To the Editor: In recent months, multiple lineages of the omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have emerged,1 with subvariants BA.1 and BA.2 showing substantial escape from neutralizing antibodies.2-5 Subvariant BA.2.12.1 is now the dominant strain in the United States, and BA.4 and BA.5 are dominant in South Africa (Figure 1A). Subvariants BA.4 and BA.5 have identical sequences of the spike protein.
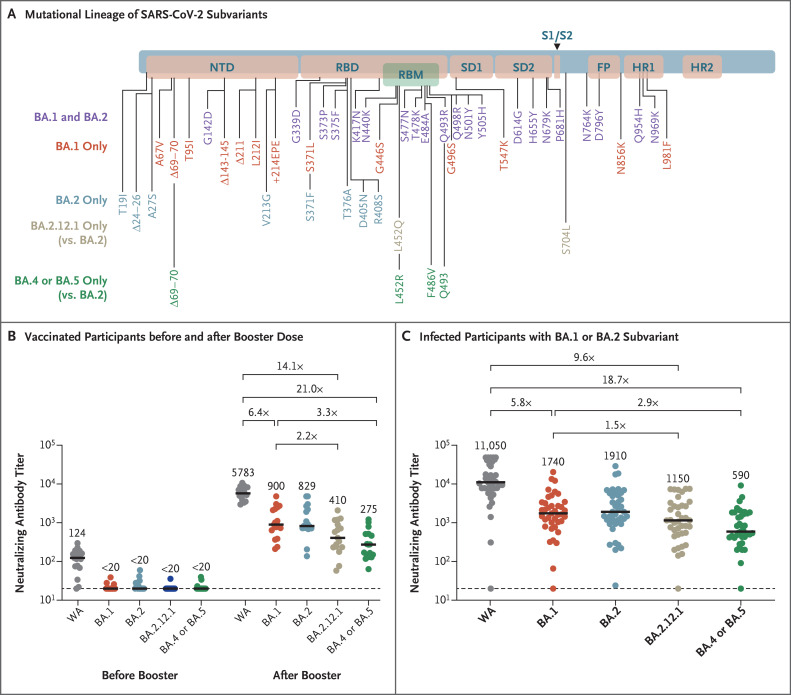
Figure 1. Omicron Subvariant Mutations and Neutralizing Antibody Responses.
Panel A shows the lineage of mutations that have been identified in the omicron BA.1, BA.2, BA.2.12.1, and BA.4 or BA.5 subvariants of SARS-CoV-2, as compared with the reference WA1/2020 isolate. BA.4 and BA.5 have identical sequences of the spike protein and thus have been grouped together. FP denotes fusion peptide, HR1 heptad repeat 1, HR2 heptad repeat 2, NTD N-terminal domain, RBD receptor-binding domain, RBM receptor-binding motif, SD1 subdomain 1, and SD2 subdomain 2. Panel B shows neutralizing antibody titers as determined by luciferase-based pseudovirus neutralization assays in samples obtained from 27 participants 6 months after receipt of the two-dose BNT162b2 messenger RNA vaccine series and 2 weeks after the third (booster) dose. Panel C shows neutralizing antibody titers in participants who had been infected with the BA.1 or BA.2 subvariant. All the infected participants had been vaccinated except for 1 participant who had a negative neutralizing antibody titer. In 9 participants, two or three time points after infection are shown. Neutralizing antibody titers were measured against the SARS-CoV-2 reference isolate WA1/2020 and the omicron BA.1, BA.2, BA.2.12.1, and BA.4 or BA.5 subvariants. In Panels B and C, medians (black bars) are shown numerically, and factor differences from other subvariants are indicated; the dashed horizontal line indicates the lower limit of detection for the assay.
We evaluated neutralizing antibody titers against the reference WA1/2020 isolate of SARS-CoV-2 along with omicron subvariants BA.1, BA.2, BA.2.12.1, and BA.4 or BA.5 in 27 participants who had been vaccinated and boosted with messenger RNA vaccine BNT162b2 (Pfizer–BioNTech) and in 27 participants who had been infected with the BA.1 or BA.2 subvariant a median of 29 days earlier (range, 2 to 113) (Tables S1 and S2 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). In the vaccine cohort, participants were excluded if they had a history of SARS-CoV-2 infection or a positive result on nucleocapsid serologic analysis or if they had received another vaccine against coronavirus disease 2019 (Covid-19) or an immunosuppressive medication.
Six months after the initial two BNT162b2 immunizations, the median neutralizing antibody pseudovirus titer was 124 against WA1/2020 but less than 20 against all the tested omicron subvariants (Figure 1B). Two weeks after administration of the booster dose, the median neutralizing antibody titer increased substantially, to 5783 against the WA1/2020 isolate, 900 against the BA.1 subvariant, 829 against the BA.2 subvariant, 410 against the BA.2.12.1 subvariant, and 275 against the BA.4 or BA.5 subvariant. These data show that as compared with the response against the WA1/2020 isolate, the neutralizing antibody titer was lower by a factor of 6.4 against BA.1, by a factor of 7.0 against BA.2, by a factor of 14.1 against BA.2.12.1, and by a factor of 21.0 against BA.4 or BA.5. In addition, as compared with the median neutralizing antibody titer against the BA.1 subvariant, the median titer was lower by a factor of 2.2 against the BA.2.12.1 subvariant and by a factor of 3.3 against the BA.4 or BA.5 subvariant.
Among the participants who had been infected with the BA.1 or BA.2 subvariant of omicron, all but one had been vaccinated against Covid-19. Because of the variation in sampling after the onset of infection, some samples may not reflect peak neutralizing antibody titers (Table S2). Among the participants with a history of Covid-19, the median neutralizing antibody titer was 11,050 against the WA1/2020 isolate, 1740 against the BA.1 subvariant, 1910 against the BA.2 subvariant, 1150 against the BA.2.12.1 subvariant, and 590 against the BA.4 or BA.5 subvariant (Figure 1C). These data show that as compared with the WA1/2020 isolate, the median neutralizing antibody titer was lower by a factor of 6.4 against BA.1, by a factor of 5.8 against BA.2, by a factor of 9.6 against BA.2.12.1, and by a factor of 18.7 against BA.4 or BA.5. In addition, as compared with the median titers against the BA.1 subvariant, the median titer was lower by a factor of 1.5 against the BA.2.12.1 subvariant and by a factor of 2.9 against the BA.4 or BA.5 subvariant.
These data show that the BA.2.12.1, BA.4, and BA.5 subvariants substantially escape neutralizing antibodies induced by both vaccination and infection. Moreover, neutralizing antibody titers against the BA.4 or BA.5 subvariant and (to a lesser extent) against the BA.2.12.1 subvariant were lower than titers against the BA.1 and BA.2 subvariants, which suggests that the SARS-CoV-2 omicron variant has continued to evolve with increasing neutralization escape. These findings provide immunologic context for the current surges caused by the BA.2.12.1, BA.4, and BA.5 subvariants in populations with high frequencies of vaccination and BA.1 or BA.2 infection.
Supplementary Appendix
Disclosure Forms
This letter was published on June 22, 2022, at NEJM.org.
Footnotes
Supported by a grant (CA260476) from the National Institutes of Health (NIH), by the Massachusetts Consortium for Pathogen Readiness, and by the Ragon Institute. Dr. Barouch is supported by the Musk Foundation. Dr. Collier is supported by the Reproductive Scientist Development Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, by a grant (HD000849) from the Burroughs Wellcome Fund, and by a grant (AI69309) from the NIH.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature 2022;603:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022;602:654-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2022;602:676-681. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N Engl J Med 2022;386:1579-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature 2022;604:553-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.