ABSTRACT
Background
It is unknown whether the Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with other blood pressure (BP) variables, beyond mean systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Objectives
The study aimed to study the associations between the DASH dietary pattern and daytime and nighttime mean BPs and BP variance independent of the mean (VIM).
Methods
A sample of 324 Chinese adults aged ≥ 60 y who were not on BP-lowering medications were included in the analysis. The DASH score was calculated using data collected by a validated FFQ. The 24-h ambulatory BP was measured and the mean and VIM SBP and DBP were calculated for both the daytime (06:00–21:59) and nighttime periods (22:00–05:59). Multivariable linear models were constructed to assess associations between the DASH dietary pattern and daytime and nighttime BP outcomes, adjusting for sociodemographic factors, lifestyle, BMI, and hypertension (clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg), and sleep parameters (only for nighttime BP outcomes). An interaction term between DASH score and hypertension status was added to explore the potential differential association in normotensive and hypertensive individuals.
Results
Every 1-unit increase in the DASH score was associated with a 0.18-unit (95% CI: −0.34, −0.01 unit) and a 0.22-unit (95% CI: −0.36, −0.09 unit) decrease in nighttime VIM SBP and nighttime VIM DBP, respectively. DASH score was not associated with any daytime BP outcomes, nighttime mean SBP, or nighttime mean DBP. A significant interaction (DASH score × hypertension status) was detected for VIM SBP (P-interaction = 0.04), indicating a differential association between DASH score and nighttime VIM SBP by hypertension status.
Conclusions
Independently of sleep parameters and other factors, the DASH dietary pattern is associated with lower nighttime BP variability in elderly adults.
Keywords: DASH dietary pattern, ambulatory blood pressure, blood pressure variability, Chinese, older adults
Introduction
In 2017, high blood pressure (BP) is the leading risk factor for mortality, accounting for 10.4 million deaths and 218 million disability-adjusted life-years globally (1). Most of the evidence that has assessed the BP-attributable disease or mortality burden has been based on mean BP measures, which are typically measured during the day. Nighttime BP (2, 3), which normally falls by 10%–20% from diurnal BP, and nondipping nocturnal BP (2) are also risk factors for cardiovascular events and mortality. In addition, BP variability, measured as the variance in BP over time, has been recognized to have its own merits independent of mean BP in predicting the prognosis of hypertensive patients undergoing antihypertensive drug treatment (4), and all-cause mortality and cardiovascular diseases in both general and hypertensive patients (5). Increased short-term BP variability during the day or night is associated with cardiovascular mortality and cardiovascular events (5), although data are still limited and primarily based on 3 large studies (6–8).
The Dietary Approaches to Stop Hypertension (DASH) diet includes multiple food and nutrient components emphasizing fruits, vegetables, low-fat dairy products, whole grains, poultry, fish, and nuts. It also emphasizes a reduction in SFAs, red meat, sweets, and beverages containing added sugars. Several large randomized controlled feeding trials testing the original DASH diet (9), the DASH diet with sodium reduction (10), and the DASH diet with modified macronutrient content (11) have documented strong and consistent evidence of BP reduction. A meta-analysis including these earlier trials and 17 more recent randomized controlled trials concluded that the DASH diet was related to a reduced systolic blood pressure (SBP) and diastolic blood pressure (DBP) by 5.2 mm Hg (95% CI: −7.0, −3.4 mm Hg) and 2.6 mm Hg (95% CI: −3.5, −1.7 mm Hg), respectively (12). Because of its well-established BP-lowering effect, the DASH diet has been widely recommended by the National High Blood Pressure Education Program (13) and the American Heart Association (14).
What remains unclear is how the DASH dietary pattern may influence BP beyond the typical daytime mean BPs. A few feeding trials in hypertensive patients have shown that the DASH diet affects the “around-the-clock” reduction in BP, which was assessed by ambulatory blood pressure (ABP) monitoring over a 24-h period (15–17). Some studies reported a similar effect on nighttime BP, which was measured as the nighttime mean BP (15, 17), hour by hour mean nighttime BP (15), or nighttime BP dipping as seen in African Americans (18) and participants with low-grade albuminuria (17). There is still a lack of data relating the DASH diet with other BP metrics, including BP variability. Because associations between the DASH diet and BP outcomes may vary by baseline BP level, it is critical to examine the associations in a mixed overall sample, as well as in subgroups by hypertension status. The goal of this study is to examine how the DASH diet is related to the mean and variability of both daytime and nighttime BP in an elderly Chinese sample, including those with or without hypertension.
Methods
Study population
Data were based on a subcohort of the Kailuan study in the Kailuan community, Tangshan City, Hebei Province, China. Since 2006–2007 (the baseline cohort), participants have been enrolled from 11 hospitals providing health care to the Kailuan community. About one-third of the participants in the Kailuan Cohort were shift-working coal miners and ∼80% of the baseline participants were men. At the baseline and subsequent biennial follow-ups, standardized questionnaires were administered to collect demographic data, disease history, medication use, and physical activity; as well, clinical and laboratory examinations were conducted. Diet was assessed in the 2014 follow-up survey using a standardized FFQ. In 2014–2015, a random subgroup of participants from the Kailuan Cohort and patients with hypertension were selected to conduct ABP monitoring for 24 h. The mean ± SD duration between the dietary and ABP assessments was 13.2 ± 7.9 mo. To avoid influence of night work shifts and/or antihypertensive medication use, we only included participants who were aged 60 y or older (at or beyond the retirement age for men in China) and who were not on BP-lowering medications, according to the self-reported medication use questions in 2014. We further excluded participants who had missing dietary assessment data (n = 28) and insufficient BP records (defined as having valid BP measures < 70% for 24-h measures, <10 times for daytime measures, <5 times for nighttime measures, or having no valid recording for >2 h; n = 51) during ABP monitoring. The total sample size included in the analysis was 324. The prevalence of hypertension in participants > 60 y old was 57.9% in the Kailuan Cohort and 69.1% in our analytic sample. The study was approved by the Ethics Committee of the Kailuan Medical Group, Kailuan Company.
Dietary assessment and DASH score calculation
A self-administered semiquantitative FFQ including 40 food items was used to assess usual dietary intake in the past year. This questionnaire has been validated in the general Chinese population and has been used in the 2002 Chinese National Nutrition Survey (19). Participants were asked to report frequency of food consumption (options included never or rarely and times per month, week, and day; all answers were then converted to times per day) and the typical amount of food consumption per eating event (converted to g/d). Similar to a prior study (20), the habitual daily consumption of each food was calculated by multiplying the frequency and amount consumed each time. For condiments, participants were asked to estimate the average amount of each condiment consumed at the household level per month (which was then divided by 30 to convert to the total amount at the household level per day) and the typical number of people who ate condiment in the household. The average daily consumption of each condiment per each household member was calculated as the total amount per day divided by the number of people in the household who consumed it (g/d). Nutrient content was calculated based on the Chinese Food Composition Table (21). The DASH score was calculated from 9 dietary components, including vegetables, fruits, dairy, beans, whole grains, meat, total fat, sodium, and sugar-sweetened beverages (22, 23). For each of these components, participants’ intake was grouped into quintiles with 1 representing the lowest intake quintile and 5 representing the highest intake quintile. Because lower intakes of meat, total fat, sodium, and sugar-sweetened beverages are recommended in the DASH diet, these 4 dietary components were reverse coded, with 1 representing the highest intake quintile (least optimal) and 5 representing the lowest intake quintile (most optimal). The DASH score was calculated as the sum of the quintile scores of all dietary components, ranging from 9 to 45.
ABP monitoring
ABP was assessed by either the SunTech Oscar 2 24-HR Ambulatory Blood Pressure (ABP) Monitor (SunTech Medical, Inc.) or the Spacelabs 90217 Ultralite Ambulatory Blood Pressure Monitor (OSI Systems, Inc.). A trained physician put the ABP monitor onto the participant's nondominant upper arm and participants were asked to wear the ABP monitor over a 24-h period. The ABP monitor measured BP at 15-min intervals during the daytime period (between 06:00 and 21:59) and at 30-min intervals during the nighttime period (between 22:00 and 05:59).
The mean daytime and nighttime SBP and DBP were calculated separately.
BP variance independent of the mean (VIM) is a measure of BP variability and was calculated separately by time of day for both SBP and DBP, so that the variables were daytime VIM SBP, daytime VIM DBP, nighttime VIM SBP, and nighttime VIM DBP.
The following formula was used to calculate VIM for each participant i (24):
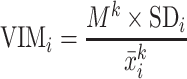 |
(1) |
where M is the sample mean of SBP or DBP during the period of interest (day or night); 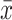 is the mean of SBP or DBP for each participant i over the period of interest; SD is the SD of SBP or DBP over the period of interest for each participant i; and k is a constant estimated by plotting SD of SBP or DBP against
is the mean of SBP or DBP for each participant i over the period of interest; SD is the SD of SBP or DBP over the period of interest for each participant i; and k is a constant estimated by plotting SD of SBP or DBP against 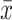 for all participants as:
for all participants as:
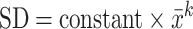 |
(2) |
VIM SBP/DBP may be superior to the CV in prognostic implications because it removes the correlation with mean BP (25). Calculating daytime and nighttime VIM SBP/DBP separately may also avoid the influence of the “dipping” phenomenon that has been known to reduce the prognostic power of 24-h ABP measurements (25). Higher VIM values indicate greater BP variability.
Clinic BP measurement
Clinic BP was measured in the physician's office by a calibrated desk mercury sphygmomanometer on the participant's right upper arm, in the morning. Participants were asked to fast before the physical checkup and to refrain from smoking, tea, coffee, and alcohol for 30 min before measurement. They were also rested in a seating position for 5 min. Three BP measurements were taken with 5-min intervals between any 2 consecutive measurements. The mean of the 3 measures for SBP and DBP was calculated. Hypertension was defined as clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg.
Assessment of covariates
Demographic data, socioeconomic status, and lifestyle risk factors were collected in the biennual questionnaire. Specifically, we collected information on age, sex (male/female), education (elementary school or lower, middle school, or high school or above), current smoking status (yes/no), current alcohol intake (yes/no), and total energy intake calculated from the FFQ. We also measured weight and height, which were used to calculate BMI (in kg/m2) and to categorize weight status (normal: BMI < 24; overweight: 24 ≤ BMI < 28; and obesity: BMI ≥ 28) (26). Physical activity was assessed by the Chinese version of the International Physical Activity Questionnaire (IPAQ-C) and was grouped into high (≥1 h/d of moderate physical activity or ≥30 min/d vigorous physical activity), moderate (≥30 min/d and < 1 h/d moderate physical activity), and low activity (<30 min/d moderate physical activity), according to an established guideline for the IPAQ-C (27). We also assessed the self-reported nighttime sleep quality by asking questions regarding nighttime sleep duration (total hours), current snoring status (categorized as no if less than once per week or as yes if once or more per week), and 5 questions regarding insomnia symptoms adapted from the Health Professionals Follow-up Study (28), including 1) difficulty falling asleep (yes/no); 2) difficulty maintaining sleep during the night (yes/no); 3) early-morning awakenings (yes/no); 4) nonrestorative sleep (yes/no); and 5) excessive daytime sleepiness (yes/no). We defined insomnia as having ≥1 of the first 3 nighttime symptoms and ≥1 of the last 2 daytime symptoms.
Statistical analysis
All analyses were performed on Stata/SE 15.0 (StataCorp). Normally distributed continuous variables were presented as mean ± SD and categorical variables were presented as n (%). Pairwise Pearson correlation coefficients were calculated between the BP outcomes. The Bonferroni-adjusted significance level at P = 0.05/28 (where n = 28 is the number of pairs) was used to determine statistical significance of the correlation coefficients. We applied the restricted cubic spline with 3 knots to test linearity between DASH score and the BP outcomes and all of the Wald tests for linearity were nonsignificant (P = 0.29–0.94), suggesting that we could not reject the null hypotheses that each examined BP outcome follows a linear function of the DASH score. Therefore, multivariable linear regression was used to analyze the association between the DASH score tertiles and the BP outcomes. Covariates for daytime BP outcomes included age (60–64, 65–69, or ≥70 y), sex (men or women), total energy intake (kcal/d), BMI (<24.0, 24.0–27.9, or ≥28.0), current smoking status (yes/no), current alcohol intake (yes/no), physical activity (low compared with moderate or high), education, and hypertension status. For the nighttime BP outcomes, the 3 sleep variables of nighttime sleep duration (<7 h, 7–8 h, or >8 h), snoring (yes/no), and insomnia symptoms (yes/no) were in addition adjusted for. To understand how hypertension status may modify the association between the DASH score and BP outcomes, a continuous-by-categorical interaction term was included in the models. The statistical significance level was set at P < 0.05.
Results
Table 1 presents the characteristics of the study participants. The mean age was 66.3 ± 6.0 y. Two-thirds (66.7%) of the participants were male and a little more than two-thirds (69.1%) had hypertension. The mean BMI was 25.4 ± 3.3 and 18.5% of the participants had obesity (BMI ≥ 28.0). About one-third (35.1%) of the participants usually slept <7 h in the night and 12.0% of the participants reported insomnia-like symptoms. The mean DASH score was 28.2 ± 3.7 and the mean total energy intake was 2186 ± 1375 kcal/d. By hypertension status (n = 100, normotensive; n = 224, hypertensive), we observed significant group differences (normotensive compared with hypertensive) in BMI (24.7 ± 3.1 compared with 25.7 ± 3.4; P = 0.009), difficulty initiating sleep (15.0% compared with 7.1%; P = 0.026), early-morning awakenings (17.0% compared with 9.4%; P = 0.049), nonrestorative sleep (17.0% compared with 9.4%; P = 0.049), excessive daytime sleepiness (18.8% compared with 10.5%; P = 0.044), and total energy intake (1930 ± 1248 compared with 2300 ± 1415 kcal; P = 0.025).
TABLE 1.
Sample characteristics for elderly Chinese adults by hypertension status1
| All | Normotensive (clinic SBP < 140 mm Hg or DBP < 90 mm Hg) | Hypertensive (clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg) | P | |
|---|---|---|---|---|
| n | 324 | 100 (30.9) | 224 (69.1) | |
| Age, y | 66.3 ± 6.0 | 65.4 ± 6.1 | 66.7 ± 5.9 | 0.075 |
| 60–64 | 133 (41.1) | 48 (48.0) | 85 (38.0) | 0.183 |
| 65–69 | 113 (34.9) | 33 (33.0) | 80 (35.7) | |
| ≥70 | 78 (24.1) | 19 (19.0) | 59 (26.3) | |
| Male | 216 (66.7) | 63 (63.0) | 153 (68.3) | 0.350 |
| Education | ||||
| Elementary school or lower | 42 (13.0) | 12 (12.0) | 30 (13.4) | 0.882 |
| Middle school | 257 (79.3) | 81 (81.0) | 176 (78.6) | |
| High school or above | 25 (7.7) | 7 (7.0) | 18 (8.0) | |
| Current smokers | 69 (21.3) | 20 (20.0) | 49 (21.9) | 0.703 |
| Current alcohol drinker | 105 (32.4) | 27 (27.0) | 78 (34.8) | 0.165 |
| Moderate or high physical activity2 | 38 (11.7) | 7 (7.0) | 31 (13.8) | 0.077 |
| BMI, kg/m2 | 25.4 ± 3.3 | 24.7 ± 3.1 | 25.7 ± 3.4 | 0.009 |
| Normal (<24.0) | 115 (35.5) | 44 (44.0) | 71 (31.7) | 0.061 |
| Overweight (24.0–27.9) | 149 (46.0) | 43 (43.0) | 106 (47.3) | |
| Obesity (≥28.0) | 60 (18.5) | 13 (13.0) | 47 (21.0) | |
| Nighttime sleep duration, h | 6.8 ± 1.2 | 6.7 ± 1.2 | 6.8 ± 1.2 | 0.626 |
| <7 | 104 (32.1) | 40 (40.0) | 64 (28.6) | 0.116 |
| 7–8 | 155 (47.8) | 41 (41.0) | 114 (50.9) | |
| >8 | 65 (20.1) | 19 (19.0) | 46 (20.5) | |
| Snoring | 62 (19.1) | 21 (21.0) | 41 (18.3) | 0.569 |
| Insomnia-related symptoms | ||||
| Difficulty initiating sleep | 31 (9.6) | 15 (15.0) | 16 (7.1) | 0.026 |
| Difficulty maintaining sleep | 47 (14.5) | 18 (18.0) | 29 (13.0) | 0.233 |
| Early-morning awakenings | 38 (11.7) | 17 (17.0) | 21 (9.4) | 0.049 |
| Nonrestorative sleep | 38 (11.7) | 17 (17.0) | 21 (9.4) | 0.049 |
| Excessive daytime sleepiness | 41 (12.7) | 18 (18.8) | 23 (10.5) | 0.044 |
| Insomnia | 39 (12.0) | 17 (17.0) | 22 (9.8) | 0.067 |
| DASH score | 28.2 ± 3.7 | 27.7 ± 4.0 | 28.5 ± 3.5 | 0.096 |
| Total energy, kcal/d | 2186 ± 1375 | 1930 ± 1248 | 2300 ± 1415 | 0.025 |
n = 324. Values are mean ± SD or n (%). DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure.
According to a published cutoff (27), categorization of physical activity is defined as high (≥1 h/d of moderate physical activity or ≥30 min/d vigorous physical activity), moderate (≥30 min/d and < 1 h/d moderate physical activity), and low activity (<30 min/d moderate physical activity).
Table 2 presents the Pearson correlation coefficients (ρ) between the BP outcomes. Mean SBP and DBP measured during the day and night were significantly correlated with each other (ρ = 0.467–0.811). The VIM SBP and VIM DBP correlation coefficients during the daytime and nighttime were 0.726 and 0.711, respectively (all P < 0.002). The correlations were smaller between nighttime VIM SBP and daytime VIM SBP (ρ = 0.332), nighttime VIM SBP and daytime VIM DBP (ρ = 0.256), nighttime VIM DBP and daytime VIM SBP (ρ = 0.252), and nighttime VIM DBP and daytime VIM DBP (ρ = 0.294). As expected, none of the correlations between the mean and variability measure were statistically significant (ρ = −0.04 to 0.148, P = 0.21–1.00).
TABLE 2.
Pearson correlation coefficients between daytime and nighttime BP measures in elderly Chinese adults1
| Mean BP | BP variability | |||||||
|---|---|---|---|---|---|---|---|---|
| Daytime SBP | Daytime DBP | Nighttime SBP | Nighttime DBP | Daytime VIM SBP | Daytime VIM DBP | Nighttime VIM SBP | Nighttime VIM DBP | |
| Mean BP | ||||||||
| Daytime SBP | 1.000 | — | — | — | — | — | — | — |
| Daytime DBP | 0.6532 | 1.000 | — | — | — | — | — | — |
| Nighttime SBP | 0.8112 | 0.4672 | 1.000 | — | — | — | — | — |
| Nighttime DBP | 0.5202 | 0.7102 | 0.7382 | 1.000 | — | — | — | — |
| BP variability | ||||||||
| Daytime VIM SBP | 0.072 | 0.041 | 0.091 | 0.062 | 1.000 | — | — | — |
| Daytime VIM DBP | 0.134 | 0.060 | 0.148 | 0.056 | 0.7262 | 1.000 | — | — |
| Nighttime VIM SBP | −0.004 | −0.033 | −0.025 | −0.041 | 0.3322 | 0.2562 | 1.000 | — |
| Nighttime VIM DBP | 0.139 | 0.108 | 0.054 | 0.038 | 0.2522 | 0.2942 | 0.7112 | 1.000 |
n = 324. Values are Pearson correlation coefficients. BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; VIM, variance independent of the mean.
Correlations are significant using the Bonferroni-adjusted significance level at P = 0.05/28.
Table 3 presents the adjusted associations between the DASH score and BP outcomes. A 1-unit increase in the DASH score was not significantly associated with mean SBP or DBP during the daytime or nighttime, although the point estimates were all negative. The DASH score was significantly and negatively associated with nighttime BP variability. For every unit increase in the DASH score, there was a 0.18-unit (95% CI: −0.34, −0.01 unit) decrease in the VIM SBP and a 0.22-unit (95% CI: −0.36, −0.09 unit) decrease in the VIM DBP.
TABLE 3.
Multivariable linear analysis for the association between the DASH score and daytime and nighttime BP outcomes in elderly Chinese adults1
| Daytime2 | Nighttime3 | |||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| Mean SBP, mm Hg | −0.23 (−0.86, 0.39) | 0.467 | −0.43 (−1.21, 0.35) | 0.281 |
| Mean DBP, mm Hg | −0.09 (−0.44, 0.26) | 0.625 | −0.26 (−0.66, 0.15) | 0.210 |
| VIM SBP | −0.10 (−0.26, 0.05) | 0.183 | −0.18 (−0.34, −0.01) | 0.036 |
| VIM DBP | −0.04 (−0.89, 0.88) | 0.564 | −0.22 (−0.36, −0.09) | 0.001 |
n = 324. BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure; VIM, variance independent of the mean.
Models for daytime BP outcomes adjusted for age, sex, total energy intake, BMI, current smoking, alcohol intake, physical activity, education, and hypertension.
Models for nighttime BP outcomes adjusted for the variables included in footnote 2 and in addition adjusted for nighttime sleep duration, snoring, and insomnia.
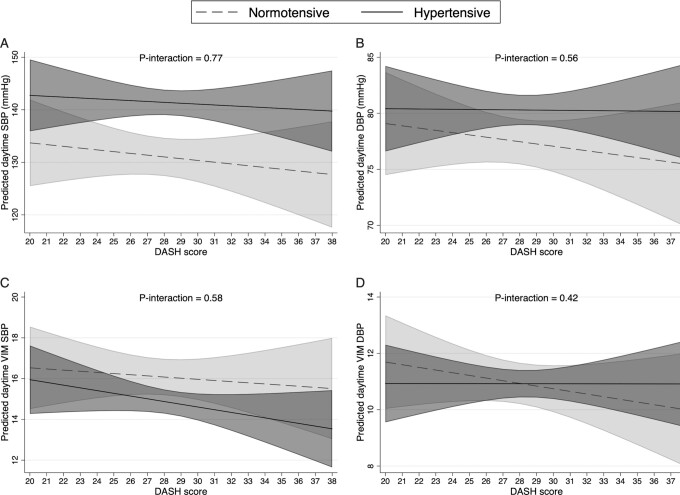
When including the interaction term between the DASH score and hypertension, no significant associations were observed between the DASH score and any of the daytime BP outcomes in either the normotensive or hypertensive individuals (Figure 1). Also no interaction was detected (all P-interaction > 0.4).
FIGURE 1.
The associations between DASH score and daytime mean SBP (A), daytime mean DBP (B), daytime VIM SBP (C), and daytime VIM DBP (D) in normotensive (clinic SBP < 140 mm Hg or DBP < 90 mm Hg, n = 100) and hypertensive (clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, n = 224) elderly Chinese adults. Lines represent the estimated linear relation. Shaded areas represent the 95% CI at each level of the DASH score. Models adjusted for age, sex, total energy intake, BMI, current smoking, alcohol intake, physical activity, and education. DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure; VIM, variance independent of the mean.
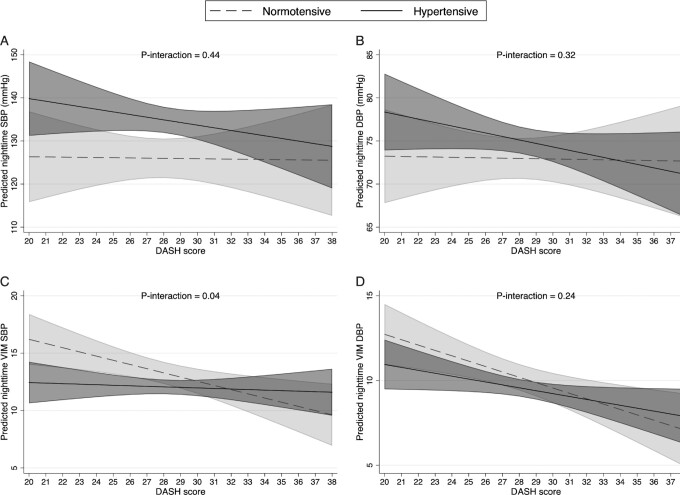
For nighttime BP outcomes (Figure 2), the estimated association between the DASH score and VIM SBP (Figure 2C) was −0.36 (95% CI: −0.61, −0.11; P = 0.004) in normotensive participants; however, the association was not significant in hypertensive individuals (β = −0.05, 95% CI: −0.25, 0.15; P = 0.648). There was a significant interaction between the DASH score and nighttime VIM SBP (P-interaction = 0.04). Although the interaction was not significant (P-interaction = 0.24), a greater negative association was observed for nighttime VIM DBP (Figure 2D), comparing normotensive (β = −0.32, 95% CI: −0.52, −0.11; P = 0.002) with hypertensive individuals (β = −0.17, 95% CI: −0.34, −0.01; P = 0.04). The associations were not significant for nighttime mean SBP or DBP and no interaction was detected with hypertension status for nighttime mean BPs.
FIGURE 2.
The associations between DASH score and nighttime mean SBP (A), nighttime mean DBP (B), nighttime VIM SBP (C), and nighttime VIM DBP (D) in normotensive (clinic SBP < 140 mm Hg or DBP < 90 mm Hg, n = 100) and hypertensive (clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, n = 224) elderly Chinese adults. Lines represent the estimated linear relation. Shaded areas represent the 95% CI at each level of the DASH score. Models adjusted for age, sex, total energy intake, BMI, current smoking, alcohol intake, physical activity, education, nighttime sleep duration, snoring, and insomnia. DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; SBP, systolic blood pressure; VIM, variance independent of the mean.
Discussion
In this study, we found that the DASH diet in older Chinese adults was associated with reduced nighttime BP variability, particularly among adults without hypertension. The observed association was adjusted for demographic and lifestyle factors, BMI, energy intake, and sleep parameters. Future intervention trials are warranted to investigate whether the DASH diet has extended benefits in stabilizing BP, which would likely have additional protective value for cardiovascular health (29) and survival (30) in older adults.
Significant associations were only observed between the DASH score and the 2 nighttime BP variability outcomes and, unlike we expected, not for any of the mean BP outcomes. We did see that the DASH diet score was associated with mean SBP/DBP in the expected direction. The nonsignificant results could be due to our small sample size, the lack of DASH score variability in our population, or reverse causality between eating pattern and daytime BP, which may have brought the observed association toward the null. Our findings suggest that nighttime BP VIM is a more sensitive, and potentially less biased, BP measure for the DASH diet. Although not significant in our study, findings from randomized controlled trials seem to support that the DASH diet has a greater BP-lowering effect in hypertensive rather than normotensive subjects (8). Hypertension treatment may also modify the DASH diet's effect on BP. Comparing the effect of the DASH diet in hypertensive adults with or without treatment and normotensive adults in a meta-analysis (28), the greatest reduction of mean SBP and DBP was seen in untreated hypertensive adults and the smallest seen in treated hypertensive adults. Although we only included individuals who were not undergoing treatment of hypertension at the initial survey into our analysis, treatment status may have changed thereafter, attenuating the association by averaging the untreated and treated adults. The same meta-analysis also formally tested the potential modifying impact of hypertension status and concluded that the DASH's BP-lowering effect was independent of baseline BP levels (31). Similarly, our study did not observe any significant interaction between DASH and hypertension status on mean SBP/DBP.
Interestingly, the DASH diet was associated with nighttime DBP variability, even after further adjustment of the 3 nocturnal sleep variables, including sleep duration, snoring, and insomnia. It is recommended that adults aged 60–64 y sleep for 7–9 h/night and that adults 65 y and older sleep 7–8 h/night (32). Among the older Chinese population (60 y and older), the pooled estimated mean sleep duration was 6.82 h/d (95% CI: 6.59, 7.05 h/d) (33). The mean sleep duration was similar in our sample (6.8 ± 1.2 h) and about one-third of the older adults slept less than the recommended duration. Short sleep duration could confound the relation between diet and nighttime BP. Meta-analyses of human intervention studies estimated that partial sleep deprivation (≤5.5 h/night) increases the mean energy intake by 204–385 kcal/d (34, 35). On the other hand, data primarily from observational studies indicate that diet may also influence sleep duration and quality, suggesting a cyclic relation between sleep and diet (36). In addition, short sleep duration is associated with increased risk of future hypertension by 23% (95% CI: 6%, 42%), according to the pooled analysis of 6 longitudinal studies (37). Snoring appears to be another independent risk factor for the development of hypertension, as observed in prospective studies in both men (38) and women (39). Reported snoring and difficulty initiating sleep were significantly associated with the DASH score in our sample (data not shown). Current evidence suggests a positive association of insomnia and its symptoms, such as difficulty initiating sleep, with elevated BP (29). When these sleep parameters were in addition adjusted in our model, we did see a slight attenuation in the β-coefficients of 3 out of 4 nighttime BP outcomes (data not shown). It is possible that sleep partially mediates the observed associations between the DASH dietary pattern and nighttime BP outcomes in the overall sample. Because the insomnia-related symptoms were more prevalent in hypertensive individuals, they may have mediated the adjusted associations so that those observed for normotensive individuals appeared stronger.
The significant decrease in SBP/DBP variability associated with the DASH score was more consistently observed in normotensive individuals during nighttime. Are there additional explanations for the absence of associations in the hypertensive individuals? First, as previously mentioned, hypertensive individuals may have begun treatment since their inclusion in the study, which would alter their expected patterns of BP. For example, among the most commonly used antihypertensive medicine classes in China (30), calcium channel blockers and diuretics alone or in addition to other drugs have significant lowering effects on short-term BP variability (40). Second, hypertensive individuals may have changed their lifestyle behaviors since their diagnosis. Compared with the normotensive older adults, the mean DASH score (mean ± SD: 28.5 ± 3.5 compared with 27.7 ± 4.0; P = 0.096) and the proportion of moderate or high physical activity (13.8% compared with 7.0%, P = 0.077) were higher in hypertensive individuals, although the difference did not reach statistical significance. Adoption of a healthier lifestyle after a hypertension diagnosis has been observed in China (41) and in other populations (42). Screening for hypertension and awareness of BP-lowering behaviors, without actual behavior-changing interventions, have been associated with future SBP reduction in 2 y (43). Collectively, both of the foregoing explanations could help reduce variability in the DASH score distribution and could attenuate the observed association between the DASH diet and the participants’ BP profiles.
To our knowledge, this study is the first to explore the relations between the DASH diet and daytime and nighttime BP profiles that include not only the mean, but also BP variability. However, there are some limitations to note. We cannot make any causal inference regarding the DASH dietary pattern and nighttime BP variability, given the cross-sectional nature of this single study. The DASH diet score was relatively high and unvarying in our sample. Aging adults tend to consume less total energy (44) and to eat healthier, as observed in their reduction in unhealthy fat intake (45) and overall dietary pattern (46). These observations could explain the DASH diet score's low variability in our older adult sample. Ambulatory daytime BP may be influenced by many factors, including body position and physical and mental activity (2), which we did not assess for further control in the analysis. Although it is believed nighttime BP is less affected by the factors aforementioned, the overnight BP profile could be influenced by central and reflex autonomic modulation, effects of hormone fluctuations, or other intrinsic and external factors (47). Residual confounding cannot be excluded even though nutritional status, lifestyle, and sleep behaviors have been controlled for in this study. Circadian stage– and sex-dependent BP response to the DASH intervention has been observed (48), indicating sex differences in BP variability in relation to the DASH dietary pattern. However, ∼70% of our sample were male. Whether the association observed from our male-dominant sample exists in the female population remains to be investigated. The generalizability of our findings, therefore, should not be extrapolated to other populations with differing characteristics (e.g., female, younger age, other racial/ethnic groups, populations with more variability in DASH score distribution, or different baseline disease conditions).
In conclusion, our study findings support an association between the DASH-style diet and stabilized nighttime BP. Future DASH feeding trials should consider monitoring BP during sleep and including measures of BP variability, with the aim to investigate their potential causal relation.
Acknowledgments
We thank Kiara Smith for proofreading the manuscript. The authors’ responsibilities were as follows—MN and XG: conceived of the research question; MG, AX, SW, and XG: designed the study and collected the data; MN, YW, and XZ: performed the analysis; MN, YW, PMK-E, MG, AX, SW, and XG: interpreted the data; MN, YW, and CS: performed the literature review; MN: wrote the first draft of the manuscript; MN and SW: had primary responsibility for the final content; and all authors: revised the manuscript and read and approved the final manuscript.
Notes
Supported by the Broadhurst Career Development Professorship for the Study of Health Promotion and Disease Prevention, College of Health and Human Development, The Pennsylvania State University (to MN). The Hershey Company Endowment in Nutrition supported the publication of the manuscript.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ABP, ambulatory blood pressure; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; IPAQ-C, Chinese version of the International Physical Activity Questionnaire; SBP, systolic blood pressure; VIM, variance independent of the mean.
Contributor Information
Muzi Na, Department of Nutritional Sciences, Penn State College of Health and Human Development, University Park, PA, USA.
Yanxiu Wang, Department of Cardiology, Kailuan General Hospital, Hebei United University, Tangshan, China.
Xinyuan Zhang, Department of Nutritional Sciences, Penn State College of Health and Human Development, University Park, PA, USA.
Christopher Sarpong, Department of Biology, Penn State Eberly College of Science, University Park, PA, USA.
Penny M Kris-Etherton, Department of Nutritional Sciences, Penn State College of Health and Human Development, University Park, PA, USA.
Ming Gao, Department of Cardiology, Kailuan General Hospital, Hebei United University, Tangshan, China.
Aijun Xing, Department of Cardiology, Kailuan General Hospital, Hebei United University, Tangshan, China.
Shouling Wu, Department of Cardiology, Kailuan General Hospital, Hebei United University, Tangshan, China.
Xiang Gao, Department of Nutrition and Food Hygiene, School of Public Health, Fudan University, Shanghai, China.
References
- 1. GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57(1):3–10. [DOI] [PubMed] [Google Scholar]
- 3. Yang W-Y, Melgarejo JD, Thijs L, Zhang Z-Y, Boggia J, Wei F-Fet al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322(5):409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375(9718):938–48. [DOI] [PubMed] [Google Scholar]
- 5. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJet al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård Ket al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55(4):1049–57. [DOI] [PubMed] [Google Scholar]
- 7. Palatini P, Reboldi G, Beilin LJ, Casiglia E, Eguchi K, Imai Yet al. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure–International study. Hypertension. 2014;64(3):487–93. [DOI] [PubMed] [Google Scholar]
- 8. Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PWet al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21(12):2251–7. [DOI] [PubMed] [Google Scholar]
- 9. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FMet al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–24. [DOI] [PubMed] [Google Scholar]
- 10. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha Det al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 11. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ERet al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. [DOI] [PubMed] [Google Scholar]
- 12. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113(1):1–15. [DOI] [PubMed] [Google Scholar]
- 13. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TAet al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–8. [DOI] [PubMed] [Google Scholar]
- 14. Van Horn L, Carson JAS, Appel LJ, Burke LE, Economos C, Karmally Wet al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–e29. [DOI] [PubMed] [Google Scholar]
- 15. Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TMet al. Effect of dietary patterns on ambulatory blood pressure: results from the Dietary Approaches to Stop Hypertension (DASH) Trial. Hypertension. 1999;34(3):472–7. [DOI] [PubMed] [Google Scholar]
- 16. Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin P-Het al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tyson CC, Barnhart H, Sapp S, Poon V, Lin PH, Svetkey LP. Ambulatory blood pressure in the dash diet trial: effects of race and albuminuria. J Clin Hypertens (Greenwich). 2018;20(2):308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prather AA, Blumenthal JA, Hinderliter AL, Sherwood A. Ethnic differences in the effects of the DASH diet on nocturnal blood pressure dipping in individuals with high blood pressure. Am J Hypertens. 2011;24(12):1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y-p, Wang D, He Y-n, Hu X, Zhai F, Yang Xet al. Comparative study on the results of energy and nutrients intakes investigated by different evaluation methods. Chinese Journal of Prevention and Control of Chronic Non Communicable Diseases. 2007;15(2):79. [Google Scholar]
- 20. Gao Y, Cui L-F, Sun Y-Y, Yang W-H, Wang J-R, Wu S-Let al. Adherence to the Dietary Approaches to Stop Hypertension diet and hyperuricemia: a cross-sectional study. Arthritis Care Res (Hoboken). 2021;73(4):603–11. [DOI] [PubMed] [Google Scholar]
- 21. Yang Y, Wang G, Pan X. China food composition. Beijing, China: Peking University Medical Press; 2002. [Google Scholar]
- 22. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 23. US Department of Health and Human Services, NIH, National Heart, Lung, and Blood Institute (NHLBI) . Your guide to lowering your blood pressure with DASH: DASH eating plan, lower your blood pressure. Bethesda, MD: US Department of Health and Human Services, NIH, NHLBI; 2006. [Google Scholar]
- 24. Selvarajah V, Pasea L, Ojha S, Wilkinson IB, Tomlinson LA. Pre-dialysis systolic blood pressure-variability is independently associated with all-cause mortality in incident haemodialysis patients. PLoS One. 2014;9(1):e86514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(10):e006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Kong L. Chinese adults guidelines for the prevention and control of overweight and obesity. Beijing, China: People's Medical Publishing House; 2006. [Google Scholar]
- 27. Macfarlane DJ, Lee CCY, Ho EYK, Chan K, Chan DTS. Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport. 2007;10(1):45–51. [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer Met al. Association between insomnia symptoms and mortality: a prospective study of US men. Circulation. 2014;129(7):737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jarrin DC, Alvaro PK, Bouchard M-A, Jarrin SD, Drake CL, Morin CM. Insomnia and hypertension: a systematic review. Sleep Med Rev. 2018;41:3–38. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Zet al. Status of hypertension in China: results from the China Hypertension Survey, 2012–2015. Circulation. 2018;137(22):2344–56. [DOI] [PubMed] [Google Scholar]
- 31. Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LIet al. Dietary Approaches to Stop Hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos Let al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3. [DOI] [PubMed] [Google Scholar]
- 33. Lu L, Wang S-B, Rao W-W, Ungvari GS, Ng CH, Chiu HFKet al. Sleep duration and patterns in Chinese older adults: a comprehensive meta-analysis. Int J Biol Sci. 2017;13(6):682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al Khatib H, Harding SV, Darzi J, Pot GK. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur J Clin Nutr. 2017;71(5):614–24. [DOI] [PubMed] [Google Scholar]
- 35. Fenton S, Burrows T, Skinner J, Duncan M. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies. J Hum Nutr Diet. 2021;34(2):273–85. [DOI] [PubMed] [Google Scholar]
- 36. Zuraikat FM, Wood RA, Barragán R, St-Onge M-P. Sleep and diet: mounting evidence of a cyclical relationship. Annu Rev Nutr. 2021;41:309–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li Jet al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14(4):324–32. [DOI] [PubMed] [Google Scholar]
- 38. Lindberg E, Janson C, Gislason T, Svardsudd K, Hetta J, Boman G. Snoring and hypertension: a 10 year follow-up. Eur Respir J. 1998;11(4):884–9. [DOI] [PubMed] [Google Scholar]
- 39. Hu FB, Willett WC, Colditz GA, Ascherio A, Speizer FE, Rosner Bet al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150(8):806–16. [DOI] [PubMed] [Google Scholar]
- 40. Levi-Marpillat N, Macquin-Mavier I, Tropeano A-I, Parati G, Maison P. Antihypertensive drug classes have different effects on short-term blood pressure variability in essential hypertension. Hypertens Res. 2014;37(6):585–90. [DOI] [PubMed] [Google Scholar]
- 41. Zhao M, Konishi Y, Glewwe P. Does information on health status lead to a healthier lifestyle? Evidence from China on the effect of hypertension diagnosis on food consumption. J Health Econ. 2013;32(2):367–85. [DOI] [PubMed] [Google Scholar]
- 42. Scheltens T, Beulens J, Verschuren W, Boer J, Hoes A, Grobbee Det al. Awareness of hypertension: will it bring about a healthy lifestyle?. J Hum Hypertens. 2010;24(9):561–7. [DOI] [PubMed] [Google Scholar]
- 43. Chen S, Sudharsanan N, Huang F, Liu Y, Geldsetzer P, Bärnighausen T. Impact of community based screening for hypertension on blood pressure after two years: regression discontinuity analysis in a national cohort of older adults in China. BMJ. 2019;366:l4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: summary. J Gerontol A Biol Sci Med Sci. 2001;56(Suppl 2):89–94. [DOI] [PubMed] [Google Scholar]
- 45. Hallfrisch J, Muller D, Drinkwater D, Tobin J, Andres R. Continuing diet trends in men: the Baltimore Longitudinal Study of Aging (1961–1987). J Gerontol. 1990;45(6):M186–91. [DOI] [PubMed] [Google Scholar]
- 46. Harrington JM, Dahly DL, Fitzgerald AP, Gilthorpe MS, Perry IJ. Capturing changes in dietary patterns among older adults: a latent class analysis of an ageing Irish cohort. Public Health Nutr. 2014;17(12):2674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10(3):143–55. [DOI] [PubMed] [Google Scholar]
- 48. Saka F, Cornelissen G. Chronobiologic assessment of the effect of the DASH diet on blood pressure. J Hum Hypertens. 2021;35(8):678–84. [DOI] [PubMed] [Google Scholar]