To the Editor: Emerging subvariants of the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have reignited concern about further immune escape. Specifically, BA.2.12.1, which is on the rise in the United States, has two more mutations (L452Q and S704L) than BA.2 (Fig. S1A in the Supplementary Appendix, available with the full text of this letter at NEJM.org).1 In addition, BA.4 and BA.5 (hereafter, BA.4/5), which bear identical spike proteins, have become the dominant strains in South Africa.2 Here, we examine neutralizing-antibody titers in serum samples obtained from vaccinated persons who had received a single booster dose of the same vaccine used in the two-dose series and who had been previously infected with SARS-CoV-2.
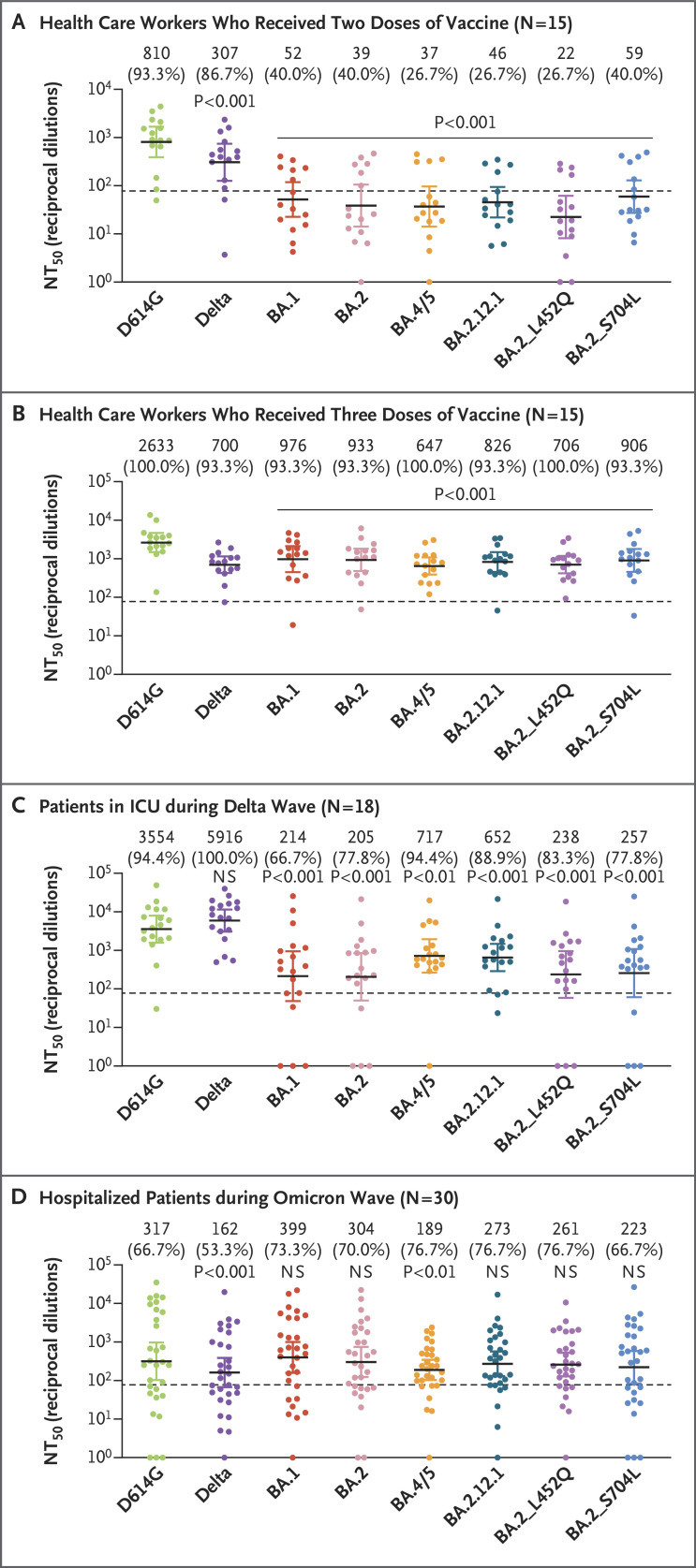
We first used a pseudotyped lentivirus neutralization assay to examine the neutralizing-antibody resistance to these subvariants in 4 health care workers who had received two doses of the mRNA-1273 vaccine (Moderna) and 11 health care workers who had received two doses of the BNT162b2 vaccine (Pfizer–BioNTech) (Table S1).3 The titers of these viruses in HEK293T-ACE2 and CaLu-3 cells are shown in Figures S1B and S1C. The BA.4/5 and BA.2.12.1 subvariants had neutralization resistance that was similar to that of the BA.1 and BA.2 subvariants (Figure 1A and Figs. S2A and S2B). However, the 4 health care workers who had received three doses of the mRNA-1273 vaccine and the 11 health care workers who had received three doses of the BNT162b2 vaccine had dramatically increased neutralizing-antibody titers overall. As compared with the response against the ancestral SARS-CoV-2 strain bearing the D614G mutation, neutralizing-antibody titers were 4.1 times as low against the BA.4/5 variant and 3.2 times as low against the BA.2.12.1 variant (P<0.001 for both comparisons), and the titers were approximately 2.8 times as low against the BA.1 and BA.2 variants (Figure 1B and Figs. S2C and S2D). The neutralizing-antibody resistance of BA.2_L452Q was stronger than that of BA.2_S704L, which had neutralizing-antibody titers that were approximately 3.7 times and 2.9 times as low, respectively, as those against the D614G strain (P<0.001 for both comparisons) (Figure 1B).
Figure 1. Stronger Immune Escape with the BA.4/5 and BA.2.12.1 Omicron Subvariants than with BA.1 and BA.2.
Shown are neutralizing-antibody titers against virus pseudotyped with spike protein from ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) bearing the D614G mutation, along with the B.1.617.2 (delta) variant, the BA.1, BA.2, BA.4/5, and BA.2.12.1 subvariants of the B.1.1.529 (omicron) variant, and the BA.2 single mutants BA.2_ L452Q and BA.2_S704L in serum samples obtained from health care workers 3 to 4 weeks after they had received the second dose of messenger RNA (mRNA) vaccine (Panel A) and from health care workers after they had received the third (booster) dose of the same mRNA vaccine (Panel B). Also shown are neutralizing-antibody titers in serum samples obtained from patients with coronavirus disease 2019 (Covid-19) in the intensive care unit (ICU) during the delta wave of the Covid-19 pandemic (Panel C) and from patients who were hospitalized with Covid-19 but were not admitted to the ICU during the BA.1 wave of the pandemic (Panel D). In all panels, dots indicate individual samples, and the horizontal dashed lines indicate the limit of detection. The geometric mean values for the 50% neutralization titers (NT50) are shown at the top of the plots along with the percentage of persons with NT50 values above the limit of detection. 𝙸 bars represent 95% confidence intervals. The statistical significance of the neutralizing-antibody titers against the subvariants relative to those against the ancestral SARS-CoV-2 strain bearing the D614G mutation was determined by means of one-way repeated-measures analysis of variance with Bonferroni’s multiple testing correction. NS denotes not significant.
In serum samples obtained from patients who were hospitalized in the intensive care unit (ICU) during the delta wave of the coronavirus disease 2019 pandemic, neutralization of the delta variant was 66.5% higher than that of the D614G variant (Figure 1C). BA.4/5 and BA.2.12.1 had less escape from neutralization in serum samples obtained during the delta wave; geometric mean values for neutralization titers were 5.0 times as low against the BA.4/5 variant as against the D614G variant (P<0.01), and the values were 5.5 times as low against the BA.2.12.1 variant as against the D614G variant (P<0.001) (Figure 1C). However, the neutralizing-antibody titers were 14.9 times as low against the BA.2 L452Q variant as against the D614G variant (P<0.001), and the titers were 13.8 times as low against the BA.2_S704L variant as against the D614G variant (P<0.001) (Figure 1C) — even lower than the titers against BA.4/5 and BA.2.12.1. During the delta wave, unvaccinated patients had a neutralizing-antibody response that was more biased toward neutralization of the delta variant than that in vaccinated patients (Fig. S3A and S3B).
In 30 persons who were infected with the omicron variant and who were hospitalized but not admitted to the ICU, neutralizing-antibody titers against BA.4/5 and BA.2.12.1 were 37.8% and 10.2% lower, respectively, than those against BA.2 (P>0.05 for both comparisons). The BA.2 single mutants (i.e., BA.2_L452Q and BA.2_S704L) had neutralizing-antibody escape that was similar to that of the BA.4/5 and BA.2.12.1 subvariants, with neutralizing-antibody titers that were 14.1% and 26.6% lower, respectively, than those against BA.2 (P>0.05 for both comparisons) (Figure 1D). Notably, 2 of 30 BA.1-infected but unvaccinated patients (Patients U12 and U13) had high neutralizing-antibody titers against all the variants except BA.4/5, whereas patients who had received a booster dose had broader neutralization against all the variants examined (Fig. S3C and S3D). Overall, these results showed that infection during the BA.1 wave did not appear to offer effective protection against the newly emerged sublineages.
In this study, we characterized infection-induced immunity and vaccine-induced immunity against newly emerged omicron subvariants. Booster vaccination provided sufficient neutralizing-antibody titers against the BA.4/5 and BA.2.12.1 subvariants, albeit to a lower extent than against BA.1 and BA.2.4,5 These findings underscore the importance of booster vaccination for protection against emerging variants.
Supplementary Appendix
Disclosure Forms
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This letter was published on June 15, 2022, at NEJM.org.
Footnotes
Supported by a fund provided by an anonymous donor to Ohio State University (to Dr. Liu); an award (U54CA260582, to Drs. Lozanski, Saif, Oltz, Gumina, and Liu) from the National Cancer Institute of the National Institutes of Health (NIH); a grant (R01 AI150473, to Dr. Liu) from the NIH; a Glenn Barber Fellowship (to Mr. Evans) from the Ohio State University College of Veterinary Medicine; grants (to Dr. Gumina) from the Robert J. Anthony Fund for Cardiovascular Research and the JB Cardiovascular Research Fund; a grant (R01 HD095881, to Dr. Saif) from the NIH; and grants (UL1TR002733 and KL2TR002734, to Dr. Bednash) from the National Center for Advancing Translational Sciences.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Centers for Disease Control and Prevention. COVID data tracker: variant proportions (https://covid.cdc.gov/covid-data-tracker/#variant-proportions).
- 2.National Institute for Communicable Diseases South Africa. SARS-CoV-2 genomic surveillance update: tracking SARS-CoV-2 variants (https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/sars-cov-2-genomic-surveillance-update/).
- 3.Zeng C, Evans JP, Pearson R, et al. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight 2020;5(22):e143213-e143213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JP, Zeng C, Qu P, et al. Neutralization of SARS-CoV-2 omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe 2022. April 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022;386:1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.