Abstract
Primary immunodeficiencies (PID) are heterogeneous inborn errors of the immune system. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is curative and safe at the pediatric age but remains underperformed in adults. We report our experience on 32 consecutive adult patients with various PID including 17 (53%) with a combined immune deficiency, six (19%) with a disease of immune dysregulation and nine (28%) with a chronic granulomatous disease (CGD) who underwent an allo-HSCT between 2011 and 2020. The median age at transplant was 27 years (17–41). All assessable patients engrafted. The majority of patients received a fludarabine-Busulfan (FB) based regimen (FB2-3 in 16, FB4 in 12). Overall survival (OS) was 80.4% (100% for CGD and 74% for other PID patients) at 9 months and beyond (median follow-up 51.6 months). Six patients died, all in the first-year post-transplant. Cumulative incidences of grade II–IV acute GVHD/chronic GVHD were 18%/22%. Stem cell source, GVHD prophylaxis and conditioning intensity had no impact on OS. All surviving patients had over 90% donor chimerism, immune reconstitution, no sign of active PID related complications and were clinically improved. Allo-HSCT is effective in young adults PID patients with an acceptable toxicity and should be discussed in case of life-threatening PID.
Subject terms: Stem-cell therapies, Haematological diseases
Introduction
Primary immunodeficiencies (PID) are heterogeneous inborn disorders affecting the immune system [1, 2]. PID patients experience a wide variety of clinical manifestations ranging from infections to autoimmune, inflammatory and/or malignant complications. Initial symptoms and clinical diagnosis often occur during childhood but may be delayed until adulthood [3].
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is curative for pediatric PID patients with a good safety record. Severe combined immune deficiency (SCID) is the most severe form of PID and is usually lethal within the first year of life [4]. Outcome after allo-HSCT of SCID patients is dramatically improved with upfront early transplantation [5, 6]. Moreover, patients with combined immune deficiency (CID), primary immune regulation disorders (PIRD) and numerous other PID types will also require allo-HSCT because of the severity of the clinical phenotype usually during childhood [7–12]. Allo-HSCT may be indicated when prognosis is known to be pejorative based on the clinical and genetic diagnosis or when clinical manifestations lead to organ damage that will ultimately impact prognosis [13]. In case of unequivocal transplant indication, it should be performed sooner rather than later to prevent organ damage from repeated or severe infections or immune mediated complications thereby improving transplant outcomes [14].
However, a number of PID patients will require allo-HSCT during adulthood either because of delayed onset of symptoms or increasing severity of clinical manifestations later in life, leading to a delayed diagnosis. Alternatively, suitable donors may only become available at later times in life [15]. Likewise, the improvement of life expectancy due to efficient supportive care may allow a number of PID patients to reach adulthood and become eligible for the procedure. As such, it is likely that adult physicians will be increasingly exposed to PIDs patients who while require an allo-HSCT [16, 17].
Few studies have reported experiences of allo-HSCT in adult PID patients and included mostly CGD patients. A recent large study from the EBMT registry has reported the outcome of allograft for CGD and has included 77 adults [18]. Another recent study has also reported a favorable outcome of a cohort 29 patients including 11 CGD and 18 various other PID patients [19]. Two smaller studies have reported 18 Adolescent Young Adult (AYA) patients and 14 Adult patients with Common Variable Immune Deficiency (CVID) and various PID respectively [20, 21].
Allo-HSCT for adult PID patients is probably underperformed as transplant related morbidity and mortality may exceed the benefit of curing PID and may discourage physicians outside expert centers. The paucity of publications underlines the lack of experience and the need for new reports in order to establish guidelines in this setting notably regarding the trigger to consider allo-HSCT.
Patients and method
Study design
We performed a single center retrospective study on 32 consecutive patients with various PID who underwent an allo-HSCT between 2011 and 2020. This study was approved by the scientific board of the French reference centre for primary Immune Deficiency (CEREDIH). All patients provided signed informed consent.
Immunological reconstitution and oxidative burst in CGD patients
All patients were evaluated for immunological reconstitution by flow cytometry of lymphocyte subsets, serum immunoglobulin levels and antibody response to vaccines. Phagocyte oxidative burst (dihydrorhodamine, DHR) was analyzed in CGD patients. Details are presented in the supplementary methods section.
Chimerism follow-up
Chimerism was analyzed on whole blood by quantitative real-time polymerase chain reaction for insertion/deletion polymorphism as previously described [22].
Definition of the endpoints and statistical analysis
Data are presented as frequency (percent) or median (range). The endpoints studied were overall survival (OS), GVHD free, relapse free overall survival (GRFS), transplant related mortality (TRM), acute and chronic graft versus host disease (aGVHD and cGVHD respectively). Endpoints were analyzed at the reference date of January 1st, 2021. Follow-up durations were computed as the time interval between date of transplant to the date of event, date of last follow-up or reference date, whichever occurred first. Cumulative incidence of aGVHD and cGVHD were analyzed with death as competing risk. OS and GRFS were estimated using Kaplan–Meier product-limit estimator. All tests were two-sided and P values <0.05 were considered as indicating significant association.
Results
Patient characteristics/Indication for allo-HSCT
Tables 1, 2 Thirty patients out of 32 (93.9%) were males. The median age at time of transplant was 26.9 years (range, 17–41). Seventeen patients (53%) had CID, six (19%) had PIRD and nine (28%) had CGD. Twenty-six (81%) patients had a genetic diagnosis. Nine (28%) patients had a history of lymphoma prior to allo-HSCT with a median of 7.1 mo. (range 4.8–14.8) prior to allo-HSCT and had received immuno-chemotherapy. Nineteen (59%) had a history of autoimmune/ inflammatory complication including auto immune cytopenia (n = 10), inflammatory bowel disease (IBD, n = 5), vasculitis (n = 3) arthritis (n = 2) and myopericarditis (n = 1), of whom 13 were active at the time of transplantation. Seven (22%) patients were splenectomised prior to allo-HSCT with a median age at splenectomy of eight years (range 3–36). At time of allo-HSCT, 16 patients had an active infection including invasive fungal infections (IFI, n = 6), biliary tract cryptosporidial cholangitis (n = 3), bacterial infections (n = 6), and viral infections (VZV and HPV, n = 3). Nineteen (59%) were on IgRT prior to allo-HSCT. Sixteen (50%) patients had a high-risk Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) score (i.e., ≥3) [23].
Table 1.
Patient’s characteristics.
| N = 32 (%) | |
|---|---|
| Gender, no (%) | |
| Female | 2 (6.2%) |
| Male | 30 (93.8%) |
| PID subtype, no (%): | |
| CID | 17 (53.1%) |
| PIRD | 6 (18.8%) |
| CGD | 9 (28.1%) |
| Age (years) of first symptoms (median, range) | 1.70 [0.06;34.3] |
| Age (years) at clinical diagnosis (median, range) | 3.81 [0.27;34.8] |
| Time between first symptoms and diagnosis (median, range) | 0.41 [0.00;14.2] |
| Age at transplant (median, range) | 26.9 [17.2;41.4] |
| Delay between diagnosis and allo-HSCT (median, range) | 19.4 [2.03;38.3] |
| Active infection at time of allo-HSCT | 16 (50.0%) |
| History of AI | 20 (62.5%) |
| Active AI at time of allo-HSCT, no (%) | 13/20 (65.0%) |
| History of lymphoma, no (%) | 9 (28.1%) |
| Preallo-HSCT chemotherapy, no (%) | 9 (28.1%) |
| IgRT prior to allo-HSCT, no (%) | 9 (59.4%) |
| Splenectomy, no (%) | 7 (21.9%) |
| Age at splenectomy (median, range) | 8.00 [3.00;36.0] |
| HTC-CI score, no (%) | |
| High (>2) | 16 (50%) |
| Intermediate (1–2) | 13 (40.6%) |
| Low (0) | 3 (9.38%) |
| Main organ contributing to HCT-CI score (%) | |
| Pulmonary | 16 (50%) |
| Hepatitis | 4 (12.5%) |
| Active IBD | 4 (12.5%) |
AI autoimmunity/autoinflammation (AI), HCT-CI hematopoietic cell transplantation-comorbidity index.
Table 2.
Patient list and indication for allo-HSCT.
| Patient ID/gender | PID subtype | PID name | Gene | Age at first symptoms/ clinical diagnosis (years) | Main complications before allo-HSCT | Indication for allo-HSCT |
|---|---|---|---|---|---|---|
| UPN 1/M | CID | CID | RAG 1 | 10/10 | Recurrent bacterial infections (ULRTI/meningitis)/colitis/LPD. Splenectomy | Worsening pancytopenia |
| UPN 2/M | PIRD | ALPS | TNFRSF | 5/16 | Recurrent AI cytopenia. Splenomegaly. Splenectomy | Refractory pancytopenia |
| UPN 3/M | PIRD | IDR with colitis | IL10RB | 1/15 | Relapsing DLBCL. Colitis | DLBCL (CR4) |
| UPN 4/M | CID | XL-HIGM | CD40L | 1/2 | Recurrent bacterial infections (ULRTI). Toxoplasmosis. Cholangitis. | Cryptosporidium cholangitis |
| UPN 5/M | CID | WAS | WASP | 2/3 | Recurrent bacterial infections (ULRTI). Vasculitis. AI cytopenia. DLBCL. Splenectomy | DLBCL (CR1) |
| UPN 6/M | CID | WAS | WASP | 1/3 | Recurrent bacterial infections (ULRTI). Vasculitis. Nephropathy. Renal transplantation | Vasculitis |
| UPN 8/M | CID | MST1 deficiency | MST1 | 6/8 | Recurrent bacterial infections (ULRTI). Disseminated moluscum | Worsening moluscum |
| UPN 9/M | CID | XL-HIGM | CD40L | 0/0 | Recurrent bacterial infections (ULRTI). PJP. Cholangitis | Cryptosporidium cholangitis |
| UPN 11/M | CID | CID | CD3E | 0/1 | Recurrent bacterial infections (ULRTI). DLBCL | DLBCL (CR1) |
| UPN 12/M | CID | APDS-1 | PIK3CD | 1/4 | Recurrent bacterial infections (ULRTI). AI cytopenia. Disseminated VZV. Splenectomy | Disseminated VZV |
| UPN 13/M | PIRD | unk | unk | 0/9 | Recurrent bacterial infections (ULRTI). AI cytopenia | Refractory pancytopenia |
| UPN 14/M | CID | WAS | WASP | 1/2 | Recurrent bacterial infections (ULRTI). Vasculitis. AI cytopenia. Splenectomy | Vasculitis |
| UPN 15/F | CID | CID | PRKDC | 1/9 | Recurrent bacterial infections (ULRTI). IFI. Arthritis. DLBCL | DLBCL (CR1) |
| UPN 16/M | Phag | CGD | CYB | 1/1 | IFI | CNS IA |
| UPN 17/M | Phag | CGD | CYB | 11/11 | IFI. Colitis. | IA |
| UPN 18/M | CID | WAS | WASP | 0/0 | Recurrent bacterial infections (ULRTI). AI cytopenia. Arthritis Disseminated moluscum. DLBCL. Splenectomy | DLBCL (CR1) |
| UPN 19/M | CID | CID | unk | 0/1 | Recurrent bacterial infections (ULRTI). Recurrent EBV reactivation. HLH. Relapsing DLBCL | DLBCL (CR2) |
| UPN 20/F | CID | CID | unk | 34/35 | Recurrent bacterial infections (ULRTI). Recurrent EBV T cell LPD | EBV positive T cell LPD |
| UPN 21/M | PIRD | PIRD | unk | 18/18 | Recurrent bacterial and fungal infection. Recurrent AI neutropenia. Recurrent T cell LPD. | Refractory neutropenia |
| UPN 22/M | PIRD | XLP-1 | SH2D1A | 11/11 | Recurrent bacterial infections (ULRTI). Severe aplastic anemia. Liver nodular regenerative hyperplasia. Recurrent EBV LPD. BL. | Recurrent EBV LPD |
| UPN 23/M | CID | XL-HIGM | CD40L | 0/5 | Recurrent bacterial infections (ULRTI). Toxoplasmosis. Cholangitis | Cryptosporidium cholangitis |
| UPN 24/M | CID | ADA deficiency | ADA | 1/1 | Recurrent bacterial infections (ULRTI). Recurrent EBV HL. Liposarcoma | Recurrent EBV HL (CR2) |
| UPN 25/M | Phag | CGD | CYB | 3/3 | IFI | lung and cardiac IA |
| UPN 26/M | Phag | CGD | CYB | 2/2 | IFI | Lung IA |
| UPN 27/M | CID | CID | unk | 2/3 | Recurrent bacterial infections (ULRTI). AI cytopenia. Recurrent T cell LPD. Splenectomy | Refractory neutropenia |
| UPN 28/M | CID | CID | unk | 2/3 | Recurrent bacterial infections (ULRTI). Meningitis. Colitis | Refractory colitis |
| UPN 29/M | PIRD | XLP-1 | SH2D1A | 4/4 | Recurrent bacterial infections (ULRTI). HLH. AI neutropenia. | Refractory neutropenia |
| UPN 30/M | Phag | CGD | CYB | 5/5 | Recurrent bacterial and fungal infections. Actinomycosis. Colitis | Actynomycosis |
| UPN 31/M | Phag | CGD | CYB | 2/2 | Recurrent bacterial and fungal infections. Inflammatory myopericarditis | Inflammatory myopericarditis |
| UPN 32/M | Phag | CGD | CYB | 1/1 | Recurrent bacterial and fungal infections. HLH | IFI. HLH |
| UPN 33/M | Phag | CGD | CYB | 14/14 | Recurrent bacterial and fungal infections. Colitis. ILD | ILD |
| UPN 34/M | Phag | CGD | CYB | 6/7 | Recurrent bacterial and fungal infections. | Lung and thyroid IA |
APDS Activated PI3K delta syndrome, ALPS autoimmune lymphoproliferative syndrome, AI autoimmune, CID combined immunodeficiency, CGD Chronic granulomatous disease, CR complete remission, CMV cytomegalovirus, DLBCL diffuse large B-cell lymphoma, EBV Epstein Barr virus, HLH Hemophagocytic lymphohistiocytosis, IDR Immune deregulation, IFI invasive fungal infection, IA invasive aspergillosis, ILD Interstitial lung disease, LPD lymphoproliferative disease, PIRD primary immune regulation disorders, Phag phagocyte impairment, PJP Pneumocystis jirovecii Pneumonia, ULRTI upper and low respiratory tractus infection, Unk unknown, VZV varicella-zoster virus, WAS Wiskott–Aldrich syndrome, XL-HIGM X-linked hyper IgM syndrome, XLP-1 X-linked lymphoproliferative disease type 1.
Transplant modalities
Tables 3 and 4 According to the EBMT classification, 24 (78%) received a reduced toxicity conditioning (RTC) and seven (22%) a reduced intensity conditioning (RIC). The remaining patient received a full myeloablative conditioning (MAC) [24]. The majority of patients (n = 28, 87.5%) received a Fludarabine- Busulfan (FB) based platform including reduced intensity (FB2, Busulfan = 6.4 mg/kg i.v., n = 4), sub-myeloablative reduced-toxicity dose (FB3, Busulfan = 9.6 mg/kg i.v., n = 12) and myeloablative reduced-toxicity dose (FB4, Busulfan = 12.8 mg/kg i.v., n = 12) [25, 26]. Therapeutic dose monitoring (TDM) of busulfan was performed only for the first three patients with CGD (UPN 16, 17 and 25). Twenty-two (69%) patients received in vivo T depletion (alemtuzumab or thymoglobulin). Alemtuzumab was administrated according to T. Güngör publication [12]. GVHD prophylaxis with cyclosporine was used in almost all patients. The stem cell source was mobilized peripheral blood stem cells (PBSC) in 23 patients (72%) and bone marrow (BM) in nine (28%). Donors were HLA matched sibling donors (MSD) for nine (28%), matched unrelated donors (MUD) for 17 (53%), mismatched unrelated (one class I mismatch, MMUD) for four (12%) and haplo-identical for two (6%).
Table 3.
Transplant characteristics.
| N (%) | |
|---|---|
| Patients | N = 32 |
| Donor typea | |
| MSD | 9 (28.1) |
| MUD | 17 (53.1) |
| MMUD (one class I mismatch) | 4 (12.5) |
| HAPLO | 2 (6.3) |
| Donor gender: | |
| F | 11 (34.4) |
| M | 21 (65.6) |
| Donor CMV status | |
| Negative | 14 (43.8) |
| Positive | 18 (56.2) |
| Conditioning type | |
| BuCy | 1 (3.12) |
| FB4 | 12 (37.5) |
| FB3 | 12 (37.5) |
| FB2 | 4 (12.5) |
| RFC | 1 (3.12) |
| T1B2F | 1 (3.12) |
| Flu-Cy-TBI 2 Gy | 1 (3.12) |
| Flu-Bu subgroup (N = 28) | |
| FB2-3 | 16 (57.1%) |
| FB4 | 12 (42.9%) |
| Stem cells source | |
| BM | 9 (28.1%) |
| PBSC | 23 (71.9%) |
| T cell depletion/repletion | |
| None | 8 (25.0%) |
| Alemtuzumab | 11 (34.4%) |
| ATG | 11 (34.4%) |
| PTCy | 2 (6.25) |
| GVHD prophylaxisb | |
| CsA-MMF | 17 (53.1) |
| CsA-MTX | 13 (40.6) |
| TAC-MMF | 1 (3.12) |
| TAC-MTX | 1 (3.12) |
Alem alemtuzumab, ATG anti-thymo-globulin, B IV busulfan, BM bone marrow, C cyclophosphamide, CsA cyclosporine, F fludarabine, HAPLO sibling haplo-identical donor, MSD matched sibling donor, MUD matched related donor, MMUD mismatched unrelated donor, MMF mycophenolate mofetil, MTX methotrexate, PBSC peripheral blood stem cell, PTCy post-transplant cyclophosphamide, R Rituximab, TAC tacrolimus, T Thiotepa.
aMolecular high-resolution typing of HLA-A, -B, -C, -DQ, and -DRB1 alleles was performed for each patient and donor.
bIn the absence of GVHD, MMF was stopped at D + 100 and cyclosporine was tapered at six months and discontinued at 1-year post allo-HSCT.
Table 4.
Detailed transplantation procedures and causes of death.
| Patient ID | Age at alloSCT (years) | HCT-CI score | Active infection type at time of alloSCT | HLA match | Conditioning | Stem cell source | T cell depletion | GVHD prophylaxis | Cause of death (days post SCT) | Organ toxicity (days post SCT) |
|---|---|---|---|---|---|---|---|---|---|---|
| UPN 1 | 20 | 0 | MSD | FB4 | BM | CsA-MTX | ||||
| UPN 2 | 26 | 1 | MSD | BuCy | BM | Alem | CsA-MTX | MOF (24) | Hepatic (SOS) grade 5 (20) | |
| UPN 3 | 17 | 1 | MSD | RFC | PBSC | TAC-MTX | ||||
| UPN 4 | 20 | 2 | Cryptosporidiosis | MMUD (mM DQ) | FB4 | PBSC | ATG | CsA-MMF | ||
| UPN 5 | 23 | 1 | MUD | FB4 | PBSC | ATG | CsA-MTX | |||
| UPN 6 | 22 | 2 | MUD | FB4 | PBSC | ATG | CsA-MTX | |||
| UPN 8 | 21 | 1 | Papovavirus. Moluscum | MSD | FB4 | BM | CsA-MMF | |||
| UPN 9 | 20 | 4 | Cryptosporidiosis | MMUD (mM A) | FB4 | PBSC | ATG | CsA-MTX | Hepatic grade 3 (4) | |
| UPN 11 | 30 | 2 | MSD | FB4 | PBSC | CsA-MTX | Pulmonary grade 3 (15) | |||
| UPN 12 | 18 | 4 | VZV | MSD | FB4 | PBSC | CsA-MMF | Refractory aGVHD (79) | ||
| UPN 13 | 28 | 2 | Bacterial cellulitis | MUD | FB4 | PBSC | CsA-MTX | |||
| UPN 14 | 23 | 2 | MUD | FB4 | PBSC | CsA-MTX | ||||
| UPN 15 | 22 | 5 | Cerebral IFI | HAPLO | Baltimore | PBSC | PTCy | CsA-MMF | PML (36) | |
| UPN 16 | 19 | 4 | Lung IFI | MUD | FB3 | BM | Alem | CsA-MMF | ||
| UPN 17 | 28 | 5 | MUD | FB3 | BM | Alem | CsA-MMF | |||
| UPN 18 | 23 | 1 | Bacterial pneumonia. Molluscum | MMUD (mM C) | FB3 | PBSC | ATG | CsA-MTX | ||
| UPN 19 | 35 | 4 | IFI. Bacterial pneumonia. Papovavirus. Moluscum | MUD | FB3 | BM | CsA-MTX | |||
| UPN 20 | 37 | 0 | MSD | FB4 | PBSC | ATG | CsA-MTX | |||
| UPN 21 | 32 | 0 | MUD | FB2 | PBSC | ATG | CsA-MTX | |||
| UPN 22 | 33 | 5 | MUD | FB2 | PBSC | ATG | CsA-MMF | Refractory cGVHD (285) | Hepatic grade 3 (1) | |
| UPN 23 | 37 | 6 | Cryptosporidiosis | MUD | FB2 | PBSC | ATG | TAC-MMF | Hepatic grade 3 (7) | |
| UPN 24 | 28 | 4 | HAPLO | T1B2F | PBSC | PTCy | CsA-MMF | Acute cardiac dysfunction (24) | Cardiac grade 5 (8) | |
| UPN 25 | 21 | 3 | lung and cardiac IFI | MUD | FB3 | PBSC | Alem | CsA-MMF | ||
| UPN 26 | 32 | 3 | Lung IFI | MSD | FB3 | BM | ATG | CsA-MMF | ||
| UPN 27 | 41 | 3 | MSD | FB4 | PBSC | ATG | CsA-MTX | Refractory aGVHD (52) | ||
| UPN 28 | 28 | 5 | Bacterial pneumonia | MUD | FB2 | PBSC | Alem | CsA-MMF | ||
| UPN 29 | 31 | 4 | Bacterial pneumonia | MUD | FB3 | PBSC | Alem | CsA-MMF | ||
| UPN 30 | 18 | 1 | Skin infection. Actynomycosis | MUD | FB3 | BM | Alem | CsA-MMF | ||
| UPN 31 | 28 | 2 | MUD | FB3 | PBSC | Alem | CsA-MMF | |||
| UPN 32 | 39 | 6 | MUD | FB3 | PBSC | Alem | CsA-MMF | |||
| UPN 33 | 36 | 4 | MMUD (mM DQ) | FB3 | BM | Alem | CsA-MMF | |||
| UPN 34 | 24 | 1 | Lung and thyroid IFI | MUD | FB3 | PBSC | Alem | CsA-MMF |
Alem alemtuzumab, ATG anti-thymo-globulin, B IV busulfan, BM bone marrow, C cyclophosphamide, CsA cyclosporine, F fluarabine, IFI invasive fungal infection, HCT-CI hematopoietic cell transplantation-comorbidity index, HAPLO sibling haplo-identical donor, MSD matched sibling donor, MUD matched related donor, MMUD mismatched unrelated donor, MMF mycophenolate mofetil, MTX methotrexate, MOF multi-organ failure, NA not applicable, PML Progressive multifocal leukoencephalopathy, PBSC peripheral blood stem cell, R Rituximab, SOS sinusoidal obstruction syndrome.
Engraftment
Thirty-one evaluable patients engrafted (no graft failure and one early death). The median time to neutrophil (>0.5 G/l) and platelet recovery (>50 G/l) was 17 days and 16 days respectively. As expected, we observed a shorter time (although not statically significant) to neutrophil and platelet engraftments in PBSCs compared to BM recipients (median time to neutrophil engraftment 16 days versus 23 days in for PBSC and BM respectively, p = 0.12; median time to platelet engraftment 14 days versus 25 days for PBSC and BM respectively, p = 0.24).
Graft versus host disease
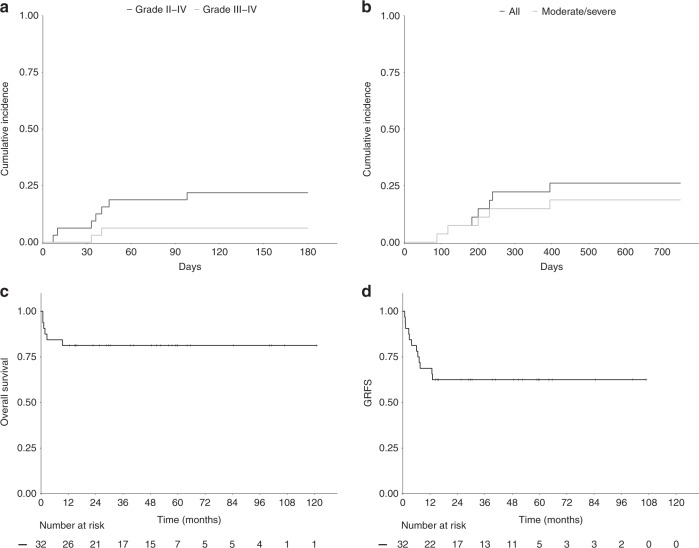
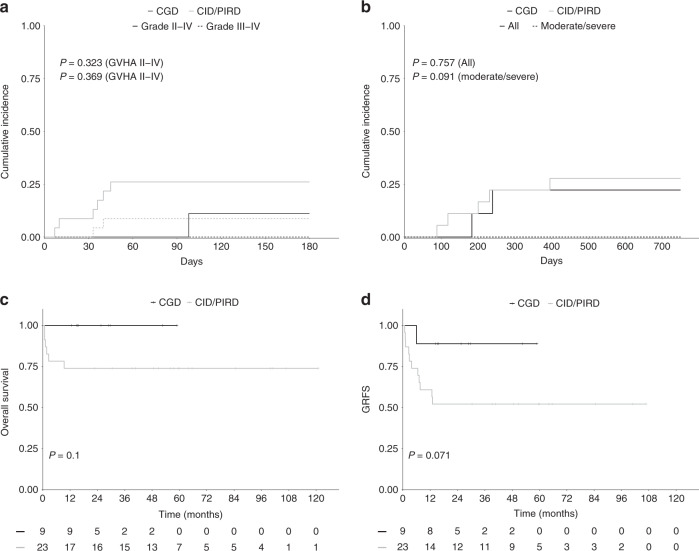
Seven patients developed grade II–IV aGVHD (6 skin, 1 gut) including 2 grade III–IV aGVHD (1 skin and 1 gut). The cumulative incidence rate at D + 100 of grade II–IV aGVHD and grade III–IV aGVHD were 18.7% (95% CI 5.2–32.2) and 6.3% (95% CI 4.7–31.3) respectively (Fig. 1a). cGVHD was evaluated in the 27 patients who survived beyond D + 100 and occurred in seven patients (two mild, three moderate, two severe). Among those, one patient died from cGVHD and one was still under immunosuppressive treatment at last follow-up. The 1-year cumulative incidence rate of cGVHD and moderate to severe cGVHD was 22% (95% CI, 6.3–37.6) and 15% (95% CI, 1.5–28.5) respectively (Fig. 1b). Cumulative incidences of aGVHD and cGVHD did not differ with respect to PID category (Fig. 2a, b), donor type, stem cell source, serotherapy, pre-transplant malignancy and HCT-CI. However, pre-transplant splenectomy in patients with CID or PIDR was associated with significantly more acute and chronic GVHD. Indeed, the cumulative incidence rate of grade II–IV aGVHD and grade III–IV aGVHD at D + 100 was 57.0% and 28.5% respectively for patients who had prior splenectomy compared to 12.5% and 0% respectively for patients who did not (p = 0.026 and 0.029). The 1-year cumulative incidence rate of cGVHD and moderate/severe cGVHD was 50.0% for patients who had prior splenectomy compared to 10.0% for patients who did not (p = 0.04) (supplementary fig. 1A and B).
Fig. 1. Acute GVHD, Chronic GVHD, Overall survival and GRFS in the whole cohort.
a Cumulative incidences of grade II–IV and severe (grade III–IV) aGVHD, (b) Cumulative incidences of cGVHD and moderate to severe cGVHD, (c, d). Kaplan–Meier survival curves of OS and GRFS in the whole cohort.
Fig. 2. Acute GVHD, Chronic GVHD, Overall survival and GRFS according to PID subtype.
a Cumulative incidences of grade II–IV and severe (grade III–IV) aGVHD, (b) Cumulative incidences of cGVHD and moderate to severe cGVHD, (c, d). Kaplan–Meier survival curves of OS and GRFS according to IUIS classification: CID and PIRD (n = 23) versus CGD (n = 9).
Transplant related mortality and organ toxicity (Tables 3 and 4)
Six patients died, all in the 1st year post transplant (five before D + 100). The causes of death were steroid refractory aGVHD (n = 2), cGVHD (n = 1), multi-organ (MOF, n = 1) failure following refractory veno-occlusive disease/sinusoidal obstruction syndrome (SOS), infection (n = 1), cardiac toxicity (n = 1). The cumulative incidence of TRM was 15% (95% CI 2.6–27.4) at three months and 19% (95% CI 4.7–31.3) at one year. Five of the six patients had an HCT-CI score ≥3. The two patients (UPN 15, DNA-PKcs deficiency and UPN 24 ADA deficiency) who had received a haplo-identical transplant died before D + 30 from acute cardiac dysfunction (UPN 15) and progressive multifocal leukoencephalopathy (PML, UPN 24). The PML was, upon review, probably present before allo-HSCT.
One patient (UPN3, ALPS) died from multi-organ failure after MUD transplant following Bu-Cy conditioning. Two CID patients (UPN 12 and 27) died from steroid refractory aGVHD after MSD transplants following FB4 conditioning. One patient with XLP1 (UPN 22) died from cGVHD 9.4 months after a MMUD transplant following FB2 regimen. Outside lethal complications, we observed four transient organ specific toxicities including 3 grade 3 hepatic (elevated liver enzymes and bilirubin) despite defibrotide prophylaxis in all cases (UPN 9, 22 and 23) and one grade 3 pulmonary (massive hemoptysis in the context of bronchiectasis, UPN 11).
OS and GRFS
Twenty-six patients were alive with a median follow-up of 51.6 months (range 12.6–120.9). OS was 80.4% at nine months and beyond. The composite endpoint GRFS was 75% at 12 months and beyond (Fig. 1c, d). OS at nine months and beyond was 100% and 74% for CGD patients and other PIDs respectively. GRFS at 12 months and beyond was 90% and 52% for CGD patients and other PIDs respectively (Fig. 2c, d). There was no impact of genetic diagnosis, donor type (MSD vs MUD), stem cell source, HTC-CI score, pre-transplant malignancy, serotherapy on OS and GRFS. Pre-transplant splenectomy in the CID and PIDR cohort was associated with a worse GRFS but not OS. Indeed, the GRFS and OS was 14.3% and 57.1% respectively for patients who had prior splenectomy compared to 81.2% and 81.2% respectively for patients who did not (p = 0.0008 and p = 0.23) (Supplementary fig. 1C, D).
Outcome of patients receiving a FB based conditioning regimen
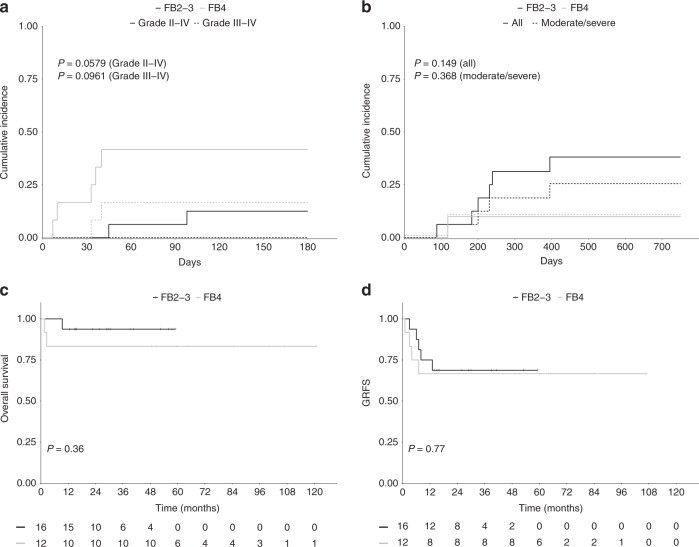
Among the 26 patients who received a FB platform (16 FB2-3 and 12 FB4) conditioning, eight experienced grade II–IV aGVHD (2 grade III–IV) and seven cGVHD (two mild, three moderate, two severe). The cumulative incidence rate of aGVHD at D + 100 and cGVHD at 1 year were respectively 25% and 23%. We observed a lower incidence of aGVHD in the FB2-3 group compared to the FB4 group with notably no grade III–IV aGVHD in the FB2-3 group and two fatal cases of aGVHD in the FB4 group. The cumulative incidence rates of grade II–IV aGVHD and grade III–IV aGVHD at D + 100 was 12.5% and 0% in the FB2-3 group compared to 41.5% and 16.5% in the FB4 group (p = 0.058) (Fig. 3a). The cumulative incidence of cGVHD at 1-year was 31% in the FB2-3 group compared to 10% in the FB4 group (18.8% and 10% respectively for moderate to severe cGVHD) (Fig. 3b). There was no difference in terms of OS (90% and 83% in the FB2-3 and FB4 groups respectively), GRFS (73% and 72%) and TRM (1 death in the FB2-3 group and two deaths in the FB4 group) (Fig. 3c, d).
Fig. 3. Acute GVHD, Chronic GVHD, Overall survival and GRFS according to Busulfan dosage (n = 28).
a Cumulative incidences of grade II–IV and severe (grade III–IV) aGVHD, (b) Cumulative incidences of cGVHD and moderate to severe cGVHD, (c, d). Kaplan–Meier survival curves of OS and GRFS according to FB group (FB2-3, n = 16; FB4, n = 12).
Outcome of PID related complications after allo-HSCT
Infectious complications
Complete resolution of active pre-transplant infections (n = 16) was observed in all patients by D + 100 except for 2 with severe bronchiectasis and Pseudomonas aeruginosa colonization. All patients with IFI at time of allo-HSCT (n = 6) discontinued antifungal therapy after calcineurin inhibitor cessation. Two patients with disseminated molluscum at time of allo-HSCT completed cleared their skin lesions at day 55 and 73 respectively despite still being under immunosuppression. The spectrum of infections after allo-HSCT differed from that of the pre-transplant period. Eleven patients developed CMV reactivation (no CMV disease), which were all treated successfully with standard antiviral therapy and four developed asymptomatic EBV reactivation (plasma viral load >4 log), which resolved in all cases after 1 or 2 infusions of Rituximab. One CGD patient developed a proven pulmonary mucormycosis at D + 35. One patient with Wiskott–Aldrich syndrome developed a Mycobacterium avium intracellulare bronchiolitis at D + 120. These 2 patients were successfully treated without relapse.
PID-associated colitis/autoimmunity
Active autoimmune/inflammatory complications at time of allo-HSCT resolved in affected patients (n = 13) and none of the six additional patients with a history of autoimmunity and/or inflammation relapsed after allo-HSCT.
Lymphoma
Of the nine patients with a history of lymphoma, only one relapsed at day +90, while still on immunosuppression with cyclosporine and with full donor chimerism, and remains in complete remission more than six years after salvage chemo-radiotherapy (four cycles of association of cytarabin and cisplatin followed by radiotherapy).
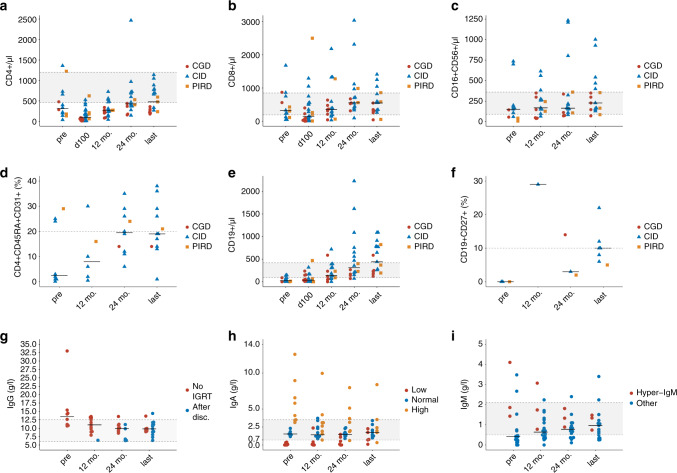
Immune reconstitution (Fig. 4 and Supplementary Table 1–3)
Fig. 4. Immune reconstitution.
Immune reconstitution after allo-HSCT. Each dot represents a patient at a given timepoint. The horizontal black bar within the scatter plot indicates the median value for the given timepoint. a–c Reconstitution of CD4+, CD8+ and NK in cells/µl for patients with CGD (red), CID (blue) and PIRD (orange). For details, see supplementary table 2. d Reconstitution of recent thymic emigrant naive CD4+ in % for patients with CGD (red), CID (blue) and PIRD (orange). The horizontal dashed grey line indicates the lower limit of naive CD4+ cells (20%) and memory B-cells (10%). e, f Reconstitution of CD19+ B cells in cells/µl and memory B-cells in for patients with CGD (red), CID (blue) and PIRD (orange). The horizontal dashed grey line indicates the lower limit of memory B-cells (10%). g Serum IgG levels in g/l. Values for patients never on IgRT are shown in red starting before transplantation and at 1 year, 2 years and last follow-up. Patients on IGRT are shown in blue starting at least 6 months after weaning of immunoglobulins (one patient at one year, five patients at two years and 12 patients at last follow-up. h Serum IgA levels in g/l. Patients with initial low IgA counts (<0.7 g/l) are shown in red. Patients with initial IgA between 0.7 and 2.5 g/l are shown in blue and patients with initial IgA levels above 2.5 g/l are shown in orange. Overall, IgA levels remained stable from the entire cohort (median IgA 1.53 g/l pre-transplant and 1.41 g/l, 1.48 g/l and 1.75 g/l at one year, two years and last follow-up. Patients with low IgA pre-transplant (median IgA 0.04, range 0–0.39), increased steadily to 0.13 g/l (range 0–2.36), 0.31 g/l (range (0–1.47) and 0.77 (range 0.1–2.26) at 1 year, 2 years and last follow-up. i Serum IgM levels in g/l. Patients with hyper-IgM syndrome are shown in red and patients with other PID are shown in blue. Across both groups, median IgM increased from 0.42 g/l (range 0–4.1) pre-transplant, to 0.64 g/l (range 0–3.1) at 1 year, 0.78 g/l (range 0.11–2.4) at two years and 0.97 g/l (range 0.25–3.4) at last follow-up. In patients with hyper-IgM syndrome, median pre-transplant IgM levels was 1.86 g/l (range 1.43–4.1). At 1-year, median IgM was 1.74 g/l (range 0.78–3.1), at two years median IgM was 1.33 g/l (range 0.89–1.81) and at last follow-up median IgM was 1.34 g/l (range 0.74–1.48). Most patients with other PID had low pre-transplant IgM levels with a median IgM of 0.4 g/l (range 0–3.48) and progressively increased to normal or near normal values (median IgM 0.87, range 0.25–3.4) at last follow-up.
Median last follow-up evaluation time was 45 months (range 13–100) for immune cell reconstitution and 50 months (range 13–94) for immunoglobulin levels.
T-cell reconstitution
T-cell reconstitution occurred progressively in all patients with a median count of CD4 positive cells of 100/µl (range 6–624), 270/µl (range 43–725), 427/µl (range 146–2467) and 477/µl (range 179–1138) on D + 100, 1-year, 2-years and at last follow-up respectively. Median CD8+ cells were 142/µl (range 2–2500), 349/µl (range 32–2181) and 548/µl (range 41–1411) on D + 100 and at one year and last follow-up respectively (Fig. 4a, b).
Reconstitution was also observed in the naive CD4+ and CD8+ subsets (12 patients analyzed). Median pre-transplant CD4+CD45RA+CD31+ cells was 2.5% (range 0.1–29) and increased to 8% (range 0.5–30) and 19.5% (6–35) at one and two year respectively. Naive CD4+ remained stable between two years post-transplant and at last follow-up (Fig. 4d). Naive CD8+ reconstitution was slightly slower with a pre-transplant median of 20% (range 2–39), and medians of 5% (range 0.1–18.5), 9.5% (range 3–50) and 14% (range 1–53) CD8+CD45RA+CCR7+ cells at one year, two year and last follow-up respectively.
B-cell reconstitution
B-cells reconstitution was delayed with median of 34 (range 0–468) CD19+ cells/µl on day +100, 137 (range 0–718) at year 1 and 440 (range 120–1092) at last follow-up. Memory B-cell reconstitution was evaluated in nine patients. At last follow-up, median CD19+CD27+ was 10% (range 5–22) (Fig. 4e, f).
Immunoglobulin reconstitution
Humoral reconstitution was evaluated in 24 patients. Only values of patients never on IGRT (n = 12) or at least six months after IGRT discontinuation (n = 12) were analyzed. Median IgG levels was 13.4 g/l (range 10.7–33) before transplantation, 11 g/l (range 6.4–13.49), 9.37 (range 6.31–11.17) and 9.86 (range 6.36–14.4) at one year, two year and last follow-up respectively (Fig. 4g). IgA and M reconstitutions are depicted in Fig. 4h, i. Thirteen patients discontinued IgRT (median time to discontinuation 22.1 mo., range 6–40.7).
Vaccine response
antibody response to tetanus toxoid and pneumococcal polysaccharide was available after vaccination for 19 patients (six CGD and 13 CID) and all developed protective titres. Seven patients were evaluable for antibody response to SARS-COV2 after two doses of COVID RNA vaccine (six CID and one CGD). All developed high IgG titres to SARS-COV2 Spike protein (median IgG 32453, range 15,886–40,000).
Donor chimerism
Chimerism was evaluated at D + 100, one year and at last follow-up (median time 29 mo., range 11–73) in 28 patients. The frequency of patients achieving >90% donor chimerism at D + 100, one year and at last follow-up, was 92.5% (>99%, 44.4%), 92.3% (>99%, 57.7%) and 94.6% (>99%, 67.9%) respectively (Supplementary Table 3). The frequency of patients achieving 50–89% donor chimerism at D + 100, 1 year and at last follow-up was 7.4%, 7.7% and 3.6% respectively.
Recovery of a normal oxidative burst in CGD patients
All CGD patients had null DHR + cells prior to transplantation. Median DHR + cells were 88% (range 79–99), 95.5% (range 83–98), 95 (range 89–99) and 95 (range 92–99) at d + 30, d + 180, 1 year and last follow-up (15 mo., range 13–50) respectively (data not shown).
Quality of life (QoL)
Among the 26 patients who survived at least one-year post allo-HSCT, 22 (84.5%) had been hospitalized at least two weeks in the year preceding transplantation for PID related complications. Between D + 100 and 1-year post-transplant, seven patients (27%) were hospitalised (four for infectious complications, one each for lymphoma relapse, GVHD and diabetes). During the second-year post-transplant, only three patients (11.5%) required hospitalisations (all for infections). No hospitalisation for PID nor transplant related causes occurred after two years post transplantation. Immunosuppressive therapy was discontinued in 24 patients with a median of 11.6 mo. (range 4.1–90.3). Two patients are still on immunosuppressive therapy (one for moderate cGVHD and one for kidney transplantation predating allo-HSCT). All but one of the 14 surviving patients who received IgRT prior to transplant discontinued immunoglobulins. At one-year post allo-HSCT, all surviving patients had an ECOG status of zero. Furthermore, all patients resumed their normal education or employment course within 1 year of transplantation.
Discussion
This study reports the outcome of 32 consecutive adult patients with various life-threatening PID who received an allo-HSCT during a ten-year period with a median follow-up >4 year after allo-HSCT. It represents a large unbiased cohort and includes a large majority of CID and PIRD patients (72% of the cohort).
Findings of this study confirm that allo-HSCT is relatively safe and efficient for adult patients with PID, particularly following RIC or RTC. We show especially that FB2-3 conditioning regimen allow a durable engraftment with favorable toxicity profile compared to myeloablative regimens, which is consistent with previous reports in pediatric patients [12, 27]. Only the first three patients with CGD had TDM as per protocol. Because we found no significant differences in engraftment and toxicity, we chose not to continue with this routine.
Neither HTC-CI score nor type of donor (MSD versus MUD) or serotherapy (none vs ATG, vs alemtuzumab) influence OS or GRFS. However, we observed that, in the “CID and PIDR” cohort, pre-transplant splenectomy was associated with more acute and chronic GVHD which translates in worse GRFS but OS. No conclusions can be drawn regarding the feasibility of haplo-identical transplantation given the limited number of patients [28]. Complications observed after allo-HSCT were comparable to those observed in the context of hematological malignancies (GVHD, CMV, EBV reactivations) and easily manageable. We did not observe an increased mortality rate with regards to the comorbidity profile of this PID cohort as reflected by the high HCT-CI score. Indeed, a recent study, with mostly pediatric patients shows that high HCT-CI score correlates with poor outcome even in non-malignant hematological disease such as PID [29]. In our study, of the six deaths, three occurred after a myeloablative conditioning, two in the context of haplo-identical SCT. The only patient who died after a RIC had very long history of PID related complications. With regards to our findings this patient would have been transplanted sooner nowadays.
The triggers for allo-HSCT included severe infections, lymphomas and autoimmunity/ autoinflammation which all rapidly resolved without recurrence after allo-HSCT (mostly before D + 100). Specifically, severe infections, such as IFI, do not preclude allo-HSCT in patients with PID. The majority of the patients who were on IgRT for years to decades were able discontinue immunoglobulins and had a satisfactory immune recovery with positive vaccine responses. Remarkably, thymopoiesis was apparent with recovery of significant proportions of recent thymic emigrant naive T cells, and we demonstrated that recovery of memory B cells was possible. Moreover, in addition to the reversion of the clinical and immunological phenotypes after allo-HSCT, we observed a rapid recovery of patients and improved QoL.
Our results build upon those of other groups [19] to clarify the role of allo-HSCT in the treatment of adult patients with severe PID. With respect to delayed PID complications as well as late transplant complications, the long follow-up strengthens our conclusions. Furthermore, it provides new findings notably for CID patients conditioned mainly with the homogeneous FB platform. These results led us to adopt FB3 conditioning regimen, which has a similar toxicity profile compared with Flu-Mel [30, 31], as our standard conditioning for these patients.
In conclusion, we demonstrate that reduced toxicity allo-HSCT is relatively safe, can cure the PID and prevent further organ damage. We also demonstrate that pre-transplant active PID related complications, such as inflammatory colitis or severe active infections, are not associated with significant post-transplant complications and should not dissuade physicians from opting for allo-HSCT in this setting. The extremely favorable outcome for CGD patients in particular pleads for the upfront use of allo-HSCT in most adult with this PID.
Supplementary information
Supplementary tables 1-4 and supplementary figure legend
Author contributions
AM and FS designed and supervised the study and wrote the manuscript. AM, NM, BN, FL, EC, HS, MC, LJC, OL, CP, DM, OH and AF provided clinical care for the patients included in the study. MJ performed post-vaccination serological assays. VA performed chimerism analysis. PvE performed HLA typing and donor selection and CP performed genetic analysis and immunological cellular reconstitution.
Data availability
Correspondence and requests for materials should be addressed to Ambroise Marçais or Felipe Suarez.
COMPETING INTERESTS
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ambroise Marçais, Email: ambroise.marcais@aphp.fr.
Felipe Suarez, Email: felipe.suarez@aphp.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-022-01739-x.
References
- 1.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. 2020;40:24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40:66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol 2018; 14. 10.1186/s13223-018-0290-5. [DOI] [PMC free article] [PubMed]
- 4.Mitchell R. Hematopoietic stem cell transplantation beyond severe combined immunodeficiency: seeking a cure for primary immunodeficiency. J Allergy Clin Immunol Pr. 2019;7:776–85. doi: 10.1016/j.jaip.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Fischer A, Landais P, Friedrich W, Morgan G, Gerritsen B, Fasth A, et al. European experience of bone-marrow transplantation for severe combined immunodeficiency. Lancet Lond Engl. 1990;336:850–4. doi: 10.1016/0140-6736(90)92348-L. [DOI] [PubMed] [Google Scholar]
- 6.Pai S-Y, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N. Engl J Med. 2014;371:434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrua F, Galimberti S, Courteille V, Slatter MA, Booth C, Moshous D, et al. Hematopoietic stem cell transplantation for CD40 ligand deficiency: results from an EBMT/ESID-IEWP-SCETIDE-PIDTC study. J Allergy Clin Immunol. 2019;143:2238–53. doi: 10.1016/j.jaci.2018.12.1010. [DOI] [PubMed] [Google Scholar]
- 8.Fischer A, Landais P, Friedrich W, Gerritsen B, Fasth A, Porta F, et al. Bone marrow transplantation (BMT) in Europe for primary immunodeficiencies other than severe combined immunodeficiency: a report from the European Group for BMT and the European Group for Immunodeficiency. Blood. 1994;83:1149–54. doi: 10.1182/blood.V83.4.1149.1149. [DOI] [PubMed] [Google Scholar]
- 9.Antoine C, Müller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet Lond Engl. 2003;361:553–60. doi: 10.1016/S0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 10.Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood. 2011;118:1675–84. doi: 10.1182/blood-2010-11-319376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins A-M, et al. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128:440–8. doi: 10.1182/blood-2016-03-704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güngör T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383:436–48. doi: 10.1016/S0140-6736(13)62069-3. [DOI] [PubMed] [Google Scholar]
- 13.Castagnoli R, Delmonte OM, Calzoni E, Notarangelo LD. Hematopoietic stem cell transplantation in primary immunodeficiency diseases: current status and future perspectives. Front Pediatr. 2019;7:295. doi: 10.3389/fped.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8. doi: 10.1182/blood.V99.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Morris EC, Albert MH. Allogeneic HSCT in adolescents and young adults with primary immunodeficiencies. Front Pediatr. 2019;7:437. doi: 10.3389/fped.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlogis V, Mahlaoui N, Auquier P, Pellier I, Fouyssac F, Vercasson C, et al. Physical health conditions and quality of life in adults with primary immunodeficiency diagnosed during childhood: A French Reference Center for PIDs (CEREDIH) study. J Allergy Clin Immunol. 2017;139:1275–1281.e7. doi: 10.1016/j.jaci.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Barlogis V, Mahlaoui N, Auquier P, Fouyssac F, Pellier I, Vercasson C, et al. Burden of poor health conditions and quality of life in 656 children with primary immunodeficiency. J Pediatr. 2018;194:211–217.e5. doi: 10.1016/j.jpeds.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa R, Wang J, Blok H-J, Hazelaar S, Neven B, Moshous D, et al. Hematopoietic cell transplantation in chronic granulomatous disease: a study of 712 children and adults. Blood. 2020;136:1201–11. doi: 10.1182/blood.2020005590. [DOI] [PubMed] [Google Scholar]
- 19.Fox TA, Chakraverty R, Burns S, Carpenter B, Thomson K, Lowe D, et al. Successful outcome following allogeneic hematopoietic stem cell transplantation in adults with primary immunodeficiency. Blood. 2018;131:917–31. doi: 10.1182/blood-2017-09-807487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehr C, Gennery AR, Lindemans C, Schulz A, Hoenig M, Marks R, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135:988–997.e6. doi: 10.1016/j.jaci.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Albert MH, Hauck F, Wiebking V, Aydin S, Notheis G, Koletzko S, et al. Allogeneic stem cell transplantation in adolescents and young adults with primary immunodeficiencies. J Allergy Clin Immunol Pr. 2018;6:298–301.e2. doi: 10.1016/j.jaip.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–25. doi: 10.1182/blood.V99.12.4618. [DOI] [PubMed] [Google Scholar]
- 23.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bourgeois A, Labopin M, Blaise D, Ceballos P, Vigouroux S, Peffault de Latour R, et al. Reduced-intensity versus reduced-toxicity myeloablative fludarabine/busulfan-based conditioning regimens for allografted non-Hodgkin lymphoma adult patients: a retrospective study on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Ann Oncol J Eur Soc Med Oncol. 2017;28:2191–8. doi: 10.1093/annonc/mdx274. [DOI] [PubMed] [Google Scholar]
- 26.Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Hamladji RM, Blaise D, Socié G, et al. Comparing i.v. BU dose intensity between two regimens (FB2 vs FB4) for allogeneic HCT for AML in CR1: a report from the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2014;49:1170–5. doi: 10.1038/bmt.2014.133. [DOI] [PubMed] [Google Scholar]
- 27.Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with Alemtuzumab, Fludarabine, and Melphalan reduced-intensity conditioning hematopoietic cell transplantation in patients with nonmalignant diseases reveals good outcomes and that the risk of mixed chimerism depends on underlying disease, stem cell source, and Alemtuzumab regimen. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:1460–70. doi: 10.1016/j.bbmt.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neven B, Diana J-S, Castelle M, Magnani A, Rosain J, Touzot F, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for primary immunodeficiencies and inherited disorders in children. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2019;25:1363–73. doi: 10.1016/j.bbmt.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Thakar MS, Broglie L, Logan B, Artz A, Bunin N, Burroughs LM, et al. The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood. 2019;133:754–62. doi: 10.1182/blood-2018-09-876284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura A, Aoki Y, Ishiwata Y, Ichimura T, Ueyama J, Kawahara Y et al. Hematopoietic cell transplantation with reduced intensity conditioning using fludarabine/busulfan or fludarabine/melphalan for primary immunodeficiency diseases. J Clin Immunol 2021. 10.1007/s10875-021-00966-z. [DOI] [PubMed]
- 31.Peric Z, Labopin M, Peczynski C, Polge E, Cornelissen J, Carpenter B, et al. Comparison of reduced-intensity conditioning regimens in patients with acute lymphoblastic leukemia >45 years undergoing allogeneic stem cell transplantation-a retrospective study by the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2020;55:1560–9. doi: 10.1038/s41409-020-0878-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables 1-4 and supplementary figure legend
Data Availability Statement
Correspondence and requests for materials should be addressed to Ambroise Marçais or Felipe Suarez.