Abstract
Background
Limited evidence is available on the real-world effectiveness of the BNT162b2 vaccine against coronavirus disease 2019 (Covid-19) and specifically against infection with the omicron variant among children 5 to 11 years of age.
Methods
Using data from the largest health care organization in Israel, we identified a cohort of children 5 to 11 years of age who were vaccinated on or after November 23, 2021, and matched them with unvaccinated controls to estimate the vaccine effectiveness of BNT162b2 among newly vaccinated children during the omicron wave. Vaccine effectiveness against documented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and symptomatic Covid-19 was estimated after the first and second vaccine doses. The cumulative incidence of each outcome in the two study groups through January 7, 2022, was estimated with the use of the Kaplan–Meier estimator, and vaccine effectiveness was calculated as 1 minus the risk ratio. Vaccine effectiveness was also estimated in age subgroups.
Results
Among 136,127 eligible children who had been vaccinated during the study period, 94,728 were matched with unvaccinated controls. The estimated vaccine effectiveness against documented infection was 17% (95% confidence interval [CI], 7 to 25) at 14 to 27 days after the first dose and 51% (95% CI, 39 to 61) at 7 to 21 days after the second dose. The absolute risk difference between the study groups at days 7 to 21 after the second dose was 1905 events per 100,000 persons (95% CI, 1294 to 2440) for documented infection and 599 events per 100,000 persons (95% CI, 296 to 897) for symptomatic Covid-19. The estimated vaccine effectiveness against symptomatic Covid-19 was 18% (95% CI, −2 to 34) at 14 to 27 days after the first dose and 48% (95% CI, 29 to 63) at 7 to 21 days after the second dose. We observed a trend toward higher vaccine effectiveness in the youngest age group (5 or 6 years of age) than in the oldest age group (10 or 11 years of age).
Conclusions
Our findings suggest that as omicron was becoming the dominant variant, two doses of the BNT162b2 messenger RNA vaccine provided moderate protection against documented SARS-CoV-2 infection and symptomatic Covid-19 in children 5 to 11 years of age. (Funded by the European Union through the VERDI project and others.)
Throughout the coronavirus disease 2019 (Covid-19) pandemic, children 5 to 11 years of age have been susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but have generally presented with asymptomatic infection or milder symptoms than those observed in adults.1-3 Changes in dominant viral variants have influenced the burden and severity of disease among younger children. For example, the spread of the B.1.617.2 (delta) variant increased the incidence of pediatric Covid-19–related hospitalizations in the United States.4
After vaccine trials involving adults and adolescents demonstrated safety and efficacy, a randomized trial of a lower-dosage formulation of the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) in children 5 to 11 years of age was initiated (ClinicalTrials.gov number, NCT04816643). On the basis of findings from safety and immunogenicity assessments, a dose level of 10 μg was selected as the dose for the vaccine efficacy trial.5 The trial, conducted when the delta variant gradually became dominant, showed a vaccine efficacy of 90.7% (95% confidence interval [CI], 67.7 to 98.3) against symptomatic Covid-19 from 7 to 126 days after the second dose.5 In the fall of 2021, the Food and Drug Administration, the European Medicines Agency, and the Israeli Ministry of Health authorized the BNT162b2 vaccine for administration in children 5 to 11 years of age,6 and on November 23, 2021, Israeli health care organizations began administering the 10-μg vaccine dose to children in this age group.7 The spread of infection with the B.1.1.529 (omicron) variant coincided with the vaccination campaign for children 5 to 11 years of age.7 By the end of December 2021, the omicron variant was the dominant strain in Israel.8
Randomized trials of the BNT162b2 vaccine in children 5 to 11 years of age were conducted during a period when variants other than omicron were dominant, including the delta variant. Therefore, it is important to evaluate vaccine effectiveness against the currently dominant omicron variant. Initial assessments of the effectiveness of the BNT162b2 vaccine against omicron among adults, adolescents, and children have shown that the protection conferred by the vaccine was substantially lower against infection and moderately lower (with greater variability) against hospitalization than the protection the vaccine conferred against previous variants.9-17 In the current study, we aimed to estimate the short-term vaccine effectiveness of BNT162b2 against documented infection with SARS-CoV-2 and symptomatic Covid-19 among children 5 to 11 years of age during a period when omicron was the prevalent variant.
Methods
Data Source
Clalit Health Services (CHS) is the largest integrated health care service provider in Israel, with more than 4.7 million active members. CHS maintains a comprehensive health care data warehouse that combines hospital and community medical records, including laboratory and imaging data and data on medication use and health care utilization. Covid-19–related data are collected by the Israeli Ministry of Health for all members. During the study period, children were required to be tested when they had contact with an infected person or if they opted to take part in any activity that required a “green pass” — a certificate issued by the Israeli Ministry of Health that allowed a person who had been vaccinated against Covid-19, had recently been infected and recovered from Covid-19, or had tested negative for Covid-19 on a recent (within the previous 24 hours) antigen test or a recent (within the previous 72 hours) polymerase-chain-reaction (PCR) test to partake in activities such as cultural events or travel abroad.
Study Design and Population
We conducted an observational cohort study emulating a target trial. We enrolled children 5 to 11 years of age who were vaccinated on or after November 23, 2021, when vaccination became available for this age group in Israel. We matched each vaccinated child with an unvaccinated control on the date of vaccination (the recruitment date). The study period ended on January 7, 2022, when a new testing policy was implemented in Israel (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). This new policy required unvaccinated persons to be tested in an official setting supervised by the Ministry of Health, whereas vaccinated persons were permitted to conduct rapid antigen tests at home. We examined testing rates of both vaccinated and unvaccinated populations before and after the change in testing policy on January 7, 2022, to gauge the effect of this change.
We evaluated vaccine effectiveness against documented SARS-CoV-2 infection, as confirmed by a positive PCR test, and against symptomatic Covid-19, which was defined as a PCR-confirmed infection with a report of any Covid-19 symptom in the patient’s electronic health record. Details of outcome definitions are provided in Table S1. We estimated vaccine effectiveness for documented infection and symptomatic Covid-19 over two separate periods — from 14 to 27 days after the first dose and from 7 to 21 days after the second dose. To assess the changing dominance of omicron variant infections in Israel over the study period, we estimated the daily proportion of omicron cases on the basis of a sample of positive PCR test results that had been collected and sequenced by the Israeli Ministry of Health.
Children were eligible for the study if they were 5 to 11 years of age at the time of recruitment; had at least 12 months of continuous membership in CHS before recruitment; had no previous PCR, serology, or antigen test that was positive for SARS-CoV-2; had a valid residence location and assignment to a population sector; were not homebound because of medical reasons; and had no interaction with the health care system (physician appointment, hospitalization, or laboratory testing) in the 3 days preceding the recruitment date, since such interaction would potentially be an indication of a developing case of symptomatic Covid-19. On each day of recruitment, all newly vaccinated children who met the inclusion criteria were matched one to one with eligible unvaccinated children on the basis of age, sex, population sector, residential area, number of influenza vaccines received in the past 5 years, overweight status, and number of diagnosis codes in the patient’s medical record that were considered by the physician to represent chronic conditions. Thousands of diagnosis codes are included in the variable that captured background conditions; therefore, when describing the study population, we provided estimates of grouped diagnoses and key single conditions that were defined by the Centers for Disease Control and Prevention as risk factors for severe Covid-19. Definitions of population characteristic variables are provided in Table S2. This study was approved by the institutional review board at CHS, and an exemption from the requirement for informed consent from the participants was granted.
All the authors conceived of and designed the study. A subgroup of authors collected and analyzed the data and wrote the manuscript. All the authors critically reviewed the manuscript. The first and last authors supervised the study and vouch for the accuracy and completeness of the data and that the conduct of the analyses followed the study design. No one who is not an author participated in the writing of the manuscript. The funding institutions did not dictate the design of the study, have access to the data, or influence the decision to submit the manuscript for publication.
Statistical Analysis
In the estimation of the per-protocol effect of vaccination, data from both members of a matched pair were censored if and when the control received a vaccination; such censoring was done in order to maintain the comparability of the two study groups with respect to the matching factors. The control for whom data were censored could be rerecruited as a vaccinated person if a new matched unvaccinated control was found. Follow-up also ended when a person had an outcome or died or the end of the study period was reached (in the latter two circumstances, the patient data were considered to have been censored).
The Kaplan–Meier estimator was used to estimate the cumulative incidence (risk) of each outcome in the vaccinated and unvaccinated groups. Risk ratios and risk differences were calculated by dividing and subtracting the period-specific risk estimates, respectively. Vaccine effectiveness was defined as 1 minus the risk ratio, and 95% confidence intervals were estimated with the use of the nonparametric percentile bootstrap method with 500 repetitions. Estimates in each period included only matched pairs in which both members of the pair were still undergoing follow-up at the beginning of the period relevant for that analysis. We also conducted subgroup analyses according to age (5 or 6 years, 7 to 9 years, and 10 or 11 years), examining the cumulative incidence of and comparing the vaccine effectiveness against documented and symptomatic infections 7 to 21 days after the second dose. All analyses were performed with the use of R software, version 4.1.0.18
Results
Study Population
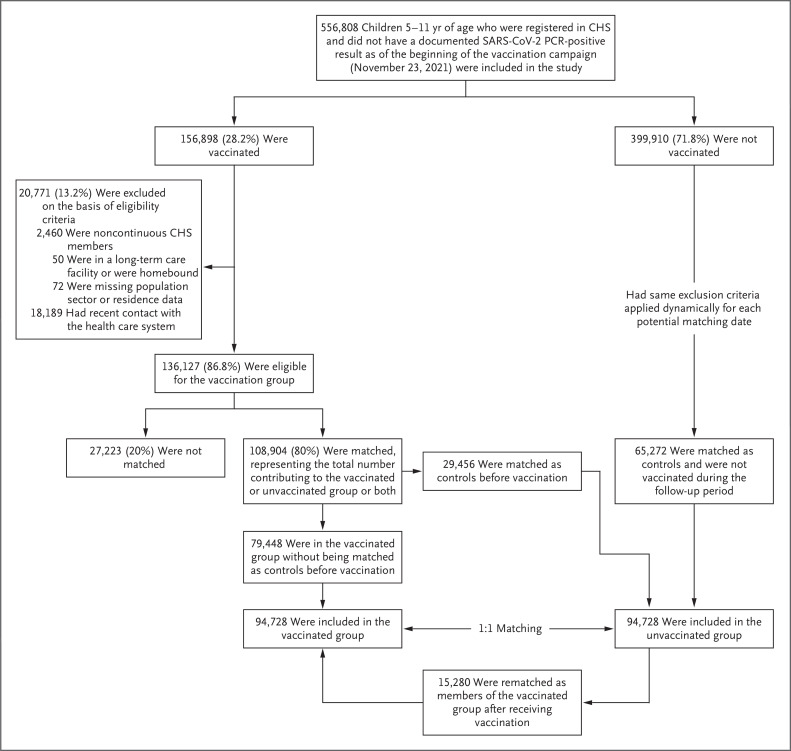
A total of 156,898 children covered by CHS were vaccinated during the study period; of these children, 136,127 were eligible for the study. After matching, the final study population comprised 94,728 vaccinated children and 94,728 unvaccinated matched controls (Figure 1). The median age of the children in the study population was 8 years (interquartile range, 7 to 10), and in each study group, 49% of the children were female and 17% were overweight or obese (Table 1). The study groups were identical in terms of the distribution of matching criteria and similar with respect to the various risk factors for severe Covid-19. Before January 7, 2022, Covid-19 testing rates were similar in the vaccinated and unvaccinated groups; after January 7, differences in testing rates were observed between the two groups (Fig. S2).
Figure 1. Enrollment and Matching.
Of 136,127 eligible children who had been vaccinated, 108,904 (80%) were successfully matched and included in at least one of the study groups: 94,728 were included in the vaccinated group, of whom 79,448 were originally included as members in the vaccinated group and 15,280 were initially matched as members of the unvaccinated group and then were rematched as members of the vaccinated group after receiving their first dose. A total of 29,456 children were originally included as members of the unvaccinated group but were vaccinated during the study follow-up period. Of these children, 15,280 were successfully rematched as members of the vaccinated group (for whom a new matched unvaccinated control was found); 14,176 were not. CHS denotes Clalit Health Services, PCR polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Table 1. Demographic and Clinical Characteristics of Vaccinated Children and Unvaccinated Controls at Baseline.*.
| Variable | Total Population Eligible for the Vaccinated Group (N=136,127) |
Vaccinated Group (N=94,728) |
Unvaccinated Group (N=94,728) |
|---|---|---|---|
| Median age (IQR) — yr | 8 (7–10) | 8 (7–10) | 8 (7–10) |
| Age distribution — no. (%) | |||
| 5 yr | 15,052 (11) | 10,576 (11) | 10,576 (11) |
| 6 yr | 18,412 (14) | 12,910 (14) | 12,910 (14) |
| 7 yr | 19,390 (14) | 13,577 (14) | 13,577 (14) |
| 8 yr | 19,770 (15) | 13,786 (15) | 13,786 (15) |
| 9 yr | 20,332 (15) | 14,088 (15) | 14,088 (15) |
| 10 yr | 20,924 (15) | 14,455 (15) | 14,455 (15) |
| 11 yr | 22,247 (16) | 15,336 (16) | 15,336 (16) |
| Female sex — no. (%) | 66,164 (49) | 46,083 (49) | 46,083 (49) |
| Population sector — no. (%) | |||
| General Jewish | 113,970 (84) | 79,560 (84) | 79,560 (84) |
| Arab | 16,098 (12) | 11,180 (12) | 11,180 (12) |
| Ultra-Orthodox Jewish | 6,059 (4.5) | 3,988 (4.2) | 3,988 (4.2) |
| Influenza vaccinations in the previous 5 yr — no. (%) | |||
| 0–2 | 77,556 (57) | 56,056 (59) | 56,056 (59) |
| ≥3 | 58,571 (43) | 38,672 (41) | 38,672 (41) |
| Overweight or obese status — no. (%)† | 26,350 (19) | 15,802 (17) | 15,802 (17) |
| No. of chronic diagnostic codes — no. (%) | |||
| 0 | 87,466 (64) | 63,641 (67) | 63,641 (67) |
| 1 | 27,756 (20) | 17,845 (19) | 17,845 (19) |
| ≥2 | 20,905 (15) | 13,242 (14) | 13,242 (14) |
| CDC risk factors for severe Covid-19 — no. (%) | |||
| Cancer | 80 (<0.1) | 48 (<0.1) | 62 (<0.1) |
| Chronic kidney disease | 2,566 (1.9) | 1,671 (1.8) | 1,714 (1.8) |
| Heart disease | 136 (<0.1) | 76 (<0.1) | 101 (0.1%) |
| Cerebrovascular disease | 80 (<0.1) | 53 (<0.1) | 91 (<0.1) |
| Neurologic disease | 1,281 (0.9) | 797 (0.8) | 954 (1.0) |
| Liver disease | 82 (<0.1) | 53 (<0.1) | 57 (<0.1) |
| Asthma | 5,429 (4.0) | 3,444 (3.6) | 3,646 (3.8) |
| Diabetes mellitus | |||
| Type 1 | 121 (<0.1) | 77 (<0.1) | 86 (<0.1) |
| Type 2 | 25 (<0.1) | 19 (<0.1) | 19 (<0.1) |
| Immunosuppression | 1,533 (1.1) | 986 (1.0) | 1,042 (1.1) |
| Hypertension | 48 (<0.1) | 30 (<0.1) | 35 (<0.1) |
| Solid-organ transplantation | 13 (<0.1) | 10 (<0.1) | 10 (<0.1) |
The 15,280 children who were first recruited as unvaccinated controls and then subsequently rerecruited as vaccinated children are counted in both groups. CDC denotes the Centers for Disease Control and Prevention, Covid-19 coronavirus disease 2019, and IQR interquartile range.
Overweight or obese status was defined as a body-mass index at the 85th percentile or higher (according to CDC growth charts).
Estimated Vaccine Effectiveness
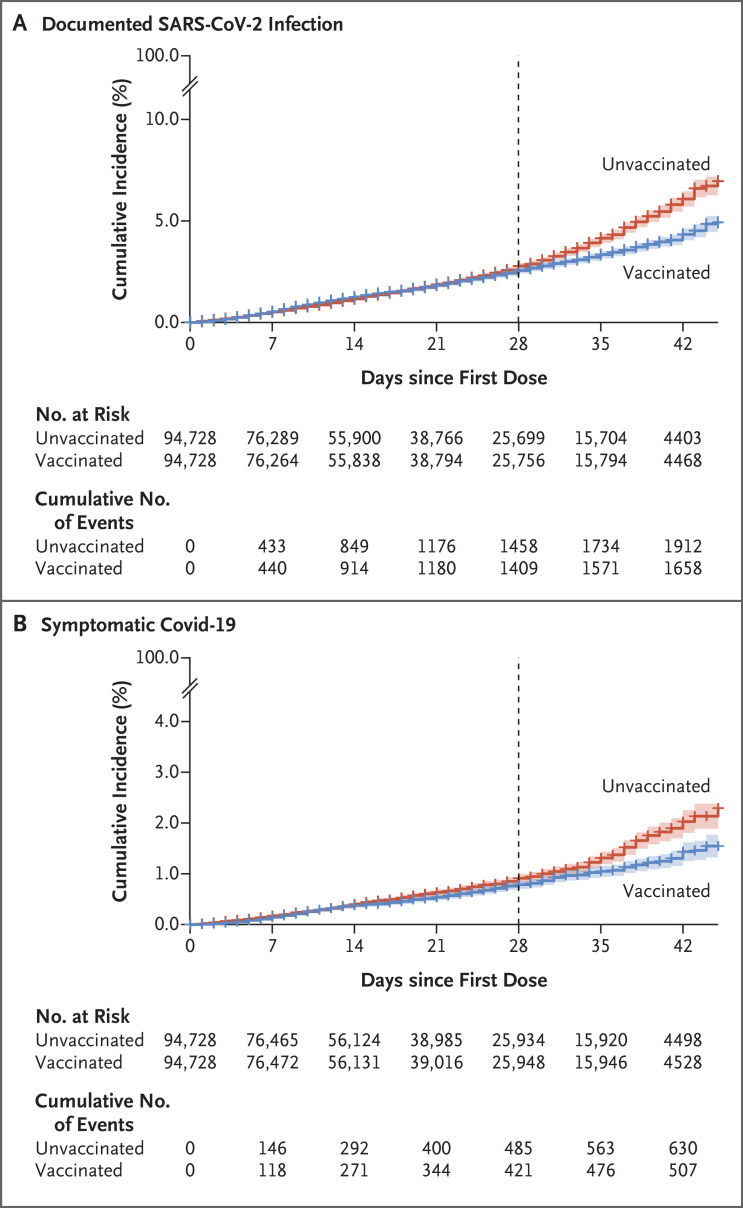
The median duration of follow-up after the first dose was 17 days (interquartile range, 8 to 29). The incidence of documented SARS-CoV-2 infection and symptomatic Covid-19 was similar in the vaccinated and unvaccinated groups at the beginning of the follow-up period and began to diverge noticeably for both outcomes after day 28 (Figure 2). Vaccine effectiveness against documented infection was 17% (95% CI, 7 to 25) at 14 to 27 days after the first dose and 51% (95% CI, 39 to 61) at 7 to 21 days after the second dose (Table 2). Vaccine effectiveness against symptomatic Covid-19 was 18% (95% CI, −2 to 34) at 14 to 27 days after the first dose and 48% (95% CI, 29 to 63) at 7 to 21 days after the second dose. The between-group risk differences after the second vaccine dose were estimated to be 1905 events per 100,000 persons (95% CI, 1294 to 2440) for documented infections and 1599 events per 100,000 persons (95% CI, 296 to 897) for symptomatic Covid-19.
Figure 2. Cumulative Incidence of Documented SARS-CoV-2 Infection and Symptomatic Covid-19.
Panel A shows the cumulative incidence of documented SARS-CoV-2 infection, as confirmed by a positive PCR test. Panel B shows the cumulative incidence of symptomatic Covid-19. The dashed vertical line at day 28 represents 7 days after the second vaccine dose was scheduled to be administered and marks the time at which the main follow-up period starts (in the vaccine effectiveness analysis, the follow-up period for children who did not receive the second dose at the designated time was shifted according to the time they received the second dose). Shaded areas indicate the 95% confidence intervals, and plus signs censored data.
Table 2. Vaccine Effectiveness against Documented SARS-CoV-2 Infection and Symptomatic Covid-19.*.
| Outcome | Total Population in Each Study Group† |
Events in the Unvaccinated Group |
Events in the Vaccinated Group |
Risk in the Unvaccinated Group‡ |
Risk in the Vaccinated Group‡ |
Vaccine Effectiveness (95% CI) |
Risk Difference (95% CI) |
|---|---|---|---|---|---|---|---|
| number | events/100,000 | percent | events/100,000 | ||||
| Documented SARS-CoV-2 infection§ | |||||||
| At 14 to 27 days after the first dose | 55,510 | 608 | 507 | 1528 | 1272 | 17 (7 to 25) | 255 (102 to 408) |
| At 7 to 21 days after the second dose | 22,109 | 423 | 201 | 3754 | 1849 | 51 (39 to 61) | 1905 (1294 to 2440) |
| Symptomatic Covid-19 | |||||||
| At 14 to 27 days after the first dose | 56,015 | 198 | 154 | 488 | 401 | 18 (−2 to 34) | 87 (−9 to 187) |
| At 7 to 21 days after the second dose | 22,386 | 133 | 68 | 1244 | 645 | 48 (29 to 63) | 599 (296 to 897) |
SARS-CoV-2 denotes severe acute respiratory syndrome coronavirus 2.
The total population in each study group represents the total number of children in each study group at the first day of the relevant follow-up period.
Risk was estimated with the use of the Kaplan–Meier estimator.
Documented SARS-CoV-2 infection was confirmed on polymerase-chain-reaction testing.
The earliest possible date of an outcome event after the second dose for any of the children in the study would have been December 21, 2021. The omicron variant was responsible for an estimated two thirds of new infections on that date and rapidly became more dominant over the subsequent days (Table S3). By December 31, 2021, more than 85% of the cases of SARS-CoV-2 infection that were sequenced were identified as omicron infections.
Among children 5 or 6 years of age, vaccine effectiveness against documented infection was 68% (95% CI, 43 to 84) at 7 to 21 days after the second dose. Among children 7 to 9 years of age, effectiveness was 56% (95% CI, 41 to 68), and among children 10 or 11 years of age, effectiveness was 38% (95% CI, 18 to 53) (Table 3). A similar trend was observed against symptomatic Covid-19, with an estimated vaccine effectiveness of 69% (95% CI, 30 to 91) among children 5 or 6 years of age, 49% (95% CI, 6 to 76) among children 7 to 9 years of age, and 36% (95% CI, 0 to 61) among children 10 or 11 years of age.
Table 3. Vaccine Effectiveness against Documented SARS-CoV-2 Infection and Symptomatic Covid-19 at 7 to 21 Days after the Second Dose, Stratified According to Age Subgroup.
| Outcome | Total Population in Each Study Group* |
Events in the Unvaccinated Group |
Events in the Vaccinated Group |
Risk in the Unvaccinated Group† |
Risk in the Vaccinated Group† |
Vaccine Effectiveness (95% CI) |
Risk Difference (95% CI) |
|---|---|---|---|---|---|---|---|
| number | events/100,000 | percent | events/100,000 | ||||
| Documented SARS-CoV-2 infection‡ | |||||||
| Age 5 or 6 yr | 5418 | 71 | 23 | 2867 | 922 | 68 (43 to 84) | 1944 (977 to 2915) |
| Age 7 to 9 yr | 9324 | 177 | 75 | 3575 | 1559 | 56 (41 to 68) | 2016 (1279 to 2764) |
| Age 10 or 11 yr | 7367 | 175 | 103 | 4586 | 2850 | 38 (18 to 53) | 1736 (703 to 2753) |
| Symptomatic Covid-19 | |||||||
| Age 5 or 6 yr | 5468 | 26 | 10 | 1190 | 367 | 69 (30 to 91) | 822 (224 to 1444) |
| Age 7 to 9 yr | 9445 | 45 | 20 | 971 | 491 | 49 (6 to 76) | 480 (39 to 919) |
| Age 10 or 11 yr | 7473 | 62 | 38 | 1614 | 1029 | 36 (0 to 61) | 585 (−3 to 1195) |
The total population in each study group represents the total number of children in each study group at the first day of the relevant follow-up period.
Risk was estimated with the use of the Kaplan–Meier estimator.
Documented SARS-CoV-2 infection was confirmed on polymerase-chain-reaction testing.
Discussion
This observational study of effectiveness of the BNT162b2 vaccine among children 5 to 11 years of age showed a vaccine effectiveness of 51% against documented SARS-CoV-2 infection and 48% against symptomatic Covid-19 at 7 to 21 days after the second dose. Our results also suggested that effectiveness may be greater among younger children (5 or 6 years of age) than older children (10 or 11 years of age).
In our study, the estimated vaccine effectiveness of 48% against symptomatic Covid-19 when the omicron variant was dominant was lower than the estimated vaccine efficacy of 90.7% (95% CI, 67.7 to 98.3) in the randomized trial by Walter et al.5 when the delta variant gradually became dominant. Previous studies also showed relatively low vaccine effectiveness against omicron infection, ranging from 30 to 68% among adults, adolescents, and children.9,10,12-16 Yet, estimates of effectiveness among adults and adolescents may not apply to young children, because both vaccine dosage and immunity in children differ from those in adolescents and adults.3 Two studies have estimated vaccine effectiveness against omicron infections in children. In the PROTECT (Pediatric Research Observing Trends and Exposures in COVID-19 Timelines) prospective cohort study that involved a limited sample of 1052 children 5 to 11 years of age who were tested weekly for SARS-CoV-2, the estimated effectiveness against omicron infection was 31% (95% CI, 9 to 48).16 In preliminary findings of a retrospective study involving children 5 to 11 years of age, effectiveness against infection was estimated to be 68% (95% CI, 63 to 72) in December 2021 but declined rapidly to 12% (95% CI, 6 to 16) in January 2022.14 However, this study did not adjust for many of the confounding variables that could affect these estimates.
Although previous studies involving adults and adolescents have shown a lower risk of documented infection or symptomatic Covid-19 among vaccinated persons than among unvaccinated persons starting at approximately 12 to 14 days after the first dose of an mRNA vaccine,19,20 we observed minimal differences in risk between vaccinated and unvaccinated children after the first dose, with a majority of the difference observed after the second vaccine dose. The randomized trial by Walter et al.5 did not provide additional evidence of the early efficacy after the first dose, because the trial results documented only one event in both study groups before day 28. The difference between the studies involving adults and those involving children with respect to the time point at which the incidence curves of the vaccinated and unvaccinated groups diverge might be related to the lower dose of the pediatric formulation.
We observed a trend toward higher effectiveness against documented infection and symptomatic Covid-19 in the youngest age group (5 or 6 years of age) than in the oldest age group (10 or 11 years of age), which is consistent with recent findings showing that the protection provided by the pediatric formulation may be lower in the older children (within the range of 5 to 11 years of age) than in the adolescents who received a higher dosage.14,16 One of the two studies compared vaccine effectiveness among 11-year-old children with that among adolescents 12 or 13 years of age who received different dose formulations, given the age threshold for vaccine administration.14 The other study estimated a vaccine effectiveness against omicron infections of 31% (95% CI, 9 to 48) among children 5 to 11 years of age and of 59% (95% CI, 22 to 79) among adolescents 12 to 15 years of age.16
The current study has several limitations. First, the study period and individual follow-up times are limited owing to testing policy changes that took effect in Israel on January 7, 2022. Therefore, this study addresses only the question of short-term effectiveness soon after vaccination. Furthermore, many of the children in our study cohort did not receive a second dose within the study follow-up period. Examination of vaccine effectiveness against more severe outcomes such as hospitalization were not possible, because they were very rare in the study population (two hospitalizations in the unvaccinated group and one hospitalization in the vaccinated group). As in any observational study, the possibility of residual confounding was present (e.g., confounding by behavioral factors such as mask-wearing for which no data are available). However, the similarity in the incidence of outcome events between the study groups at the beginning of the follow-up period suggests that such residual confounding is probably minimal. Finally, because we had only a subsample of sequenced PCR results, we were only able to approximate the daily proportion of omicron cases among those infected but could not fully discern the differences in vaccine effectiveness attributable to variant type in our study cohort.
Overall, vaccination with BNT162b2 showed moderate protection against documented SARS-CoV-2 infection and symptomatic Covid-19 among children 5 to 11 years of age. However, protection was not uniform across all ages in this cohort; a trend toward greater protection was observed in the younger age subgroups. Future studies are needed to evaluate longer-term vaccine effectiveness among children and to further investigate the potential dose effect in children of various ages and in other key subpopulations.
Supplementary Appendix
Disclosure Forms
The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
This article was published on June 29, 2022, at NEJM.org.
Footnotes
Supported by the European Union through the VERDI project (grant number, 101045989), the U.K. Medical Research Council (grant number, MC_UU_00004/03), and the Morris-Singer Fund. The funding institutions did not dictate the design of the study, have access to the data, or influence the decision to submit the manuscript for publication.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Considering the impact of COVID-19 on children (https://www.euro.who.int/en/health-topics/Life-stages/child-and-adolescent-health/covid-19-and-children).
- 2.World Health Organization. COVID-19 disease in children and adolescents: scientific brief. September 29, 2021. (https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1).
- 3.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 2020. December 1 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 4.Siegel DA, Reses HE, Cool AJ, et al. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0-17 years — United States, August 2020–August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1249-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022;386:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodworth KR, Moulia D, Collins JP, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5-11 years — United States, November 2021. MMWR Morb Mortal Wkly Rep 2021;70:1579-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israeli Ministry of Health. Vaccines: COVID-19 vaccine for children. 2021. (https://corona.health.gov.il/en/vaccine-for-covid/under-12/).
- 8.Our World in Data. SARS-CoV-2 variants in analyzed sequences, Israel (https://ourworldindata.org/grapher/covid-variants-area?country=~ISR).
- 9.UK Health Security Agency. COVID-19 vaccine surveillance report week 5. February 3, 2022. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1052353/Vaccine_surveillance_report_-_week_5.pdf).
- 10.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. December 14, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.14.21267615v1). preprint.
- 11.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022;386:494-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med 2022;28:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. January 28, 2022. (https://www.medrxiv.org/content/10.1101/2021.12.30.21268565v2). preprint. [DOI] [PMC free article] [PubMed]
- 14.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5-11 and 12-17 years in New York after the emergence of the Omicron variant. February 28, 2022. (https://www.medrxiv.org/content/10.1101/2022.02.25.22271454v1). preprint. [DOI] [PMC free article] [PubMed]
- 15.Veneti L, Berild JD, Watle SV, et al. Vaccine effectiveness with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine against reported SARS-CoV-2 Delta and Omicron infection among adolescents, Norway, August 2021 to January 2022. March 25, 2022. (https://www.medrxiv.org/content/10.1101/2022.03.24.22272854v1). preprint. [DOI] [PMC free article] [PubMed]
- 16.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 years — PROTECT Cohort, July 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med 2022;386:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Foundation. The R project for statistical computing (https://www.R-project.org).
- 19.Reis BY, Barda N, Leshchinsky M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med 2021;385:2101-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.