Abstract
A randomized control trial was conducted to investigate the effects of skin-to-skin, chest-to-chest contact (kangaroo care, KC) in mother-infant dyads on patterns of infant brain activity and associated mother-infant neurohormone releases. 33 mother-infant dyads participated during pregnancy (29–38 weeks gestation), at neonatal and 3-month periods. Overall, analyses indicated that: 1) infants in the KC group showed left frontal brain activation patterns (asymmetry and coherence) associated with KC training; 2) KC produced moderate to large increases in oxytocin levels; and 3) KC yielded moderate decreases in cortisol reactivity. Findings suggest KC may garner favorable neuro-maturational and neurobiological outcomes for dyads.
Mother-infant touch and contact have been shown to precipitate optimal neuro-developmental regulation in early infancy (Feldman & Eidelman, 2003; Kaffashi, Scher, Ludington-Hoe, & Loparo, 2013; Scher et al., 2009). In particular, Kangaroo Care (KC), a procedure defined as skin-to-skin, chest-to-chest contact within the dyad after birth (Ludington-Hoe, 2008), has been implicated in promoting neurophysiological development. Ludington-Hoe (2012) contends that KC stimulates the ventral area of the skin, facilitating vagal-induced hormonal cascades which downregulate physiological stress responses and in turn promotes neuro-regulation. Missing from past research is consideration of brain development in normative samples. With the aim of filling this gap in the literature, we examined EEG patterns along with basal oxytocin and cortisol reactivity in infants as a function of participation in 6-weeks of KC compared to standard-care during the first 3 months of life.
Electrophysiological measures can elucidate the contribution of experience on neurodevelopment (Fox & Rutter, 2010). Frontal EEG asymmetry patterns in the right or left hemisphere of the brain are an indicator of neuro-maturation (Zhu et al., 2011). In particular, the left hemisphere in the frontal region is implicated in higher-order cognitive and emotional regulatory skills (Gartstein, Hancock, Potapova, Calkins, & Bell, 2019). Since maturation of the frontal lobe takes place postnatally, environmental input is important (Bell & Fox, 1996; Carrion et al., 2001). Hill et al. (2010) contend that the infant brain is vulnerable to environmental insult and our own studies indicate that maternal depression negatively impacts EEG power and asymmetry from as early as 1-month of age (Jones, Field, Fox, Lundy, & Davalos, 1997). However, brain plasticity research that links physiological maturation to the context of development would suggest that positive experiences can direct the developmental trajectory in an adaptive direction as well. For example, variations in maternal caretaking have been linked to infant EEG power (Bernier, Calkins, & Bell, 2016) and asymmetry patterns (Hane, Henderson, Reeb-Sutherland, & Fox, 2010; Jones, McFall, & Diego, 2004) with positive interactions being associated with more optimal EEG power scores across the first 2 years. The functional connectivity between regions of the brain may be informative as well, with increases in EEG coherence associated with enhanced localization of abilities contributing to more efficient information processing across development (Cuevas, Bell, Marcovitch, & Calkins, 2012). In the current study, we examined the impact of KC as a beneficial caretaking experience on resting EEG power/asymmetry and coherence in infants as well as on affiliative and stress hormones.
Oxytocin (OT) is considered an affiliative hormone associated with caregiving and affectionate behavior (Hammock, 2015; Jones & Sloan, 2018) whereas cortisol reactivity is implicated in the stress response system. KC affects the release of these two hormones differentially. Previous studies have found that because of the extended tactile contact, KC increases oxytocin levels of mother-infant dyads (Kommers et al., 2018). Further, interpersonal touch has been demonstrated as an effective method of diminishing behavioral and physiological stress responses (Feldman, Singer, & Zagoory-Sharon, 2010; Gray, Miller, Philip, & Blass, 2002) specifically through the release of oxytocin (Feldman, Gordon, & Zagoory-Sharon, 2010). Thus, we also examined whether exposure to extended tactile stimulation via KC increased peripheral basal OT and downregulated cortisol reactivity.
The current study focused on neurodevelopment in full-term infants as a consequence of KC use in a longitudinal randomized, controlled trial. Part of the novelty of the current study is examining the potential association between KC and brain development, specifically measures of EEG asymmetry/power and coherence. We hypothesized that KC experience would be associated with greater relative left frontal asymmetry and increased coherence between brain regions. Second, based on previous research with preterm infants (Kommers et al., 2018; Uvnäs-Moberg, 2003), it was hypothesized that KC would positively influence tonic oxytocin levels in mothers as well as their full-term infants. Last, we expected that KC experience would impact infant cortisol reactivity due to the effects of KC promoting infant stress regulation (Cong, Ludington-Hoe, & Walsh, 2011; Gitau et al., 2002; Modi & Glover, 1998; Swinth, Anderson, & Hadeed, 2003).
The procedures for this study were reviewed and approved by Florida Atlantic University’s IRB and all mothers signed consent for their own and their infant’s participation. Pregnant mothers were recruited between 29–38 weeks gestation and group assignment was determined through stratified random sampling. Data were collected from 33 mother-infant dyads (N = 16 KC group; N = 17 control group). Infants were full-term (> 37 weeks GA) and the two groups did not differ on demographic characteristics (see Table 1).
Table 1.
Demographic characteristics of the infant participants and their families.
| KC (N = 16) | Control (N = 17) | |
|---|---|---|
| Family Characteristics | ||
| Race/Ethnicity (Ns and %) | ||
| Caucasian | 14 (88 %) | 11 (64 %) |
| African-American | 0 (0 %) | 1 (6 %) |
| Hispanic | 1 (6 %) | 3 (18 %) |
| More than one group | 1 (6 %) | 2 (12 %) |
| Parity (Ns and %) | 9 (56 %) | 8 (48 %) |
| One child | ||
| Two children | 5 (32 %) | 5 (29 %) |
| More than 2 Children | 2 (12 %) | 4 (23 %) |
| Parent Age (in years; M and SDs) | 30.29 (4.40) | 30.08 (5.14) |
| Maternal Age | ||
| Paternal Age | 34.60 (7.81) | 31.00 (5.09) |
| Maternal Education (in years; M and SD) | 15.88 (2.47) | 16.12 (1.99) |
| SES* (M and SD) | 30.18 (11.00) | 23.27 (15.25) |
| Infant Characteristics | ||
| Sex (Ns and %) | ||
| Female | 6 (38 %) | 9 (53 %) |
| Male | 10 (62 %) | 8 (47 %) |
| Delivery Type (Ns and %) | ||
| Vaginal | 15 (94 %) | 14 (83 %) |
| C-section | 1 (6 %) | 3 (17 %) |
| Age (in days; M and SDs) | ||
| Newborn Visit | 7.60 (3.32) | 9.50 (3.29) |
| 3-month Visit | 105.10 (15.74) | 102.13 (13.38) |
Note: SES numbers are for the Hollingshead Four Factor Index, 18–43 corresponding to middle class/upper middle class.
Mothers assigned to the KC group were given a KC wrap (Nurtured by Design, The Kangaroo Zak) and were taught proper KC procedures by a certified trainer at the prenatal visit. Mothers were asked to use KC, skin-to-skin, chest-to-chest contact with her infant, for 1 h per day for 6 weeks and provided with journals in which to record the frequency of KC use. Mothers in the control group were given infant feeding pillows (Boppy) and journals in which they were asked to record infant feedings for 6-weeks. Three mothers were not included in the final dataset (N = 2 KC for refusing the KC training and N = 1 in the control group that dropped out before the end of the study).
A stretch Lycra cap (Electro Cap, Inc.) with the international 10–20 system was used to measure EEG activity during a 5-minute quiet-alert state at 3-months. Mid-frontal (F3 and F4), lateral frontal (F7 and F8), central (C3 and C4), parietal (P3 and P4) and occipital (O1 and O2) sites were referenced to the vertex (Cz). EEG was collected using James Long Inc. system of data collection and analysis (see Jones et al., 2004 for exact procedures). Artifact editing was performed and interrater reliability was suitable (Cohen’s K = .82). Three measures of EEG were computed for analysis, including EEG power (in picowatt ohms within the 6−9 Hz frequency band and transformed via a natural log procedure, Ln), EEG asymmetry (Ln(right) minus Ln(left) for each homologous electrode site) and EEG coherence for each electrode pair within a hemisphere (i.e., F3 +F7, F3 + C3, F7 +C3, F3 +P3, F7 +P3, F3 +O1, F7 +O1, C3 +P3 C3+ O1, P3 +O1 and F4 +F8, F4 +C4, F8 + C4, F4 + P4, F8 + P4, F4 + O2, F8 +O2, C4 + P4 C4+ O2, P4 +O2 (Cuevas et al., 2012)).
OT was measured by collecting maternal (at the prenatal and 3-month assessments) and infant (at the neonatal and 3-month visits) urine over 4 consecutive days (Thermo Fisher Scientific Inc., PA, USA). After initial collection, urine samples were stored in freezers and subsequently transported to an ultra-low temperature freezer at −80 °C. OT samples were assayed and analyzed at the University of Wisconsin, Primate Lab using procedures outlined by Seltzer, Ziegler, and Pollak (2010). Table 2 include means and standard deviations based on group assignment and frequency of KC use, respectively. All raw OT scores were normalized using ln transformations.
Table 2.
Means and Standard Deviation of Raw oxytocin (OT) values of mothers and infants.
| Kangaroo Care | Control Group | |
|---|---|---|
| Prenatal OT mother | 10.94(4.44) N = 18 | 20.54(25.71) N = 14 |
| Newborn OT | 9.84(14.31) N = 12 | 6.60(5.13) N = 8 |
| 3 mo. OT mother | 10.08(6.95) N = 9 | 7.37(4.01) N = 9 |
| 3 mo. OT infant | 16.52(25.53) N = 9 | 6.58(4.91) N = 8 |
Infant cortisol reactivity was measured as the difference in salivary cortisol concentrations before (upon arrival to the lab) and 20 min (the elapsed time for cortisol levels to peak following a stimulus (Gunnar & Quevedo, 2007)) after exposure to an acute stressor (arm restraint task) at 3-months (Table 2a includes Ms and SDs). The arm restraint task has been used to assess temperamental reactivity and frustration (Jones et al., 2004; Stifter & Jain, 1996). The infant was placed in an infant seat and the mother was instructed to silently maintain a neutral face while holding down the arms of her infant for 90-seconds (restraint stressor task); this was performed after other tasks. Cortisol was measured by collecting infant saliva samples (passive drool) using a nalgene cryogenic vial (Thermo Scientific, NY, USA). Samples were immediately placed in a −20 °C freezer and moved to the storage freezer after the visit. Samples were assayed using a commercial cortisol EIA kit (Salimetrics, PA, USA).
Our data analysis plan drove the examination of the KC versus the control group yet preliminary scrutiny during data collection and reported variation in maternal use of KC compelled the KC group to be split-up in follow-up analyses into two separate groups based on maternal compliance with task demands ((for a total of three groups: 1) recommended-use KC (> 1h. per day for 6 weeks) (N = 8); 2) low-use KC (< 1 h per day for 6 weeks) (N = 6); and 3) control group (N = 16)). In addition, our data analysis plan also tested the bio-hormonal hypotheses with common null hypothesis significance testing (NHST) and explored the strength of the relationship between variables using effect sizes (Cohen’s d) due to our small sample (33 mothers and infants).
To examine the effect of KC on EEG asymmetry scores, a 2 group (KC versus control) by region (mid-frontal, lateral frontal, central, parietal, occipital) repeated measures MANOVA was conducted and yielded a multivariate Group by Region interaction effect, F(4,18) = 3.15, p = .04, ɳ2 = .41. Subsequent ANOVAs, however, showed no group differences for each region and no significant hemispheric differences were obtained for EEG power, ps >.05 (2 ×5 ×2; Group by Region by Hemisphere).
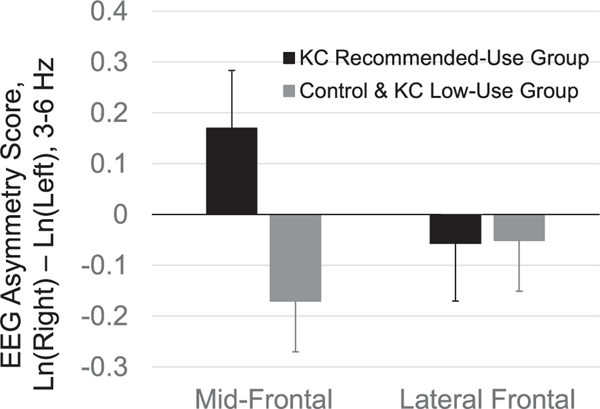
In order to examine the recommended-use KC group, we performed a Group (with 3 groups) by Region analysis on the EEG asymmetry scores. This test yielded a significant interaction effect, F(4,17) = 2.99, p = .049, ɳ2 = .41 however, the low-use KC group contained a minimal number of participants with usable EEG data (N = 3). Notably, the data for the low-use KC group infants showed right mid-frontal asymmetry (M = −.38, SD = .37) like the control group (M = −.13, SD = .19), with the only group exhibiting left frontal EEG asymmetry being the recommended-use KC group (M = .18, SD = .33). A subsequent analysis that combined the low-use KC group with the control group and compared those infants’ EEG asymmetry patterns to the recommended-use KC group yielded a significant effect for the mid-frontal region, using post hoc t-test analysis, t(26) = 1.90, p = .05, one-tailed (Fig. 1). Since multivariate hemisphere effects were non-significant, no follow-up analyses were conducted for EEG power.
Fig. 1.
Frontal EEG asymmetry scores as a function of KC training and use in early infancy.
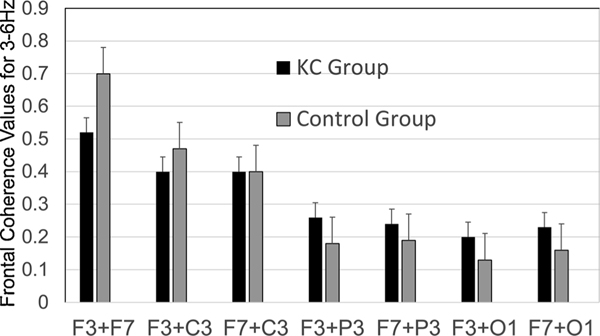
A Group by Regional coherence pair (10 pairs) by Hemisphere analysis demonstrated significant effects for hemisphere for each pair, F(9,15) = 2.78, p = .04, ɳ2 = .63. We also found an overall interaction for Group by Regional coherence pairs utilizing data that was obtained from the frontal region (7 pairs), F(6,138) = 5.31, p = .036, ɳ2 = .18. While there were no significant effects for individual pairs (ps > .05), examination of the means showed higher coherence scores for the control group in close region pairs (F3 + F7; F4 + F8; F3 + C3 and F4 + C4) but frontal regions pairs showed higher coherence values in distant pairs in the KC group (F3 + P3; F7 + P3; F3 + O1, F7 + O1 and F4 + P4; F8 + P4; F4 + O2, F8 + O2). A Group by Regional pair conducted separately for each hemisphere showed a significant effect for the left hemisphere in the frontal region, F(6,144) = 2.75, p = .015, ɳ2 = .10 but not for the right hemisphere, p > .05 (Fig. 2). KC-use groups yield non-significant effects for coherence measures.
Fig. 2.
Frontal coherence values for 3–6 Hz.
For oxytocin analyses, the effect size estimates revealed medium to large effects for oxytocin increases in mother-infant dyads. Negligible and small effect sizes were found at the prenatal (d = .24) and newborn (d = .06) measurements yet this was expected as the KC procedure had not been implemented. At 3-months, the data showed that effect size estimates for oxytocin increases were large, (d = .62 for infants and d = .84 for mothers) in the KC group (relative to the control group) (Table 3). Independent samples t-tests failed to reveal significant differences in mother-infant oxytocin levels between groups.
Table 3.
Effect size estimates using Cohen’s d (small effect = .2, moderate effect = .5, large effect = .8); 95 % confidence intervals in parentheses.
| KC v. CG | KC Recommended-Usev. CG | |
|---|---|---|
| Cortisol Reactivity | −.18 | −.45 |
| (−.56, .93) | (−1.22, .31) | |
| v = .14 | v = .15 | |
| p = .24 | p = .03 | |
| OT prenatal | .24 | .03 |
| (−.47, −.96) | (−.76 .81) | |
| v = .13 | v = .16 | |
| p = .83 | p = .49 | |
| OT 3 mo. mother | .84 | .60 |
| (−.53, 2.20) | (−.35, 1.54) | |
| v = .4834 | v = .232 | |
| p = .43 | p = .17 | |
| OT newborn | .06 | .01 |
| (−.86, .97) | (−.94, .96) | |
| v = .22 | v = .24 | |
| p = .90 | p = .98 | |
| OT 3 mo. infant | .62 | .44 |
| (−.80, 2.03) | (−.55, 1.43) | |
| v = .52 | v = .26 | |
| p = .28 | p = .25 |
Note. KC = kangaroo care group; CG = control group; KC Recommended-Use= group using kangaroo care for recommended amount of time (1 hr. per day for 6 weeks); OT = oxytocin; v =variance of the effect size.
Effect size estimates using Cohen’s d indicated that recommended-use of KC had a moderate effect on lowering infant cortisol reactivity (d=−.45). Comparing the 3 groups, we found a trend for cortisol reactivity across groups, F(2, 24) = 2.74, p = .085. The recommended-use KC group had the lowest average for infant cortisol reactivity (M=−.28 micrograms/deciliter, indicating an average decrease in cortisol from baseline to task) followed by the control group (M = .12 μg/deciliter). An independent samples t-test was conducted to examine differences in cortisol reactivity between the recommended-use KC group and the control group. This test revealed a significant difference between these 2 groups, t(20) = 2.24, p = .036. However, no significant differences were found when comparing the recommended-use and low-use KC group or when comparing the low-use KC group to the control group.
Overall, the analyses indicated that: 1a) infants with recommended-use KC experience showed left frontal EEG asymmetry and; 1b) KC-exposed infants showed significant effects for left frontal (but not for the right hemisphere) EEG coherence pairs in comparison to the control group; 2) KC experience produced moderate to large effects on increasing oxytocin levels in mothers and infants at 3 months postpartum; and 3) KC yielded moderate effects on decreasing cortisol reactivity in infants after exposure to an acute stressor for only the recommended-use KC group.
Part of the novelty of the current study was examining if KC influenced markers of neuro-maturation in healthy full-term infants. Although we did not find differences in infant resting frontal EEG asymmetry (rather KC appeared to impact multiple regions) we did find that frontal asymmetry was influenced by KC use; with the recommended-use KC group associated with left frontal asymmetry versus right frontal asymmetry in the control and low-use KC groups. Prior research supports the current finding demonstrating a link between positive dimensions of maternal caregiving behavior and left hemisphere neurodevelopment (Bernier et al., 2018; Gartstein et al., 2019), with maternal warmth and sensitivity predicting greater regulatory abilities (Frick et al., 2018) and secure attachment (Bigelow et al., 2010).
EEG coherence yielded an overall effect but primarily for the left hemisphere, with higher coherence in nearby leads (frontal-to-central sites) for the control group and increases in coherence across longer distances (frontal-to-parietal; frontal-to-occipital sites) for the KC group. EEG coherence has been rarely studied in infants younger than 5-months-old (Cuevas et al., 2012), however, the findings may suggest that KC influences the generalized functional connectivity between the left frontal region and more distant areas of the infant’s brain. Notably, left frontal brain activation has been associated with more neuro-maturation, approach motivation, and positive emotions (Harmon-Jones, Gable, & Peterson, 2010; Poole & Gable, 2014; Zhu et al., 2011).
Further, oxytocin levels of mother-infant dyads in the KC group were higher than the control group. Researchers have theorized a bio-mechanism exists between KC-use and subsequent oxytocin increase in infants (Feldman, 2007). The current study extends this theory with evidence that KC accounts for increases in oxytocin in mothers and their full-term infants. Kommers et al. (2018) found increases in salivary basal oxytocin after one session of KC. Similarly, our findings indicated that minimal KC-use was associated with higher basal oxytocin level of mothers and infants at 3-months postpartum. Moreover, decreases in cortisol reactivity were found but only for the recommended-use KC group indicating that consistent KC use in early infancy may be associated with enhanced stress regulation.
The findings presented need to be interpreted along with recognition of the limitations of the study. Our sample was small and further studies are needed to address the stability of the physiological outcomes of KC with a larger sample size. Future studies also should employ a more regulated system for monitoring maternal KC use across development as most participants estimated their use of KC rather than using the provided journal.
Due to the lack of studies examining oxytocin in human infant urine (but see White-Traut, Powlesland, Gelhar, Chatterton, & Morris, 1998) and because of the measure’s novelty there exist concerns in reliability, stability and comparability of infant oxytocin levels to adult levels. So while there is a lack of agreement on whether urinary oxytocin levels are comparable to salivary or blood plasma levels (Feldman, Gordon, & Zagoory-Sharon, 2011; Hoffman, Brownley, Hamer, & Bulik, 2012), we chose this method as it is less invasive and previous research has demonstrated oxytocin levels can be detected and analyzed in urine samples (for review see Ziegler, 2018). Finally MacLean et al. (2019) have suggested that the complexity of the hormone discrepancies between measurement methods does not nullify the importance of information garnered from any given method. We agree with this and posit that the current study provides evidence that collecting and subsequently analyzing infant urine for peripheral oxytocin levels is feasible and worthwhile for understanding infant developmental outcomes.
Like urinary oxytocin measures, EEG coherence was also challenging to interpret as few studies have measured coherence in infants at this early age period. While we associated KC with infant EEG, the meaning of its use for impacting neuro-maturation is still elusive. Does higher coherence associated with anterior to posterior sites mean greater neuro-maturation for the left hemisphere for the KC group? Or does greater coherence indicate more or less specificity in patterns of brain development? We could not determine this by our measures.
The current study provides evidence that the physiology of mothers and their full-term infants is influenced by obtaining KC training and utilizing it during the postpartum period. KC is most often provided to preterm infants in order to enhance the development of an immature nervous system; yet, even full-term infants have protracted frontal lobe development. Our findings suggest that full-term infants and their mothers may benefit from the experience of extended KC use. In particular the left frontal area of the brain appears to be stimulated and dyads show increased oxytocin along with decreases in stress reactivity, suggesting regulatory abilities are prompted by experiences with positive caregiving in infancy. Taken together, the findings indicate that KC training and level of use by caregivers during infancy can favorably influence both neurodevelopmental trajectories and infant neurobiological functioning.
Table 2a.
Cortisol levels of infants at 3 month visit.
| KC Recommended-Use | KC Low-Use | Control Group | |
|---|---|---|---|
| Cortisol Pre-task | .60(.48) N = 8 | .60(.55) N = 5 | .84(.42) N = 17 |
| Cortisol Post-task | .73(.44) N = 7 | .38(.32) N = 5 | .70(.30) N = 15 |
| Cortisol Reactivity | −.28(.34) N = 7 | .22(.55) N = 5 | .12(.40) N = 15 |
Note. Method accuracy, determined by spike and recovery, and linearity, determined by serial dilution, are 100.8 % and 91.7 %. Samples from two participants were discarded due to lack of saliva. Samples were assessed for pH and all were found to be within normal range. All but one saliva samples were taken between 10 a.m. and 12:30 p.m.
Acknowledgements
We would like to sincerely thank all the mothers and infants who participated in this study. We would also like to thank Maria Corbett-Dominguez, Ph.D. for her help with the cortisol analysis. A special thanks to Yamile Jackson of Nurtured by Design and Susan Ludington-Hoe for their guidance and help on implementing kangaroo care.Another special thanks to Toni Ziegler at the Assay Services Unit of the University of Wisconsin Primate Research Center, NIH funding: P51OD011106, who helped us with the urinary oxytocin analysis.
Funding
This study was a part of the first author’s dissertation and was funded by a Dissertation Year Award through the FAU Research Enhancement Program awarded to the first author and a CESCOS seed grant and a FAU Division of Research Seed Grant awarded to the second and third authors
Footnotes
CRediT authorship contribution statement
Jillian S. Hardin: Conceptualization, Methodology, Resources, Data curation, Writing - original draft, Writing - review & editing, Funding acquisition, Project administration. Nancy Aaron Jones: Conceptualization, Methodology, Validation, Resources, Writing review & editing, Funding acquisition, Supervision. Krystal D. Mize: Conceptualization, Methodology, Writing - review & editing, Funding acquisition. Melannie Platt: Writing - review & editing, Project administration.
Declaration of Competing Interest
None.
References
- Bell MA, & Fox NA (1996). Crawling experience is related to changes in cortical organization during infancy: Evidence from EEG coherence. Developmental Psychobiology, 29, 551–561. [DOI] [PubMed] [Google Scholar]
- Bernier A, Calkins SD, & Bell MA (2016). Longitudinal associations between the quality of mother-infant interactions and brain development across infancy. Child Development, 87(4), 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Dégeilh F, Leblanc É, Daneault V, Bailey HN, & Beauchamp MH (2018). Mother–Infant interaction and child brain morphology: A multidimensional approach to maternal sensitivity. Infancy Advance online publication. [DOI] [PubMed]
- Bigelow AE, MacLean K, Proctor J, Myatt T, Gillis R, & Power M. (2010). Maternal sensitivity throughout infancy: Continuity and relation to attachment security. Infant Behavior & Development, 33(1), 50–60. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. (2001). Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry, 50(12), 943–951. [DOI] [PubMed] [Google Scholar]
- Cong X, Ludington-Hoe SM, & Walsh S. (2011). Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biological Research in Nursing, 13(2), 204–216. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA, Marcovitch S, & Calkins SD (2012). Electroencephalogram and heart rate measures of working memory at 5 and 10 months of age. Developmental Psychology, 48(4), 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. (2007). Maternal-infant contact and child development: Insights from the kangaroo care intervention. In L’Abate L. (Ed.). Low-cost approaches to promote physical and mental health: Theory, research, and practice (pp. 323–351). New York, NY: Springer Science and Business Media. [Google Scholar]
- Feldman R, & Eidelman AI (2003). Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Developmental Psychobiology, 43, 109–119. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O. (2010). The cross-generation transmission of oxytocin in humans. Hormones and Behavior, 58, 669–676. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O. (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent–Infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–761. 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Singer M, & Zagoory-Sharon O. (2010). Touch attenuates infant’s physiological reactivity to stress. Developmental Science, 13(2), 271–278. [DOI] [PubMed] [Google Scholar]
- Fox NA, & Rutter M. (2010). Introduction to the special section on the effects of early experience on development. Child Development, 81, 23–27. 10.1111/j.1467-8624.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- Frick MA, Forslund T, Fransson M, Johansson M, Bohlin G, & Brocki KC (2018). The role of sustained attention, maternal sensitivity, and infant temperament in the development of early self‐regulation. British Journal of Psychology, 109(2), 277–298. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Hancock GR, Potapova NV, Calkins SD, & Bell MA (2019). Modeling development of frontal electroencephalogram (EEG) asymmetry: Sex differences and links with temperament. Developmental Sciencee 12891. 10.1111/desc.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Modi N, Gianakoulopoulos X, Bond C, Glover V, & Stevenson J. (2002). Acute effects of maternal skin-to-skin contact and massage on salivary cortisol in preterm babies. Journal of Reproductive and Infant Psychology, 20(2), 83–88. [Google Scholar]
- Gray L, Miller LW, Philip BL, & Blass EM (2002). Breastfeeding is analgesic in healthy newborns. Pediatrics, 109(4), 590–593. [DOI] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K. (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. [DOI] [PubMed] [Google Scholar]
- Hammock EAD (2015). Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology Reviews, 40, 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Henderson HA, Reeb-Sutherland BC, & Fox NA (2010). Ordinary variations in human maternal caregiving in infancy and biobehavioural development in early childhood: a follow-up study. Developmental Psychobiology, 52, 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, & Peterson CK (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology, 84(3), 451–462. 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, & Van Essen D. (2010). Similar patterns of cortical expansion during human development and evolution. Proceedings of the National Academy of Sciences, 107(29), 13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman ER, Brownley KA, Hamer RM, & Bulik CM (2012). Plasma, salivary, and urinary oxytocin in anorexia nervosa: A pilot study. Eating Behaviors, 13(3), 256–259. 10.1016/j.eatbeh.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, & Davalos M. (1997). EEG activation in 1-month-old infants of depressed mothers. Development and Psychopathology, 9, 491–505 PMID: 9327235. [DOI] [PubMed] [Google Scholar]
- Jones NA, McFall BA, & Diego MA (2004). Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: The mediating role of infant temperament. Biological Psychology, 67, 103–124. [DOI] [PubMed] [Google Scholar]
- Jones NA, & Sloan A. (2018). Neuro-hormones and temperament interact during infant development. Philosophical Transactions Biological Sciences, 18(54), 10.1098/rstb.2017.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffashi F, Scher MS, Ludington-Hoe SM, & Loparo KA (2013). An analysis of kangaroo care using neonatal EEG complexity: A preliminary study. Clinical Neurophysiology, 124, 238–246. [DOI] [PubMed] [Google Scholar]
- Kommers D, Broeren M, Oei G, Feijs L, Andriessen P, & Bambang Oetomo S. (2018). Oxytocin levels in the saliva of preterm infant twins during Kangaroo Care. Biological Psychology, 137, 18–23. [DOI] [PubMed] [Google Scholar]
- Ludington-Hoe SM (2008). A clinical guideline for implementation of Kangaroo Care with premature infants of 30 or more weeks’ postmenstrual age. Advances in Neonatal Care, 8(3), s3–s23.18818541 [Google Scholar]
- Ludington-Hoe SM (2012). United States Institute for Kangaroo Care, 2012 Annual National Intensive Certified Kangaroo Caregiver Course, Cleveland, Ohio. [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, & Carter CS (2019). Challenges for measuring oxytocin: The blind man and the elephant? Psychoneuroendocrinology, 107, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi N, & Glover V. (1998). Non-pharmacological reduction of hypercortisolaemia in preterm infants. Infant Behavior & Development, 21, 86. [Google Scholar]
- Poole BD, & Gable PA (2014). Affective motivational direction drives asymmetric frontal hemisphere activation. Experimental Brain Research, 232(7), 2121–2130. 10.1007/s00221-014-3902-4. [DOI] [PubMed] [Google Scholar]
- Scher MS, Ludington-Hoe SM, Kaffashi F, Johnson MW, Holditch-Davis D, & Loparo KA (2009). Neurophysiologic assessment of brain maturation after an eight week trial of skin-to-skin contact on preterm infants. Journal of Clinical Neurophysiology, 120(10), 1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, & Pollak SD (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society of London Series B, Biological Sciences, 277(1694), 2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter C, & Jain A. (1996). Psychophysiological correlates of infant temperament: Stability of behavior and autonomic patterning from 5 to 18 months. Developmental Psychobiology, 29(4), 379–391. [DOI] [PubMed] [Google Scholar]
- Swinth JY, Anderson GC, & Hadeed AJ (2003). Kangaroo Care with a preterm infant: Before, during and after mechanical ventilation. Neonatal Network, 22(6), 33–46. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. (2003). The Oxytocin Factor. Tapping the hormone of calm, love, and healing. Cambridge, MA: Da Capo Press. [Google Scholar]
- White-Traut R, Powlesland J, Gelhar D, Chatterton R, & Morris M. (1998). Methodologic issues in the measurement of oxytocin in human neonates. Journal of Nursing Measurement, 6(2), 155–174. [PubMed] [Google Scholar]
- Zhu C, Guo X, Jin Z, Sun J, Qui Y, Zhu Y, et al. (2011). Influences on brain development and ageing on cortical interactive networks. Clinical Neurophysiology, 122, 278–293. [DOI] [PubMed] [Google Scholar]
- Ziegler TE (2018). Measuring peripheral oxytocin and vasopressin in nonhuman primates. American Journal of Primatology, 80, e22871. 10.1002/ajp.22871. [DOI] [PubMed] [Google Scholar]