Abstract
Background
The protection conferred by natural immunity, vaccination, and both against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with the BA.1 or BA.2 sublineages of the omicron (B.1.1.529) variant is unclear.
Methods
We conducted a national, matched, test-negative, case–control study in Qatar from December 23, 2021, through February 21, 2022, to evaluate the effectiveness of vaccination with BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna), natural immunity due to previous infection with variants other than omicron, and hybrid immunity (previous infection and vaccination) against symptomatic omicron infection and against severe, critical, or fatal coronavirus disease 2019 (Covid-19).
Results
The effectiveness of previous infection alone against symptomatic BA.2 infection was 46.1% (95% confidence interval [CI], 39.5 to 51.9). The effectiveness of vaccination with two doses of BNT162b2 and no previous infection was negligible (−1.1%; 95% CI, −7.1 to 4.6), but nearly all persons had received their second dose more than 6 months earlier. The effectiveness of three doses of BNT162b2 and no previous infection was 52.2% (95% CI, 48.1 to 55.9). The effectiveness of previous infection and two doses of BNT162b2 was 55.1% (95% CI, 50.9 to 58.9), and the effectiveness of previous infection and three doses of BNT162b2 was 77.3% (95% CI, 72.4 to 81.4). Previous infection alone, BNT162b2 vaccination alone, and hybrid immunity all showed strong effectiveness (>70%) against severe, critical, or fatal Covid-19 due to BA.2 infection. Similar results were observed in analyses of effectiveness against BA.1 infection and of vaccination with mRNA-1273.
Conclusions
No discernable differences in protection against symptomatic BA.1 and BA.2 infection were seen with previous infection, vaccination, and hybrid immunity. Vaccination enhanced protection among persons who had had a previous infection. Hybrid immunity resulting from previous infection and recent booster vaccination conferred the strongest protection. (Funded by Weill Cornell Medicine–Qatar and others.)
Qatar endured a wave of the omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 that started on December 19, 2021, and peaked in mid-January 2022.2-4 The wave was first dominated by the BA.1 subvariant, but within a few days after the onset of the wave, the BA.2 subvariant predominated (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Although BA.1 and BA.2 remain classified as subvariants of omicron, considerable genetic distance exists between them.5 The protection against these subvariants provided by previous immunity — and whether immunity is induced by previous infection, vaccination, or a hybrid of both — remains to be established.
With the use of data from December 23, 2021, through February 21, 2022, we investigated the protection conferred by previous infection from variants other than omicron, vaccination with two or three doses of the coronavirus disease 2019 (Covid-19) messenger RNA (mRNA) vaccines BNT162b2 (Pfizer–BioNTech)6 or mRNA-1273 (Moderna),7 and hybrid immunity (previous infection and vaccination). Effectiveness against symptomatic BA.1 infection, symptomatic BA.2 infection, and any symptomatic omicron infection was assessed. Protection against any severe (acute-care hospitalization),8 critical (hospitalization in an intensive care unit),8 or fatal9 case of Covid-19 due to BA.1, BA.2, or any omicron infection was also assessed.
Methods
Study Population and Data Sources
The study was conducted in the resident population of Qatar. We analyzed information from the national, federated databases regarding Covid-19 vaccination, laboratory testing, hospitalization, and death. These data were retrieved from the integrated nationwide digital-health information platform. The databases included all SARS-CoV-2–related data and associated demographic information since the start of the pandemic. These databases include, with no missing information, results of all polymerase-chain-reaction (PCR) testing and, more recently, rapid antigen testing conducted at health care facilities on or after January 5, 2022.
All PCR testing (but not rapid antigen testing) performed in Qatar is classified on the basis of symptoms and the reason for testing. Of all the PCR tests conducted during this study, 19.2% were performed because of clinical symptoms. Qatar has an unusually young, diverse population — only 9% of its residents are 50 years of age or older, and 89% are expatriates from more than 150 countries.10 Qatar launched its Covid-19 vaccination program in December 2020 with the BNT162b2 and mRNA-1273 vaccines.11 Further descriptions of the study population and the national databases have been reported previously.4,10-15
Study Design
The study assessed the effectiveness of previous infection, vaccination with BNT162b2 or mRNA-1273, and hybrid immunity (previous infection and vaccination) against symptomatic infection with BA.1, BA.2, and any omicron infection.2,15-18 We used a test-negative, case–control design, in which effectiveness estimates were derived by comparing the odds of previous infection or vaccination or both among case participants (persons with a positive PCR test) with that among controls (PCR-negative persons).2,15-18 We also assessed effectiveness against any severe, critical, or fatal case of Covid-19.
To estimate the effectiveness against symptomatic infection, we exactly matched cases and controls that were identified from December 23, 2021, through February 21, 2022. Case participants and controls were matched in a 1:1 ratio according to sex, 10-year age group, nationality, and calendar week of PCR test. Matching was performed to control for known differences in the risk of SARS-CoV-2 exposure in Qatar.10,19,20 Matching according to these factors was previously shown to provide adequate control of differences in the risk of SARS-CoV-2 exposure in studies of different designs, all of which involved control groups, such as test-negative, case–control studies.11,12,15,21,22 To assess effectiveness against any severe, critical, or fatal case of Covid-19, we used a 1:5 matching ratio to improve the statistical precision of the estimates.
Only the first PCR-positive test that was identified for an individual participant during the study period was included, but all PCR-negative tests were included. Controls included persons with no record of a PCR-positive test during the study period. Only PCR tests conducted because of clinical symptoms were used in the analyses.
SARS-CoV-2 reinfection is conventionally defined as a documented infection that occurs at least 90 days after an earlier infection, to avoid misclassification of prolonged PCR positivity as reinfection if a shorter time interval is used.2,23 Previous infection was therefore defined as a PCR-positive test that occurred at least 90 days before the PCR test used in the study. Tests for persons who had PCR-positive tests that occurred within 90 days before the PCR test used in the study were excluded. Accordingly, previous infections in this study were considered to be due to variants other than omicron, since they occurred before the omicron wave in Qatar.2-4
PCR tests for persons who received vaccines other than BNT162b2 or mRNA-1273 and tests for persons who received mixed vaccines were excluded from the analyses. Tests that occurred within 14 days after a second dose or 7 days after a third dose of vaccine were excluded. These inclusion and exclusion criteria were implemented to allow for build-up of immunity after vaccination4,14 and to minimize different types of potential bias, as informed by earlier analyses in the same population.12,22 Every control that met the inclusion criteria and that could be matched to a case was included in the analyses.
We compared five groups with the group that had no previous infection and no vaccination. The five groups were characterized by type of exposure: previous infection and no vaccination, two-dose vaccination and no previous infection, two-dose vaccination and previous infection, three-dose vaccination and no previous infection, and three-dose vaccination and previous infection. The groups were defined on the basis of the status of previous immunologic events (previous infection or vaccination) at the time of the PCR test.
Classification of severe,8 critical,8 and fatal9 Covid-19 cases followed World Health Organization guidelines, and assessments were made by trained medical personnel with the use of individual chart reviews as part of a national protocol applied to hospitalized patients with Covid-19. Details regarding Covid-19 severity, criticality, and fatality classification are provided in Section S1 in the Supplementary Appendix.
Laboratory Methods and Subvariant Ascertainment
The large omicron wave in Qatar started on December 19, 2021, and peaked in mid-January 2022.2-4 A total of 315 random SARS-CoV-2–positive specimens collected from December 19, 2021, through January 22, 2022, underwent viral whole-genome sequencing on a GridION sequencing device (Nanopore Technologies). Of these specimens, 300 (95.2%) were confirmed to be omicron infections and 15 (4.8%) to be delta (or B.1.617.2)1 infections.2-4 Of the 286 omicron infections with confirmed subvariant status, 68 (23.8%) were BA.1 and 218 (76.2%) were BA.2.
We used the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific), which tests for the spike (S) gene of SARS-CoV-2 and the 69-70del mutation in the S gene,24 to identify BA.1 and BA.2 infections. An S-gene target failure was used as a proxy for BA.1 infection, and a non–S-gene target failure was used as a proxy for BA.2 infection. Additional details regarding laboratory methods for real-time reverse-transcriptase–quantitative PCR testing are provided in Section S2.
Oversight
This retrospective study was approved by the institutional review boards at Hamad Medical Corporation and Weill Cornell Medicine–Qatar, with a waiver of informed consent. The reporting of this study follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Table S1). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. All the authors contributed to data collection and acquisition, discussion and interpretation of the results, and the writing of the manuscript. All the authors read and approved the final manuscript.
Statistical Analysis
Although all records of PCR testing were examined for selection of cases and controls, only matched samples were analyzed. Cases and controls were described with the use of frequency distributions and measures of central tendency and compared with the use of standardized mean differences. The standardized mean difference was defined as the difference between the mean value of a covariate in one group and the corresponding mean value of a covariate in the other group, divided by the pooled standard deviation, with values of less than 0.1 indicating adequate matching.25
Odds ratios, which compared the odds of previous infection or vaccination or both among cases with that among controls, and associated 95% confidence intervals were derived with the use of conditional logistic regression. This analytic approach, which also incorporated matching according to calendar week of PCR test, minimizes potential bias due to variation in epidemic phase16,26 and roll-out of vaccination during the study period.16,26 Confidence intervals were not adjusted for multiplicity and therefore should not be used to infer definitive differences among exposure groups. Interactions were not investigated. Effectiveness and associated 95% confidence intervals were calculated as 1 minus the odds ratio of previous infection or vaccination or both among cases as compared with controls.16,17 The reference group for all estimates included persons with no previous infection and no vaccination.
An additional analysis was conducted to investigate the effects of previous infection, two-dose vaccination, and three-dose vaccination as a function of time since the immunologic event (previous infection or vaccination). This analysis used the same approach as the primary analysis, but with stratification according to time since the most recent immunologic event.
A person was considered to have had a previous positive test if that test was positive by PCR assay. A sensitivity analysis of effectiveness against any symptomatic omicron infection was conducted, but with previous positive testing being based on positive PCR as well as positive rapid antigen tests, to investigate whether exclusion of rapid antigen–positive tests could have biased our estimates. Statistical analyses were performed with the use of Stata/SE software, version 17.0 (StataCorp).
Results
Study Population
From December 23, 2020 (the date that vaccination began in Qatar), through February 21, 2022 (the end of the study), 1,306,862 persons received at least two doses of BNT162b2, and 341,438 of these received a third (booster) dose. The median date of the first dose was May 3, 2021, the median date of the second dose was May 24, 2021, and the median date of the third dose was December 25, 2021. The median interval between the first and second doses was 21 days (interquartile range, 21 to 22), and between the second and third doses was 251 days (interquartile range, 233 to 274). The narrow interquartile range between the first and second doses reflects strict adherence to national policy.
During the study period, 893,671 persons received two doses of mRNA-1273, and 135,050 of these received a third dose. The median date of the first dose was May 28, 2021, the median date of the second dose was June 27, 2021, and the median date of the third dose was January 12, 2022. The median interval between the first and second doses was 28 days (interquartile range, 28 to 30), and between the second and third doses was 236 days (interquartile range, 213 to 260).
The study was based on the total population of Qatar; therefore, the population is representative of the internationally diverse, but young and predominantly male, population of the country (Table S2). Figure S2 shows the process for selecting the populations for the analysis of BNT162b2, and Table 1 shows the characteristics of these populations. Figure S3 shows the process for selecting the populations for the analysis of mRNA-1273, and Table S4 shows the characteristics of these populations.
Table 1. Characteristics of the Matched Case Participants and Controls According to Omicron Infection in the BNT162b2 Analysis.*.
| Characteristic | Omicron BA.1 Infection | Omicron BA.2 Infection | Any Omicron Infection | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case Participants (N=6264) |
Controls (N=6264) |
SMD | Case Participants (N=20,693) |
Controls (N=20,693) |
SMD | Case Participants (N=25,288) |
Controls (N=25,288) |
SMD | |
| Median age (IQR) — yr | 32 (22–42) | 32 (22–42) | 0.00† | 33 (21–43) | 33 (21–43) | 0.00† | 32 (20–42) | 32 (20–42) | 0.00† |
| Age group — no. (%) | |||||||||
| <20 yr | 1394 (22.3) | 1394 (22.3) | 0.00 | 4,742 (22.9) | 4,742 (22.9) | 0.00 | 6,185 (24.5) | 6,185 (24.5) | 0.00 |
| 20–29 yr | 1207 (19.3) | 1207 (19.3) | 3,873 (18.7) | 3,873 (18.7) | 4,776 (18.9) | 4,776 (18.9) | |||
| 30–39 yr | 1759 (28.1) | 1759 (28.1) | 5,581 (27.0) | 5,581 (27.0) | 6,675 (26.4) | 6,675 (26.4) | |||
| 40–49 yr | 944 (15.1) | 944 (15.1) | 2,982 (14.4) | 2,982 (14.4) | 3,620 (14.3) | 3,620 (14.3) | |||
| 50–59 yr | 610 (9.7) | 610 (9.7) | 2,191 (10.6) | 2,191 (10.6) | 2,493 (9.9) | 2,493 (9.9) | |||
| 60–69 yr | 251 (4.0) | 251 (4.0) | 915 (4.4) | 915 (4.4) | 1,053 (4.2) | 1,053 (4.2) | |||
| ≥70 yr | 99 (1.6) | 99 (1.6) | 409 (2.0) | 409 (2.0) | 486 (1.9) | 486 (1.9) | |||
| Sex — no. (%) | |||||||||
| Male | 3003 (47.9) | 3003 (47.9) | 0.00 | 11,191 (54.1) | 11,191 (54.1) | 0.00 | 13,596 (53.8) | 13,596 (53.8) | 0.00 |
| Female | 3261 (52.1) | 3261 (52.1) | 9,502 (45.9) | 9,502 (45.9) | 11,692 (46.2) | 11,692 (46.2) | |||
| Nationality — no. (%)‡ | |||||||||
| Bangladeshi | 88 (1.4) | 88 (1.4) | 0.00 | 452 (2.2) | 452 (2.2) | 0.00 | 558 (2.2) | 558 (2.2) | 0.00 |
| Egyptian | 378 (6.0) | 378 (6.0) | 1,209 (5.8) | 1,209 (5.8) | 1,443 (5.7) | 1,443 (5.7) | |||
| Filipino | 690 (11.0) | 690 (11.0) | 1,922 (9.3) | 1,922 (9.3) | 2,365 (9.4) | 2,365 (9.4) | |||
| Indian | 677 (10.8) | 677 (10.8) | 2,503 (12.1) | 2,503 (12.1) | 3,093 (12.2) | 3,093 (12.2) | |||
| Nepalese | 57 (0.9) | 57 (0.9) | 282 (1.4) | 282 (1.4) | 312 (1.2) | 312 (1.2) | |||
| Pakistani | 131 (2.1) | 131 (2.1) | 653 (3.2) | 653 (3.2) | 753 (3.0) | 753 (3.0) | |||
| Qatari | 2453 (39.2) | 2453 (39.2) | 8,011 (38.7) | 8,011 (38.7) | 9,572 (37.9) | 9,572 (37.9) | |||
| Sri Lankan | 58 (0.9) | 58 (0.9) | 258 (1.2) | 258 (1.2) | 287 (1.1) | 287 (1.1) | |||
| Sudanese | 310 (4.9) | 310 (4.9) | 910 (4.4) | 910 (4.4) | 1,098 (4.3) | 1,098 (4.3) | |||
| Other§ | 1422 (22.7) | 1422 (22.7) | 4,493 (21.7) | 4,493 (21.7) | 5,807 (23.0) | 5,807 (23.0) | |||
| Calendar week of PCR test — no. (%) | |||||||||
| Dec. 23–29, 2021 | 1145 (18.3) | 1145 (18.3) | 0.00 | 2,494 (12.1) | 2,494 (12.1) | 0.00 | 5,748 (22.7) | 5,748 (22.7) | 0.00 |
| Dec. 30, 2021–Jan. 5, 2022 | 3570 (57.0) | 3570 (57.0) | 11,136 (53.8) | 11,136 (53.8) | 11,677 (46.2) | 11,677 (46.2) | |||
| Jan. 6–12, 2022 | 999 (15.9) | 999 (15.9) | 3,940 (19.0) | 3,940 (19.0) | 4,040 (16.0) | 4,040 (16.0) | |||
| Jan. 13–19, 2022 | 310 (4.9) | 310 (4.9) | 1,752 (8.5) | 1,752 (8.5) | 1,954 (7.7) | 1,954 (7.7) | |||
| Jan. 20–26, 2022 | 94 (1.5) | 94 (1.5) | 698 (3.4) | 698 (3.4) | 886 (3.5) | 886 (3.5) | |||
| Jan. 27–Feb. 2, 2022 | 72 (1.1) | 72 (1.1) | 393 (1.9) | 393 (1.9) | 520 (2.1) | 520 (2.1) | |||
| Feb. 3–9, 2022 | 38 (0.6) | 38 (0.6) | 138 (0.7) | 138 (0.7) | 233 (0.9) | 233 (0.9) | |||
| Feb. 10–16, 2022 | 27 (0.4) | 27 (0.4) | 107 (0.5) | 107 (0.5) | 163 (0.6) | 163 (0.6) | |||
| Feb. 17–20, 2022 | 9 (0.1) | 9 (0.1) | 35 (0.2) | 35 (0.2) | 67 (0.3) | 67 (0.3) | |||
Case participants had test results that were positive for severe acute respiratory syndrome coronavirus 2 by polymerase-chain-reaction (PCR) assay, and controls had test results that were negative by PCR assay. Case participants and controls were matched exactly in a 1:1 ratio according to sex, 10-year age group, nationality, and calendar week of PCR test. Unless otherwise specified, the standardized mean difference (SMD) was defined as the difference between the mean value of a covariate in one group and the corresponding mean value of a covariate in the other group, divided by the pooled standard deviation. An SMD of less than 0.1 indicates adequate matching. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
The SMD is the difference in the mean age between groups, divided by the pooled standard deviation.
Nationalities were chosen to represent the most populous groups in Qatar.
The category of other nationalities includes 57 other nationalities in Qatar among case participants and controls in the analysis of BA.1 infection, 71 other nationalities among case participants and controls in the analysis of BA.2 infection, and 78 other nationalities among case participants and controls in the analysis of any omicron infection.
Effectiveness of Previous Infection and BNT162b2 Vaccination against BA.1 Infection
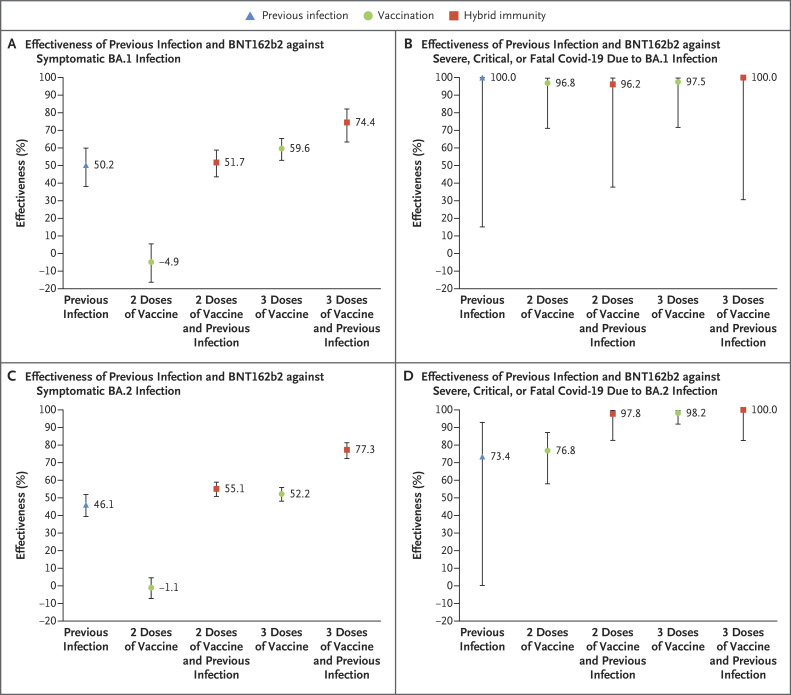
The effectiveness of previous infection and no vaccination against symptomatic BA.1 infection was 50.2% (95% confidence interval [CI], 38.1 to 59.9) (Figure 1A and Table 2). The median interval between the previous infection and the PCR test used in the study was 324.5 days (range, 91 to 643; interquartile range, 274 to 497).
Figure 1. Effectiveness of Previous Infection, Vaccination with BNT162b2, and Hybrid Immunity against Symptomatic Omicron BA.1 and BA.2 Infection and against Severe, Critical, or Fatal Covid-19.
𝙸 bars indicate 95% confidence intervals. Covid-19 denotes coronavirus disease 2019.
Table 2. Effectiveness of Previous Infection, Vaccination with BNT162b2, and Hybrid Immunity against Symptomatic Omicron Infections and against Severe, Critical, or Fatal Covid-19.*.
| Infection and Immune Status | Case Participants with Symptomatic Infection (PCR-Positive)† | Controls (PCR-Negative)† | Effectiveness against Symptomatic Infection (95% CI) | Case Participants with Severe, Critical, or Fatal Covid-19‡ | Controls (PCR- Negative)‡ | Effectiveness against Severe, Critical, or Fatal Covid-19 (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed§ | Exposed | Unexposed§ | Exposed | Unexposed§ | Exposed | Unexposed§ | |||
| number | percent | number | percent | |||||||
| BA.1 infection | ||||||||||
| Previous infection and no vaccination | 149 | 1738 | 255 | 1536 | 50.2 (38.1 to 59.9) | 0 | 12 | 6 | 11 | 100.0 (15.1 to 100.0)¶ |
| Two doses and no previous infection | 3,449 | 1738 | 2,762 | 1536 | −4.9 (−16.4 to 5.4) | 5 | 12 | 39 | 11 | 96.8 (71.1 to 99.6) |
| Two doses and previous infection | 402 | 1738 | 688 | 1536 | 51.7 (43.5 to 58.7) | 1 | 12 | 8 | 11 | 96.2 (37.7 to 99.8) |
| Three doses and no previous infection | 479 | 1738 | 892 | 1536 | 59.6 (52.9 to 65.3) | 2 | 12 | 20 | 11 | 97.5 (71.7 to 99.8) |
| Three doses and previous infection | 47 | 1738 | 131 | 1536 | 74.4 (63.4 to 82.2) | 0 | 12 | 7 | 11 | 100.0 (30.6 to 100.0)¶ |
| BA.2 infection | ||||||||||
| Previous infection and no vaccination | 565 | 6051 | 895 | 5372 | 46.1 (39.5 to 51.9) | 3 | 43 | 17 | 50 | 73.4 (0.2 to 92.9) |
| Two doses and no previous infection | 10,880 | 6051 | 8,846 | 5372 | −1.1 (−7.1 to 4.6) | 41 | 43 | 168 | 50 | 76.8 (58.0 to 87.1) |
| Two doses and previous infection | 1,160 | 6051 | 2,108 | 5372 | 55.1 (50.9 to 58.9) | 1 | 43 | 41 | 50 | 97.8 (82.6 to 99.7) |
| Three doses and no previous infection | 1,884 | 6051 | 2,983 | 5372 | 52.2 (48.1 to 55.9) | 3 | 43 | 98 | 50 | 98.2 (91.9 to 99.6) |
| Three doses and previous infection | 153 | 6051 | 489 | 5372 | 77.3 (72.4 to 81.4) | 0 | 43 | 23 | 50 | 100.0 (82.6 to 100.0)¶ |
| Any omicron infection | ||||||||||
| Previous infection and no vaccination | 637 | 7837 | 1,113 | 6904 | 50.8 (45.4 to 55.7) | 4 | 100 | 24 | 139 | 71.6 (15.7 to 90.4) |
| Two doses and no previous infection | 13,033 | 7837 | 10,600 | 6904 | −0.2 (−5.5 to 4.9) | 63 | 100 | 320 | 139 | 73.5 (60.5 to 82.2) |
| Two doses and previous infection | 1,360 | 7837 | 2,501 | 6904 | 55.5 (51.8 to 59.0) | 3 | 100 | 79 | 139 | 94.3 (81.3 to 98.3) |
| Three doses and no previous infection | 2,234 | 7837 | 3,586 | 6904 | 54.0 (50.4 to 57.3) | 12 | 100 | 164 | 139 | 92.5 (84.4 to 96.3) |
| Three doses and previous infection | 187 | 7837 | 584 | 6904 | 76.3 (71.7 to 80.1) | 0 | 100 | 47 | 139 | 100.0 (91.8 to 100.0)¶ |
A symptomatic infection was defined as a PCR-positive nasopharyngeal swab specimen that was obtained because of the presence of symptoms consistent with a respiratory tract infection. Effectiveness was estimated with the use of a test-negative, case–control study design.16,18 The widths of the confidence intervals have not been adjusted for multiplicity and should not be used to infer definitive differences among exposure groups. Severity,8 criticality,8 and fatality9 were defined according to World Health Organization guidelines. Covid-19 denotes coronavirus disease 2019.
Case participants and controls were exactly matched in a 1:1 ratio according to sex, 10-year age group, nationality, and calendar week of PCR test.
Case participants and controls were exactly matched in a 1:5 ratio according to sex, 10-year age group, nationality, and calendar week of PCR test.
Unexposed was defined as no previous infection and no vaccination.
The confidence interval was estimated with the use of McNemar’s test for matched pairs.
The effectiveness of two doses of BNT162b2 and no previous infection was negligible (−4.9%; 95% CI, −16.4 to 5.4). The median interval between the second dose and the PCR test used in the study was 268 days (range, 15 to 394; interquartile range, 211 to 293). The effectiveness of three doses and no previous infection was 59.6% (95% CI, 52.9 to 65.3). The median interval between the third dose and the PCR test used in the study was 42 days (range, 7 to 291; interquartile range, 28 to 62).
The effectiveness of hybrid immunity (previous infection and two doses of BNT162b2) was 51.7% (95% CI, 43.5 to 58.7), which was similar to the effectiveness of previous infection alone. The effectiveness of previous infection and three doses of BNT162b2 was the highest, at 74.4% (95% CI, 63.4 to 82.2).
Previous infection, vaccination, and hybrid immunity all showed strong effectiveness (>90%) against severe, critical, or fatal Covid-19 due to BA.1 infection, but some of the 95% confidence intervals were wide because of small case numbers (Figure 1B and Table 2). The severity of BA.1 infections was low, and only 0.3% (95% CI, 0.2 to 0.4) of infections progressed to severe, critical, or fatal Covid-19.
Effectiveness of Previous Infection and BNT162b2 Vaccination against BA.2 Infection
The effectiveness of previous infection and no vaccination against symptomatic BA.2 infection was 46.1% (95% CI, 39.5 to 51.9) (Figure 1C and Table 2). The median interval between the previous infection and the PCR test used in the study was 319 days (range, 90 to 662; interquartile range, 275 to 499).
The effectiveness of two doses of BNT162b2 and no previous infection was negligible (−1.1%; 95% CI, −7.1 to 4.6). The median interval between the second dose and the PCR test used in the study was 270 days (range, 14 to 399; interquartile range, 213 to 296). The effectiveness of three doses of BNT162b2 and no previous infection was 52.2% (95% CI, 48.1 to 55.9). The median interval between the third dose and the PCR test used in the study was 43 days (range, 7 to 322; interquartile range, 26 to 65).
The effectiveness of previous infection and two doses of BNT162b2 was 55.1% (95% CI, 50.9 to 58.9), which is similar to the effectiveness of previous infection alone. The effectiveness of previous infection and three doses of BNT162b2 was the highest, at 77.3% (95% CI, 72.4 to 81.4).
Previous infection, vaccination, and hybrid immunity all showed strong effectiveness (>70%) against severe, critical, or fatal Covid-19 due to BA.2, but some of the 95% confidence intervals were wide because of small case numbers (Figure 1D and Table 2). The severity of BA.2 infections was low, and only 0.3% (95% CI, 0.2 to 0.3) of infections progressed to severe, critical, or fatal Covid-19.
Effectiveness of Previous Infection and BNT162b2 Vaccination against Any Omicron Infection
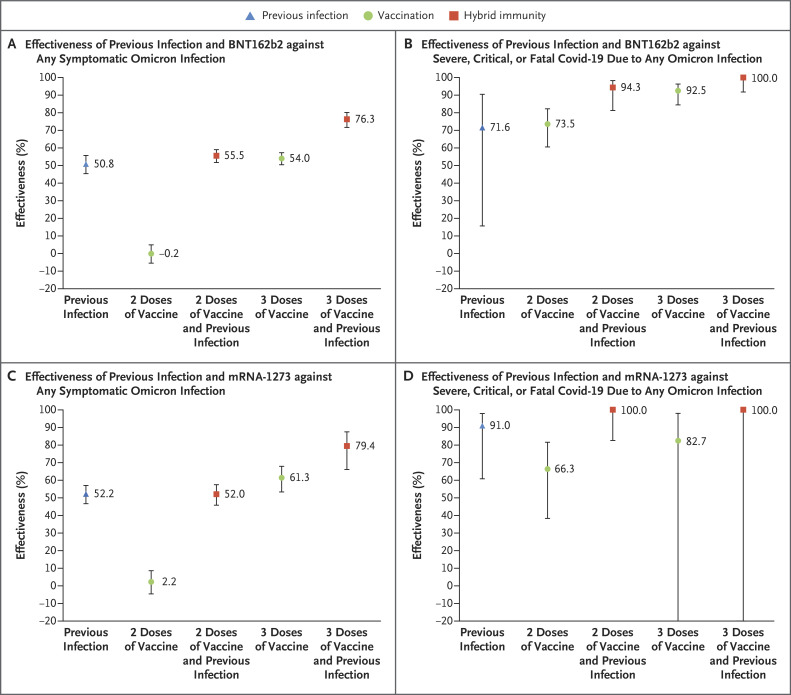
The effectiveness of previous infection, BNT162b2 vaccination, and hybrid immunity against any symptomatic omicron infection showed similar patterns to those against BA.1 and BA.2 (Figure 2A and Table 2). The effectiveness against severe, critical, or fatal Covid-19 due to any omicron infection also showed similar patterns to those against these outcomes due to BA.1 and BA.2 (Figure 2B and Table 2).
Figure 2. Effectiveness of Previous Infection, Vaccination with BNT162b2 or mRNA-1273, and Hybrid Immunity against Any Symptomatic Omicron Infection and against Severe, Critical, or Fatal Covid-19.
𝙸 bars indicate 95% confidence intervals.
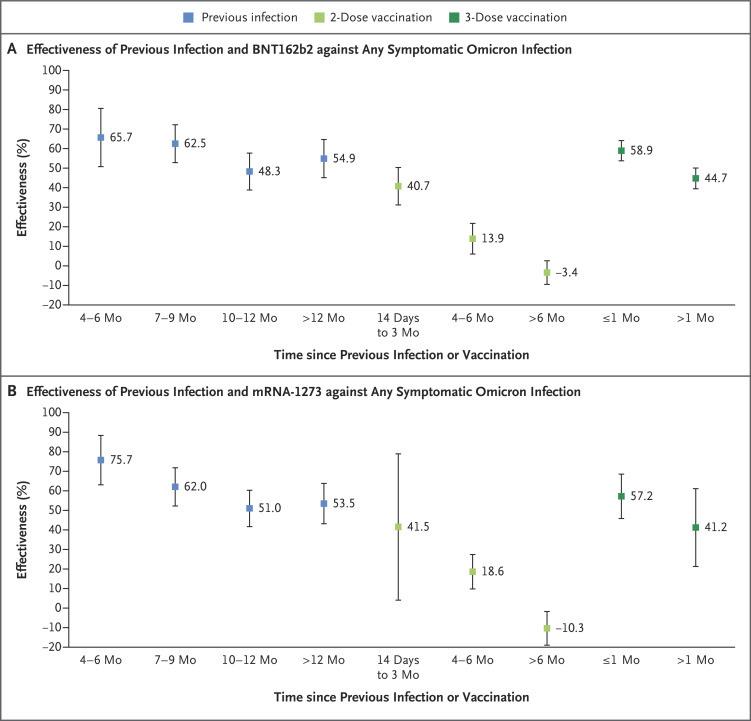
The analysis of the effectiveness of previous infection, two-dose vaccination, and three-dose vaccination as a function of time since the immunologic event (previous infection or vaccination) showed rapidly waning vaccine protection after the second and third doses but slowly waning protection from previous infection (Figure 3). A sensitivity analysis in which previous positive testing included both PCR-positive and rapid antigen–positive results showed similar findings to those of the main analyses, which indicates that exclusion of previous rapid antigen–positive tests may not have biased our estimates (Table S3).
Figure 3. Effectiveness of Previous Infection, Vaccination, and Hybrid Immunity against Any Symptomatic Omicron Infection According to Time since Previous Infection or Vaccination.
𝙸 bars indicate 95% confidence intervals.
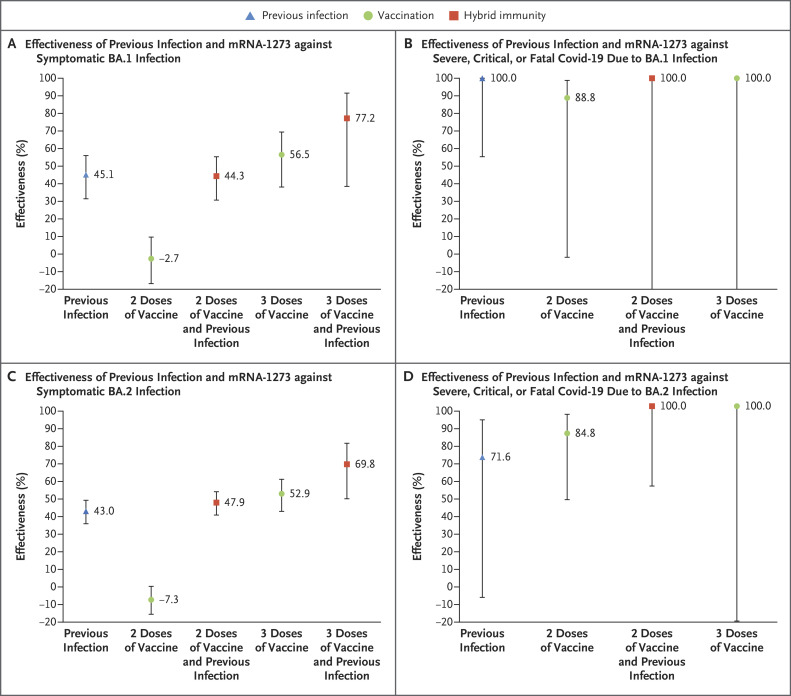
Effectiveness of Previous Infection and mRNA-1273 Vaccination against BA.1, BA.2, and Any Omicron Infection
The effectiveness of previous infection, vaccination, and hybrid immunity in the analysis of mRNA-1273 showed similar patterns to those of the analysis of BNT162b2 (Figure 2 and Figure 4). Additional information is provided in Table S5.
Figure 4. Effectiveness of Previous Infection, Vaccination with mRNA-1273, and Hybrid Immunity against Symptomatic Omicron BA.1 and BA.2 Infection and against Severe, Critical, or Fatal Covid-19.
𝙸 bars indicate 95% confidence intervals.
Discussion
Previous infection with a variant other than omicron was associated with an approximately 50% reduced risk of infection. No difference in the protection of previous infection against BA.1 and BA.2 was discernable. Two-dose vaccination and no previous infection had negligible effectiveness against BA.1 and BA.2, but most persons received their second dose more than 8 months earlier. These findings are explained by the short-lived protection of primary-series vaccination against omicron infections3,27 and the more durable protection from natural infection,2,28 as confirmed by the additional analysis of protection as a function of time after previous infection or vaccination (Figure 3).
Booster vaccination was associated with an approximately 60% reduced risk of infection. No difference in the protection of booster vaccination against BA.1 and BA.2 was discernable. However, most persons received their third dose less than 45 days earlier, perhaps explaining the relatively high effectiveness.3
The protection conferred by hybrid immunity of previous infection and two-dose vaccination was similar to that of previous infection alone, at approximately 50%, which suggests that this protection originated from the previous infection and not from vaccination. This finding is also explained by the short-lived protection of primary-series vaccination against omicron infections.3,27
However, the highest effectiveness was seen with hybrid immunity from previous infection and recent booster vaccination (approximately 80%). This finding provides evidence for the benefit of vaccination, even for persons with a previous infection. Strikingly, this protection is what one would expect if previous infection and booster vaccination each acted independently. Because previous infection reduced the risk of infection by 50% and booster vaccination reduced it by 60%, the reduction in the risk of infection for both combined, if they acted fully independently, would be 1−(1−0.5)×(1−0.6)=0.8, which is an 80% reduction, just as observed. Although this effect needs to be further investigated, this finding may suggest that the combined effect of these two forms of immunity against omicron infection reflects neither synergy nor redundancy of the individual biologic effects of each.
Even though the five forms of immunity investigated showed large differences in protection against symptomatic infection that ranged from 0 to 80%, they all showed strong protection against Covid-19–related hospitalization and death, at an effectiveness of more than 70%. This suggests that any form of previous immunity, whether induced by previous infection or vaccination, is associated with strong and durable protection against Covid-19–related hospitalization and death. Notably, there was no evidence for a difference in severity between BA.1 and BA.2 infections in the study samples.
No notable differences were observed between the effects of BNT162b2 and mRNA-1273 vaccination. The results confirmed other findings that we reported recently, including a protection of approximately 50% for previous infection against reinfection with BA.1,2 a protection of approximately 50% for mRNA boosters as compared with primary series,4 and the finding that mRNA vaccines have negligible effectiveness against omicron infection 6 or more months after the second dose.3
This study has limitations. Ascertainment of BA.1 and BA.2 infections was based on proxy criteria, but this method of ascertainment is well established.24,29,30 Some omicron infections may have been misclassified delta infections, but the incidence of delta was limited during the study period (Section S2). Ascertainment of BA.1 and BA.2 infections was not possible for a minority of infections. However, this may not have biased our results, since both infections with and without BA.1 or BA.2 ascertainment had a similar distribution among exposure categories (Table S6).
Although matching was performed according to sex, age, and nationality, matching was not possible for other factors, such as coexisting conditions. However, matching according to these factors provided demonstrable control of bias in our earlier studies.11,12,15,21,22 The analysis of effectiveness according to time since the most recent immunologic event is possibly at higher risk than the primary analysis for bias because of confounding, since persons who were vaccinated earliest were more likely to have coexisting conditions or to work in high-risk occupations. Effectiveness was assessed with the use of an observational, test-negative, case–control design rather than a design in which cohorts of individual persons were followed up. However, the cohort study design applied earlier to the same population yielded findings similar to those of the test-negative design.14,15,31 Moreover, our recent study of the effectiveness of boosters relative to primary series used a cohort study design and generated results consistent with the results reported here.4
Nonetheless, one cannot rule out the possibility that in real-world data, bias could arise in unexpected ways or from unknown sources, such as subtle differences or changes in test-seeking behavior. For example, with the large omicron wave, use of rapid antigen testing was expanded to supplement PCR testing in Qatar starting on January 5, 2022, and especially so for some of the routine testing such as post-travel testing. However, rapid antigen testing was broadly implemented and probably did not differentially affect PCR testing to introduce bias, as supported by the sensitivity analysis (Table S3) and the minimal differences between PCR and rapid antigen tests according to exposure category (Table S7). With the small proportion of the population of Qatar being 50 years of age or older,10 our findings may not be generalizable to other countries in which elderly citizens constitute a larger proportion of the population.
Notwithstanding these limitations, findings were consistent with those of other studies of vaccine effectiveness against omicron infection (BA.1 or BA.2 subvariants were not specified).27,32-36 Moreover, with the mass scale of PCR testing in Qatar,12 the likelihood of bias is perhaps minimized. Extensive sensitivity and additional analyses were conducted to investigate effects of potential bias in our earlier studies that used similar methods. These included different adjustments and controls in the analysis and different study inclusion and exclusion criteria to investigate whether effectiveness estimates could have been biased.12,22 These analyses showed consistent findings.2,3,12,17,22
No notable differences were observed in the effectiveness against BA.1 and BA.2 of previous infection, vaccination, and hybrid immunity. Protection from previous infection with variants other than omicron against reinfection was moderate and durable, but protection of primary-series vaccination against infection was negligible by 6 months after the second dose. Recent booster vaccination had moderate effectiveness, whereas hybrid immunity from previous infection and recent booster vaccination conferred the strongest protection against infection, at approximately 80%. All five forms of immunity were associated with strong and durable protection against Covid-19–related hospitalization and death.
Acknowledgments
We thank the many dedicated persons at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine–Qatar for their diligent efforts and contributions to make this study possible.
Supplementary Appendix
Disclosure Forms
This article was published on June 15, 2022, at NEJM.org.
Footnotes
Supported by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar; the Ministry of Public Health; Hamad Medical Corporation; and Sidra Medicine. The Qatar Genome Program and the Qatar University Biomedical Research Center provided the reagents needed for the viral genome sequencing.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Tracking SARS-CoV-2 variants. 2020. (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/).
- 2.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med 2022;386:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun 2022;13:3082-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med 2022;386:1804-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Karuppanan K, Subramaniam G. Omicron (BA.1) and sub-variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. February 11, 2022. (https://www.biorxiv.org/content/10.1101/2022.02.11.480029v1). preprint. [DOI] [PMC free article] [PubMed]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Living guidance for clinical management of COVID-19. November 23, 2021. (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2).
- 9.World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. April 20, 2020. (https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-%28coding%29-of-covid-19-as-cause-of-death).
- 10.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep 2021;11:6233-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med 2022;386:799-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385(24):e83-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemaitelly H, Bertollini R, Abu-Raddad LJ. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Engl J Med 2021;385:2585-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021;385:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27:1614-1621. [DOI] [PubMed] [Google Scholar]
- 16.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31:2165-2168. [DOI] [PubMed] [Google Scholar]
- 17.Ayoub HH, Tomy M, Chemaitelly H, et al. Estimating protection afforded by prior infection in preventing reinfection: applying the test-negative study design. January 3, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.02.22268622v1). preprint. [DOI] [PMC free article] [PubMed]
- 18.Verani JR, Baqui AH, Broome CV, et al. Case-control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine 2017;35:3295-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyle PV, Chemaitelly H, Ben Hadj Kacem MA, et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. iScience 2021;24:102646-102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Thani MH, Farag E, Bertollini R, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis 2021;8(8):ofab221-ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Raddad LJ, Chemaitelly H, Yassine HM, et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med 2021;28(7):taab083-taab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med 2022;386:1091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res 2022;209:112911-112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 34. January 14, 2022. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050236/technical-briefing-34-14-january-2022.pdf). [Google Scholar]
- 25.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228-1234. [Google Scholar]
- 26.Jacoby P, Kelly H. Is it necessary to adjust for calendar time in a test negative design?: responding to: Jackson ML, Nelson JC. The test negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31(April (17)):2165-8. Vaccine 2014;32:2942-2942. [DOI] [PubMed] [Google Scholar]
- 27.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022;386:1532-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Protection of prior natural infection compared to mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar. March 18, 2022. (https://www.medrxiv.org/content/10.1101/2022.03.17.22272529v1). preprint. [DOI] [PMC free article] [PubMed]
- 29.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: a nationally representative cohort study. PLoS Med 2021;18(12):e1003879-e1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 2021;372:n579-n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butt AA, Chemaitelly H, Al Khal A, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest 2021;131(23):e153662-e153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against omicron or delta infection. January 1, 2022. (https://www.medrxiv.org/content/10.1101/2021.12.30.21268565v1). preprint. [DOI] [PMC free article] [PubMed]
- 33.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. December 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v1). preprint.
- 34.Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. February 26, 2022. (https://www.medrxiv.org/content/10.1101/2022.02.25.22271494v1). preprint. [DOI] [PMC free article] [PubMed]
- 35.Šmíd M, Berec L, Májek O, et al. Protection by vaccines and previous infection against the omicron variant of SARS-CoV-2. February 25, 2022. (https://www.medrxiv.org/content/10.1101/2022.02.24.22271396v1). preprint. [DOI] [PMC free article] [PubMed]
- 36.Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med 2022;28:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.