | 177 ± 81.0 | 152 ± 67.8 | 157 ± 69.8 | 0.440 | −18.9 | −48.2, 10.5 | 0.201 |
| LDL-5, nmol/L | 138 ± 96.4 | 134 ± 81.3 | 141 ± 80.2 | 203 ± 98.6 | 129 ± 90.3 | 115 ± 68.1 | 0.859 | −80.0 | −117, −43.3 | <0.001 |
| LDL-6, nmol/L | 233 ± 139 | 252 ± 112 | 262 ± 111 | 329 ± 116 | 247 ± 134 | 221 ± 88.6 | 0.491 | −102 | −153, −49.9 | <0.001 |
Values are mean ± SD unless otherwise indicated. TG, triglyceride.
Main lipoprotein fractions
Although total triglyceride was similar in young and older adults at baseline, older adults exhibited significantly higher triglyceride content of LDL and HDL (Table 3, Figure 1A). The cholesterol contents of VLDL, IDL, LDL, and HDL were not remarkably different between young and old, with the exception of HDL free cholesterol, which was significantly higher in older than in young adults (Table 3, Figure 1B–C). Older adults also exhibited a nonsignificant trend (P = 0.072) for lower apoA-2 content of HDL (Table 3, Figure 1E). Triglyceride content of IDL decreased and LDL triglyceride content increased more in the n–3 PUFA group than in placebo (Table 3, Figure 1A). Total and free cholesterol content of VLDL decreased, whereas HDL free cholesterol increased, to a greater extent in n–3 PUFA than in placebo (Table 3, Figure 1B–C). The apoA-1 and apoA-2 content of HDL decreased in older adults after n–3-PUFA supplementation, to a greater extent than after placebo (Table 3, Figure 1E).
TABLE 3.
Main plasma lipoprotein fractions of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 PUFA vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Old n–3 PUFA (n = 22) | Old placebo (n = 22) | Young vs. old | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | Baseline | Follow-up | Baseline | Follow-up | P value | difference | 95% CI | P value | |
| Triglycerides, VLDL, mg/dL | 60.9 ± 35.1 | 53.8 ± 34.0 | 56.9 ± 35.6 | 42.4 ± 17.5 | 51.7 ± 37.2 | 46.0 ± 17.0 | 0.365 | 4.97 | −3.8, 13.7 | 0.259 |
| Triglycerides, IDL, mg/dL | 8.28 ± 7.40 | 7.76 ± 6.75 | 7.96 ± 6.85 | 3.29 ± 2.63 | 7.98 ± 7.64 | 6.96 ± 3.10 | 0.740 | 3.67 | 2.03, 5.31 | <0.001 |
| Triglycerides, LDL, mg/dL | 13.5 ± 4.96 | 15.9 ± 3.94 | 15.4 ± 3.61 | 17.7 ± 5.11 | 16.6 ± 4.74 | 15.9 ± 3.38 | 0.019 | −2.66 | −4.66, −0.664 | 0.010 |
| Triglycerides, HDL, mg/dL | 8.77 ± 2.71 | 10.1 ± 2.48 | 9.52 ± 1.83 | 9.36 ± 2.06 | 10.6 ± 3.05 | 10.6 ± 2.67 | 0.028 | 0.735 | −0.582, 2.05 | 0.266 |
| Cholesterol, VLDL, mg/dL | 15.3 ± 7.96 | 16.3 ± 10.4 | 16.6 ± 10.1 | 10.9 ± 6.38 | 16.4 ± 12.01 | 14.6 ± 6.47 | 0.637 | 3.74 | 0.736, 6.75 | 0.016 |
| Cholesterol, IDL, mg/dL | 7.01 ± 4.65 | 8.04 ± 5.10 | 7.84 ± 4.55 | 6.05 ± 4.36 | 8.56 ± 6.00 | 6.89 ± 4.46 | 0.363 | 0.517 | −1.77, 2.8 | 0.650 |
| Cholesterol, LDL, mg/dL | 96.4 ± 26.2 | 101 ± 28.8 | 99.5 ± 26.4 | 106 ± 29.8 | 104 ± 34.4 | 103 ± 27.7 | 0.484 | −5.98 | −18, 6.08 | 0.322 |
| Cholesterol, HDL, mg/dL | 51.8 ± 10.5 | 55.5 ± 14.2 | 54.4 ± 12.3 | 58.6 ± 13.5 | 57.1 ± 16.7 | 60.0 ± 13.9 | 0.208 | −0.499 | −6.15, 5.15 | 0.859 |
| Free cholesterol, VLDL, mg/dL | 7.35 ± 3.39 | 7.99 ± 3.96 | 8.12 ± 3.99 | 5.99 ± 2.44 | 8.02 ± 4.41 | 7.41 ± 2.58 | 0.461 | 1.46 | 0.365, 2.56 | 0.010 |
| Free cholesterol, IDL, mg/dL | 2.12 ± 1.36 | 2.36 ± 1.49 | 2.33 ± 1.36 | 1.50 ± 1.17 | 2.51 ± 1.69 | 2.04 ± 1.27 | 0.459 | 0.463 | −0.182, 1.11 | 0.155 |
| Free cholesterol, LDL, mg/dL | 28.3 ± 6.41 | 30.0 ± 7.67 | 29.3 ± 7.08 | 31.0 ± 7.88 | 30.7 ± 9.19 | 30.5 ± 7.04 | 0.306 | −1.32 | −4.37, 1.74 | 0.390 |
| Free cholesterol, HDL, mg/dL | 11.5 ± 3.12 | 13.6 ± 3.41 | 12.9 ± 3.28 | 15.8 ± 3.75 | 14.1 ± 3.66 | 14.9 ± 3.56 | 0.006 | −1.97 | −3.23, −0.707 | 0.003 |
| Phospholipids, VLDL, mg/dL | 16.6 ± 7.40 | 16.3 ± 8.69 | 16.8 ± 9.05 | 13.3 ± 5.35 | 16.2 ± 9.47 | 14.8 ± 5.21 | 0.891 | 1.75 | −0.575, 4.08 | 0.136 |
| Phospholipids, IDL, mg/dL | 4.67 ± 2.79 | 4.55 ± 2.86 | 4.47 ± 2.91 | 3.24 ± 1.88 | 4.80 ± 3.10 | 4.07 ± 1.88 | 0.855 | 0.768 | −0.342, 1.88 | 0.170 |
| Phospholipids, LDL, mg/dL | 55.8 ± 12.4 | 58.8 ± 14.5 | 57.9 ± 13.1 | 60.5 ± 15.0 | 60.3 ± 17.4 | 60.3 ± 13.5 | 0.341 | −1.92 | −7.9, 4.05 | 0.519 |
| Phospholipids, HDL, mg/dL | 75.0 ± 15.9 | 79.8 ± 17.8 | 77.1 ± 16.2 | 76.4 ± 16.9 | 82.5 ± 20.3 | 86.9 ± 18.0 | 0.227 | 6.63 | −0.631, 13.9 | 0.072 |
| ApoA-1, HDL, mg/dL | 135 ± 20.6 | 142 ± 26.4 | 140 ± 21.8 | 134 ± 22.3 | 144 ± 32.8 | 151 ± 26.4 | 0.178 | 14.5 | 3.07, 26 | 0.014 |
| ApoA-2, HDL, mg/dL | 29.6 ± 4.24 | 27.6 ± 5.16 | 28.0 ± 3.75 | 24.8 ± 3.00 | 27.5 ± 6.99 | 28.2 ± 5.21 | 0.072 | 3.55 | 1.14, 5.95 | 0.005 |
| ApoB, VLDL, mg/dL | 6.41 ± 2.81 | 7.10 ± 3.22 | 7.16 ± 3.19 | 5.69 ± 2.35 | 7.17 ± 3.61 | 6.68 ± 2.30 | 0.325 | 0.980 | 0.034, 1.92 | 0.043 |
| ApoB, IDL, mg/dL | 2.81 ± 1.54 | 3.34 ± 1.60 | 3.25 ± 1.45 | 2.85 ± 1.69 | 3.55 ± 1.88 | 3.15 ± 1.64 | 0.144 | 0.137 | −0.733, 1.01 | 0.752 |
| ApoB, LDL, mg/dL | 61.1 ± 17.9 | 65.2 ± 17.1 | 64.2 ± 15.9 | 68.8 ± 19.8 | 67.0 ± 20.3 | 65.8 ± 15.4 | 0.296 | −5.11 | −12.4, 2.21 | 0.166 |
Values are mean ± SD unless otherwise indicated.
FIGURE 1.
Cross-sectional comparisons of main plasma lipid parameters measured by NMR in young (n = 30) and older (n = 54) adults (left column), and the effects of 6 mo of placebo (n = 22, middle column) and n–3-PUFA supplementation (n = 22, right column) on triglycerides (A), esterified cholesterol (B), free cholesterol (C), phospholipids (D), apoA (E), apoB (F), and lipoprotein particle number (G). A total of 5 participants dropped out after randomization (2 placebo, 3 n–3 PUFA). Data are shown as mean ± SD. *Significant (P value < 0.05) differences between young and older adults observed at baseline. ΩSignificant differences between treatment groups found by ANCOVA.
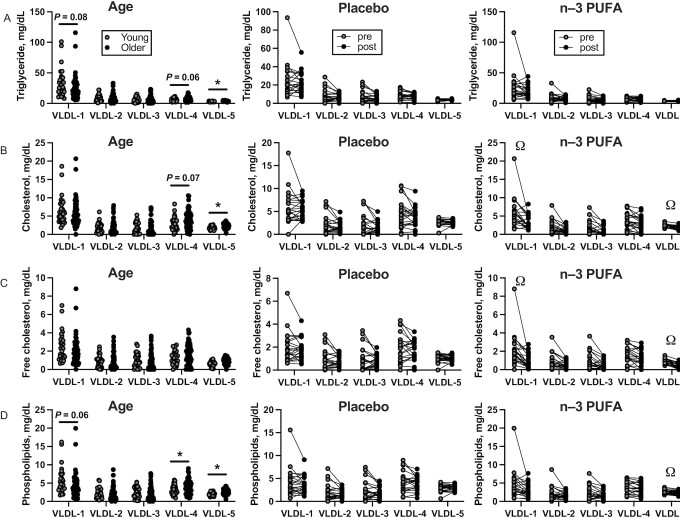
VLDL subfraction
The composition of 5 different VLDL particles, numbered in order of increasing density and decreasing size, was analyzed by NMR. Before the intervention, older adults exhibited lower triglyceride and phospholipid content of the large, least-dense VLDL-1 subfraction than young adults (Table 4, Figure 2A, C). In contrast, older adults exhibited higher triglyceride, esterified cholesterol, and phospholipid content of the small, less dense VLDL-4 and VLDL-5 particles than young adults (Table 4, Figure 2A, B, and D). Several VLDL subfraction parameters were found to change similarly in both groups, including decreased triglyceride, cholesterol (free and esterified), and phospholipid content of VLDL particles (Table 4, Figure 2A–D). Several VLDL particle parameters demonstrated notable treatment effects, including cholesterol content of the VLDL-1 and VLDL-5 particles, based on significant baseline-adjusted mean differences between treatment groups (Table 4, Figure 2B, C).
TABLE 4.
VLDL subfractions of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 PUFA vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Old n–3 PUFA (n = 22) | Old placebo (n = 22) | Young vs. old | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | Baseline | Follow-up | Baseline | Follow-up | P value | difference | 95% CI | P value | |
| Triglycerides, VLDL-1, mg/dL | 33.0 ± 22.7 | 24.7 ± 19.4 | 27.4 ± 22.3 | 19.0 ± 9.80 | 22.6 ± 18.9 | 20.2 ± 8.86 | 0.079 | 2.42 | −2.37, 7.21 | 0.314 |
| Triglycerides, VLDL-2, mg/dL | 8.50 ± 5.54 | 7.67 ± 6.69 | 8.04 ± 7.08 | 7.42 ± 3.38 | 7.36 ± 7.41 | 5.88 ± 3.24 | 0.564 | −1.37 | −3.03, 0.303 | 0.106 |
| Triglycerides, VLDL-3, mg/dL | 6.14 ± 4.41 | 6.26 ± 5.76 | 6.35 ± 5.49 | 5.23 ± 3.33 | 6.28 ± 6.89 | 4.74 ± 3.47 | 0.920 | −0.466 | −2.00, 1.07 | 0.543 |
| Triglycerides, VLDL-4, mg/dL | 5.86 ± 2.75 | 7.30 ± 3.56 | 7.19 ± 2.91 | 6.53 ± 2.91 | 7.57 ± 4.55 | 6.90 ± 3.28 | 0.059 | 0.164 | −1.25, 1.58 | 0.816 |
| Triglycerides, VLDL-5, mg/dL | 2.95 ± 0.53 | 3.77 ± 0.69 | 3.63 ± 0.52 | 3.80 ± 0.61 | 3.97 ± 0.84 | 4.08 ± 0.75 | <0.001 | 0.029 | −0.234, 0.292 | 0.824 |
| Cholesterol, VLDL-1, mg/dL | 6.41 ± 3.74 | 5.60 ± 3.70 | 5.88 ± 4.03 | 3.35 ± 1.92 | 5.41 ± 3.71 | 4.98 ± 1.78 | 0.338 | 1.74 | 0.775, 2.71 | 0.001 |
| Cholesterol, VLDL-2, mg/dL | 1.85 ± 1.34 | 1.84 ± 1.84 | 1.86 ± 1.88 | 1.14 ± 0.80 | 1.85 ± 2.11 | 1.39 ± 1.08 | 0.990 | 0.255 | −0.216, 0.726 | 0.280 |
| Cholesterol, VLDL-3, mg/dL | 1.81 ± 1.53 | 1.75 ± 2.11 | 1.77 ± 2.06 | 0.85 ± 1.13 | 1.77 ± 2.49 | 1.13 ± 1.27 | 0.887 | 0.279 | −0.278, 0.836 | 0.318 |
| Cholesterol, VLDL-4, mg/dL | 2.81 ± 1.83 | 3.77 ± 2.54 | 3.68 ± 2.20 | 2.72 ± 2.06 | 4.01 ± 3.14 | 3.41 ± 2.25 | 0.070 | 0.519 | −0.500, 1.54 | 0.310 |
| Cholesterol, VLDL-5, mg/dL | 1.79 ± 0.44 | 2.43 ± 0.68 | 2.31 ± 0.52 | 1.84 ± 0.52 | 2.60 ± 0.84 | 2.63 ± 0.56 | <0.001 | 0.641 | 0.394, 0.889 | <0.001 |
| Free cholesterol, VLDL-1, mg/dL | 2.46 ± 1.56 | 1.93 ± 1.52 | 2.09 ± 1.74 | 0.93 ± 0.80 | 1.79 ± 1.41 | 1.61 ± 0.75 | 0.138 | 0.734 | 0.297, 1.17 | 0.002 |
| Free cholesterol, VLDL-2, mg/dL | 0.91 ± 0.64 | 0.83 ± 0.82 | 0.86 ± 0.84 | 0.48 ± 0.38 | 0.80 ± 0.89 | 0.62 ± 0.42 | 0.663 | 0.159 | −0.027, 0.346 | 0.092 |
| Free cholesterol, VLDL-3, mg/dL | 0.94 ± 0.77 | 0.87 ± 0.94 | 0.89 ± 0.94 | 0.47 ± 0.52 | 0.85 ± 1.08 | 0.60 ± 0.52 | 0.695 | 0.146 | −0.095, 0.388 | 0.228 |
| Free cholesterol, VLDL-4, mg/dL | 1.25 ± 0.74 | 1.62 ± 1.12 | 1.56 ± 0.98 | 1.19 ± 0.89 | 1.69 ± 1.36 | 1.48 ± 0.98 | 0.113 | 0.218 | −0.240, 0.676 | 0.342 |
| Free cholesterol, VLDL-5, mg/dL | 0.64 ± 0.29 | 0.96 ± 0.40 | 0.89 ± 0.33 | 0.43 ± 0.28 | 1.07 ± 0.52 | 1.08 ± 0.33 | <0.001 | 0.565 | 0.408, 0.722 | <0.001 |
| Phospholipids, VLDL-1, mg/dL | 5.67 ± 3.54 | 4.17 ± 3.38 | 4.64 ± 3.89 | 3.06 ± 1.83 | 3.79 ± 3.24 | 3.29 ± 1.45 | 0.059 | 0.447 | −0.413, 1.31 | 0.300 |
| Phospholipids, VLDL-2, mg/dL | 2.33 ± 1.41 | 1.93 ± 1.74 | 2.04 ± 1.88 | 1.63 ± 0.89 | 1.83 ± 1.88 | 1.41 ± 0.89 | 0.281 | −0.164 | −0.605, 0.277 | 0.457 |
| Phospholipids, VLDL-3, mg/dL | 2.35 ± 1.49 | 2.16 ± 1.92 | 2.19 ± 1.92 | 1.63 ± 1.17 | 2.13 ± 2.20 | 1.60 ± 1.22 | 0.636 | −0.010 | −0.576, 0.556 | 0.972 |
| Phospholipids, VLDL-4, mg/dL | 3.05 ± 1.27 | 3.82 ± 1.92 | 3.77 ± 1.59 | 3.33 ± 1.59 | 3.94 ± 2.44 | 3.63 ± 1.69 | 0.053 | 0.223 | −0.544, 0.99 | 0.561 |
| Phospholipids, VLDL-5, mg/dL | 2.07 ± 0.45 | 2.69 ± 0.75 | 2.58 ± 0.61 | 2.28 ± 0.61 | 2.85 ± 0.94 | 2.87 ± 0.66 | <0.001 | 0.434 | 0.175, 0.693 | 0.002 |
Values are mean ± SD unless otherwise indicated.
FIGURE 2.
Cross-sectional comparisons of plasma VLDL subfractions measured by NMR in young (n = 30) and older (n = 54) adults (left column), and the effects of 6 mo of placebo (n = 22, middle column) and n–3-PUFA supplementation (n = 22, right column) on triglycerides (A), esterified cholesterol (B), free cholesterol (C), and phospholipids (D). VLDL particles are numbered 1–4 in order of increasing density and decreasing size. A total of 5 participants dropped out after randomization (2 placebo, 3 n–3 PUFA). Data are shown as mean ± SD. *Significant (P value < 0.05) differences between young and older adults observed at baseline. ΩSignificant differences between treatment groups found by ANCOVA.
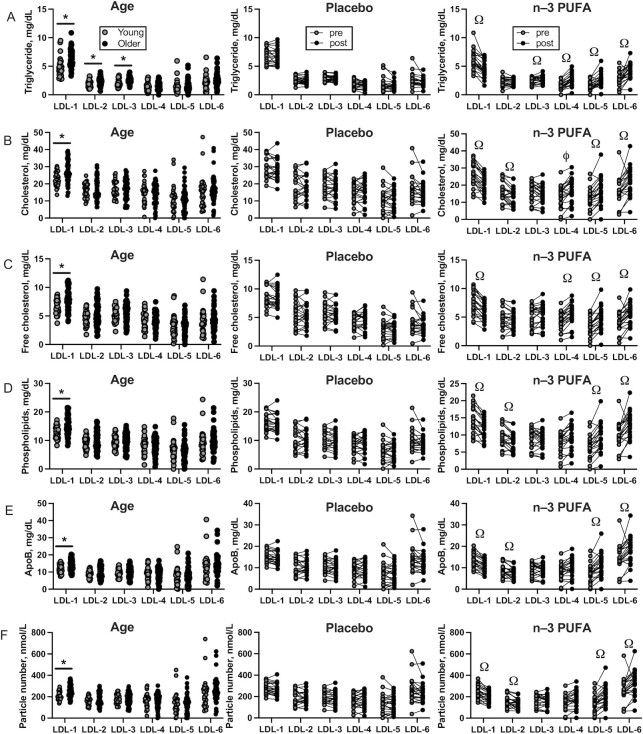
LDL subfraction
A total of 6 LDL particles of differing density and size were evaluated. Triglyceride content of large LDL particles (LDL-1, LDL-2, LDL-3) was significantly higher in older than in young adults, with no differences by age in the small, dense LDL particles (Table 5, Figure 3A). The cholesterol (esterified and free), phospholipid, and apoB contents were significantly higher in older than in young adults for LDL-1 particles, but similar for LDL-2, -3, -4, -5, and -6 (Table 5, Figure 3B–E). After the intervention, older adults treated with n–3-PUFAs exhibited decreased triglyceride content of large LDL-1 particles and increased triglyceride content of smaller LDL-4, LDL-5, and LDL-6 particles compared with placebo (Table 5, Figure 3A). A similar general pattern was observed for cholesterol, phospholipid, and apoB contents where the n–3-PUFA group showed decreased content in large, less-dense LDL particles (LDL-1, LDL-2, LDL-3) and increased content in smaller, denser LDL-4, LDL-5, and LDL-6 particles compared with the placebo group (Table 5, Figure 3B–E).
TABLE 5.
Plasma LDL subfractions of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 PUFA vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Old n–3 PUFA (n = 22) | Old placebo (n = 22) | Young vs. old | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | Baseline | Follow-up | Baseline | Follow-up | P value | difference | 95% CI | P value | |
| Triglycerides, LDL-1, mg/dL | 4.82 ± 1.67 | 6.37 ± 1.49 | 6.11 ± 1.69 | 4.54 ± 1.41 | 6.85 ± 1.36 | 6.75 ± 1.36 | <0.001 | 1.67 | 1.14, 2.21 | <0.001 |
| Triglycerides, LDL-2, mg/dL | 1.93 ± 0.58 | 2.36 ± 0.70 | 2.19 ± 0.75 | 2.24 ± 0.61 | 2.56 ± 0.70 | 2.55 ± 0.66 | 0.006 | 0.058 | −0.187, 0.302 | 0.636 |
| Triglycerides, LDL-3, mg/dL | 2.06 ± 0.62 | 2.64 ± 0.54 | 2.48 ± 0.56 | 2.75 ± 0.61 | 2.88 ± 0.52 | 2.89 ± 0.47 | <0.001 | −0.198 | −0.375, −0.021 | 0.029 |
| Triglycerides, LDL-4, mg/dL | 1.47 ± 0.76 | 1.65 ± 0.70 | 1.55 ± 0.70 | 2.59 ± 1.03 | 1.70 ± 0.75 | 1.66 ± 0.61 | 0.292 | −1.05 | −1.42, −0.691 | <0.001 |
| Triglycerides, LDL-5, mg/dL | 1.47 ± 1.10 | 1.63 ± 0.98 | 1.60 ± 0.80 | 2.62 ± 1.17 | 1.63 ± 1.27 | 1.43 ± 0.75 | 0.517 | −1.20 | −1.67, −0.733 | <0.001 |
| Triglycerides, LDL-6, mg/dL | 2.25 ± 1.23 | 2.55 ± 1.14 | 2.67 ± 1.26 | 4.29 ± 1.36 | 2.47 ± 1.27 | 2.16 ± 0.80 | 0.268 | −2.05 | −2.65, −1.45 | <0.001 |
| Cholesterol, LDL-1, mg/dL | 22.5 ± 4.10 | 26.6 ± 7.20 | 25.6 ± 6.99 | 20.3 ± 5.49 | 28.3 ± 8.02 | 28.3 ± 6.94 | 0.006 | 6.44 | 3.61, 9.27 | <0.001 |
| Cholesterol, LDL-2, mg/dL | 16.1 ± 4.09 | 17.3 ± 6.68 | 16.3 ± 5.67 | 13.6 ± 5.21 | 18.8 ± 7.79 | 19.1 ± 7.36 | 0.369 | 3.49 | 1.32, 5.66 | 0.002 |
| Cholesterol, LDL-3, mg/dL | 17.0 ± 4.65 | 16.8 ± 6.21 | 16.0 ± 5.77 | 14.7 ± 5.53 | 17.6 ± 7.27 | 17.9 ± 6.89 | 0.844 | 1.94 | −0.34, 4.23 | 0.093 |
| Cholesterol, LDL-4, mg/dL | 14.8 ± 5.90 | 13.9 ± 6.17 | 13.8 ± 6.43 | 16.8 ± 7.13 | 13.7 ± 6.14 | 14.4 ± 6.52 | 0.536 | −2.29 | −4.91, 0.337 | 0.086 |
| Cholesterol, LDL-5, mg/dL | 11.4 ± 7.54 | 10.9 ± 6.56 | 11.5 ± 6.66 | 16.7 ± 8.02 | 10.4 ± 7.04 | 9.55 ± 5.58 | 0.777 | −6.35 | −9.25, −3.45 | <0.001 |
| Cholesterol, LDL-6, mg/dL | 15.3 ± 9.25 | 16.3 ± 7.64 | 17.1 ± 7.74 | 22.8 ± 8.07 | 16.0 ± 8.96 | 14.0 ± 6.00 | 0.582 | −8.29 | −11.9, −4.72 | <0.001 |
| Free cholesterol, LDL-1, mg/dL | 6.54 ± 1.25 | 7.81 ± 2.17 | 7.52 ± 2.02 | 6.03 ± 1.55 | 8.30 ± 2.53 | 8.26 ± 2.11 | 0.004 | 1.82 | 0.949, 2.70 | <0.001 |
| Free cholesterol, LDL-2, mg/dL | 4.66 ± 1.24 | 5.13 ± 2.13 | 4.86 ± 1.83 | 4.53 ± 1.59 | 5.54 ± 2.53 | 5.65 ± 2.20 | 0.271 | 0.624 | −0.043, 1.29 | 0.066 |
| Free cholesterol, LDL-3, mg/dL | 5.15 ± 1.27 | 5.43 ± 1.80 | 5.19 ± 1.78 | 5.52 ± 1.78 | 5.70 ± 2.02 | 5.82 ± 1.88 | 0.448 | −0.112 | −0.723, 0.499 | 0.714 |
| Free cholesterol, LDL-4, mg/dL | 4.34 ± 1.44 | 4.28 ± 1.50 | 4.23 ± 1.59 | 5.57 ± 1.83 | 4.25 ± 1.55 | 4.40 ± 1.59 | 0.869 | −1.19 | −1.85, −0.526 | 0.001 |
| Free cholesterol, LDL-5, mg/dL | 3.17 ± 1.88 | 3.11 ± 1.50 | 3.21 ± 1.55 | 4.81 ± 2.02 | 3.00 ± 1.64 | 2.80 ± 1.31 | 0.889 | −1.87 | −2.59, −1.15 | <0.001 |
| Free cholesterol, LDL-6, mg/dL | 4.01 ± 2.23 | 4.04 ± 1.84 | 4.19 ± 1.78 | 5.55 ± 1.92 | 3.97 ± 2.20 | 3.40 ± 1.50 | 0.940 | −2.04 | −2.94, −1.15 | <0.001 |
| Phospholipids, LDL-1, mg/dL | 12.8 ± 2.14 | 15.1 ± 3.66 | 14.5 ± 3.66 | 11.9 ± 2.86 | 16.0 ± 3.99 | 16.1 ± 3.52 | 0.002 | 3.29 | 1.89, 4.70 | <0.001 |
| Phospholipids, LDL-2, mg/dL | 9.12 ± 2.03 | 9.89 ± 3.41 | 9.37 ± 2.95 | 8.13 ± 2.67 | 10.7 ± 3.94 | 10.8 ± 3.71 | 0.260 | 1.69 | 0.632, 2.75 | 0.002 |
| Phospholipids, LDL-3, mg/dL | 9.50 ± 2.25 | 9.52 ± 3.09 | 9.09 ± 2.91 | 8.60 ± 2.77 | 10.0 ± 3.56 | 10.1 ± 3.42 | 0.973 | 0.883 | −0.224, 1.99 | 0.115 |
| Phospholipids, LDL-4, mg/dL | 8.37 ± 2.93 | 7.97 ± 3.18 | 7.90 ± 3.28 | 9.41 ± 3.61 | 7.86 ± 3.19 | 8.23 ± 3.24 | 0.568 | −1.14 | −2.50, 0.209 | 0.095 |
| Phospholipids, LDL-5, mg/dL | 6.42 ± 3.73 | 6.20 ± 3.32 | 6.45 ± 3.33 | 9.26 ± 3.99 | 5.93 ± 3.66 | 5.60 ± 2.77 | 0.783 | −3.28 | −4.72, −1.84 | <0.001 |
| Phospholipids, LDL-6, mg/dL | 9.04 ± 4.41 | 9.58 ± 3.75 | 9.90 ± 3.66 | 12.9 ± 3.99 | 9.46 ± 4.50 | 8.57 ± 2.86 | 0.555 | −4.11 | −5.89, −2.33 | <0.001 |
| ApoB, LDL-1, mg/dL | 11.6 ± 2.16 | 13.9 ± 3.41 | 13.3 ± 3.42 | 10.9 ± 2.86 | 14.9 ± 3.66 | 14.8 ± 3.33 | 0.001 | 2.91 | 1.62, 4.20 | <0.001 |
| ApoB, LDL-2, mg/dL | 8.91 ± 2.01 | 9.70 ± 3.33 | 9.15 ± 2.95 | 7.59 ± 2.53 | 10.5 ± 3.80 | 10.6 ± 3.61 | 0.242 | 2.02 | 0.942, 3.10 | <0.001 |
| ApoB, LDL-3, mg/dL | 9.79 ± 2.36 | 9.76 ± 3.30 | 9.29 ± 3.10 | 8.85 ± 3.05 | 10.2 ± 3.85 | 10.3 ± 3.61 | 0.956 | 0.778 | −0.421, 1.98 | 0.197 |
| ApoB, LDL-4, mg/dL | 9.15 ± 3.49 | 8.50 ± 3.77 | 8.41 ± 3.85 | 9.75 ± 4.46 | 8.34 ± 3.71 | 8.66 ± 3.85 | 0.439 | −1.04 | −2.65, 0.576 | 0.201 |
| ApoB, LDL-5, mg/dL | 7.59 ± 5.30 | 7.40 ± 4.47 | 7.74 ± 4.41 | 11.2 ± 5.44 | 7.10 ± 4.97 | 6.32 ± 3.75 | 0.859 | −4.40 | −6.42, −2.38 | <0.001 |
| ApoB, LDL-6, mg/dL | 12.8 ± 7.64 | 13.9 ± 6.16 | 14.4 ± 6.10 | 18.1 ± 6.38 | 13.6 ± 7.36 | 12.1 ± 4.88 | 0.491 | −5.58 | −8.43, −2.74 | <0.001 |
Values are mean ± SD unless otherwise indicated.
FIGURE 3.
Cross-sectional comparisons of plasma LDL subfractions measured by NMR in young (n = 30) and older (n = 54) adults (left column), and the effects of 6 mo of placebo (n = 22, middle column) and n–3-PUFA supplementation (n = 22, right column) on triglycerides (A), esterified cholesterol (B), free cholesterol (C), phospholipids (D), apoB (E), and lipoprotein particle number (F). LDL particles are numbered 1–6 in order of increasing density and decreasing size. A total of 5 participants dropped out after randomization (2 placebo, 3 n–3 PUFA). Data are shown as mean ± SD. *Significant (P value < 0.05) differences between young and older adults observed at baseline. ΩSignificant differences between treatment groups found by ANCOVA.
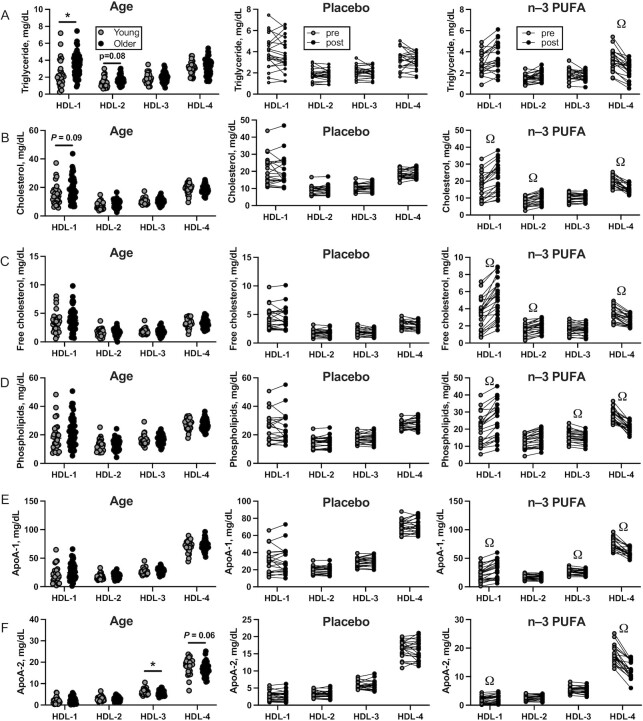
HDL subfraction
Four distinct HDL particle subfractions were evaluated. Older adults exhibited higher triglyceride content in large, least-dense HDL-1 particles and a nonsignificant trend (P = 0.084) for higher triglyceride content in HDL-2 particles than young adults (Table 6, Figure 4A). Esterified and free cholesterol content of HDL subfractions was similar in young and older adults except for a trend (P = 0.086) for higher cholesterol in the large HDL-1 particles in older adults (Table 6, Figure 4B). Phospholipids, apoA-1, and apoA-2 contents were similar in young and older adults with the exception of apoA-2 contents of HDL-3 and HDL-4, which were lower in older than in young adults (Table 6, Figure 4F). When compared with the placebo group, older adults treated with n–3 PUFAs exhibited a significant decrease in triglyceride content of small, least dense HDL-4 particles (Table 6, Figure 4A). A consistent pattern was observed for the effect of n–3-PUFA supplementation on the cholesterol, phospholipid, apoA-1, and apoA-2 contents of HDL particles where the content was increased in the larger HDL-1 and HDL-2 particles but reduced in the smaller, denser HDL-3 and HDL-4 particles (Table 6, Figure 4C–F).
TABLE 6.
Plasma HDL subfractions of young and older adults at baseline and older adults before and after 6 mo of placebo or n–3-PUFA supplementation1
| Old n–3 PUFA vs. old placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Old n–3 PUFA (n = 22) | Old placebo (n = 22) | Young vs. old | Adjusted mean | |||||||
| Young (n = 30) | Old (n = 54) | Baseline | Follow-up | Baseline | Follow-up | P value | difference | 95% CI | P value | |
| Triglycerides, HDL-1, mg/dL | 2.43 ± 1.59 | 3.30 ± 1.30 | 2.90 ± 0.98 | 3.51 ± 1.31 | 3.65 ± 1.55 | 3.67 ± 1.69 | 0.009 | - 0.344 | −1.14, 0.45 | 0.387 |
| Triglycerides, HDL-2, mg/dL | 1.40 ± 0.58 | 1.61 ± 0.50 | 1.48 ± 0.38 | 1.74 ± 0.47 | 1.71 ± 0.61 | 1.74 ± 0.56 | 0.084 | −0.103 | −0.397, 0.192 | 0.485 |
| Triglycerides, HDL-3, mg/dL | 1.84 ± 0.58 | 1.93 ± 0.55 | 1.85 ± 0.52 | 1.73 ± 0.42 | 2.01 ± 0.61 | 2.03 ± 0.52 | 0.468 | 0.23 | −0.019, 0.471 | 0.069 |
| Triglycerides, HDL-4, mg/dL | 3.07 ± 0.80 | 3.20 ± 0.97 | 3.25 ± 0.98 | 2.30 ± 0.84 | 3.23 ± 0.98 | 3.11 ± 0.80 | 0.542 | 0.83 | 0.507, 1.15 | <0.001 |
| Cholesterol, HDL-1, mg/dL | 15.1 ± 7.28 | 18.3 ± 8.30 | 16.6 ± 7.64 | 22.0 ± 8.86 | 20.0 ± 8.91 | 20.5 ± 9.29 | 0.086 | −4.45 | −7.87, −1.02 | 0.012 |
| Cholesterol, HDL-2, mg/dL | 7.63 ± 2.50 | 8.30 ± 2.83 | 7.82 ± 2.63 | 10.0 ± 3.24 | 8.78 ± 3.19 | 9.35 ± 2.95 | 0.281 | −1.463 | −2.68, −0.246 | 0.020 |
| Cholesterol, HDL-3, mg/dL | 10.1 ± 2.08 | 10.2 ± 2.51 | 10.0 ± 2.25 | 10.2 ± 2.16 | 10.4 ± 3.0 | 11.1 ± 2.53 | 0.898 | 0.68 | −0.363, 1.72 | 0.196 |
| Cholesterol, HDL-4, mg/dL | 19.1 ± 3.41 | 18.9 ± 2.82 | 19.9 ± 2.95 | 15.3 ± 2.35 | 18.4 ± 2.53 | 18.8 ± 2.81 | 0.793 | 4.414 | 3.12, 5.71 | <0.001 |
| Free cholesterol, HDL-1, mg/dL | 3.40 ± 1.79 | 3.86 ± 1.92 | 3.40 ± 1.83 | 5.18 ± 2.20 | 4.20 ± 2.02 | 4.34 ± 2.16 | 0.281 | −1.60 | −2.37, −0.824 | <0.001 |
| Free cholesterol, HDL-2, mg/dL | 1.69 ± 0.64 | 1.63 ± 0.64 | 1.48 ± 0.66 | 1.92 ± 0.70 | 1.73 ± 0.66 | 1.82 ± 0.66 | 0.751 | −0.31 | −0.583, −0.029 | 0.031 |
| Free cholesterol, HDL-3, mg/dL | 1.81 ± 0.53 | 1.76 ± 0.62 | 1.68 ± 0.61 | 1.57 ± 0.56 | 1.85 ± 0.70 | 1.87 ± 0.66 | 0.724 | 0.19 | −0.067, 0.447 | 0.144 |
| Free cholesterol, HDL-4, mg/dL | 3.35 ± 0.81 | 3.27 ± 0.78 | 3.38 ± 0.80 | 2.60 ± 0.66 | 3.22 ± 0.80 | 3.19 ± 0.75 | 0.659 | 0.69 | 0.4, 0.98 | <0.001 |
| Phospholipids, HDL-1, mg/dL | 18.6 ± 9.80 | 22.1 ± 10.0 | 19.6 ± 9.29 | 25.1 ± 10.7 | 24.2 ± 10.6 | 25.3 ± 11.2 | 0.128 | −3.95 | −8.02, 0.13 | 0.057 |
| Phospholipids, HDL-2, mg/dL | 12.4 ± 4.12 | 13.3 ± 4.10 | 12.5 ± 3.85 | 14.5 ± 4.50 | 14.1 ± 4.60 | 15.1 ± 4.22 | 0.348 | −0.721 | −2.48, 1.04 | 0.414 |
| Phospholipids, HDL-3, mg/dL | 16.5 ± 3.54 | 16.5 ± 3.76 | 16.1 ± 3.52 | 14.7 ± 3.14 | 17.0 ± 4.31 | 18.1 ± 3.99 | 0.974 | 2.79 | 1.25, 4.32 | 0.001 |
| Phospholipids, HDL-4, mg/dL | 27.4 ± 3.73 | 27.3 ± 3.52 | 28.1 ± 3.80 | 20.8 ± 2.77 | 26.9 ± 3.10 | 27.7 ± 3.89 | 0.857 | 7.56 | 5.86, 9.26 | <0.001 |
| ApoA-1, HDL-1, mg/dL | 20.2 ± 14.2 | 25.4 ± 13.9 | 21.9 ± 12.8 | 30.6 ± 15.8 | 28.2 ± 14.6 | 30.0 ± 16.2 | 0.111 | −6.801 | −12.3, −1.3 | 0.017 |
| ApoA-1, HDL-2, mg/dL | 17.1 ± 4.75 | 18.3 ± 4.54 | 17.5 ± 4.22 | 17.5 ± 4.13 | 19.2 ± 5.07 | 20.4 ± 4.64 | 0.253 | 1.69 | −0.095, 3.47 | 0.063 |
| ApoA-1, HDL-3, mg/dL | 26.7 ± 5.13 | 27.7 ± 5.33 | 27.2 ± 5.02 | 25.6 ± 4.50 | 28.3 ± 6.10 | 30.1 ± 5.86 | 0.426 | 3.72 | 1.55, 5.89 | 0.001 |
| ApoA-1, HDL-4, mg/dL | 70.6 ± 9.97 | 71.7 ± 9.37 | 74.6 ± 9.66 | 59.6 ± 7.50 | 69.9 ± 8.82 | 71.4 ± 9.19 | 0.633 | 14.48 | 10.3, 18.6 | <0.001 |
| ApoA-2, HDL-1, mg/dL | 1.97 ± 1.44 | 2.16 ± 1.31 | 1.74 ± 1.17 | 2.35 ± 1.41 | 2.53 ± 1.31 | 2.63 ± 1.45 | 0.535 | −0.46 | −1, 0.083 | 0.095 |
| ApoA-2, HDL-2, mg/dL | 3.05 ± 1.06 | 2.86 ± 0.97 | 2.59 ± 0.84 | 2.73 ± 0.94 | 3.11 ± 1.13 | 3.19 ± 1.17 | 0.394 | 0.113 | −0.414, 0.640 | 0.667 |
| ApoA-2, HDL-3, mg/dL | 6.11 ± 1.32 | 5.49 ± 1.35 | 5.33 ± 1.17 | 5.26 ± 1.17 | 5.66 ± 1.69 | 5.83 ± 1.74 | 0.043 | 0.38 | −0.375, 1.13 | 0.315 |
| ApoA-2, HDL-4, mg/dL | 18.1 ± 3.63 | 16.7 ± 3.10 | 17.7 ± 3.24 | 12.3 ± 2.91 | 16.0 ± 3.05 | 16.0 ± 3.14 | 0.060 | 4.72 | 3.20, 6.23 | <0.001 |
Values are mean ± SD unless otherwise indicated.
FIGURE 4.
Cross-sectional comparisons of plasma HDL subfractions measured by NMR in young (n = 30) and older (n = 54) adults (left column), and the effects of 6 mo of placebo (n = 22, middle column) and n–3-PUFA supplementation (n = 22, right column) on triglycerides (A), esterified cholesterol (B), free cholesterol (C), phospholipids (D), apoA-1 (E), and apoA-2 (F). HDL particles are numbered 1–4 in order of increasing density and decreasing size. A total of 5 participants dropped out after randomization (2 placebo, 3 n–3 PUFA). Data are shown as mean ± SD. *Significant (P value < 0.05) differences between young and older adults observed at baseline. ΩSignificant differences between treatment groups found by ANCOVA.
Discussion
This study shows that n–3-PUFA supplementation in healthy older adults leads to modest reductions in triglycerides and VLDL cholesterol content but marked shifts in the distribution and composition of LDL and HDL particles. Triglyceride, cholesterol, phospholipid, and apoB contents in the large, least-dense LDL particles decreased in older adults supplemented with n–3 PUFAs, whereas the smaller, densest LDL particles increased in this group. Furthermore, the number of small, dense LDL particles increased in the n–3 PUFA group. In HDL particles, n–3 PUFAs increased cholesterol, phospholipid, apoA-1, and apoA-2 contents of larger particles but reduced these parameters in the smaller, densest HDL particles. The increase in small, dense LDL particles by n–3 PUFAs could be interpreted as detrimental to older adults based on the atherogenic nature of these particles. On the other hand, the key finding that n–3 PUFAs increase the lipid and protein contents of large HDL particles and reduce small, dense HDL particles is suggestive of potential cardioprotective effects in older adults. Collectively, these data motivate continued investigation into the potential beneficial or detrimental effects of n–3-PUFA supplementation in healthy older adults without hypertriglyceridemia.
The triglyceride-lowering effects of n–3 PUFAs are well documented in people with hypertriglyceridemia (1, 2), but the current study shows that n–3 PUFAs can lower circulating triglycerides by ∼24% in healthy older adults with normal baseline triglyceride concentrations. It is unclear if this confers any health benefit to older adults, but a more detailed examination of the composition, size, and density of blood lipoproteins may help better evaluate the utility of n–3-PUFA supplementation in the absence of major risk factors for ASCVD other than chronological age. The overall triglyceride-lowering effect of n–3 PUFAs appears to be due to the additive effects of reduced triglyceride content of the largest, least dense VLDL-1, VLDL-2, and LDL-1 particles, which carry the bulk of triglyceride of all lipoprotein subfractions. Other lipoprotein particles exhibited dissimilar changes in triglyceride content with n–3-PUFA supplementation, particularly the smaller, denser VLDL and LDL particles and larger HDL particles. One of the main ways that n–3 PUFAs are believed to reduce circulating triglycerides is through their effect on lowering hepatic VLDL production, as previously demonstrated in dyslipidemic individuals (16, 17). The absence of any significant changes in total apoB-100 concentration or particle number with n–3-PUFA supplementation in the current study led us to consider the possibility that clearance of apoB-containing lipoprotein may explain the observed reduction in triglycerides. Although we did not measure VLDL flux in the present study, the absence of any change in apoB content of LDL or IDL suggests that increased catabolism of apoB-containing lipoproteins is an unlikely explanation. We observed that VLDL apoB concentrations decreased after n–3-PUFA supplementation, and although the reduction was not significantly different from what was observed in the placebo group, there was a trend for a greater reduction (20%) with n–3 PUFA than with placebo (7%). The possibility remains that the mechanism of triglyceride reduction by n–3 PUFAs is distinct in people with dyslipidemia compared with those with normal triglycerides, as demonstrated when VLDL apoB flux was determined by reinjecting autologous isotope-labeled lipoprotein (17). Indeed, hypertriglyceridemic patients reduced VLDL production, whereas patients with normal triglycerides increased clearance (17).
Among the major circulating lipoproteins, HDL is of particular interest because of its negative association with heart disease and important antiatherogenic effects through its role in scavenging cholesterol and transporting it to the liver for hepatobiliary secretion. In the current study we observed a significant increase in total HDL cholesterol that was similar in n–3-PUFA- and placebo-treated older adults. This observation is consistent with prior studies in mildly hyperlipidemic individuals (18, 19) but in contrast to other studies where HDL cholesterol increased after n–3-PUFA supplementation (20, 21). The composition and distribution of the distinct HDL subclasses provide additional insight beyond total HDL cholesterol concentrations given that the largest HDL particles are, in particular, inversely associated with ASCVD (22, 23). We observed a distinct shift in the composition of HDL subclasses with n–3-PUFA supplementation that was highly dependent on the size and density of the HDL particle. n–3 PUFAs increased the triglyceride, cholesterol, phospholipid, and apoA contents of the larger, less dense HDL-1 and HDL-2 particles and decreased these parameters in the smaller, denser HDL-3 and HDL-4 particles. This finding is consistent with an observational study in monozygotic twins where n–3-PUFA intake was associated with higher proportions of larger HDL particles and lower proportions of smaller HDL particles (24) and prospective studies of n–3-PUFA supplementation in healthy and hyperlipidemic humans (21, 25). It is likely that the shift in composition of HDL particles is a reflection of the prevailing number of HDL particles in circulation, but in the absence of specific measurements of HDL particle number, we are unable to determine the extent to which HDL composition shifts independently of particle number. The new data from the present study support that n–3-PUFA supplementation shifts the distribution of HDL particles toward a cardioprotective profile in healthy older adults without hyperlipidemia.
The relative distribution of LDL particles is also linked with cardiovascular disease (CVD) risk; in particular, the small, dense LDL particles are believed to more easily penetrate the walls of blood vessels and exhibit other atherogenic properties such as prolonged clearance from circulation, susceptibility to oxidation, and affinity for proteoglycans (26, 27). We previously reported a paradoxical increase in the purportedly atherogenic small, dense LDL particles in a small cohort of healthy older adults after an open-label n–3-PUFA supplementation study (14)—a finding that contrasted with previous reports of reduced small, dense LDL particles after n–3-PUFA supplementation (28–30). Here we confirm our earlier finding in a larger independent cohort of older adults with a rigorous randomized, placebo-controlled, double-blind study over 6 mo. Although total LDL cholesterol was unchanged in response to n–3 PUFAs, the LDL subfraction analysis revealed a notable decrease in the free and esterified cholesterol content in the larger particles and an increase in the free and esterified cholesterol content in the small, dense LDL particles with n–3-PUFA supplementation. In addition to the changes in the LDL-cholesterol concentration, there was also a remarkable increase in small, dense LDL-5 and LDL-6 particle number and an elevation in the apoB content associated with higher LDL-5 and LDL-6 particle numbers in older adults after n–3-PUFA supplementation. Although these observations contrast with some published data (28–30), they are consistent with other reports (14, 31, 32) and underscore an important lack of consensus in the literature that deserves additional consideration. A lingering question is to what extent these shifts in LDL particle distribution with n–3 PUFAs can be considered detrimental or benign. Increased concentrations of small, dense LDL have been associated with elevated apoB concentrations and reduced HDL-cholesterol concentrations, which are positive risk factors for ischemic heart disease (33) and myocardial infarction (34). Furthermore, increased concentrations of small LDL particles combined with elevated apoB concentration lead to a 6-fold higher risk of developing ischemic heart disease (26). Whether the observed increases in small, dense LDL particles in the absence of any reduction in HDL or increase in apoB translate into any meaningful CVD risk is uncertain.
This study has a number of limitations that should be considered. The older adults included in this trial were screened to exclude a variety of common age-related chronic conditions, and the results may not be generalizable to the overall population of older adults, particularly those with hyperlipidemia. It is also important to consider that the n–3-PUFA product used contained an ∼2:1 ratio of EPA to DHA, which differs from typical fish oil products (1.5:1 EPA:DHA) and other FDA-approved formulations such as Lovaza® (1.2:1 EPA:DHA) or Vascepa® (ethyl-EPA). It is known that EPA and DHA have distinct biological effects (35), and the proportion of EPA and DHA in dietary supplements should be considered when comparing the results of this study with others in the precedent literature. Additional limitations of this work are that we did not adjust P values for multiple comparisons, which increases the risk of making type I errors, and that this is an exploratory report of outcomes that were not defined a priori.
In summary, this study demonstrates that n–3-PUFA supplementation in healthy older adults without dyslipidemia leads to modest reductions in triglycerides and VLDL cholesterol content and notable shifts in the distribution and composition of HDL and LDL particles. Although the observed increase in abundance of small, dense LDL particles could be construed as an undesirable risk factor for ASCVD, the absence of any increase in apoB, reduction in total triglycerides, maintenance of total HDL, HDL particle profile, and reduced systolic blood pressure altogether suggest a potential cardioprotective benefit of n–3-PUFA supplementation in healthy older adults. An important lingering question is whether these shifts in lipoprotein subclasses translate into any meaningful benefit to older adults in the absence of major risk factors for ASCVD, particularly in consideration of the cost, sustainability, and environmental impact of widespread use of marine-derived ω-3 fatty acid supplements.
Supplementary Material
Acknowledgments
We thankRoberta Soderberg, Vicky Wade, Bonnie Arendt, and Zachary Ryan for expert technical support of the studies. Kevin Gries, Corey Hart, Adrian Vella, Michael Jensen, and Sree Nair provided support for the studies. Carol Ewing Garber provided mentoring and guidance throughout the project. The authors’ responsibilities were as follows—HEK and IRL: designed the research; DM, IV, and IRL: analyzed the data; DM and IRL: wrote the paper; IV and HEK: edited the paper; IRL: had primary responsibility for the final content; and all authors: conducted the research and read and approved the final manuscript.
Notes
Supported by National Center for Advancing Translational Sciences grant UL1 TR002377 and National Institute on Aging grant R01 AG054454 (to IRL). HEK was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant T32 AR056950 for the Musculoskeletal Research Training Program.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; IRB, Institutional Review Board; MVPA, moderate-to-vigorous physical activity; 1H-NMR, proton nuclear magnetic resonance.
Contributor Information
Darya Moosavi, Endocrine Research Unit, Division of Endocrinology, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA; Department of Biobehavioral Sciences, Teachers College, Columbia University, New York, NY, USA.
Ivan Vuckovic, Division of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN, USA.
Hawley E Kunz, Endocrine Research Unit, Division of Endocrinology, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Ian R Lanza, Endocrine Research Unit, Division of Endocrinology, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Data Availability
Data described in the article (de-identified) will be made available upon request pending application to and approval of the corresponding author.
References
- 1. von Lossonczy TO, Ruiter A, Bronsgeest-Schoute HC, van Gent CM, Hermus RJ. The effect of a fish diet on serum lipids in healthy human subjects. Am J Clin Nutr. 1978;31(8):1340–6. [DOI] [PubMed] [Google Scholar]
- 2. Harris WS, Connor WE, McMurry MP. The comparative reductions of the plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oils. Metabolism. 1983;32(2):179–84. [DOI] [PubMed] [Google Scholar]
- 3. Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CKet al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140(12):e673–e91. [DOI] [PubMed] [Google Scholar]
- 4. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan Wet al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin sensitizing effects. Cell. 2010;142(5):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LRet al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62(5):1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–4. [DOI] [PubMed] [Google Scholar]
- 7. Gerling CJ, Mukai K, Chabowski A, Heigenhauser GJF, Holloway GP, Spriet LLet al. Incorporation of omega-3 fatty acids into human skeletal muscle sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front Physiol. 2019;10:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barry AR, Dixon DL. Omega-3 fatty acids for the prevention of atherosclerotic cardiovascular disease. Pharmacotherapy. 2021;41(12):1056–65. [DOI] [PubMed] [Google Scholar]
- 9. Lalia AZ, Lanza IR. Insulin-sensitizing effects of omega-3 fatty acids: lost in translation?. Nutrients. 2016;8(6):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lalia AZ, Dasari S, Robinson MM, Abid H, Morse DM, Klaus KAet al. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging. 2017;9(4):1096–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herbst EAF, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski Aet al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol. 2014;592(6):1341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJet al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito Det al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012;95(2):428–36. [DOI] [PubMed] [Google Scholar]
- 14. Xyda SE, Vuckovic I, Petterson XM, Dasari S, Lalia AZ, Parvizi Met al. Distinct influence of omega-3 fatty acids on the plasma metabolome of healthy older adults. J Gerontol A Biol Sci Med Sci. 2020;75(5):875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Persson XM, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51(9):2761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n–3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77(2):300–7. [DOI] [PubMed] [Google Scholar]
- 17. Nestel PJ, Connor WE, Reardon MF, Connor S, Wong S, Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984;74(1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Padro T, Vilahur G, Sánchez-Hernández J, Hernández M, Antonijoan RM, Perez Aet al. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J Lipid Res. 2015;56(5):1043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JDet al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–94. [DOI] [PubMed] [Google Scholar]
- 20. Suzukawa M, Abbey M, Howe PR, Nestel PJ. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. J Lipid Res. 1995;36(3):473–84. [PubMed] [Google Scholar]
- 21. Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, Sun GY. Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism. 2004;53(6):749–54. [DOI] [PubMed] [Google Scholar]
- 22. Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MCet al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24(11):2181–7. [DOI] [PubMed] [Google Scholar]
- 23. Johansson J, Carlson LA, Landou C, Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11(1):174–82. [DOI] [PubMed] [Google Scholar]
- 24. Bogl LH, Maranghi M, Rissanen A, Kaprio J, Taskinen M-R, Pietiläinen KH. Dietary omega-3 polyunsaturated fatty acid intake is related to a protective high-density lipoprotein subspecies profile independent of genetic effects: a monozygotic twin pair study. Atherosclerosis. 2011;219(2):880–6. [DOI] [PubMed] [Google Scholar]
- 25. Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini Fet al. An ω-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 2004;53(2):153–8. [DOI] [PubMed] [Google Scholar]
- 26. Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJet al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation. 1997;95(1):69–75. [DOI] [PubMed] [Google Scholar]
- 27. Sancho-Rodríguez N, Avilés-Plaza FV, Granero-Fernández E, Hernández-Martínez AM, Albaladejo-Otón MD, Martínez-Mernández Pet al. Observational study of lipid profile and LDL particle size in patients with metabolic syndrome. Lipids Health Dis. 2011;10(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilkinson P, Leach C, Ah-Sing EE, Hussain N, Miller GJ, Millward DJet al. Influence of α-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis. 2005;181(1):115–24. [DOI] [PubMed] [Google Scholar]
- 29. Minihane AM, Khan S, Leigh-Firbank EC, Talmud P, Wright JW, Murphy MCet al. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler Thromb Vasc Biol. 2000;20(8):1990–7. [DOI] [PubMed] [Google Scholar]
- 30. Baumstark MW, Frey I, Berg A, Keul J. Influence of n-3 fatty acids from fish oils on concentration of high- and low-density lipoprotein subfractions and their lipid and apolipoprotein composition. Clin Biochem. 1992;25(5):338–40. [DOI] [PubMed] [Google Scholar]
- 31. Mostad IL, Bjerve KS, Lydersen S, Grill V. Effects of marine n-3 fatty acid supplementation on lipoprotein subclasses measured by nuclear magnetic resonance in subjects with type II diabetes. Eur J Clin Nutr. 2008;62(3):419–29. [DOI] [PubMed] [Google Scholar]
- 32. Sullivan DR, Sanders TA, Trayner IM, Thompson GR. Paradoxical elevation of LDL apoprotein B levels in hypertriglyceridaemic patients and normal subjects ingesting fish oil. Atherosclerosis. 1986;61(2):129–34. [DOI] [PubMed] [Google Scholar]
- 33. Tornvall P, Karpe F, Carlson LA, Hamsten A. Relationships of low density lipoprotein subfractions to angiographically defined coronary artery disease in young survivors of myocardial infarction. Atherosclerosis. 1991;90(1):67–80. [DOI] [PubMed] [Google Scholar]
- 34. Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260(13):1917–21. [PubMed] [Google Scholar]
- 35. Kunz HE, Dasari S, Lanza IR. EPA and DHA elicit distinct transcriptional responses to high-fat feeding in skeletal muscle and liver. Am J Physiol Endocrinol Metab. 2019;317(3):E460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article (de-identified) will be made available upon request pending application to and approval of the corresponding author.