To the Editor: The value of variant-adapted vaccines that are capable of inducing a higher and broader immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at booster vaccination is currently being evaluated.1-4 We conducted a multicenter, randomized, single-blind trial to assess the immunogenicity and safety of two adjuvanted recombinant vaccines and the messenger RNA (mRNA) vaccine BNT162b2 (Pfizer–BioNTech) administered as a booster (ClinicalTrials.gov number, NCT05124171; EudraCT number, 2021-004550-33).
We randomly assigned adults with no previous diagnosis of coronavirus disease 2019 (Covid-19) who had received primary vaccination with two doses of BNT162b2 (with the second dose received between 3 and 7 months earlier) to receive booster vaccination with one of the following: a third dose of BNT162b2, a dose of the Sanofi–GSK SARS-CoV-2 adjuvanted recombinant protein MVD614 vaccine (monovalent formulation based on the original strain), or a dose of the SARS-CoV-2 adjuvanted recombinant protein MVB.1.351 vaccine (monovalent beta formulation; beta-adjuvanted vaccine). The primary end point was the percentage of participants who had an increase in the neutralizing-antibody titer by a factor of at least 10 between day 0 and day 15 after receipt of the booster as measured by microneutralization against the original D614 (wild-type) strain of SARS-CoV-2 or the B.1.351 (beta) variant. The primary end point was assessed in the per-protocol population, which included all the participants who underwent randomization and did not meet any of the exclusion criteria (see the protocol, available with the full text of this letter at NEJM.org). The main other prespecified immunologic end points were the rate of increase between day 0 and day 15 in neutralizing-antibody titers against the original D614 strain and the beta, B.1.617.2 (delta), and B.1.1.529 (omicron) BA.1 variants; the geometric mean of anti-spike (anti-S1) IgG levels; and interferon-γ– and interleukin-2–secreting CD4+ T-cell response against the original strain and the omicron variant in each randomized group.
Among the 247 participants who underwent randomization between December 8, 2021, and January 14, 2022, a total of 24 participants were excluded, mainly owing to preexisting immunity against SARS-CoV-2. The per-protocol population included 223 participants: 76 in the MVD614 group, 71 in the MVB.1.351 group, and 76 in the BNT162b2 group (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The mean age of the participants was 40.6 years, and 40% were women. The baseline characteristics of the participants did not differ among the trial groups (Table S1).
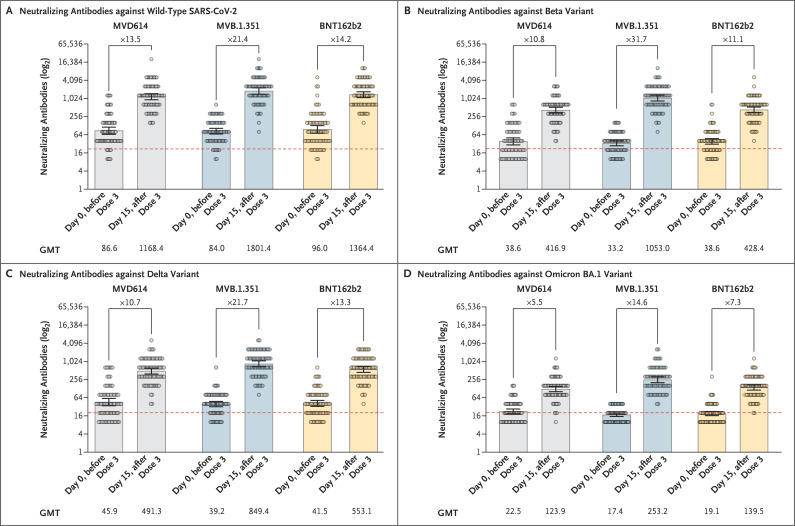
The percentage of participants who had an increase in the neutralizing-antibody titer by a factor of at least 10 between day 0 and day 15 for the original strain was 55% (95% confidence interval [CI], 43 to 67) in the MVD614 group, 76% (95% CI, 64 to 85) in the MVB.1.351 group, and 63% (95% CI, 51 to 74) in the BNT162b2 group. For the beta variant, the corresponding percentages were 45% (95% CI, 33 to 57), 85% (95% CI, 74 to 92), and 51% (95% CI, 40 to 63).
The MVB.1.351 vaccine elicited higher neutralizing-antibody titers than the other vaccines against the original strain and against the beta, delta, and omicron BA.1 variants (Figure 1 and Table S2). The geometric mean titer of anti-S1 increased from 277.1 binding antibody units (BAU) per milliliter at day 0 to 1875.1 BAU per milliliter at day 15 in the MVD614 group, from 206.8 to 2240.8 BAU per milliliter in the MVB.1.351 group, and from 253.6 to 2405.4 BAU per milliliter in the BNT162b2 group (Table S3). The MVB.1.351 vaccine also induced high levels of specific polyfunctional CD4+ type 1 helper T-cell response (Figs. S3 and S4). The reactogenicity profiles of the three booster vaccines did not differ among the trial groups (Table S4).
Figure 1. Neutralizing Antibodies against Wild-type SARS-CoV-2 and the Beta, Delta, and Omicron BA.1 Variants at Days 0 and 15 after Receipt of a Third Vaccine Dose (Per-Protocol Population).
Shown are geometric mean titers (GMTs) of neutralizing antibodies against the original D614 (wild-type) strain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the beta (B.1.351), delta (B.1.617.2), and omicron (B.1.1.529) BA.1 variants, as assessed at days 0 and 15 after the receipt of a third vaccine dose (booster). Participants who had received two doses of the BNT162b2 vaccine were randomly assigned to receive one of the following as a booster dose: the MVD614 vaccine (monovalent parental formulation), the MVB.1.351 vaccine (beta-adjuvanted formulation), or a third dose of the BNT162b2 vaccine. The per-protocol population included all the participants who underwent randomization and did not meet any of the exclusion criteria. The factor increases in each trial group are shown for the change from day 0 to day 15. 𝙸 bars indicate 95% confidence intervals, and circles the titers in individual participants. The dashed line represents the positivity threshold on the neutralization assay.
Over the short term, heterologous boosting with the beta-adjuvanted MVB.1.351 vaccine resulted in a higher neutralizing-antibody response against the beta variant as well as against the original strain and the delta and omicron BA.1 variants than did the mRNA vaccine BNT162b2 or the MVD614 formulation. The use of new vaccines that contain beta spike protein may be an interesting strategy for broader protection against SARS-CoV-2 variants.
Protocol
Supplementary Appendix
Disclosure Forms
This letter was published on June 29, 2022, at NEJM.org.
Footnotes
Supported by the French Ministries of Solidarity and Health and of Higher Education, Research, and Innovation.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Rubin EJ, Baden LR, Morrissey S. Audio interview: do we need new Covid-19 vaccines? N Engl J Med 2022;386(18):e52-e52. [DOI] [PubMed] [Google Scholar]
- 2.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med 2022;386:1046-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med 2021;27:2025-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.